Abstract
The ability of poliovirus that was irradiated with UV light at energies up to 2,160 ergs/mm2 to subsequently inhibit host cell protein synthesis was measured. The inactivation of the host cell shutoff function followed one-hit kinetics. Increasing irradiation did not affect the rate of inhibition until the multiplicity of infection after irradiation was reduced to approximately 1 PFU/cell. At higher functional multiplicities, the rate was unchanged, but an increasing lag before the onset of inhibition was observed with increasing irradiation. The energy levels required to inactivate virus-induced inhibition of host cell protein synthesis suggest that damage to virus RNA rather than to virus capsid proteins is responsible for the loss of function. When the inactivation of host cell shutoff was compared with the inactivation of other viral functions by UV irradiation, it correlated exactly with the loss of infectivity but not with other viral functions measured. Guanidine treatment, which prevents detectable viral RNA and protein synthesis, completely inhibited host cell shutoff by low multiplicities of unirradiated virus infection but not higher multiplicities. When a high multiplicity of virus was first reduced to a low titer by irradiation, host cell shutoff was still evident in the presence of guanidine. The results demonstrate that the complete inhibition of host cell protein synthesis can be accomplished by one infectious viral genome per cell.
Full text
PDF

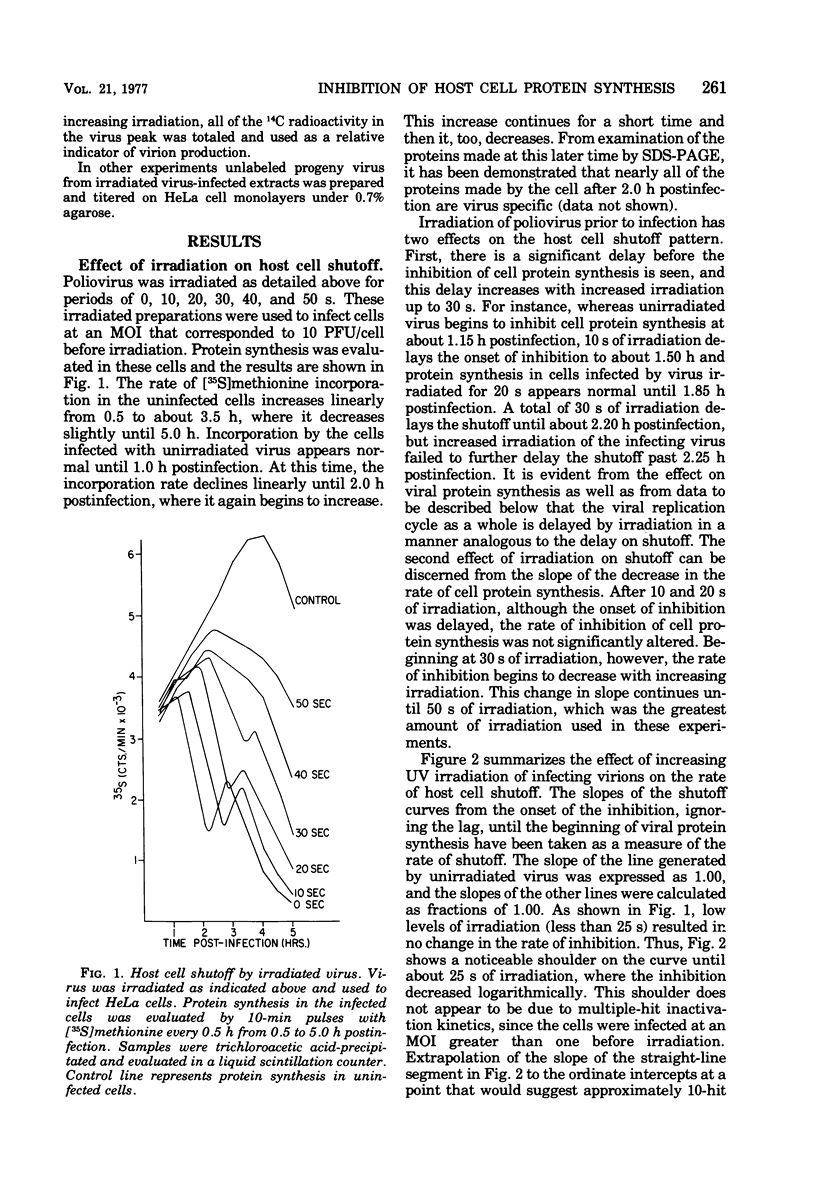
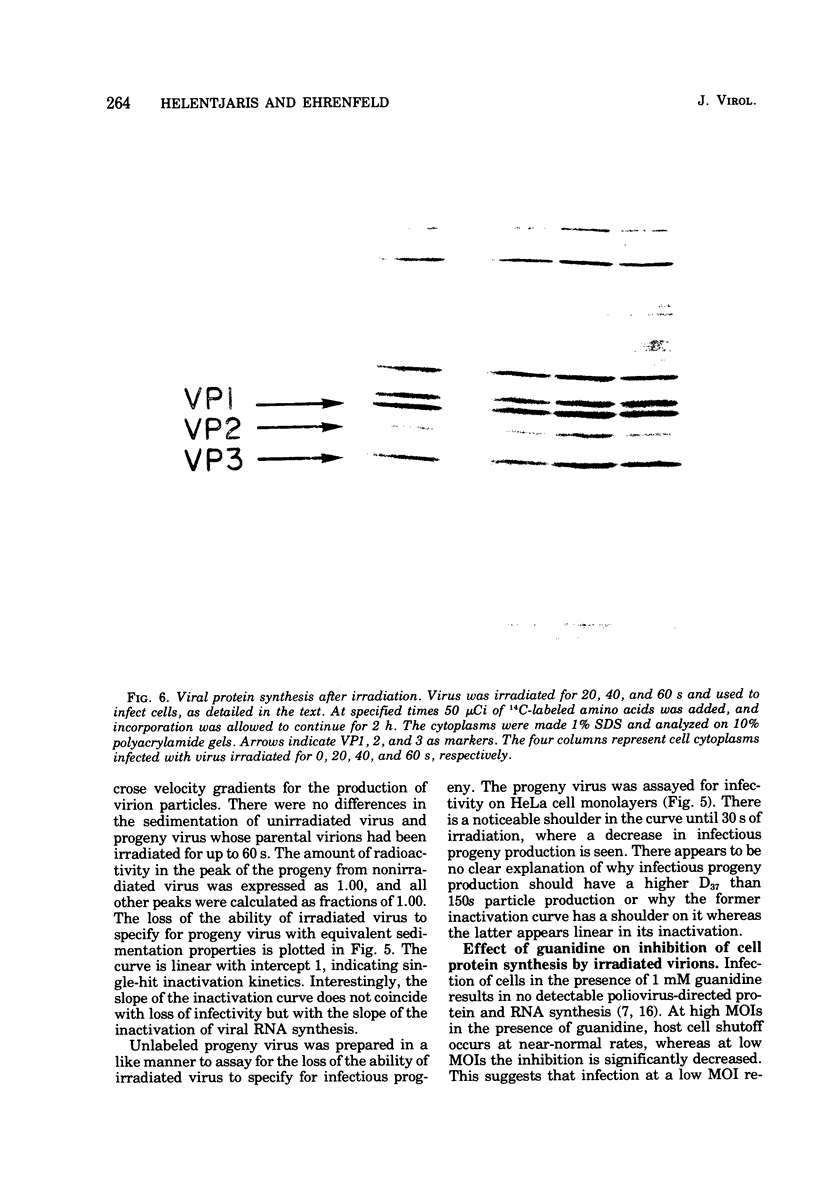
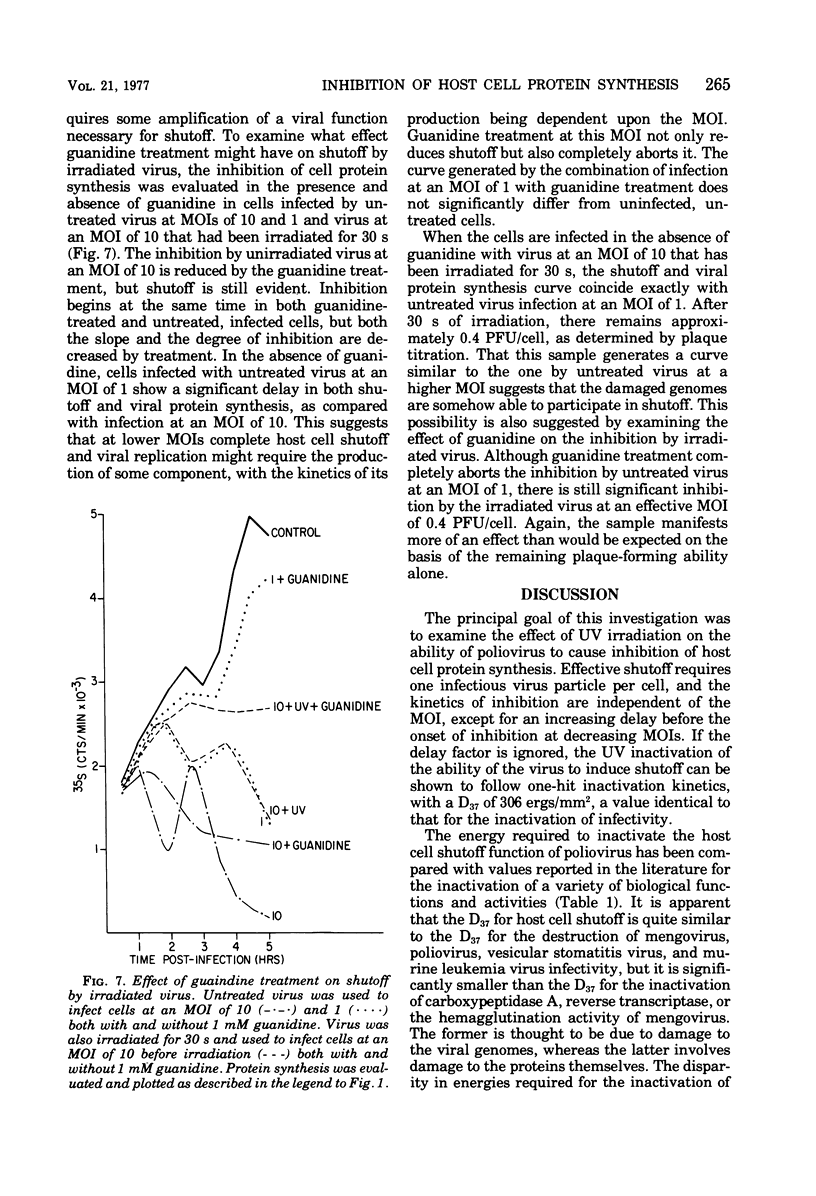


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALTIMORE D., FRANKLIN R. M., CALLENDER J. MENGOVIRUS-INDUCED INHIBITION OF HOST RIBONUCLEIC ACID AND PROTEIN SYNTHESIS. Biochim Biophys Acta. 1963 Nov 22;76:425–430. [PubMed] [Google Scholar]
- Bablanian R. Depression of macromolecular synthesis in cells infected with guanidine-dependent poliovirus under restrictive conditions. Virology. 1972 Jan;47(1):255–259. doi: 10.1016/0042-6822(72)90260-7. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. II. Nature of the defect. J Mol Biol. 1973 May 25;76(3):325–343. doi: 10.1016/0022-2836(73)90508-1. [DOI] [PubMed] [Google Scholar]
- Collins F. D., Roberts W. K. Mechanism of Mengo virus-induced cell injury in L cells: use of inhibitors of protein synthesis to dissociate virus-specific events. J Virol. 1972 Nov;10(5):969–978. doi: 10.1128/jvi.10.5.969-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN R. M., BALTIMORE D. Patterns of macromolecular synthesis in normal and virus-infected mammalian cells. Cold Spring Harb Symp Quant Biol. 1962;27:175–198. doi: 10.1101/sqb.1962.027.001.019. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. INHIBITION OF HOST CELL MACROMOLECULAR SYNTHESIS BY HIGH MULTIPLICITIES OF POLIOVIRUS UNDER CONDITIONS PREVENTING VIRUS SYNTHESIS. J Mol Biol. 1964 Apr;8:574–581. doi: 10.1016/s0022-2836(64)80012-7. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Levy H., Baron S., Kasel J. A. Mengovirus-induced cytopathic effect in L-cells: protective effect of interferon. J Virol. 1969 Oct;4(4):490–495. doi: 10.1128/jvi.4.4.490-495.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri S., Hinuma Y., Ishida N. Relation between the adsorption to cells and antigenic properties in poliovirus particles. Virology. 1968 Apr;34(4):797–799. doi: 10.1016/0042-6822(68)90101-3. [DOI] [PubMed] [Google Scholar]
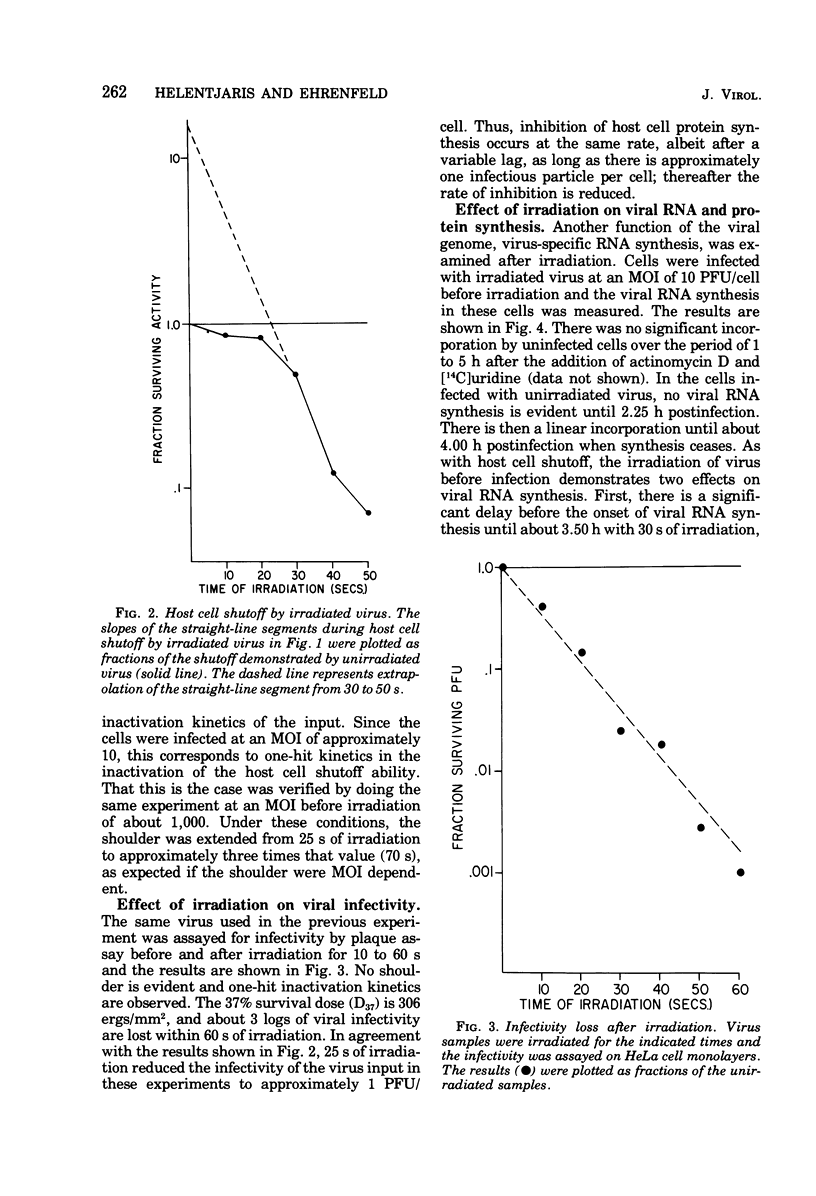
- Lawrence C., Thach R. E. Encephalomyocarditis virus infection of mouse plasmacytoma cells. I. Inhibition of cellular protein synthesis. J Virol. 1974 Sep;14(3):598–610. doi: 10.1128/jvi.14.3.598-610.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger G. G., Ling H. P., Gilden R. V., Hatanaka M. Effect of UV Light on RNA-Directed DNA Polymerase Activity of Murine Oncornaviruses. J Virol. 1975 May;15(5):1273–1275. doi: 10.1128/jvi.15.5.1273-1275.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus P. I., Sekellick M. J. Cell killing by viruses. II. Cell killing by vesicular stomatitis virus: a requirement for virion-derived transcription. Virology. 1975 Jan;63(1):176–190. doi: 10.1016/0042-6822(75)90383-9. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Plagemann P. G. Effect of ultraviolet light on mengovirus: formation of uracil dimers, instability and degradation of capsid, and covalent linkage of protein to viral RNA. J Virol. 1974 Mar;13(3):729–739. doi: 10.1128/jvi.13.3.729-739.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Inhibition of HeLa cell protein synthesis by the vaccinia virion. J Virol. 1968 Oct;2(10):1028–1037. doi: 10.1128/jvi.2.10.1028-1037.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Summers D. Effects on host cell metabolism following synchronous infection with poliovirus. Virology. 1965 Dec;27(4):614–620. doi: 10.1016/0042-6822(65)90187-x. [DOI] [PubMed] [Google Scholar]
- Piras R., Vallee B. L. Carboxypeptidase A. Quantum yields on ultraviolet irradiation. Biochemistry. 1967 Aug;6(8):2269–2272. doi: 10.1021/bi00860a001. [DOI] [PubMed] [Google Scholar]
- Racevskis J., Kerwar S. S., Koch G. Inhibition of protein synthesis in reticulocyte lysates by poliovirus. J Gen Virol. 1976 Apr;31(1):135–138. doi: 10.1099/0022-1317-31-1-135. [DOI] [PubMed] [Google Scholar]
- Shaw J. E., Cox D. C. Early inhibition of cellular DNA synthesis by high multiplicities of infectious and UV-inactivated Reovirus. J Virol. 1973 Oct;12(4):704–710. doi: 10.1128/jvi.12.4.704-710.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner-Pryor A., Cooper P. D. Temperature-sensitive poliovirus mutants defective in repression of host protein synthesis are also defective in structural protein. J Gen Virol. 1973 Nov;21(2):215–225. doi: 10.1099/0022-1317-21-2-215. [DOI] [PubMed] [Google Scholar]