Abstract
To better study the role of genetics in autism, mouse models have been developed which mimic the genetics of specific autism spectrum and related disorders. These models have facilitated research on the role genetic susceptibility factors in the pathogenesis of autism in the absence of environmental factors. Inbred mouse strains have been similarly studied to assess the role of environmental agents on neurodevelopment, typically without the complications of genetic heterogeneity of the human population. What has not been as actively pursued, however, is the methodical study of the interaction between these factors (e.g., gene and environmental interactions in neurodevelopment). This review suggests that a genetic predisposition paired with exposure to environmental toxicants play an important role in the etiology of neurodevelopmental disorders including autism, and may contribute to the largely unexplained rise in the number of children diagnosed with autism worldwide. Specifically, descriptions of the major mouse models of autism and toxic mechanisms of prevalent environmental chemicals are provided followed by a discussion of current and future research strategies to evaluate the role of gene and environment interactions in neurodevelopmental disorders.
Keywords: Autism, Mouse Model, Genetics, Environmental Pollutants
1. Introduction
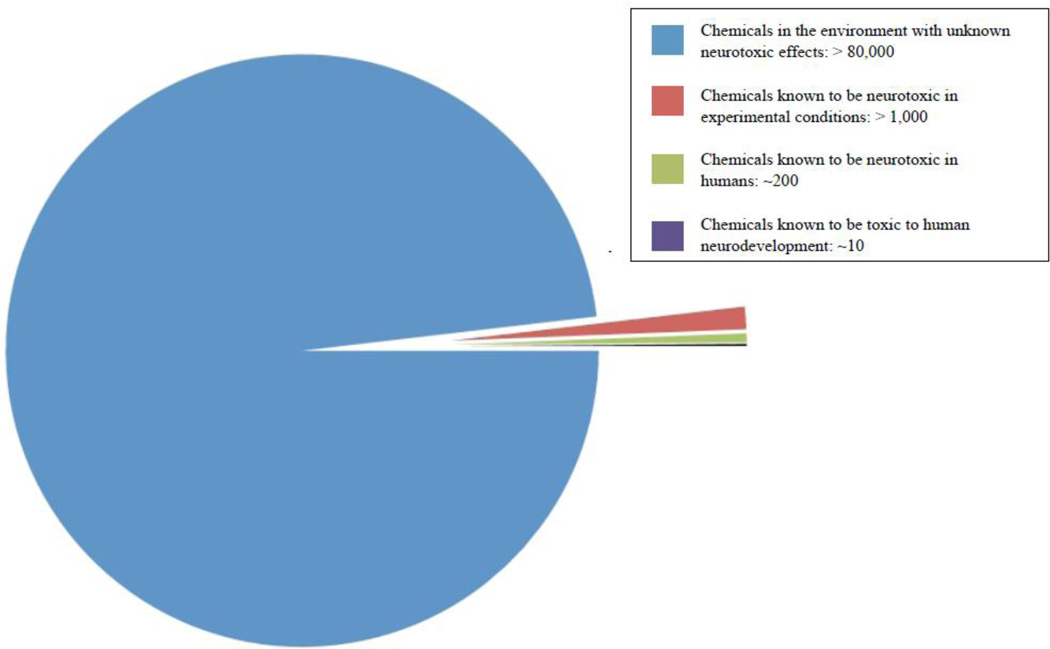
Advancement of the chemical industry in the 20th century has made exposure to hazardous chemicals and pollutants in the environment inevitable. An estimated 70,000 to 100,000 chemicals are currently registered for commercial use and information regarding their potential adverse effects on brain development is only available for a small fraction of these chemicals. Of the select chemicals that have been identified only 10 have been confirmed to be neurotoxic for human development, with an additional 200 compounds possessing neurotoxic properties, and more than 1,000 other compounds suspected to also disrupt central nervous system development based on experimental findings (Figure 1) (Grandjean and Landrigan 2006). This is particularly concerning given the continuing rise in reported cases of neurodevelopmental disorders (Schieve, Baio et al. 2010).
Figure 1.
Current knowledge of neurotoxic chemicals. Of the 80,000 plus registered chemicals known to exist in the environment, only a fraction have been evaluated for neurotoxicity, and even fewer for neurodevelopmental toxicity in humans. Although the diagram does not represent the true neurodevelopmental potential of these chemicals, it does indicate the magnitude of the environmental threat to human health and the need for continued research.
In many cases, environmental effects on brain development are preventable if vulnerable segments of the population can be identified. However, development of effective treatment strategies is only possible after the mechanisms of action of a toxicant have been elucidated. This is particularly challenging given that many neurotoxic effects often present subclinically (Grandjean and Landrigan 2006), with only a subset of exposed individuals presenting with a clinically defined developmental disorder. One plausible explanation for this discrepancy is the underlying genetic variability that may predispose one individual to a developmental disorder while providing resistance to vulnerability in another. Indeed developmental disorders such as attention deficit hyperactive disorder (ADHD) and autism spectrum disorder (ASD) have strong genetic links, despite the absence of any single gene that can account for the majority of cases. Given that at present neither environmental influences nor genetic background can independently explain the cause of neurodevelopmental disorders, it is hypothesized that the effects of environmental toxicants on development are interacting with a genetic predisposition to toxicant vulnerability.
This possibility complicates our ability to identify environmental agents that may be neurotoxic to human development because while many individuals exposed may appear typically developing, only a subset shows abnormal development because of an underlying genetic susceptibility. In order to efficiently identify potential gene-environment interactions that contribute to disease states, animal models can be used to systematically test what gene-environment combinations produce the greatest neurobehavioral deficits. However, to date the neurotoxic effects of environmental compounds and contribution of candidate genes to disease states have typically been tested independently with minimal attempts to identify how these two factors may work synergistically to disrupt CNS development. Therefore, future neurotoxicological research in animals must consider how genetic susceptibility might exacerbate the effects of environmental pollutants to produce measureable deficits in behavioral development.
To this end, the current review will focus on several classes of compounds that are persistent in the environment and are known to possess neurotoxic effects that can disrupt central nervous system development, with a focus on ASD. First, an overview of several animal models of neurodevelopmental disorders is presented along with potential mechanisms by which genes and environmental insults may converge to produce or exacerbate the behavioral phenotypes associated with developmental disorders. Then, we survey several classes of toxic compounds that are persistent in the environment, with a focus on how their mechanism of action may work in concert with genetic factors to contribute to the development of neurobehavioral disorders. Finally we discuss rational research strategies using animal models of neurodevelopmental disorders to understand neurotoxic gene-environment interactions.
2. Mouse Models
2.1 Defining Autism Spectrum Disorders
ASD are characterized by three core behavioral deficits; poor social interaction, impaired language and communication, and restrictive/repetitive behaviors (American Psychological Association 2006). These disorders are highly heritable suggesting a strong genetic component in their etiologies. However, despite this high heritability, no single gene or common genetic variants have been identified to explain the majority of ASD cases. In fact, only an estimated 5–15% can be accounted for by a known genetic defect and possessing the genotype does not always result in an ASD diagnosis (Martin and Ledbetter 2007; Caglayan 2010). This paradox has led to the notion that environmental influences, such as exposures to chemicals and pollutants, play a critical role in the development of ASD and necessitated the investigation of the effects of toxicant exposure on brain and behavior development.
Mouse models have become important tools for understanding cellular and molecular processes underlying normal brain development, as well as how genetic variation (e.g., polymorphisms, mutations, deletions and copy number variants) and exposures to toxic agents can alter development of the central nervous system (CNS) leading to neurodevelopmental disorders. By using behavioral assays in mice with face and construct validity to the behavioral deficits of ASD in humans, researchers have the benefit over prospective human studies of being able to investigate causal links between genetic or environmental insult and ASD (Silverman, Yang et al. 2010). Several mouse models are now available for elucidating the role specific gene mutations play in the etiology of some ASD. While an extensive examination of every animal model available to date is beyond the scope of this review, description of the more widely used models is presented with the goal of providing a good understanding of the types of models and behavioral measures used to study neurodevelopmental disorders.
2.2 Fragile-X Syndrome
Fragile X syndrome (FXS) is a genetic disorder resulting from large expansions of a CGG trinucleotide repeat (i.e. greater than 200) in a non-coding region of the FMR1 gene. This results in silencing of gene expression due to DNA hypermethylation and a subsequent loss of the protein product Fragile-X mental retardation protein (FMRP). FMRP is an RNA-binding protein that regulates transcription of a large number of proteins as well as protein trafficking at the synapse (Oberle, Rousseau et al. 1991; Pieretti, Zhang et al. 1991; Verkerk, Pieretti et al. 1991). The absence of FMRP results in abnormal brain development and mental retardation seen in FXS. Approximately 30% of males with FXS meet the criteria for ASD with several studies reporting prevalence ranging between 15–60% (Hatton, Sideris et al. 2006; Hunter, Rohr et al. 2010; Budimirovic and Kaufmann 2011). In many of these cases, children with FXS show mild features of autism, including deficits in play-based social behavior, poor eye contact, and preservation of speech and behavior, but with additional anxiety-like behaviors that complicate diagnosis (Hagerman 2002; Moss and Howlin 2009).
The most common mouse model of FXS is an Fmr1 knockout mouse (Fmr1KO) (Bakker, C. et al. 1994). These mice recapitulate the loss of FMRP function characteristic of FXS and have been well-characterized across numerous behavioral assays. These mice show considerable variation in phenotype that is dependent on the background mouse strain (Baker, Wray et al. 2010; Spencer, Alekseyenko et al. 2011). For example Fmr1KO mice bred from a C57Bl/6J line show increased social behavior compared to wild-type controls while those on a DBA/2J line show markedly reduced social interactions (Spencer, Graham et al. 2008; Spencer, Alekseyenko et al. 2011). The discrepancies across background strains elegantly model the variability in expression of social behavior deficits reported in the clinical population (Hessl, Dyer-Friedman et al. 2001; Hagerman 2002), and demonstrate the importance of considering how both genetic (e.g. background strain in mice and family history in humans) and environmental factors may contribute to the autism-like phenotype.
An alternative approach to studying Fmr1 function in mice stems from the development of the CGG knock-in (CGG KI) mouse where the native mouse CGG 8 repeat was replaced on Fmr1 by homologous recombination with an expanded 98 CGG repeat of human origin (Bontekoe, Bakker et al. 2001; Willemsen, Hoogeveen-Westerveld et al. 2003). Surprisingly, when these mice were bred for even longer CGG repeat expansions the gene was not hypermethylated and silenced, limiting their use as a model of FXS syndrome. However, these CGG KI mice do closely model Fragile-X premutation carriers possessing CGG expansions ranging from 55 and 200 repeats. These premutation carriers show a variety of molecular and neurological signs that are well-modeled in the CGG KI mouse, including elevated Fmr1 mRNA, somewhat reduced levels of FMRP, anxiety-like behaviors, impaired memory, and motor deficits that closely model the neurodegenerative disorder Fragile-X Associated Ataxia/Tremor Syndrome (FXTAS; for review see (Hunsaker, Arque et al. 2012)). CGG KI mice display normal social approach and social recognition behaviors that remain intact at older ages when other neuropathological markers are apparent (unpublished data). Interestingly, a case study of a small number of individuals with the Fragile X premutation living near toxic waste sites were reported to show an acceleration in the disease process, suggesting a gene-environment interaction (Paul, Pessah et al. 2010). This interesting possibility has not yet been tested in the CGG KI mouse but such studies are clearly needed.
The development of the Fmr1KO and CGG KI mouse has allowed for considerable advances in our understanding of the importance of FMRP in the pathogenesis of FXS and more generally in ASD. Using the Fmr1 null mouse, Huber et al. (Huber, Gallagher et al. 2002) identified enhanced long-term potentiation resulting from altered regulation of group 1 metabotropic glutamate receptors, leading to the development of the mGluR theory of Fragile-X (Bear, Huber et al. 2004). These discoveries have made way for novel therapeutics that have shown considerable efficacy in restoring function across a wide range of behaviors in the mouse (Bilousova, Dansie et al. 2009; Heulens, D'Hulst et al. 2012; Rotschafer, Trujillo et al. 2012; Thomas, Bui et al. 2012). For example, the combination of the anticonvulstant 2-methyl-6-phenylehynyl-pyridine (MPEP), an mGluR5 receptor antagonist, and GABAA receptor agonists have been proposed as a target treatment to restore the imbalance of excitatory and inhibitory inputs resulting from FMR1 mutation (Hagerman, Lauterborn et al. 2012). Additionally, the tetracycline analogue minocycline has shown promise in normalizing dendritic spine maturation in mice (Qin, Entezam et al. 2011) and clinical trials are currently underway (Utari, Chonchaiya et al. 2010).
2.3 Rett Syndrome
Rett Syndrome is a neurodevelopment disorder seen predominantly in females and characterized by cognitive impairments, poor expressive and receptive language and stereotypic hand movements (Rett 1968; Hagberg, Aicardi et al. 1983). Children with Rett syndrome show normal early development followed by a regressive period beginning between 6 to 18 months of age. During this period, autism-like features emerge including poor socialization and limited eye-contact. However, these features are transient, lasting months to years with eye gaze and social interaction returning by school age (PE, R et al. 2007). The majority of cases (i.e. greater than 95%) result from mutations in the methyl-CpG-binding protein 2 (MeCP2) gene located at Xq28. The MeCP2 gene encodes a transcriptional factor expressed ubiquitously across mammalian tissue with high concentrations found in the developing brain (Bienvenu and Chelly 2006). Mutations of the MeCP2 gene are generally sporadic (de novo) and include missense mutations, nonsense mutations and entire exon deletions (Percy 2011). Numerous mutant mouse models have been developed with relatively high face validity for the behavioral signs associated with Rett Syndrome.
Early attempts to delete MeCP2 resulted in embryonic lethality (Tate, Skarnes et al. 1996), which led to the development of more viable knockout mice containing deletions of exons 3 and 4 (Chen, Akbarian et al. 2001; Guy, Hendrich et al. 2001). More recent models of Rett syndrome include truncated forms of the gene (e.g., Mecp2308 mutation) and MeCP2 knock-in mice carrying Rett-associated mutations from patients (Calfa, Percy et al. 2011). Many of these mouse models show a similar delayed progression of behavioral deficits to Rett Syndrome, emphasizing the importance of the MeCP2 protein in normal brain development and function. For example, the Mecp2tm1.1Bird, Mecp2tm1Tam, and Mecp2tm1.1Jae mice develop normally with no behavioral abnormalities present until the 4th or 5th week of age at which time the mice display unusual gait, labored breathing, tremors, seizures, learning and memory deficits, low social interaction, and increased anxiety-like behaviors (Chen, Akbarian et al. 2001; Guy, Hendrich et al. 2001; Pelka, Watson et al. 2006). It is important to note that most mouse models of Rett syndrome use hemizygous male mice to study behavioral and neurological alterations despite Rett syndrome diagnosed predominantly in females. This is due in part to the wide variability and late onset of the behavioral phenotype reported in female mice (For review, see (Calfa, Percy et al. 2011)). Unfortunately, this limitation substantially undercuts the face validity of these mouse models.
The late onset of behavioral deficits reported in Rett Syndrome mice is consistent with the regressive nature of the disorder in humans leading to the hypothesis that MeCP2 function is activity-dependent. Support for this notion stems from the recent development of a mutant mouse in which the MeCP2 protein cannot be phosphorylated (MeCP2 S421) resulting in a loss of activity-dependent regulation of MeCP2 (Cohen, Gabel et al. 2011). These mice show relatively mild impairments in nervous system development (i.e. normal motor function) and are indistinguishable from wild-type littermates with regards to body weight regulation. However, MeCP2 S421 mice fail tests of sociability and novelty recognition demonstrating an activity-independent role for MeCP2 (Cohen, Gabel et al. 2011). Given that MeCP2 alters DNA methylation and more recent findings implicating a role for MeCP2 in activity-dependent synapse regulation, it is important to consider how environmental insults known to disrupt normal epigenetic function may interact with MeCP2 to further exacerbate the behavioral and neurological phenotype associated with Rett syndrome. This possibility appears likely in view of a recent study demonstrating behavioral and epigenetic effects of prenatal and perinatal exposure to the flame retardant polybrominated diphenyl ether 47 (PBDE-47) in the Mecp2308 mouse model of Rett syndrome (Woods, Vallero et al. 2012). Specifically, global hypomethylation of adult brain DNA was found in female offspring of Mecp2308 dams perinatally exposed to PBDE-47, and this coincided with reduced social behaviors and impaired spatial memory. These results suggest that individuals with Rett syndrome may be at increased risk from exposure to environmental toxicants such as PBDE-47.
2.4 Copy-number variants, Ube3a, and the GABAA β3 subunit
In addition to single gene mutations, ASD can be associated with gains, losses, or rearrangement of whole DNA segments. These DNA mutations, termed copy-number variations (CNV), can result in functional loss of multiple genes (i.e. deletion), a toxic gain of function (i.e. duplication), or dysregulation by silencing expression of nearby genes through translocation (Lupski and Stankiewicz 2005). CNVs have been linked to myriad of neuropsychiatric disorders, of which several meet criteria for an ASD (Cook and Scherer 2008). By identifying regions of the human genome susceptible to CNVs, research has uncovered several gene candidates that may contribute independently and in combination to the etiology of ASD. Of particular interest are gene candidates on chromosome 15q11–13 (Vorstman, Staal et al. 2006). Maternal duplication of 15q11–13 accounts for approximately 1–3% if individuals with ASD (Cook, Lindgren et al. 1997) while deletion of this region results in Angelman Syndrome and Prader-Willi Syndrome, both of which show behavioral phenotypes with similarities to ASD. By identifying several proteins and molecular pathways encoded on 15q11–13, numerous genetic mouse models have been developed in an attempt to understand how each of the genes individually and in concert contributes to the etiology of ASD.
Histopathological analysis of brains of ASD patients has suggested an imbalance between excitatory and inhibitory transmission which has led to the notion that the major inhibitory neurotransmitter system gamma-aminobutyric acid (GABA) may be dysregulated in ASD (for example see (Dhossche, Applegate et al. 2002; Fatemi, Halt et al. 2002; Blatt 2005)). Interestingly, a large region of chromosome 15q11–13 encodes for various subunits of the GABAA receptor. For example, the β3 subunit of the GABAA receptor has been implicated in ASD (Buxbaum, Silverman et al. 2002). Mice with a targeted deletion of gabrβ3, the gene encoding the β3 subunit (gabrb3−/−), have a high early mortality rate with only 10% of the pups surviving to adulthood. Those that survive are characterized by high frequency of seizures, learning and memory deficits, hyperactivity, and poor motor skills (Homanics, DeLorey et al. 1997; DeLorey, Handforth et al. 1998). Tests of sociability reveal that gabrβ3 knockout mice fail tests of social recognition (DeLorey, Sahbaie et al. 2008).
Another gene that has come under considerable scrutiny is the maternally derived ubiquitin-protein ligase E3A (Ube3a) located on 15q11–13. Because the paternal copy of the Ube3a is normally silenced due to imprinting, only deletion of the maternal copy of the gene is necessary to induce behavioral and brain developmental deficits (Jiang, Tsai et al. 1998). A loss of Ube3a function is associated with Angelman syndrome (Jiang, Tsai et al. 1998), a developmental disorder with clinical criteria similar to that of autism. Specifically, intellectual disabilities, poor language development, and restrictive repetitive behaviors underscore many of the core behavioral deficits in both disorders (Walz 2007). This similarity has warranted the development of Ube3a knockout mice in an attempt to better understand the neurobiological underpinnings of ASD. These mice express many of the behavioral deficits observed in individuals with ASD including motor dysfunction, increased seizure susceptibility, and learning deficits (Jiang, Tsai et al. 1998). Investigations of a mechanistic role for Ube3a in the etiology of ASD-like behaviors point to a dysregulation of synaptic plasticity (Scheiffele and Beg 2010). Specifically, Ube3a binds to Ephexin-5 and Arc, two proteins responsible for regulating the number and plasticity of synapses, respectively (Greer, Hanayama et al. 2010; Margolis, Salogiannis et al. 2010). Interestingly, dysregulation of both Ube3a and Gabrβ3 may have additive effects on communication impairments as mice with deletions spanning this entire region show altered ultrasonic vocalizations (Jiang, Pan et al. 2010). The finding that the combined expression of these genes further confers similarities to the behavioral deficits of ASD demonstrates the importance of multi-gene interactions resulting in the behavioral phenotype of ASD. Attempts to model the dysregulation of polygenic expression on human chromosome 15q11–13 has led to the development of a mouse model syntenic to a loss of 15q.
The region of chromosome 15q11–13 shows a high degree of imprinting with CNV duplications often resulting in an ASD diagnosis (Nicholls and Knepper 2001). To investigate how maternal verses paternal silencing of genes differentially regulates the etiology of ASD-like behaviors, a mouse model has been developed with either maternal or paternal duplication of mouse chromosome 7, a region equivalent to 15q in the human (Nakatani, Tamada et al. 2009). Mice expressing a paternally derived duplication (PatD/+) show deficits in all three behavioral domains modeling autism (i.e. deficits in social approach, unusual ultrasonic calling, and increased resistance to change or perseverative behaviors) (Nakatani, Tamada et al. 2009). Conversely, mice with a maternally derived duplication (MatD/+) show normal behavioral patterns compared to wild type controls (Nakatani, Tamada et al. 2009). This model is inconsistent with clinical cases in which maternal duplication accounts for high prevalence of ASD (Cook, Lindgren et al. 1997). Specifically, patients with a maternally derived duplication of chromosome 15q11–13 show a high prevalence of ASD (>85%), whereas those with a paternally derived duplications exhibit behaviors ranging from no effect to mild developmental and cognitive impairment, and rarely meet the criteria for ASD (Cook, Lindgren et al. 1997; Veenstra-Vanderweele, Christian et al. 2004; Abrahams and Geschwind 2008; Cook and Scherer 2008). While the mechanism for this discrepancy is not yet well understood, Takumi suggests that differences in mouse imprinting regions may account for some of the differences in behavioral responding between mice and humans (Takumi 2011). It is important to note that recent analysis of the mouse epigenome demonstrated differential expression of imprinting of both Ube3a and GABAA receptor subunits across regions of the mouse brain (Gregg, Zhang et al. 2010) suggesting a more complex interaction between gene expression and epigenetic modification. These discrepancies notwithstanding, the highly variable expression of ASD-like behaviors depending on the epigenetic expression of each gene speaks to the importance of examining how epigenetic modifying agents in the environment contribute to the etiology of ASD. Such agents would include, but are not limited to, histone deacetylase inhibitors (HDACi), including the anticancer drug Triclostatin A, and valproic acid, a well-known mood stabilizer and antiepileptic drug, both of which have been demonstrated to alter social-cognition in animal models (Foley, Gannon et al. 2012). Other classes of drugs that should be evaluated for toxicity, and would be particularly interesting to examine in models of Rett syndrome, include modulators of DNA methylation currently in development for treatment of various forms of cancer and autoimmune disease (Szyf 2009).
2.5 Synapse specific proteins
ASD are usually diagnosed before the age of three – a time of dramatic synapse formation, maturation and pruning. This time course of onset suggests ASD may reflect a synaptic disorder and has led to the hypothesis that ASD stems from disruptions in experience dependent synapse plasticity (Zoghbi 2003). While there are many areas of support for this notion, including reports of imbalanced excitation/inhibition across models of ASD (Gogolla, Leblanc et al. 2009), the most striking evidence stems from patients with a familial genetic variant in synapse-specific proteins. For example, cell-adhesion molecules are important for proper formation and maturation of the synapse and are associated with the pathophysiology of ASD (Ye, Liu et al. 2010). Interestingly, a small percentage of patients with an ASD show monogeneic, heritable variants of cell-adhesion molecules that are causal contributors to their behavioral abnormalities (Szatmari, Paterson et al. 2007). These proteins include two forms of the Neuroligin proteins, neurligin-3 and 4 (Jamain, Quach et al. 2003), the presynaptic binding proteins Neurexins (Feng, Schroer et al. 2006), and synapse scaffolding proteins such as SHANK3 (Gauthier, Spiegelman et al. 2009). By using murine genetics, the role of many cell-adhesion molecules in brain and behavior regulation has been examined by systematically knocking out each gene.
Neuroligins are a family of transmembrane adhesion molecules with de novo gene mutations, particularly Neuroligin 3 and 4 (NLG3 & NLG4), reported in cases of ASD (Jamain, Quach et al. 2003). In mice, knockout of either NLG3 or NLG4 alters pup ultrasonic vocalizations when separated from the dam (Radyushkin, Hammerschmidt et al. 2009). However, only knockout of NLG4, but not NLG3, results in deficits in sociability suggesting that mutation of a single NLG gene cannot alone explain the ASD-phenotype (Jamain, Radyushkin et al. 2008; Radyushkin, Hammerschmidt et al. 2009). Neuroligins are expressed post-synaptically and bind the presynaptic adhesion molecule Neurexin to form a bridge in the synaptic complex. Thus it is not surprising that disruptions of Neurexin molecules would also result in improper synapse formation and maturation. Numerous cases-reports have identified individuals with de novo mutations in the Neurexin 1 gene meeting the criteria for an ASD (Szatmari, Paterson et al. 2007; Kim, Kishikawa et al. 2008; Glessner, Wang et al. 2009). Despite the strong link between neurexin-1 function and ASD, mouse models with knockout of neurexin-1 have shown less convincing evidence for their role in regulating behaviors of the core-domains of ASD. While female neurexin knockout mice display less care for their litters (Geppert, Khvotchev et al. 1998), no apparent social deficits in overt social approach or social novelty recognition were observed (Etherton, Blaiss et al. 2009). However, neurexin knockout mice do exhibit excess grooming, a species typical behavior thought to be related to repetitive stereotypies in autism (Etherton, Blaiss et al. 2009).
In addition to the importance of cell-adhesion molecules in proper synapse formation and function, the intracellular protein complexes that regulate and anchor trans-synaptic molecules such as neuroligins and neurexins are equally important in neuronal function, brain development, and behavior. The SHANK family of proteins is important for anchoring cell-adhesion molecules and receptors to the postsynaptic membrane. Individuals with deletion of the SHANK genes (often resulting from 22q13 deletion known as Phelan-McDermid syndrome) are characterized by global developmental delays, deficits in expressive language, and often meet the criteria for ASD (Phelan 2008). Mounting evidence points to a mutation on the SHANK3 gene as sufficient to cause the neurobehavioral profile in Phelan-McDermid syndrome. This has led to the development of mouse models to study the effects of SHANK3 mutations on ASD-related behaviors. The SHANK3 protein consists of 3 isoforms (i.e. α, β, γ) that together facilitate anchoring of postsynaptic molecules. Recent attempts to elucidate the role of each of these isoforms have demonstrated that knockout of both α and β subunits together produce excessive grooming behavior and deficits in social interactions (Peca, Feliciano et al. 2011). An alternative approach to studying SHANK3 disruption used deletion of exons 4–9, which encode for the cytoskeleton binding region of the SHANK protein. These mice exhibit deficits in behavioral measures relevant to all three domains of ASD in addition to sex-dependent decreases in motor function observed in male mice (Bozdagi, Sakurai et al. 2010; Wang, McCoy et al. 2011). Although, some of these behaviors were not consistently replicable (Yang, Bozdagi et al. 2012).
Together, SHANK3, Neuroligin, and Neurexin molecules are important components of glutamatergic postsynaptic densities. Therefore disruption of any one of these proteins could disrupt glutamate signaling, including altering AMPA and mGluR5 trafficking (Uchino, Wada et al. 2006; Verpelli, Dvoretskova et al. 2011). Additionally, the SHANK3 gene is enriched with CpG islands that make this gene sensitive to DNA methylation (Ching, Maunakea et al. 2005). In fact, SHANK3 expression is dependent on proper DNA methylation to regulate tissue specific expression (Beri, Tonna et al. 2007). Similar to other ASD candidate genes, it is hypothesized that environmental alterations in the expression of SHANK3 could produce additive adverse effects on brain development.
2.7 Strain differences as a model of idiopathic ASD
Despite the high heritability of ASD, only 5–15 percent of cases can be attributed to a known genetic defect (Martin and Ledbetter 2007), with the majority of cases resulting from unknown, or idiopathic, origin. The multigenetic etiology and varying degree of symptomology in ASD may be analogous to the diverse behavioral phenotypes associated with different mouse strains. A survey of 10 of the top tier mouse strains revealed significant variation in performance across several social and learning behavioral tasks (Moy, Nadler et al. 2007). Of particular interest is the BTBR T+ tf/J (BTBR) mouse which has become a focus of research on mouse models of autism. The BTBR mouse displays deficits in behavioral tasks modeling the three core domains of ASD. In both the three-chamber social approach and visible burrow task, the BTBR mice show marked reductions in social behaviors compared to the highly social C57Bl/6J controls (Moy, Nadler et al. 2007; McFarlane, Kusek et al. 2008; Pobbe, Pearson et al. 2010). Importantly, the reductions in social behavior characteristic of the BTBR mice can be observed at an early juvenile age (McFarlane, Kusek et al. 2008), which is consistent with the deficits in social play among children with ASD. Prior to weaning, juvenile BTBR mice also express increased distress vocalizations and an altered pattern of calling when separated from the nest (Scattoni, Gandhy et al. 2008). The pattern of reduced social behaviors persists into adulthood in BTBR mice when they exhibit reduced number of vocalizations and scent marking behaviors when presented with the scent of a receptive female (Scattoni, Ricceri et al. 2011; Wohr, Roullet et al. 2011). While it is tempting to interpret these changes in vocalizations as a representative model for poor language development in children with ASD, the functional significance of mouse vocalizations and the degree to which they serve to elicit behavioral responses as a form of communication is still under investigation.
Another core behavior of ASD, restrictive/repetitive behaviors and motor stereotypies, has been modeled in the BTBR mouse. For example, the BTBR mice spend significantly more time displaying species typical grooming behaviors and engaging in compulsive burying when compared to C57Bl/6J controls (McFarlane, Kusek et al. 2008; Amodeo, Jones et al. 2012). Additionally, while BTBR mice perform well in spatial learning tasks, more complex reversal learning assays have revealed increased perseverations in a probabilistic spatial learning task (Amodeo, Jones et al. 2012). Taken together, reduced social interactions, altered calling patterns, reduced scent marking behavior, and increases in preservative behaviors in the BTBR mouse demonstrates the breadth of behavioral deficits representative of the core-signs of ASD.
The discovery of the BTBR strain as a model animal for ASD behaviors has allowed for identification and testing of novel hypothesis with regards to the etiology of the disorder. Perhaps the most apparent distinction between the BTBR and other mouse strains is the absences of a corpus callosum and markedly reduced hipppocampal commissure (Wahlsten, Metten et al. 2003). It was hypothesized that much of the behavioral phenotype unique to the BTBR mouse might be attributed to the agenesis of the corpus callosum. While studies have shown a correlation between callosal connectivity and social behavior in other mouse strains (Kim, Pickup et al. 2012), Yang et al. argue against this hypothesis as neonatal lesions of the corpus callosum fail to disrupt social approach in highly social C57Bl/6J mice (Yang, Clarke et al. 2009). An alternative hypothesis that has yet to be examined stems from a single nucleotide polymorphism in a coding region of the KMO gene encoding kynurenine 3-hydroxylase (McFarlane, Kusek et al. 2008). Disruptions of KMO can result in altered tryptophan catabolism leading to an imbalance of serotonin synthesis and excess release of excitotoxic derivatives such as quinolinic acid. Interestingly, the BTBR mouse has reduced serotonin receptor 1A and 2A densities and shows improved social behaviors when treated with the serotonin transport blocker fluoxetine (Yang, Clarke et al. 2009; Chadman 2011; Gould, Hensler et al. 2011). Finally, increasing evidence has been found in support of altered immunological function among individuals with ASD (for review see (Careaga, Van de Water et al. 2010)). Similarly, the BTBR mice exhibit elevated immunoglobulin proteins, enhanced cytokine levels, and increased MHC II expression suggesting that aberrant immune responses may contribute to the behavioral deficits in these mice (Heo, Zhang et al. 2011). Considering the multigenic nature of ASD, and the numerous biological factors that could contribute to the BTBR phenotype, this mouse and similar strains provide a novel animal model to explore how environmental insults may interact with biological dispositions to produce or exacerbate behaviors relevant to ASD. Additionally, the BTBR mouse can be used to identify potentially useful drugs for the treatment of ASD. This approach has already been successful in screening the compound GRN529, a negative allosteric modulator of mGluR5 receptors which reduced repetitive behaviors and reversed some deficits in social approach and reciprocal social interactions in BTBR mice (Silverman, Smith et al. 2012).
2.8 Timothy’s Syndrome
Recently a new developmental disorder was added to the list of monogenic diseases associated with ASD. Timothy’s syndrome (TS) is a rare autosomal dominant disorder resulting from a point mutation on a gene encoding the α-subunit of the L-type calcium CaV1.2 channel (Splawski, Timothy et al. 2004). Given the global importance of calcium in regulating cellular functions ranging from cardiac rhythmicity in the heart to neuronal transmission in the brain, it is not surprising that individuals with Timothy’s syndrome have multiorgan deficits leading to lethal cardiac arrhythmias, webbed fingers and toes, immune deficiencies, and autism-like behaviors. Initial investigations on the role of the CaV1.2 channel in brain function are already underway with the first development of a mouse model for the disorder. The TS2-neo mouse possesses an inverted neomycin cassette for exon 8A of the CaV1.2 gene and displays many of the behavioral deficits observed in humans with ASD. For example, the T2-neo pups have a significantly reduced ultrasonic vocalization call length when separated from the nest and the adults display decreased sociability. Furthermore these mice are reported to show increased marble burying and mild perseverations, despite having intact memory formation (Bader, Faizi et al. 2011). Because Timothy’s Syndrome is relatively new, there is still much work that needs to be done, both clinically and in animal models, to identify how the specific genetic deficit contributes to ASD-like behaviors. However, several persistent environmental pollutants, including brominated flame retardants and organochlorines, alter calcium signaling pathways and these agents would be predicted to be particularly toxic to individuals with Timothy syndrome, although this likely gene-environment interaction has not yet been experimentally examined. To this end, an in-depth understanding of several classes of persistent organic pollutants including their mechanisms of action and potential role in neurotoxicology is warranted.
3. Neurotoxicity of Prevalent Environmental Agents
3.1 Heavy Metals
Heavy metals are of concern in neurotoxicology due to continued occupational and residential exposures. Small amounts of heavy metals are common in the environment, and small amounts of certain heavy metals in the diet are necessary for good health. But, large amounts of any heavy metal can cause acute or chronic toxicity. The focus of this section will be on two specific heavy metals; lead (Pb) and Mercury (Hg). Lead is not usually found naturally in the environment but has become a widespread environmental contaminant from its extraction from ores and use in a variety of consumer goods. Although its use has declined over the past 30 years, harmful quantities of lead are still found in batteries, paints, metal products and ceramic glazes (Rosner and Markowitz 2007), and are still commonly detected in environment samples. Similarly, mercury is present naturally within the earth’s crust and is also widespread in the environment due to volcanic emissions, burning of waste and fossil fuels, use in electrical, medical and laboratory instrumentation and the extraction of gold.
3.1.1 Lead
The developmental neurotoxicity of elemental lead is well established (Dietrich 1991; Bellinger 2008). Children exposed to lead show significant reductions in IQ, behavioral disturbances, and altered endocrine function (Rosner and Markowitz 2007). Lead-induced brain damage preferentially occurs in the prefrontal cortex, the cerebellum and hippocampus; all areas important for cognitive function, motor skill, and memory processes. Experiments in non-human primates and rodent models have consistently shown neurodevelopmental deficits due to lead exposure, including impairments in higher-order learning (Rice 1990), memory, and attention (Chen, Ma et al. 1997; Salinas and Huff 2002).
The mechanism of lead toxicity is not fully understood. The most commonly suggested mechanism involves interference within calcium homeostasis and calcium-regulated pathways (Toscano and Guilarte 2005). This is because the chemistry of lead, a divalent cation, is similar to calcium and readily binds to oxygen and sulfur. As such, lead tends to compete with calcium for common binding sites and can interfere with calcium transport systems required for neurotransmitter release and regulation in the central nervous system. Lead is also capable of activating calcium signaling pathways. For example lead can activate protein kinase C (PKC), an enzyme involved in many cell signaling pathways, by increasing intracellular calcium levels or by mimicking the action of calcium itself (Loikkanen, Chvalova et al. 2003). Lead exposure has been shown to increase intracellular calcium levels resulting in excessive calcium influx into mitochondria leading to the production of free radicals and reactive oxygen species (Sidhu and Nehru 2003).
Along with its effects on calcium signaling, lead has been show to interfere with NMDA receptor function and long term potentiation, a mechanism of synaptic plasticity. Studies in animal models and hippocampal cultures have revealed that lead exposure results in altered NMDA receptor mRNA and protein expression, delaying or preventing the developmental switch from NR2B to NR2A receptor subunits (Toscano, Hashemzadeh-Gargari et al. 2002; Zhang, Liu et al. 2002). It has been hypothesized that these effects are due to dampened NMDA receptor activity due to the antagonism by lead (Alkondon, Costa et al. 1990; Gavazzo, Zanardi et al. 2008). In addition to effects on NMDA receptors, other studies have reported that both glutamatergic and GABAergic neurotransmission are impaired after lead exposure (Braga, Pereira et al. 1999; Lasley and Gilbert 2002) due to inhibition of presynaptic calcium channels (Peng, Hajela et al. 2002).
3.1.2 Mercury
Studies of the neurotoxicity of mercury have focused on organic forms of mercury which are the most damaging, particularly to the developing nervous system (Clarkson, Vyas et al. 2007). The best characterized form of organic mercury is methylmercury (MeHg) which has been studied in depth due to its neurotoxic effects (Grandjean, Weihe et al. 1998). Episodes of accidental human mercury poisoning were reported in Japan in the 1950s and 60s and in Iraq in the early 1970s due to consumption of contaminated food. Subsequent research on individuals exposed to mercury has provided crucial information about the dangers of mercury exposure at all stages of development (Kazantzis, Al-Mufti et al. 1976; Yorifuji, Tsuda et al. 2009)
The mechanisms by which mercury exposure results in neurodevelopmental toxicity are still not fully understood and damage may occur at several physiological levels. Specifically, MeHg has a high affinity for sulfhydryl (SH) residues in cysteine containing molecules, a common feature of many molecules, and most proteins. This has led to the hypothesis that MeHg exerts it toxic activity by suppression of cell proliferation through inhibition of specific cell cycle proteins including cyclin E. Mercury can also directly interact with the thiol group of glutathione (GSH), an antioxidant found throughout the body including the brain, decreasing the available levels of GSH and thereby contributing to the occurrence of oxidative stress (Franco, Braga et al. 2007). Further, MeHg can disrupt mitochondrial function through inhibition of respiration and ultrastructural changes (Atchison and Hare 1994; Belyaeva, Dymkowska et al. 2008), produce reactive oxygen species (ROS) through membrane lipoperoxidation (Company, Serafim et al. 2004; Messer, Lockwood et al. 2005; Yin, Milatovic et al. 2007), and interfere with normal cell cycle activity (Burke, Cheng et al. 2006).
In addition to the ability of MeHg to bind to sulfhydryl groups, research suggests that MeHg produces neurotoxicity through effects on synaptic transmission. MeHg is capable of altering synaptic release of acetylcholine (ACh) at both the neuromuscular junction as well as within cholinergic pathways in the brain (Juang and Yonemura 1975; Atchison and Narahashi 1982; Yuan and Atchison 1993). Mercury may also alter glutamatergic signaling. Although the mechanism is not well understood, evidence suggests disrupted glutamatergic signaling may be due to reduced vesicular uptake of glutamate through direct inhibition of H+-ATPase activity(Porciuncula, Rocha et al. 2003). Lastly there is also evidence that MeHg is capable of blocking excitatory postsynaptic potentials through suppression of calcium entry into nerve terminals (Atchison, Joshi et al. 1986; Shafer and Atchison 1989).
Given the link between heavy metal exposure and disrupted glutamate signaling, one might expect individuals with mutations on genes encoding molecules important for excitatory synaptic signaling (i.e. Shank 3, Timothy syndrome) to have a heightened sensitivity to the developmentally toxic effects of lead and mercury exposure. This idea could easily be tested in a variety of existing mouse models of neurodevelopmental disorders, and the results of such experiments could have important implications for defining those in the population who are at increased risk from exposure to environmental toxicants.
3.2 Organohalogens
Halogenated organic compounds contain chlorine, bromine, or fluorine atoms and are manufactured for use as pesticides, flame retardants, hydraulic fluids, and in several industrial applications. However, due to the halogenated structure many of these compounds are persistent environmental pollutants causing considerable human health problems (Mariussen and Fonnum 2006). The focus of this section will be on two prevalent organohalgen pollutants, polybrominated diphenyl ethers (PBDE) which are used as additives in electronic equipment, furniture, building material, and packaging because of their flame retardant properties (Birnbaum and Staskal 2004) and polychlorinated biphenyls (PCB) which were used in transformers and capacitors due to their dielectric and coolant properties. Both compounds share structural similarities and chemical characteristics which allow them to be both persistent in the environment and to bioaccumulate in human tissue.
3.2.1 Polybrominated Diphenyl Ether (PBDE)
There are 209 possible congeners of PBDE based on the number and placement of bromine atoms. The toxicological activity of each congener depends on the placement, geometry, and number of bromine atoms around the diphenyl ether structure (Kodavanti, Ward et al. 2005). However unlike PCBs, PBDEs have an ether bond between the two phenyl rings which prevents them from assuming a co-planar structure. For this reason PBDEs cannot activate the aryl hydrocarbon receptor (AhR) to the same degree as coplanar PCBs (Wahl, Guenther et al. 2010), however they are still able to alter gene induction and hepatic enzyme activity both in vivo and in vitro (Van den Berg, Birnbaum et al. 2006).
Several studies have demonstrated direct neurodevelopmental toxicity from perinatal exposure to PBDE (Ta, Koenig et al. ; Dingemans, Ramakers et al. 2007; Xing, Chen et al. 2009). Perinatal exposure to PBDE-47 or PBDE-209 impairs long-term potentiation, a form of synaptic plasticity associated with learning and memory (Dingemans, Ramakers et al. 2007; Xing, Chen et al. 2009). Additionally, gestational exposure to the PBDE congener, PBDE99, was shown to increase activity of glutamate-nitric oxide-cyclic guanosine monophosphate pathways in the cerebellum (Llansola, Erceg et al. 2007), pathways that are also linked to synaptic plasticity as well as the release of neurotransmitters (Boulton, Southam et al. 1995). PBDEs have also been shown to inhibit uptake of calcium in microsomes and mitochondria (Coburn, Curras-Collazo et al. 2008) as well as induce protein kinase C (PKC) translocation, arachidonic acid release via phospholipase A activation (Kodavanti and Derr-Yellin 2002; Kodavanti and Ward 2005), and stimulation of the mitogen-activated protein kinase pathway (Fan, Besas et al. 2010) (a pathway involved in the modulation of various cellular functions which is in turn modulated by both PKC and Calcium).
Additional cellular and molecular mechanisms associated with PBDE neurotoxicity include induction of apoptotic cell death in both primary neurons and cell lines due to oxidative stress (Giordano, Kavanagh et al. 2008; Yu, He et al. 2008) and altered cell migration and inhibition of neurite outgrowth and neural stem cell differentiation (Alm, Scholz et al. 2006; Zhang, Liu et al. 2010). Interference with endocrine function is another major mechanism of toxicity from PBDE exposure. In humans PBDE exposure is associated with congenital cryptorchidism (Main, Kiviranta et al. 2007) and decreased fecundability (Harley, Marks et al. 2010). In vitro studies have found that they bind to estrogen, progesterone, androgen, and glucocorticoid receptors and inhibit CYP enzymes involved in steroidogenesis (Meerts, Letcher et al. 2001; Kojima, Takeuchi et al. 2009). Epidemiological studies have shown correlations between human PBDE exposure and thyroid hormone levels (Turyk, Persky et al. 2008; Chevrier, Harley et al. 2010). PBDE structurally resembles the thyroid hormone thyroxine (T4) and can bind to transthyretin (TTR), a thyroid hormone carrier protein (Meerts, van Zanden et al. 2000), leading to a decrease in levels of T4 and hypothyroidism (Costa and Giordano 2007).
3.2.2 Polychlorinated Biphenyl (PCB)
PCBs are compounds made up of a single bonded paired phenyl ring structure with various degrees of chlorination. There are 209 possible PCB congeners with different physical and chemical properties based on the position and number of chlorine molecules. PCBs can be further subdivided into coplanar and non-coplanar congeners. Coplanar PCBs are predominant in the environment and are considered to be dioxin-like and agonists of AhR. Binding of PCB to AhR translocates it to the nucleus and activates genes known as dioxin-responsive genes. Prolonged activation of AhR has been implicated in diverse toxic endpoints (Mitchell and Elferink 2009). Early developmental exposure to PCBs can occurs through their presence in human placenta, umbilical cord serum, and breast milk, underlying concerns that exposure to these compounds may contribute to the risk for neurodevelopmental disorders (Winneke, Walkowiak et al. 2002; Jorissen 2007). Based on accidental human exposures to PCB in Japan in 1968 and Taiwan in 1979, it is known that PCBs can affect early brain development resulting in behavioral abnormalities as well as significantly lower verbal and full-scale IQ (Chen and Hsu 1994; Jacobson and Jacobson 1996). Deficits in learning and memory have been reported in animal studies after perinatal exposure to PCB (Schantz 1996; Niemi, Audi et al. 1998). Because of these latter effects, exposures to PCBs have been suggested to contribute to the increased occurrence of neurodevelopmental disorders (Jacobson and Jacobson 1996; Longnecker, Wolff et al. 2003).
PCBs have also been shown to affect cellular signaling pathways in the brain. Both in vivo and in vitro studies have shown that PCB exposure alters calcium homeostasis in neurons (Tilson and Kodavanti 1998; Kodavanti and Tilson 2000; Kim, Inan et al. 2009) leading to altered excitability and abnormal development (Kodavanti, Kannan et al. 2001; Kodavanti and Ward 2005; Kim, Inan et al. 2009). Research into the direct effects of PCB on calcium homeostasis suggests a convergent mechanism by which PCB can regulate a variety of physiological processes through activation of the ryanodine receptor (RYR) (Pessah, Hansen et al. 2006), a receptor important in the downstream signaling effects of calcium (Pessah, Cherednichenko et al. 2010). Because calcium is an important second messenger in many cellular processes, disruption of calcium homeostasis provides a direct mechanism by which PCB can impair brain development and function.
Along with the effects seen on calcium homeostasis, studies examining the effects PCBs have on neurotransmitter levels in vivo have shown that dopamine (DA) levels can be significantly altered by exposure to PCBs and these alterations are associated with neurobehavioral changes (Seegal, Bush et al. 1990; Seegal, Bush et al. 1991). In vitro studies looking at a possible direct mechanism of PCBs on neurotransmitter levels have shown that PCBs have the ability to inhibit tyrosine hydroxylase (Seegal, Bush et al. 1991), the rate-limiting enzyme in DA synthesis, and inhibit vesicular monoamine transporters thus reducing synaptosomal dopamine content (Bemis and Seegal 2004; Richardson and Miller 2004). Serotonergic, cholinergic, and norephinepherine systems have also been shown to be altered by exposure to PCBs (Seegal, Bush et al. 1985; Juarez de Ku, Sharma-Stokkermans et al. 1994; Morse, Seegal et al. 1996) but to a lesser degree than the dopamingeric system. Finally recent research has demonstrated that perinatal exposure to PCBs can increase excitotoxicity due to subsequent GABA receptor impairments (Kim, Inan et al. 2009; Kim and Pessah 2011). This is of particular interest because several neurodevelopmental syndromes, including autism and Fragile-X, have been linked to altered GABAergic signaling (Belmonte and Bourgeron 2006; Maski, Jeste et al. 2011; Rossignol 2011).
PCBs have been shown to decrease circulating thyroid hormone levels during development (Morse, Groen et al. 1993; Crofton, Kodavanti et al. 2000). This is mediated by at least three possible mechanisms including direct interaction of PCBs with the thyroid gland decreasing thyroid hormone synthesis (Collins and Capen 1980), increased biliary excretion of thyroid hormones through the induction of phase two metabolic pathways (Collins and Capen 1980; Van Birgelen, Smit et al. 1995), and displacement of thyroid hormones at the level of receptor binding (Morse, Groen et al. 1993; Chauhan, Kodavanti et al. 2000). Therefore PCBs can directly affect neurodevelopment by lowering the levels of thyroid hormone during critical periods of neurological development leading to the manifestation of developmental disorders (Delahunty, Falconer et al. 2010; La Gamma and Paneth 2012).
There is also an abundant literature establishing PCBs as potent immunotoxicants. Studies in animal models have found atrophy of the thymus induced by PCB exposure (Allen and Barsotti 1976; Smialowicz, Andrews et al. 1989), and wildlife studies have also implicated PCBs in altered immune function (Levin, Morsey et al. 2004; Keller, McClellan-Green et al. 2006). In humans, high exposures to PCBs have been linked to lower IgM and IgA levels, increases in respiratory infections (Nakanishi, Shigematsu et al. 1985), and increased incidence of cancer, particularly malignant melanoma (Loomis, Browning et al. 1997). PCB can also alter immunophenotypes, increasing the number of B cells and decreasing CD8+ and natural killer cells (Svensson, Hallberg et al. 1994). Children and newborns may have an even greater susceptibility to PCB-induced immune toxicity. In a Dutch birth cohort study, babies with higher prenatal PCB exposure had reduced MMR (measles, mumps, and rubella) reactivity after vaccination, altered lymphocyte distribution, and decreased wheeze and increase otitis media (Weisglas-Kuperus, Vreugdenhil et al. 2004). In the Faroe Islands, children with a higher exposure to PCBs exhibited a decreased antibody response to diphtheria toxoid at 18 months and a decrease response to tetanus toxoid at 7 years of age (Heilmann, Grandjean et al. 2006). Finally a study conducted in the US from 2002–2004 found that prenatal PCB exposure was associated with a small thymic index at birth. The importance of immune dysfunction in early development is underscored by a growing number of research studies linking autism with substantial deviations in immune parameters (Molloy, Morrow et al. 2006; Ashwood, Krakowiak et al. 2011; Goines, Croen et al. 2011). These findings suggest that individuals with an underlying immunological deficit who are exposed to PCBs may confer greater susceptibility to developing an ASD. Future investigations employing mouse models to study PCB exposure must consider how select mouse strains, particularly those with inherent immune dysfunction like the BTBR mouse, may result in more severe behavioral deficits.
3.3 Pesticides
The wide use of pesticides has raised significant concern over possible health effects associated with exposure to these compounds. Two classes of pesticides will be discussed in this section, pyrethroids and organophosphates, as well as fipronil a phenylpyrazole insecticide. Pyrethroid pesticides are commonly used in agricultural settings; but are also common household insecticides and pet pest control products. Their unregulated use in the home environment has increased the risk of exposure in children as well as in the general population (Ostrea, Bielawski et al. 2009; Naeher, Tulve et al. 2010). Organophosphate pesticides are among the most widely used insecticides and toxic exposure in humans is well documented (Casida and Quistad 2004). Finally fipronil, compared to pyrethroids and organophosphate pesticides, is a fairly new pesticide with relatively little known about its toxicological potential in humans. However because there is a high likelihood of exposure due to its frequent use in the home environment it is considered in this discussion.
3.3.1 Pyrethroids
Pyrethroids are synthetic derivatives of naturally occurring pyrethrins, the natural insecticidal compounds found in the chrysanthemum Chrysanthemum cineraraefolum. They are broadly classified into two types based on the presence of an α-cyano group; both types are able to produce toxicity by interaction with voltage-gated sodium channels expressed on neuronal axons. Whereas, type 1 compounds produce tremors (i.e., T-group) by prolonging channel opening to induce repetitive firing of action potentials, type 2 compounds result in choreoathetosis-salivating-seizures (i.e., C-S group) by holding channels open for an extended period of time until the neuronal membrane is depolarized to the point at which generation of action potentials fail (Shafer, Meyer et al. 2005; Ray and Fry 2006). While acute toxicity of pyrethroids is mainly mediated by prolongation of the kinetics of voltage-gated sodium channels, the effects of pyrethroids on the developing brain are more complex and can involve antagonism of GABAergic neurons, modulation of nicotinic cholinergic transmission, enhancement of noras adrenaline release, and direct actions on calcium or chloride ion channels (Tayebati, Di Tullio et al. 2009).
Studies in rodents on the developmental neurotoxicity of pyrethroids have reported increased motor activity and lack of habituation, which paralleled changes in density of muscarinic acetylcholine receptor binding after exposure (Ahlbom, Fredriksson et al. 1994; Talts, Fredriksson et al. 1998). In vitro research has also demonstrated potential developmental neurotoxicity of pyrethroids, with low levels leading to inhibition of neurite outgrowth in PC12 cells (Tran, Hoffman et al. 2006). In humans, mutations in genes encoding sodium channel subunits have been linked to neuronal hyperexcitability and epilepsy (Meisler, Kearney et al. 2001). Because pyrethroids affect the activation-inactivation kinetics of sodium channels, exposure to these compounds may be developmentally neurotoxic by this mechanism. In support of this hypothesis, phenytoin, an anticonvulsant that blocks ion conductance through sodium channels and other ion channels, disrupts nervous system structure and function (Adams, Vorhees et al. 1990). Furthermore, the use of anticonvulsants during pregnancy has been associated with adverse effects including microcephaly and intellectual impairment (Holmes, Harvey et al. 2001). In addition, deltamethrin (DM), a type 2 pyrethroid, has been shown to increase BDNF mRNA expression in cultured rat cortical neurons (Matsuya, Ihara et al. 2012). This is of interest because BDNF promotes differentiation and survival of neurons in the developing brain, and excessive levels of BDNF expression have been associated with ASD and ADHD (Shim, Hwangbo et al. 2008; Correia, Coutinho et al. 2010).
3.3.2 Organophosphate Pesticides
The primary target of organophosphate pesticides is the enzyme acetylcholinesterase (AchE), which hydrolyzes the neurotransmitter acetylcholine within the nervous system. In regards to specific toxicity, organophosphate pesticides containing a P=O moiety disrupt AChE, whereas organophosphate pesticides with a P=S moiety require bioactivity to form an “oxon” or oxygen analogue of the parent compound. A unique characteristic of organophosphate pesticides is that when they inhibit AChE, the enzyme-inhibitor complex can become “aged” leading to irreversible inhibition of AChE (Costa 2006). When AChE is inhibited it results in an accumulation of acetylcholine at cholinergic synapses, leading to over-stimulation of muscarinic and nicotinic receptors. Further, there is evidence that acetylcholine has important functions during brain development (Lauder and Schambra 1999).
Chlorpyrifos, the most extensively studied organophosphate pesticide with regards to neurodevelopmental toxicity, has been shown to cause a variety of behavioral abnormalities in both mice and rats, including impaired locomotor skills and cognitive performance (Dam, Seidler et al. 2000; Ricceri, Markina et al. 2003; Icenogle, Christopher et al. 2004; Ricceri, Venerosi et al. 2006). In concentrations comparable to those found in human meconium (Ostrea, Morales et al. 2002) Chlorpyrifos caused mitotic abnormalities and evidence of apoptosis during the neural tube development in rat embryo cultures (Roy, Andrews et al. 1998). Further, there is evidence that chlorpyrifos exposure during gestation caused reductions in brain cell number, neuritic projections and impaired synaptic communication (Qiao, Seidler et al. 2003; Qiao, Seidler et al. 2004).
Deficits elicited by prenatal exposure to chlorpyrifos are evident even at exposure levels below the threshold for detectable AChE inhibition (Dam, Seidler et al. 2000; Ricceri, Markina et al. 2003; Icenogle, Christopher et al. 2004; Ricceri, Venerosi et al. 2006). This suggests that mechanisms other than inhibition of AChE activity may, at least in part, contribute to the neurodevelopmental effects of chlorpyrifos and other organophosphate pesticides. The non-cholinergic mechanisms of chlorpyrifos are not clear, but suggested targets are pathways involved in neuronal and hormonal signaling, including the cyclic-AMP-protein kinase A cascade, receptor signaling through protein kinase C, and direct effects on expression and functioning of nuclear transcription factors mediating the switch from proliferation to differentiation, including c-fos, p53, AP-1, Sp1 and CREB (Slotkin 2004).
Epidemiological studies also support neurodevelopmental toxicity associated with organophosphate exposure. A California study found an association with reflex abnormalities in neonates and increased concentration of OP metabolites measured in maternal urine during pregnancy (Young, Eskenazi et al. 2005). Another study in New York found that maternal levels of chlorpyrifos above the limit of detection, coupled with low maternal levels of paraoxonase activity (an enzyme which hydrolyzes certain organophosphate pesticides), were associated with reduced head circumference in the infants (Eskenazi, Marks et al. 2007). In another study on the same cohort, levels of organophosphate pesticide metabolites in maternal urine were associated with anomalies of primitive reflexes in infants (Engel, Berkowitz et al. 2007). Finally, prenatal chlorpyrifos exposures were found to be inversely associated with birth weight and length (Young, Eskenazi et al. 2005). A follow up to this study on children’s cognitive and motor development at 1, 2 and 3 years of age found that at 3 years of age Psychomotor Development Index (PDI) and Mental Development Index (MDI) scores of highly exposed children differed by 7 and 3 points respectively from children with low prenatal exposure. Further the proportion of delayed children in the high-exposed group compared to the low-exposed group was five times greater for PDI and 2.4 times greater for the MDI (Rauh, Garfinkel et al. 2006).
3.3.3 Fipronil
Fipronil was developed specifically to block insect GABA receptors that differ substantially from vertebrate GABA receptors. However, there is now evidence that fipronil also interacts with vertebrate GABA receptors altering their function (Ikeda, Zhao et al. 2001). Additionally, recent reports suggest fipronil may act by interfering with MAP Kinase transduction pathways (Sidiropoulou, Sachana et al. 2011) and disrupt the balance of neuronal differentiation between dopaminergic and cholinergic phenotypes (Lassiter, MacKillop et al. 2009). Finally, notochord degeneration and locomotor defects have been found in zebra fish embryos following exposure to fipronil (Stehr, Linbo et al. 2006). In light of these findings, a closer examination of the mechanisms of action and potential neurotoxic effects of fipronil exposure are warranted.
Studies conducted on rat dorsal root ganglion (DRG) neuron cultures found that fipronil was able to suppress GABA-induced whole-cell currents in both closed and activated states (Ikeda, Zhao et al. 2001) by decreasing channel open time and the frequency of channel opening (Ikeda, Nagata et al. 2004). Exposure of GABAA receptors to fipronil and its sulphone metabolite in rat brain resulted in accumulation of GABAA receptors in a novel, long-lived blocked state. Because fipronil specifically binds to the β3 subunit of GABAergic receptors (Ratra, Kamita et al. 2001), and the subunit composition of GABAA receptors differs throughout the brain, the toxicity of fipronil is also likely to be specific to brain areas where β3 subunits are highly expressed. Therefore those individuals with greater expression of β3 subunit may suffer more severely from acute exposure to fipronil. With respect to mouse models, mice possessing increased copy-number variants of Gabaβ3 might expect to show greater behavioral deficits to specific pesticides when compared to those with a deletion of the gene. Therefore, results reported from pesticide exposure in animal models to date may under represent the potential developmental effects of fipronil and other pesticides when considering how underlying genetic variances may confer greater toxic effects. Finally, polymorphisms in Gabaβ3 in some cases of ASD may interfere with GABAergic transmission, rendering these individuals more vulnerable to the toxic effects of fipronil.
3.4 Plasticizers
A plasticizer, also known as a dispersant, is a polymer additive that serves to increase the polymer’s flexibility. Plasticizers are usually inert organic materials with high boiling points and low vapor pressures. Because of their ability to alter the physical character of the material to which they are added, plasticizers are commonly used in a large number of consumer goods resulting in human exposure. In this section we will discuss two different plasticizers: bisphenol A, one of the highest volume chemicals produced worldwide, and phthalates, a family of chemicals used widely in personal care products.
3.4.1 Bisphenol A (BPA)
Bisphenol A (BPA) is a known endocrine disrupter that was initially evaluated for possible commercial use in the 1930s as a synthetic estrogen (Vogel 2009). By the 1940s the plastic industry identified BPA as a building block for polycarbonate plastics and began using it in a variety of consumer goods such as food and beverage containers, including baby bottles. Today current estimates indicate that more than 8 billion pounds of BPA are produced annually to meet consumer demand, and approximately 100 tons are released into the atmosphere each year as a result of production (Vandenberg, Chahoud et al. 2012). Unsurprisingly there is now evidence of significant human exposure to BPA. Recent measurements by the Centers for Disease Control and Prevention (CDC) revealed that detectable levels of BPA were found in 92.6% of urine samples from more than 2500 participants of the cross sectional 2003–2004 NHANES (National Health and Nutrition Examination Survey) Study (Calafat, Ye et al. 2008). Children in the NHANES data set had the highest levels of exposure to BPA which is particularly alarming as data from animal studies has demonstrated increased vulnerability to BPA exposure during early development (Richter, Birnbaum et al. 2007). Further, BPA has been detected in pregnant women, human amniotic fluid, neonatal blood, placenta, cord blood and human breast milk (Vandenberg, Hauser et al. 2007; Vandenberg, Chahoud et al. 2012) demonstrating the presence of both pre and postnatal exposure.
The main mechanism of toxicity for BPA is through its activity as a xenoestrogen. Recent studies have demonstrated that BPA can stimulate cellular responses at very low concentrations and can have equivalent potency to estradiol (Alonso-Magdalena, Laribi et al. 2005; Zsarnovszky, Le et al. 2005; Hugo, Brandebourg et al. 2008). The mechanisms of BPA action are due to its ability to bind to classical and non-classical membrane estrogen receptors (Alonso-Magdalena, Laribi et al. 2005; Watson, Bulayeva et al. 2005) as well as the G-protein-coupled receptor 30 (Thomas and Dong 2006), and it can act through non-genomic pathways (Zsarnovszky, Le et al. 2005; Ropero, Alonso-Magdalena et al. 2006; Leranth, Hajszan et al. 2008) targeting multiple cellular sites (Ropero, Alonso-Magdalena et al. 2006). However BPA interacts differently than estradiol when it binds with estrogen receptors (Gould, Leonard et al. 1998) leading to recruitment of transcriptional co-regulators (Routledge, White et al. 2000), thus indicating that BPA is not merely an estrogen mimic.
When considering the effects of perinatal exposure to BPA, it is important to note that the fetal and neonatal liver produces high levels of alpha fetoprotein (AFP), a major estrogen binding plasma protein. AFP is thought to protect tissues from excessive exposure to estradiol (Toran-Allerand 1984). However, in contrast to estradiol, BPA has limited binding to serum proteins (Milligan, Khan et al. 1998) and therefore may have increased access to estrogen sensitive tissues of the developing fetus or neonate. For this reason BPAs actions during development may be greater than expected as it can interact with tissues not normally exposed to estrogen during critical periods of brain development. A study in rodents demonstrated that low levels of BPA resulted in masculinization of a brain region essential for cyclic gonadotropin release in the female offspring (Rubin, Lenkowski et al. 2006), and the female offspring showed masculinization of behavior in the open field. These data suggest that BPA may have been acting as an estrogen in specific regions of the developing brain important for sexual differentiation. In another study at a higher dose BPA exposure appeared to disrupt masculinization of the brain of male offspring as a result of its interference with the action of endogenous estrogens required for masculinization of the brain (Patisaul, Fortino et al. 2006).
In addition to acting as an estrogen, BPA may interfere with thyroid hormone pathways. Based on work by Heimeier and Shi, BPA may affect human embryogenesis and neonatal development through disruption or inhibition of thyroid hormone pathways (Heimeier and Shi 2010). BPA binds to thyroid hormone receptors and can act as an antagonist to inhibit transcriptional activity stimulated by the thyroid hormone triiodothryonine (T3) (Moriyama, Tagami et al. 2002; Zoeller 2005). The affinity of BPA for thyroid hormone receptors is lower than the affinity for the estrogen receptor suggesting that high levels of BPA would be required to antagonize thyroid hormone action. However, in vitro studies have demonstrated that low levels of BPA can inhibit thyroid hormone receptor-mediated gene expression by enhancing recruitment of the co-repressor N-CoR to the thyroid hormone receptor (Moriyama, Tagami et al. 2002). Studies in animals indicate that offspring born to mothers exposed to BPA in diet during gestation and lactation had increased levels of thyroxine (T4) and increased expression of a thyroid responsive gene in the brain (Zoeller, Bansal et al. 2005). Along with the antagonizing actions on thyroid hormone receptors, BPA has also been shown to bind to human glucocorticoid receptors producing agonistic effects (Prasanth, Divya et al. 2010; Sargis, Johnson et al. 2010). Specifically perinatal BPA exposure led to increased baseline corticosterone levels and increased glucocorticoid receptor levels in the hippocampus (Poimenova, Markaki et al. 2010).
Finally there is accumulating evidence indicating that developmental exposure to BPA may alter the epigenome. Several in vivo studies have demonstrated changes in DNA methylation following early exposure to BPA. For example, BPA-exposed yellow agouti mice (Avy) had a shift in coat color toward yellow after BPA exposure, with the shift due to decreased DNA methylation in 9 cytosine-guanine dinucleotide (CPG) sites in the promoter region of the Avy gene (Dolinoy, Huang et al. 2007). In another study, maternal BPA exposure changed CpG methylation in the fetal forebrain on embryonic days 12–14 (Yaoi, Itoh et al. 2008). Most recently, changes in DNA methylation of HoxA10 was reported in the uterus of CD-1 mice exposed in utero to BPA (Bromer, Zhou et al. 2010). The hypomethylation of HoxA-10 following early exposure altered the developmental programming of gene expression and affected estrogen receptor binding to the HoxA-10 estrogen receptor element resulting in increased estrogen responsiveness.
3.4.2 Phthalates
Phthalates are a group of chemicals with a di-ester structure, containing a benzene ring with two ester functional groups. These compounds are commonly used as plasticizers, but they are also used in solvents, lubricating oils, fixatives, detergents and personal care products. Annually, more than 3 million metric tons of phthalates are consumed globally (Bizzari, OppenBerg et al. 2000), and because of their widespread use they are now ubiquitous in the environment leading to exposure of humans and wildlife. DEHP is the most abundant phthalate in the environment, related to its annual 2 million ton worldwide production; however other phthalates are also extensively used (DEP, DBP, DiBuP, DnBuP, BBP, DnBP, DiBP, DiNP, and DnOP). At present only phthalates with intermediate to long alkyl side-chain length in the ortho position have been demonstrated to exert potential reproductive and developmental toxicity in humans (Fabjan, Hulzebos et al. 2006). Phthalate esters and their metabolites have been detected in human breast milk (Lottrup, Andersson et al. 2006) and amniotic fluid (Silva, Reidy et al. 2004), and have been shown to cross the placenta leading to fetal exposure (Latini, De Felice et al. 2003). Phthalate esters possess endocrine-disrupting properties (Latini 2005), and exposure to high concentrations were shown to induce fetal death, cancer, malformations, liver and kidney injury and reproductive toxicity in animals (Lovekamp-Swan and Davis 2003; Hauser, Williams et al. 2005; Latini, Del Vecchio et al. 2006). The adverse effects observed in animals raise concerns as to whether exposure to phthalate esters in the environment represents a potential health risk for humans (Kavlock, Barr et al. 2006).
The existing data are insufficient to evaluate the reproductive and developmental effects of phthalates exposure in humans, but in animals there are strong indications that phthalates have the potential to adversely affect normal development and disrupt reproductive functions (Kavlock, Barr et al. 2006). Studies in animals have shown that phthalates can have a variety of adverse effects beyond endocrine disruption, including hepatic peroxisome proliferation and cancer, changes in the kidney and thyroid (Kavlock, Barr et al. 2006), and adverse effects on systems such as thyroid signaling (Jaakkola and Knight 2008), and immune function (Meeker, Calafat et al. 2007). However, the underlying mechanisms of these effects are not clear.
Initial mechanistic studies focused on phthalates actions as environmental estrogens or anti-androgens, however using screening assays it was determined that only the parent compounds were able to bind to steroid receptors, whereas the monoesters resulting from metabolism exert little or no affinity for both estrogen and androgen receptors (David 2006). Therefore, one key mechanistic step for phthalate toxicity is formation of the monoester prior to absorption from the gastrointestinal tract. However, research has also shown that the mode of action for phthalates depends upon developmental timing and dosing level. For example exposure of fetal versus adult rat Leydig cells produces different adverse effects, with fetal Leydig cells (Barlow, McIntyre et al. 2004; Ge, Chen et al. 2007) more sensitive to exposure then adult Leydig cells (Parks, Ostby et al. 2000; Ge, Chen et al. 2007). Furthermore when exposed to high doses, phthalates inhibit testosterone production in the developing offspring ultimately delaying puberty, while low doses enhance testosterone production leading to advanced onset of puberty (Ge, Chen et al. 2007). In females it appears that phthalates are capable of reducing plasma estradiol concentrations by reducing levels of aromatase, an enzyme which converts testosterone to estradiol (Lovekamp and Davis 2001; Lovekamp-Swan and Davis 2003).
In addition to the adverse effects of phthalates on reproductive function, phthalates have also been found to modulate DNA methylation. For example, treatment of MCF7 cells with phthalates at low concentrations lead to demethylation of estrogen receptor alpha promoter-associated CpG islands, suggesting that phthalates may disrupt endocrine function by epigenetic mechanisms (Kang and Lee 2005). There is also evidence that phthalates disrupt normal thyroid hormone function, and because regulation of thyroid hormone plays a critical role in brain development disruption of its signaling could have serious consequences. Serum thyroid hormone and thyroid-stimulating hormone levels were inversely correlated with urinary phthalate concentrations comparable with levels reported for men in the general US population (Meeker, Calafat et al. 2007). An association between phthalate exposure and thyroid hormone was also found in animal and human studies (Andra and Makris 2012; Boas, Feldt-Rasmussen et al. 2012), which strengthens the suggestion that phthalates may be able to disrupt the thyroid hormone system.
Both BPA and Phthalates confer their greatest influence through disruption of endocrine function, including sex hormone signaling. This is particularly concerning given the activational effects sex hormones have on social behaviors (Eisenegger, Haushofer et al. 2011). Moreover, brain development is regulated in part by endocrine signaling, with imbalances in sex hormone availability in utero hypothesized to contribute to the development of ASD in a subset of individuals (Auyeung, Taylor et al. 2010). Interestingly, there is considerable overlap across endocrine, immune, and nervous system function such that disruption in one system could significantly alter development of the other. This complicates the question of how exposure to an environmental toxicant contributes to developmental disorders as a single compound is likely working through multiple mechanisms of action (Figure 2). Moreover, the developmental consequences of environmental toxicant exposure may be dependent on the genetic susceptibility of an individual. Therefore mouse models should be used in future work to explore the potential interactions genetic and environmental insults have on neurodevelopment by employing a systematic approach to the study of developmental neurotoxicology.
Figure 2.
Complexity of gene and environment interactions in developmental disorders. Several environmental toxins have the potential to modify gene expression via epigenetic mechanisms (e.g., DNA methylation), or by directly interfering with many critical systems for normal brain development, including the endocrine system, central nervous system, and immune system. Disruption along these pathways can result in complex behavioral outcomes, and may contribute to the etiology of neurodevelopmental disorders including autism.
4.0 Directing animal model research in neurodevelopmental toxicology
Genetic studies have not yet identified casual genetic mutations or polymorphisms that underlie the vast majority of cases of ASD. As a result, a consensus is growing that there is “genetic complexity” to autism that involves multiple genes. However, acceptance of this premise does not rule out the possible contributions of other mechanisms in the etiology of autism, including those resulting from exposure to environmental contaminants. The less than 100% concordance among monozygotic twins also strongly supports the reframing of autism as a multisystem disorder brought about by both genetic influences and environmental factors. Indeed, it is quite likely that genetic predispositions for neurodevelopmental disorders such as autism are compounded by exposure to specific environmental toxicants. More specifically, the deleterious effects of specific gene mutations on normal brain development are hypothesized to be further disrupted by the presence of one or more toxicant(s) interfering with molecular and cellular pathways necessary for proper neurodevelopment. While this hypothesis provides novel avenues of exploration for the etiology of ASD, the massive and complex gene and toxicant databases make testing every permutation of gene-environment interactions in animal models costly and unfeasible.
To overcome this obstacle, developmental toxicology must employ the use of both top-down and bottom-up approaches to narrow the focus and identify the most prevalent and potent interactions that can be reduced to testable hypotheses in model systems, such as mouse models of neurodevelopmental disorders. Top-down approaches should be based in part on epidemiological research that identifies suspicious interactions between exposure to toxicants and the incidence of neurodevelopment disorders. For example, large-scale population-based databases such as those assembled by the National Health and Nutrition Examination Survey (NHANES) and Childhood Autism Risks from Genetics and the Environment (CHARGE) can be used to identify environmental pollutants associated with developmental toxicity (Hertz-Picciotto, Croen et al. 2006; Curtin, Mohadjer et al. 2012). This epidemiological approach has already uncovered relevant environmental pollutants that have led to revealing studies in mice on plausible gene-environment interactions.
A useful example can be seen from the study of brominated flame retardants. These persistent environmental pollutants are ubiquitous in the environment (Besis and Samara 2012), accumulate in relatively high levels in many human tissues (Costa and Giordano 2007), and epidemiological data has provided evidence of its relevance to autism (Hertz-Picciotto, Bergman et al. 2011). The fact that several persistent environmental pollutants are epigenetic modifiers (Rusiecki, Baccarelli et al. 2008; Baccarelli and Bollati 2009) led do the direct investigation of the role of developmental PBDE-47 exposure on brain and behavior development in a mouse model of Rett Syndrome (Woods, Vallero et al. 2012). Perinatal exposure to PBDE-47 led to sexually dimorphic hypomethylation of brain DNA and increased deficits in social behaviors and learning and memory in a mouse model of Rett syndrome (Woods, Vallero et al. 2012). These effects coincided with an increase in methyltransferase Dnmt3a, which was suggested by the authors to be the result of a compensatory mechanism in response to both the genetic mutation and environmental exposure to PBDE-47.
Findings from genome-wide association studies (GWAS) have also provided important leads to direct research on gene-environment interactions in autism. For example, mutations in the Reelin gene have been associated with autism (Kelemenova and Ostatnikova 2009). The Reelin protein is important for normal patterning of cortical development. Abnormalities in cortical anatomy are found in autism (Lakatosova and Ostatnikova 2012), and environmental agents that may affect Reelin levels or function could contribute to or worsen the autism phenotype. For instance, Laviola and colleagues identified complex alterations brought about by the interaction between the Reelin gene and prenatal exposure to the organophosphate pesticide chlorpyrifos (Laviola, Adriani et al. 2006). Studies such as these that are driven from findings in the population represent an important top-down guided approach that should be undertaken with mouse models of autism.
While epidemiological research has uncovered novel toxicants such as occupational exposure to solvents and freeway air pollution as possible contributing factors to ASD (Volk, Hertz-Picciotto et al. 2011; McCanlies, Fekedulegn et al. 2012), large population-based studies are limited in their ability to elucidate novel gene-environment interactions. One major limitation in identifying these interactions is the availability of genotypic data among individuals in the population, which significantly restricts the ability to identify important gene interactions in the etiology of ASD. However, computational modeling, as another top-down approach, can be applied to identify potential gene-environment interactions. The development of novel computational approaches in bioinformatics has led to the construction of statistical software such as the Gene-Environment interaction Simulator 2 (GENS2) which applies a Multi-Logistic Model to simulate gene-gene and gene-environment interaction (Pinelli, Scala et al. 2012). Such tools provide a computational approach to identifying relevant gene loci that may be perturbed by specific environmental toxicants. The Comparative Toxicogenomics Database is another source of information on chemical-gene interactions that is becoming increasingly detailed and useful (Wiegers, Davis et al. 2009). Together, epidemiology, GWAS studies and computation modeling should be used to sift through the large databases and uncover specific testable interactions between autism-relevant genes and persistent environmental pollutants. The findings from these large-scale analyses can then be used to direct the use of animal models to test the predicted outcomes and identify causal mechanisms for the development of targeted therapy and treatment.
In parallel with epidemiological research to focus the search for relevant gene-environment interactions in the etiology of ASD, the development of “bottom-up” in vitro methods will also be key to systematically and efficiently identifying and testing specific hypotheses about gene-environmental interactions. In vitro toxicological approaches have been used for many years in pharmaceutical and neurotoxicological screening, where multiple toxicant combinations at varying concentrations and exposure periods can be studied to identify potential high-risk candidate toxicants. These in vitro systems include isolated cells, cell cultures and tissue slices developed from human and rodent tissues. An example of in vitro studies derived from an initial in vivo observation can be seen in a series of studies on PBDE-47 developmental neurotoxicity (Dingemans, van den Berg et al. 2011). In these studies brief exposure to polybrominated flame retardants on postnatal day 10 in C57BL/6J mice impairs memory and learning in vivo. Furthermore, in vitro hippocampal brain slices showed that long-term potentiation, a model of synaptic plasticity that is believed to underlie some forms of memory, was impaired and at the cellular level the impaired plasticity was associated with a change in NMDA receptor subunit composition (Dingemans, Ramakers et al. 2007). These types of studies are ongoing in several laboratories but relatively few studies have used this approach for screening and examining chemicals that may be specifically relevant to autism. Moreover, there are very few, if any, published in vitro studies examining the effects of an environmental toxicant in cell or brain slices isolated from mice that have been developed or used to model autism. This is clearly an area of research that needs to be developed in the neurotoxicology field.
Another potentially powerful approach is the use of inducible pluripotent stem cells (iPSCs) that can be employed to identify chemicals that interact with specific genes unique to a specific neurodevelopmental disorder. iPSC’s are pluripotent stem cells isolated from mouse and human somatic cells that have the potential to be redifferentiated into many different cell types, including neurons and astrocytes. This technology offers a powerful approach for identifying environmental chemicals that interact with specific genetic risk factors for neurodevelopmental disorders (Kumar, Aboud et al. 2012). For example, cortical precursor neurons have been derived from induced pluripotent stem cells (iPSC) isolated from individuals with Timothy’s syndrome. Phenotypic analysis of these cultured neurons revealed unique deficits in calcium signaling (Pasca, Portmann et al. 2011). Using similar techniques, specific iPSCs could be generated from a subgroup of ASD individuals with a known genetic mutation (e.g. FMR1) and tested for their response to a diverse range of toxicant exposures. This approach would serve to both screen potential gene-environment interaction as well as identify novel mechanisms of action that can be further elucidated using in vivo mouse models in which brain development and neurological function can be studied. Ultimately, both top-down and bottom-up approaches to the etiology of ASD will require the use of animal models to substantiate a causal link between gene-environment interactions and the expression of ASD-like behaviors.
The importance of animal models in evaluating the effects of gene-environment interaction on ASD-like behaviors necessitates a careful consideration of the behavioral assessments available and the methodological limitations of their use. Together, these tools must be exploited to increase efficiency while simultaneously reducing bias and confounding variables. For example, the fully automated three-chamber social approach box provides a high-throughput behavioral screening task that serves to both increase efficacy and reduce experimental bias (Nadler, Moy et al. 2004). While considerable efforts have been made to develop a battery of other mouse behavioral assays that can be used to identify deficits across the three core domains of autism (Crawley 2007; Jaubert, Golub et al. 2007; Roullet and Crawley 2011) (Table 1), these tasks vary in the degree to which they rely on subjective experimenter observations to quantify behavior and behavioral deficits that reflect the core behaviors of autism. Thus there remains an urgent need to validate and improve many of these existing tasks and to develop new behavioral assays that are fully automated and as free from investigator bias as possible.
Table 1.
Mouse behavioral tests related to deficits in the three core domains of autism spectrum disorders. For detailed descriptions and methods of each task see for example Crawley 2007; Moy, Nadler et al. 2007; Amodeo, Jones et al. 2012
| ASD Signs | Mouse Task | Behavioral Measures |
|---|---|---|
| Abnormal social approach, lack of initiation of social interaction1 | Three-chambered social approach |
|
| Deficits in developmentally appropriate play1 | Dyadic social interaction
|
|
| Deficits in understanding non-verbal communication2 | Social transmission of food preference |
|
| Poorly integrated verbal communication; stereotyped or repetitive use of speech2,3 | Ultrasonic vocalizations elicited during:
|
|
| Excessive adherence to routines or excessive resistance to change3 | Y-maze, Morris Water Maze |
|
| Stereotyped or repetitive motor movements3 | Self-groomig |
|
| Marble burying |
|
|
| Hyper- or hypo-reactivity to sensory input | Acoustic startle |
|
| Pre-pulse inhibition |
|
Core domains of ASD as noted in the DSM-IV-TR (American Psychological Association 2006)
Impaired social interactions
Deficits in communication
Restricted and repetitive behaviors
In addition to leveraging existing epidemiological and computational data to direct the selection of relevant genetic mice, a more global consideration of mouse genetics must be discussed to ensure proper controls have been met. The development of inbred mouse strains has resulted in unique mouse breeds with distinct and well-characterized genotypes. While these strain differences provide a more homogenous sample of subjects, their uses also limit the ability of mouse studies to accurately model the heterogeneity of human populations. Indeed, the effects of single gene mutations and environmental insults can have profoundly different behavioral outcomes depending on the background strain used. Therefore, one must carefully consider which strain to use for toxicology testing and behavioral profiling. Strain specific differences might result from direct gene-gene and gene-environment interactions or may simply manifest from deficits in basic sensory or motor function. For example, the C57Bl/6J mice, frequently used as wild-type controls, develop deafness in later months (Erway, Shiau et al. 1996) and the FVB/N, often used for development of transgenic mutants, possess two unrelated genes resulting in blindness (Gimenez and Montoliu 2001). Similarly, there are substantial mouse strain differences in performance in tests of social interactions (Moy, Nadler et al. 2007). These factors must be considered when deciding on the appropriateness of a particular behavioral task or the effects of a particular toxicant.
While it is important to consider controlling for strain effects by selecting mice based on inherent sensitivity, it is likely that the direct mechanisms of action of a toxicant are unknown. If an experimental design is restricted to a single mouse strain, then the possibility of uncovering alternative pathways or unidentified genetic differences that may also contribute to the behavioral outcome is severely limited. Therefore, it would be beneficial to test hypotheses across multiple strains and in mutant mice, especially if the mechanisms of action are unknown. The resulting data can more effectively reveal novel genes as protective or permissive risk factors to adverse effects of environmental toxicant exposure. One approach is to use the Mouse Phenome database from Jackson Laboratories to identify a variety of strains based on desired constraints. This method has already led to the discovery of the BTBR mouse as a novel model for ASD by surveying the top tier of recommended mouse strains (Moy, Nadler et al. 2007). Additionally, a new mouse resource known as the Collaborative Cross has been developed to overcome the limited genetic diversity of inbred mice. By crossing 8 genetically diverse strains, the Collaborative Cross can produce an unprecedented diversity of genetic interactions that recapitulates the types of genetic diversity found in humans (Churchill, Airey et al. 2004). Taken together, the use of fully automated high-throughput behavioral screening and a more diverse genetic population of mice can more efficiently and more accurately uncover relevant genetic and environmental factors contributing to ASD. Moreover, the use of epidemiological research and in vitro screening can provide specific and testable interactions that can be assessed in mouse models that will ultimately direct the development of targeted treatments and revise risk assessment strategies for ASD.
5. Conclusion
A recent study of twin pairs with ASD reported that the environmental factors common to twins could explain about 55% of the variability in behavioral deficits, and although genetic factors also play an important role, they are substantially lower in magnitude than estimates from prior twin studies of autism (Hallmayer, Cleveland et al. 2011). This finding substantiates the importance of evaluating the role of gene and environment interactions in developmental disorders. Further it suggests that there are subpopulations present with genetic predispositions for developmental disorders and that a second “hit,” such as an exposure to an environmental pollutant, may be required for the disorder to manifest.
Mouse models provide a unique tool for understanding how neurotoxicant exposures may contribute to the increased prevalence of neurodevelopmental disorders, including ASD. It is recommended that the investigation of gene-environment interactions be approached by selecting mouse models that are appropriate for the questions being asked about the toxicant under examination. However, it should be noted that toxicants can interfere with development through multiple mechanisms as summarized in this review. In addition, genetic and environmental mechanisms underlying abnormal brain development may occur via separate pathways, with damage to either alone insufficient to alter neurodevelopment. It will therefore be important to identify points of convergence between pathways in order to understand the complexity of gene-environmental interactions in autism. A similar notion was previously postulated by Vonieagu and colleagues who suggested that the behavioral and cognitive deficits in ASD may manifest from convergent gene and environmental mechanisms acting together to disrupt downstream brain connectivity (Voineagu, Wang et al. 2011). The task of sorting out the role of gene-environment interactions in autism and other neurodevelopmental disorders will be difficult, but it is hoped that the approaches put forth in this review will serve as a starting point in the process. If successful, the ensuing results should aid in the prevention and eventual development of treatments for neurodevelopmental disorders.
Highlights.
Review major mouse models used to study autism with a focus on genetic mechanisms
Review of toxic mechanisms of environmental chemicals that may contribute to autism
Discussion of research strategies to evaluate gene-environment interactions in autism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nature reviews. Genetics. 2008;9(5):341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J, Vorhees CV, et al. Developmental neurotoxicity of anticonvulsants: human and animal evidence on phenytoin. Neurotoxicol Teratol. 1990;12(3):203–214. doi: 10.1016/0892-0362(90)90092-q. [DOI] [PubMed] [Google Scholar]
- Ahlbom J, Fredriksson A, et al. Neonatal exposure to a type-I pyrethroid (bioallethrin) induces dose-response changes in brain muscarinic receptors and behaviour in neonatal and adult mice. Brain Res. 1994;645(1–2):318–324. doi: 10.1016/0006-8993(94)91666-7. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Costa AC, et al. Selective blockade of NMDA-activated channel currents may be implicated in learning deficits caused by lead. FEBS Lett. 1990;261(1):124–130. doi: 10.1016/0014-5793(90)80652-y. [DOI] [PubMed] [Google Scholar]
- Allen JR, Barsotti DA. The effects of transplacental and mammary movement of PCBs on infant rhesus monkeys. Toxicology. 1976;6(3):331–340. doi: 10.1016/0300-483x(76)90037-8. [DOI] [PubMed] [Google Scholar]
- Alm H, Scholz B, et al. Proteomic evaluation of neonatal exposure to 2,2,4,4,5-pentabromodiphenyl ether. Environmental health perspectives. 2006;114(2):254–259. doi: 10.1289/ehp.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Laribi O, et al. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environmental health perspectives. 2005;113(8):969–977. doi: 10.1289/ehp.8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association. Diagnostic and Statistical Manual of Mental Disordrs (Revised 4th ed.) Washington, DC: 2006. [Google Scholar]
- Amodeo DA, Jones JH, et al. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav Brain Res. 2012;227(1):64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andra SS, Makris KC. Thyroid disrupting chemicals in plastic additives and thyroid health. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2012;30(2):107–151. doi: 10.1080/10590501.2012.681487. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, et al. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain, behavior, and immunity. 2011;25(1):40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison WD, Hare MF. Mechanisms of methylmercury-induced neurotoxicity. FASEB J. 1994;8(9):622–629. doi: 10.1096/fasebj.8.9.7516300. [DOI] [PubMed] [Google Scholar]
- Atchison WD, Joshi U, et al. Irreversible suppression of calcium entry into nerve terminals by methylmercury. J Pharmacol Exp Ther. 1986;238(2):618–624. [PubMed] [Google Scholar]
- Atchison WD, Narahashi T. Methylmercury-induced depression of neuromuscular transmission in the rat. Neurotoxicology. 1982;3(3):37–50. [PubMed] [Google Scholar]
- Auyeung B, Taylor K, et al. Foetal testosterone and autistic traits in 18 to 24-month-old children. Mol Autism. 2010;1(1):11. doi: 10.1186/2040-2392-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Opin Pediatr. 2009;21(2):243–251. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader PL, Faizi M, et al. Mouse model of Timothy syndrome recapitulates triad of autistic traits. Proc Natl Acad Sci U S A. 2011;108(37):15432–15437. doi: 10.1073/pnas.1112667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KB, Wray SP, et al. Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain Behav. 2010;9(6):562–574. doi: 10.1111/j.1601-183X.2010.00585.x. [DOI] [PubMed] [Google Scholar]
- Bakker CE, C V, et al. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78(1):23–33. [PubMed] [Google Scholar]
- Barlow NJ, McIntyre BS, et al. Male reproductive tract lesions at 6, 12, and 18 months of age following in utero exposure to di(n-butyl) phthalate. Toxicologic pathology. 2004;32(1):79–90. doi: 10.1080/01926230490265894. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, et al. The mGluR theory of fragile X mental retardation. Trends in neurosciences. 2004;27(7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Very low lead exposures and children's neurodevelopment. Curr Opin Pediatr. 2008;20(2):172–177. doi: 10.1097/MOP.0b013e3282f4f97b. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat Neurosci. 2006;9(10):1221–1225. doi: 10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- Belyaeva EA, Dymkowska D, et al. Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol Appl Pharmacol. 2008;231(1):34–42. doi: 10.1016/j.taap.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol Sci. 2004;80(2):288–295. doi: 10.1093/toxsci/kfh153. [DOI] [PubMed] [Google Scholar]
- Beri S, Tonna N, et al. DNA methylation regulates tissue-specific expression of Shank3. J Neurochem. 2007;101(5):1380–1391. doi: 10.1111/j.1471-4159.2007.04539.x. [DOI] [PubMed] [Google Scholar]
- Besis A, Samara C. Polybrominated diphenyl ethers (PBDEs) in the indoor and outdoor environments - A review on occurrence and human exposure. Environ Pollut. 2012 doi: 10.1016/j.envpol.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Bienvenu T, Chelly J. Molecular genetics of Rett syndrome: when DNA methylation goes unrecognized. Nat Rev Genet. 2006;7(6):415–426. doi: 10.1038/nrg1878. [DOI] [PubMed] [Google Scholar]
- Bilousova TV, Dansie L, et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46(2):94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environmental health perspectives. 2004;112(1):9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzari S, OppenBerg B, et al. Chemical economics handbook. Palo Alto, CA: SRI International; 2000. Plasticizers. [Google Scholar]
- Blatt GJ. GABAergic cerebellar system in autism: a neuropathological and developmental perspective. Int Rev Neurobiol. 2005;71:167–178. doi: 10.1016/s0074-7742(05)71007-2. [DOI] [PubMed] [Google Scholar]
- Boas M, Feldt-Rasmussen U, et al. Thyroid effects of endocrine disrupting chemicals. Molecular and cellular endocrinology. 2012;355(2):240–248. doi: 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Bontekoe CJ, Bakker CE, et al. Instability of a (CGG)98 repeat in the Fmr1 promoter. Human molecular genetics. 2001;10(16):1693–1699. doi: 10.1093/hmg/10.16.1693. [DOI] [PubMed] [Google Scholar]
- Boulton CL, Southam E, et al. Nitric oxide-dependent long-term potentiation is blocked by a specific inhibitor of soluble guanylyl cyclase. Neuroscience. 1995;69(3):699–703. doi: 10.1016/0306-4522(95)00349-n. [DOI] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1(1):15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga MF, Pereira EF, et al. Nanomolar concentrations of lead inhibit glutamatergic and GABAergic transmission in hippocampal neurons. Brain Res. 1999;826(1):22–34. doi: 10.1016/s0006-8993(99)01194-4. [DOI] [PubMed] [Google Scholar]
- Bromer JG, Zhou Y, et al. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24(7):2273–2280. doi: 10.1096/fj.09-140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budimirovic DB, Kaufmann WE. What can we learn about autism from studying fragile X syndrome? Developmental neuroscience. 2011;33(5):379–394. doi: 10.1159/000330213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke K, Cheng Y, et al. Methylmercury elicits rapid inhibition of cell proliferation in the developing brain and decreases cell cycle regulator, cyclin E. Neurotoxicology. 2006;27(6):970–981. doi: 10.1016/j.neuro.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Silverman JM, et al. Association between a GABRB3 polymorphism and autism. Mol Psychiatry. 2002;7(3):311–316. doi: 10.1038/sj.mp.4001011. [DOI] [PubMed] [Google Scholar]
- Caglayan AO. Genetic causes of syndromic and non-syndromic autism. Dev Med Child Neurol. 2010;52(2):130–138. doi: 10.1111/j.1469-8749.2009.03523.x. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, et al. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environmental health perspectives. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfa G, Percy AK, et al. Experimental models of Rett syndrome based on Mecp2 dysfunction. Exp Biol Med (Maywood) 2011;236(1):3–19. doi: 10.1258/ebm.2010.010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Van de Water J, et al. Immune dysfunction in autism: a pathway to treatment. Neurotherapeutics. 2010;7(3):283–292. doi: 10.1016/j.nurt.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chemical research in toxicology. 2004;17(8):983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol Biochem Behav. 2011;97(3):586–594. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Chauhan KR, Kodavanti PR, et al. Assessing the role of ortho-substitution on polychlorinated biphenyl binding to transthyretin, a thyroxine transport protein. Toxicol Appl Pharmacol. 2000;162(1):10–21. doi: 10.1006/taap.1999.8826. [DOI] [PubMed] [Google Scholar]
- Chen HH, Ma T, et al. Developmental lead exposure and two-way active avoidance training alter the distribution of protein kinase C activity in the rat hippocampus. Neurochem Res. 1997;22(9):1119–1125. doi: 10.1023/a:1027365202328. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, et al. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27(3):327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Hsu CC. Effects of prenatal exposure to PCBs on the neurological function of children: a neuropsychological and neurophysiological study. Dev Med Child Neurol. 1994;36(4):312–320. doi: 10.1111/j.1469-8749.1994.tb11851.x. [DOI] [PubMed] [Google Scholar]
- Chevrier J, Harley KG, et al. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environmental health perspectives. 2010;118(10):1444–1449. doi: 10.1289/ehp.1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching TT, Maunakea AK, et al. Epigenome analyses using BAC microarrays identify evolutionary conservation of tissue-specific methylation of SHANK3. Nat Genet. 2005;37(6):645–651. doi: 10.1038/ng1563. [DOI] [PubMed] [Google Scholar]
- Churchill GA, Airey DC, et al. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat Genet. 2004;36(11):1133–1137. doi: 10.1038/ng1104-1133. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Vyas JB, et al. Mechanisms of mercury disposition in the body. Am J Ind Med. 2007;50(10):757–764. doi: 10.1002/ajim.20476. [DOI] [PubMed] [Google Scholar]
- Coburn CG, Curras-Collazo MC, et al. In vitro effects of environmentally relevant polybrominated diphenyl ether (PBDE) congeners on calcium buffering mechanisms in rat brain. Neurochem Res. 2008;33(2):355–364. doi: 10.1007/s11064-007-9430-x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Gabel HW, et al. Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function. Neuron. 2011;72(1):72–85. doi: 10.1016/j.neuron.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WT, Capen CC. Ultrastructural and functional alterations of the rat thyroid gland produced by polychlorinated biphenyls compared with iodide excess and deficiency, and thyrotropin and thyroxine administration. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;33(3):213–231. doi: 10.1007/BF02899183. [DOI] [PubMed] [Google Scholar]
- Collins WT, Jr, Capen CC. Biliary excretion of 125I-thyroxine and fine structural alterations in the thyroid glands of Gunn rats fed polychlorinated biphenyls (PCB) Lab Invest. 1980;43(2):158–164. [PubMed] [Google Scholar]
- Company R, Serafim A, et al. Effect of cadmium, copper and mercury on antioxidant enzyme activities and lipid peroxidation in the gills of the hydrothermal vent mussel Bathymodiolus azoricus. Mar Environ Res. 2004;58(2–5):377–381. doi: 10.1016/j.marenvres.2004.03.083. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Lindgren V, et al. Autism or atypical autism in maternally but not paternally derived proximal 15q duplication. American journal of human genetics. 1997;60(4):928–934. [PMC free article] [PubMed] [Google Scholar]
- Cook EH, Jr, Scherer SW. Copy-number variations associated with neuropsychiatric conditions. Nature. 2008;455(7215):919–923. doi: 10.1038/nature07458. [DOI] [PubMed] [Google Scholar]
- Correia CT, Coutinho AM, et al. Increased BDNF levels and NTRK2 gene association suggest a disruption of BDNF/TrkB signaling in autism. Genes, brain, and behavior. 2010;9(7):841–848. doi: 10.1111/j.1601-183X.2010.00627.x. [DOI] [PubMed] [Google Scholar]
- Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366(1–2):1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28(6):1047–1067. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain pathology. 2007;17(4):448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton KM, Kodavanti PR, et al. PCBs, thyroid hormones, and ototoxicity in rats: cross-fostering experiments demonstrate the impact of postnatal lactation exposure. Toxicol Sci. 2000;57(1):131–140. doi: 10.1093/toxsci/57.1.131. [DOI] [PubMed] [Google Scholar]
- Curtin LR, Mohadjer LK, et al. The National Health and Nutrition Examination Survey: Sample Design, 1999–2006. Vital Health Stat. 2012;2(155):1–39. [PubMed] [Google Scholar]
- Dam K, Seidler FJ, et al. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Brain Res Dev Brain Res. 2000;121(2):179–187. doi: 10.1016/s0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- David RM. Proposed mode of action for in utero effects of some phthalate esters on the developing male reproductive tract. Toxicologic pathology. 2006;34(3):209–219. doi: 10.1080/01926230600642625. [DOI] [PubMed] [Google Scholar]
- Delahunty C, Falconer S, et al. Levels of neonatal thyroid hormone in preterm infants and neurodevelopmental outcome at 5 1/2 years: millennium cohort study. J Clin Endocrinol Metab. 2010;95(11):4898–4908. doi: 10.1210/jc.2010-0743. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Handforth A, et al. Mice lacking the beta3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. J Neurosci. 1998;18(20):8505–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, et al. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behav Brain Res. 2008;187(2):207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhossche D, Applegate H, et al. Elevated plasma gamma-aminobutyric acid (GABA) levels in autistic youngsters: stimulus for a GABA hypothesis of autism. Med Sci Monit. 2002;8(8):PR1–PR6. [PubMed] [Google Scholar]
- Dietrich KN. Human fetal lead exposure: intrauterine growth, maturation, and postnatal neurobehavioral development. Fundam Appl Toxicol. 1991;16(1):17–19. doi: 10.1016/0272-0590(91)90128-q. [DOI] [PubMed] [Google Scholar]
- Dingemans MM, Ramakers GM, et al. Neonatal exposure to brominated flame retardant BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environmental health perspectives. 2007;115(6):865–870. doi: 10.1289/ehp.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MM, Ramakers GM, et al. Neonatal exposure to brominated flame retardant BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environ Health Perspect. 2007;115(6):865–870. doi: 10.1289/ehp.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MM, van den Berg M, et al. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect. 2011;119(7):900–907. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, et al. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104(32):13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenegger C, Haushofer J, et al. The role of testosterone in social interaction. Trends Cogn Sci. 2011;15(6):263–271. doi: 10.1016/j.tics.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, et al. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. American journal of epidemiology. 2007;165(12):1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Erway LC, Shiau YW, et al. Genetics of age-related hearing loss in mice. III. Susceptibility of inbred and F1 hybrid strains to noise-induced hearing loss. Hear Res. 1996;93(1–2):181–187. doi: 10.1016/0378-5955(95)00226-x. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environmental health perspectives. 2007;115(5):792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton MR, Blaiss CA, et al. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci U S A. 2009;106(42):17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabjan E, Hulzebos E, et al. A category approach for reproductive effects of phthalates. Critical reviews in toxicology. 2006;36(9):695–726. doi: 10.1080/10408440600894914. [DOI] [PubMed] [Google Scholar]
- Fan CY, Besas J, et al. Changes in mitogen-activated protein kinase in cerebellar granule neurons by polybrominated diphenyl ethers and polychlorinated biphenyls. Toxicology and applied pharmacology. 2010;245(1):1–8. doi: 10.1016/j.taap.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, et al. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52(8):805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Feng J, Schroer R, et al. High frequency of neurexin 1beta signal peptide structural variants in patients with autism. Neuroscience letters. 2006;409(1):10–13. doi: 10.1016/j.neulet.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Foley AG, Gannon S, et al. Class I histone deacetylase inhibition ameliorates social cognition and cell adhesion molecule plasticity deficits in a rodent model of autism spectrum disorder. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.05.042. [DOI] [PubMed] [Google Scholar]
- Franco JL, Braga HC, et al. Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chemical research in toxicology. 2007;20(12):1919–1926. doi: 10.1021/tx7002323. [DOI] [PubMed] [Google Scholar]
- Gauthier J, Spiegelman D, et al. Novel de novo SHANK3 mutation in autistic patients. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):421–424. doi: 10.1002/ajmg.b.30822. [DOI] [PubMed] [Google Scholar]
- Gavazzo P, Zanardi I, et al. Molecular determinants of Pb2+ interaction with NMDA receptor channels. Neurochem Int. 2008;52(1–2):329–337. doi: 10.1016/j.neuint.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Ge RS, Chen GR, et al. Biphasic effects of postnatal exposure to diethylhexylphthalate on the timing of puberty in male rats. Journal of andrology. 2007;28(4):513–520. doi: 10.2164/jandrol.106.001909. [DOI] [PubMed] [Google Scholar]
- Geppert M, Khvotchev M, et al. Neurexin I alpha is a major alpha-latrotoxin receptor that cooperates in alpha-latrotoxin action. The Journal of biological chemistry. 1998;273(3):1705–1710. doi: 10.1074/jbc.273.3.1705. [DOI] [PubMed] [Google Scholar]
- Gimenez E, Montoliu L. A simple polymerase chain reaction assay for genotyping the retinal degeneration mutation (Pdeb(rd1)) in FVB/N-derived transgenic mice. Lab Anim. 2001;35(2):153–156. doi: 10.1258/0023677011911525. [DOI] [PubMed] [Google Scholar]
- Giordano G, Kavanagh TJ, et al. Neurotoxicity of a polybrominated diphenyl ether mixture (DE-71) in mouse neurons and astrocytes is modulated by intracellular glutathione levels. Toxicology and applied pharmacology. 2008;232(2):161–168. doi: 10.1016/j.taap.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glessner JT, Wang K, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459(7246):569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Leblanc JJ, et al. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord. 2009;1(2):172–181. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines PE, Croen LA, et al. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, et al. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116(2):291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould JC, Leonard LS, et al. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Molecular and cellular endocrinology. 1998;142(1–2):203–214. doi: 10.1016/s0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, et al. Cognitive performance of children prenatally exposed to "safe" levels of methylmercury. Environ Res. 1998;77(2):165–172. doi: 10.1006/enrs.1997.3804. [DOI] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140(5):704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Zhang J, et al. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science. 2010;329(5992):643–648. doi: 10.1126/science.1190830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, et al. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27(3):322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Aicardi J, et al. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann Neurol. 1983;14(4):471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- Hagerman R. The physical and behavioral phenotype. In: Hagerman R, Hagerman P, editors. Fragile X Syndrome: Diagnosis, Treatment, and Research.3. Baltimore: The Johns Hopkins University Press; 2002. pp. 3–109. [Google Scholar]
- Hagerman R, Lauterborn J, et al. Fragile X syndrome and targeted treatment trials. Results Probl Cell Differ. 2012;54:297–335. doi: 10.1007/978-3-642-21649-7_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Archives of general psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Marks AR, et al. PBDE concentrations in women's serum and fecundability. Environmental health perspectives. 2010;118(5):699–704. doi: 10.1289/ehp.0901450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006;140A(17):1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Hauser R, Williams P, et al. Evidence of interaction between polychlorinated biphenyls and phthalates in relation to human sperm motility. Environmental health perspectives. 2005;113(4):425–430. doi: 10.1289/ehp.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann C, Grandjean P, et al. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006;3(8):e311. doi: 10.1371/journal.pmed.0030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimeier RA, Shi YB. Amphibian metamorphosis as a model for studying endocrine disruption on vertebrate development: effect of bisphenol A on thyroid hormone action. General and comparative endocrinology. 2010;168(2):181–189. doi: 10.1016/j.ygcen.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Heo Y, Zhang Y, et al. Aberrant immune responses in a mouse with behavioral disorders. PloS one. 2011;6(7):e20912. doi: 10.1371/journal.pone.0020912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Bergman A, et al. Polybrominated diphenyl ethers in relation to autism and developmental delay: a case-control study. Environ Health. 2011;10(1):1. doi: 10.1186/1476-069X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, et al. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environmental health perspectives. 2006;114(7):1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Dyer-Friedman J, et al. The influence of environmental and genetic factors on behavior problems and autistic symptoms in boys and girls with fragile X syndrome. Pediatrics. 2001;108(5):E88. doi: 10.1542/peds.108.5.e88. [DOI] [PubMed] [Google Scholar]
- Heulens I, D'Hulst C, et al. Pharmacological treatment of fragile X syndrome with GABAergic drugs in a knockout mouse model. Behav Brain Res. 2012;229(1):244–249. doi: 10.1016/j.bbr.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Holmes LB, Harvey EA, et al. The teratogenicity of anticonvulsant drugs. N Engl J Med. 2001;344(15):1132–1138. doi: 10.1056/NEJM200104123441504. [DOI] [PubMed] [Google Scholar]
- Homanics GE, DeLorey TM, et al. Mice devoid of gamma-aminobutyrate type A receptor beta3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc Natl Acad Sci U S A. 1997;94(8):4143–4148. doi: 10.1073/pnas.94.8.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, et al. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99(11):7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo ER, Brandebourg TD, et al. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environmental health perspectives. 2008;116(12):1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Arque G, et al. Mouse models of the fragile × premutation and the fragile X associated tremor/ataxia syndrome. Results Probl Cell Differ. 2012;54:255–269. doi: 10.1007/978-3-642-21649-7_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Rohr JK, et al. Co-occurring diagnoses among FMR1 premutation allele carriers. Clin Genet. 2010;77(4):374–381. doi: 10.1111/j.1399-0004.2009.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icenogle LM, Christopher NC, et al. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicol Teratol. 2004;26(1):95–101. doi: 10.1016/j.ntt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Nagata K, et al. Fipronil modulation of GABAA receptor single-channel currents. Pest Manag Sci. 2004;60(5):487–492. doi: 10.1002/ps.830. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Zhao X, et al. Fipronil modulation of gamma-aminobutyric acid(A) receptors in rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 2001;296(3):914–921. [PubMed] [Google Scholar]
- Jaakkola JJ, Knight TL. The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: a systematic review and meta-analysis. Environmental health perspectives. 2008;116(7):845–853. doi: 10.1289/ehp.10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996;335(11):783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34(1):27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Radyushkin K, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105(5):1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert PJ, Golub MS, et al. Complex, multimodal behavioral profile of the Homer1 knockout mouse. Genes Brain Behav. 2007;6(2):141–154. doi: 10.1111/j.1601-183X.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Tsai TF, et al. Imprinting in Angelman and Prader-Willi syndromes. Curr Opin Genet Dev. 1998;8(3):334–342. doi: 10.1016/s0959-437x(98)80091-9. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Pan Y, et al. Altered ultrasonic vocalization and impaired learning and memory in Angelman syndrome mouse model with a large maternal deletion from Ube3a to Gabrb3. PloS one. 2010;5(8):e12278. doi: 10.1371/journal.pone.0012278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorissen J. Literature review. Outcomes associated with postnatal exposure to polychlorinated biphenyls (PCBs) via breast milk. Advances in neonatal care : official journal of the National Association of Neonatal Nurses. 2007;7(5):230–237. doi: 10.1097/01.ANC.0000296630.03798.ba. [DOI] [PubMed] [Google Scholar]
- Juang MS, Yonemura K. Increased spontaneous transmitter release from presynaptic nerve terminal by methylmercuric chloride. Nature. 1975;256(5514):211–213. doi: 10.1038/256211a0. [DOI] [PubMed] [Google Scholar]
- Juarez de Ku LM, Sharma-Stokkermans M, et al. Thyroxine normalizes polychlorinated biphenyl (PCB) dose-related depression of choline acetyltransferase (ChAT) activity in hippocampus and basal forebrain of 15-day-old rats. Toxicology. 1994;94(1–3):19–30. doi: 10.1016/0300-483x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Kang SC, Lee BM. DNA methylation of estrogen receptor alpha gene by phthalates. Journal of toxicology and environmental health. Part A. 2005;68(23–24):1995–2003. doi: 10.1080/15287390491008913. [DOI] [PubMed] [Google Scholar]
- Kavlock R, Barr D, et al. NTP-CERHR Expert Panel Update on the Reproductive and Developmental Toxicity of di(2-ethylhexyl) phthalate. Reproductive toxicology. 2006;22(3):291–399. doi: 10.1016/j.reprotox.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Kazantzis G, Al-Mufti AW, et al. Epidemiology of organomercury poisoning in Iraq. II. Relationship of mercury levels in blood and hair to exposure and to clinical findings. Bull World Health Organ. 1976;53(suppl):37–48. [PMC free article] [PubMed] [Google Scholar]
- Kelemenova S, Ostatnikova D. Neuroendocrine pathways altered in autism. Special role of reelin. Neuro Endocrinol Lett. 2009;30(4):429–436. [PubMed] [Google Scholar]
- Keller JM, McClellan-Green PD, et al. Effects of organochlorine contaminants on loggerhead sea turtle immunity: comparison of a correlative field study and in vitro exposure experiments. Environmental health perspectives. 2006;114(1):70–76. doi: 10.1289/ehp.8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, et al. Disruption of neurexin 1 associated with autism spectrum disorder. American journal of human genetics. 2008;82(1):199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Inan SY, et al. Excitatory and inhibitory synaptic transmission is differentially influenced by two ortho-substituted polychlorinated biphenyls in the hippocampal slice preparation. Toxicology and applied pharmacology. 2009;237(2):168–177. doi: 10.1016/j.taap.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Inan SY, et al. Excitatory and inhibitory synaptic transmission is differentially influenced by two ortho-substituted polychlorinated biphenyls in the hippocampal slice preparation. Toxicol Appl Pharmacol. 2009;237(2):168–177. doi: 10.1016/j.taap.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Pessah IN. Perinatal exposure to environmental polychlorinated biphenyls sensitizes hippocampus to excitotoxicity ex vivo. Neurotoxicology. 2011;32(6):981–985. doi: 10.1016/j.neuro.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Pickup S, et al. Association between sociability and diffusion tensor imaging in BALB/cJ mice. NMR Biomed. 2012;25(1):104–112. doi: 10.1002/nbm.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti PR, Derr-Yellin EC. Differential effects of polybrominated diphenyl ethers and polychlorinated biphenyls on [3H]arachidonic acid release in rat cerebellar granule neurons. Toxicol Sci. 2002;68(2):451–457. doi: 10.1093/toxsci/68.2.451. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Kannan N, et al. Differential effects of two lots of aroclor 1254: congener-specific analysis and neurochemical end points. Environ Health Perspect. 2001;109(11):1153–1161. doi: 10.1289/ehp.011091153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti PR, Tilson HA. Neurochemical effects of environmental chemicals: in vitro and in vivo correlations on second messenger pathways. Ann N Y Acad Sci. 2000;919:97–105. doi: 10.1111/j.1749-6632.2000.tb06872.x. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Ward TR. Differential effects of commercial polybrominated diphenyl ether and polychlorinated biphenyl mixtures on intracellular signaling in rat brain in vitro. Toxicol Sci. 2005;85(2):952–962. doi: 10.1093/toxsci/kfi147. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Ward TR, et al. Polybrominated diphenyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure-activity relationships. Toxicol Sci. 2005;88(1):181–192. doi: 10.1093/toxsci/kfi289. [DOI] [PubMed] [Google Scholar]
- Kojima H, Takeuchi S, et al. Nuclear hormone receptor activity of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in transactivation assays using Chinese hamster ovary cells. Environmental health perspectives. 2009;117(8):1210–1218. doi: 10.1289/ehp.0900753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar KK, Aboud AA, et al. The potential of induced pluripotent stem cells as a translational model for neurotoxicological risk. Neurotoxicology. 2012;33(3):518–529. doi: 10.1016/j.neuro.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Gamma EF, Paneth N. Clinical importance of hypothyroxinemia in the preterm infant and a discussion of treatment concerns. Current opinion in pediatrics. 2012 doi: 10.1097/MOP.0b013e32835067cc. [DOI] [PubMed] [Google Scholar]
- Lakatosova S, Ostatnikova D. Reelin and its complex involvement in brain development and function. Int J Biochem Cell Biol. 2012;44(9):1501–1504. doi: 10.1016/j.biocel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Lasley SM, Gilbert ME. Rat hippocampal glutamate and GABA release exhibit biphasic effects as a function of chronic lead exposure level. Toxicol Sci. 2002;66(1):139–147. doi: 10.1093/toxsci/66.1.139. [DOI] [PubMed] [Google Scholar]
- Lassiter TL, MacKillop EA, et al. Is fipronil safer than chlorpyrifos? Comparative developmental neurotoxicity modeled in PC12 cells. Brain Res Bull. 2009;78(6):313–322. doi: 10.1016/j.brainresbull.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G. Monitoring phthalate exposure in humans. Clin Chim Acta. 2005;361(1–2):20–29. doi: 10.1016/j.cccn.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Latini G, De Felice C, et al. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environmental health perspectives. 2003;111(14):1783–1785. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G, Del Vecchio A, et al. In utero exposure to phthalates and fetal development. Current medicinal chemistry. 2006;13(21):2527–2534. doi: 10.2174/092986706778201666. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Schambra UB. Morphogenetic roles of acetylcholine. Environmental health perspectives. 1999;107(Suppl 1):65–69. doi: 10.1289/ehp.99107s165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Adriani W, et al. Paradoxical effects of prenatal acetylcholinesterase blockade on neuro-behavioral development and drug-induced stereotypies in reeler mutant mice. Psychopharmacology (Berl) 2006;187(3):331–344. doi: 10.1007/s00213-006-0426-z. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, et al. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci U S A. 2008;105(37):14187–14191. doi: 10.1073/pnas.0806139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Morsey B, et al. Specific non-coplanar PCB-mediated modulation of bottlenose dolphin and beluga whale phagocytosis upon in vitro exposure. J Toxicol Environ Health A. 2004;67(19):1517–1535. doi: 10.1080/15287390490486761. [DOI] [PubMed] [Google Scholar]
- Llansola M, Erceg S, et al. Prenatal exposure to polybrominated diphenylether 99 enhances the function of the glutamate-nitric oxide-cGMP pathway in brain in vivo and in cultured neurons. Eur J Neurosci. 2007;25(2):373–379. doi: 10.1111/j.1460-9568.2006.05289.x. [DOI] [PubMed] [Google Scholar]
- Loikkanen J, Chvalova K, et al. Pb2+-induced toxicity is associated with p53-independent apoptosis and enhanced by glutamate in GT1–7 neurons. Toxicol Lett. 2003;144(2):235–246. doi: 10.1016/s0378-4274(03)00220-0. [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Wolff MS, et al. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ Health Perspect. 2003;111(1):65–70. doi: 10.1289/ehp.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis D, Browning SR, et al. Cancer mortality among electric utility workers exposed to polychlorinated biphenyls. Occup Environ Med. 1997;54(10):720–728. doi: 10.1136/oem.54.10.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottrup G, Andersson AM, et al. Possible impact of phthalates on infant reproductive health. International journal of andrology. 2006;29(1):172–180. doi: 10.1111/j.1365-2605.2005.00642.x. discussion 181-175. [DOI] [PubMed] [Google Scholar]
- Lovekamp TN, Davis BJ. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicology and applied pharmacology. 2001;172(3):217–224. doi: 10.1006/taap.2001.9156. [DOI] [PubMed] [Google Scholar]
- Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environmental health perspectives. 2003;111(2):139–145. doi: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski JR, Stankiewicz P. Genomic disorders: molecular mechanisms for rearrangements and conveyed phenotypes. PLoS Genet. 2005;1(6):e49. doi: 10.1371/journal.pgen.0010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main KM, Kiviranta H, et al. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environmental health perspectives. 2007;115(10):1519–1526. doi: 10.1289/ehp.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis SS, Salogiannis J, et al. EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell. 2010;143(3):442–455. doi: 10.1016/j.cell.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. Neurochemical targets and behavioral effects of organohalogen compounds: an update. Critical reviews in toxicology. 2006;36(3):253–289. doi: 10.1080/10408440500534164. [DOI] [PubMed] [Google Scholar]
- Martin CL, Ledbetter DH. Autism and cytogenetic abnormalities: solving autism one chromosome at a time. Curr Psychiatry Rep. 2007;9(2):141–147. doi: 10.1007/s11920-007-0084-9. [DOI] [PubMed] [Google Scholar]
- Maski KP, Jeste SS, et al. Common neurological co-morbidities in autism spectrum disorders. Current opinion in pediatrics. 2011;23(6):609–615. doi: 10.1097/MOP.0b013e32834c9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuya Y, Ihara D, et al. Synthesis and biological evaluation of pyrethroid insecticide-derivatives as a chemical inducer for Bdnf mRNA expression in neurons. Bioorganic & medicinal chemistry. 2012;20(8):2564–2571. doi: 10.1016/j.bmc.2012.02.048. [DOI] [PubMed] [Google Scholar]
- McCanlies EC, Fekedulegn D, et al. Parental Occupational Exposures and Autism Spectrum Disorder. J Autism Dev Disord. 2012 doi: 10.1007/s10803-012-1468-1. In Press. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, et al. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7(2):152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, et al. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environmental health perspectives. 2007;115(7):1029–1034. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts IA, Letcher RJ, et al. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environmental health perspectives. 2001;109(4):399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts IA, van Zanden JJ, et al. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56(1):95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Meisler MH, Kearney J, et al. Identification of epilepsy genes in human and mouse. Annu Rev Genet. 2001;35:567–588. doi: 10.1146/annurev.genet.35.102401.091142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer RL, Lockwood PE, et al. Mercury (II) alters mitochondrial activity of monocytes at sublethal doses via oxidative stress mechanisms. J Biomed Mater Res B Appl Biomater. 2005;75(2):257–263. doi: 10.1002/jbm.b.30263. [DOI] [PubMed] [Google Scholar]
- Milligan SR, Khan O, et al. Competitive binding of xenobiotic oestrogens to rat alpha-fetoprotein and to sex steroid binding proteins in human and rainbow trout (Oncorhynchus mykiss) plasma. General and comparative endocrinology. 1998;112(1):89–95. doi: 10.1006/gcen.1998.7146. [DOI] [PubMed] [Google Scholar]
- Mitchell KA, Elferink CJ. Timing is everything: consequences of transient and sustained AhR activity. Biochem Pharmacol. 2009;77(6):947–956. doi: 10.1016/j.bcp.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy CA, Morrow AL, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172(1–2):198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87(11):5185–5190. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- Morse DC, Groen D, et al. Interference of polychlorinated biphenyls in hepatic and brain thyroid hormone metabolism in fetal and neonatal rats. Toxicol Appl Pharmacol. 1993;122(1):27–33. doi: 10.1006/taap.1993.1168. [DOI] [PubMed] [Google Scholar]
- Morse DC, Seegal RF, et al. Long-term alterations in regional brain serotonin metabolism following maternal polychlorinated biphenyl exposure in the rat. Neurotoxicology. 1996;17(3–4):631–638. [PubMed] [Google Scholar]
- Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. J Intellect Disabil Res. 2009;53(10):852–873. doi: 10.1111/j.1365-2788.2009.01197.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176(1):4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3(5):303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Naeher LP, Tulve NS, et al. Organophosphorus and pyrethroid insecticide urinary metabolite concentrations in young children living in a southeastern United States city. The Science of the total environment. 2010;408(5):1145–1153. doi: 10.1016/j.scitotenv.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Shigematsu N, et al. Respiratory involvement and immune status in yusho patients. Environmental health perspectives. 1985;59:31–36. doi: 10.1289/ehp.59-1568074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani J, Tamada K, et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11–13 duplication seen in autism. Cell. 2009;137(7):1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls RD, Knepper JL. Genome organization, function, and imprinting in Prader-Willi and Angelman syndromes. Annu Rev Genomics Hum Genet. 2001;2:153–175. doi: 10.1146/annurev.genom.2.1.153. [DOI] [PubMed] [Google Scholar]
- Niemi WD, Audi J, et al. PCBs reduce long-term potentiation in the CA1 region of rat hippocampus. Exp Neurol. 1998;151(1):26–34. doi: 10.1006/exnr.1998.6793. [DOI] [PubMed] [Google Scholar]
- Oberle I, Rousseau F, et al. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252(5010):1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- Ostrea EM, Jr, Bielawski DM, et al. Combined analysis of prenatal (maternal hair and blood) and neonatal (infant hair, cord blood and meconium) matrices to detect fetal exposure to environmental pesticides. Environmental research. 2009;109(1):116–122. doi: 10.1016/j.envres.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrea EM, Morales V, et al. Prevalence of fetal exposure to environmental toxins as determined by meconium analysis. Neurotoxicology. 2002;23(3):329–339. doi: 10.1016/s0161-813x(02)00077-3. [DOI] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000;58(2):339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- Pasca SP, Portmann T, et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med. 2011;17(12):1657–1662. doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, et al. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28(1):111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Paul R, Pessah IN, et al. Early onset of neurological symptoms in fragile X premutation carriers exposed to neurotoxins. Neurotoxicology. 2010;31(4):399–402. doi: 10.1016/j.neuro.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PE S, R D, et al. Pervasive developmental disorders and childhood psychosis. In: RM K, RE B, HB J, BF S, editors. Nelson Textbook of Pediatrics. 18th ed. Philadelphia, Pa: Saunders Elsevier; 2007. [Google Scholar]
- Peca J, Feliciano C, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472(7344):437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelka GJ, Watson CM, et al. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain. 2006;129(Pt 4):887–898. doi: 10.1093/brain/awl022. [DOI] [PubMed] [Google Scholar]
- Peng S, Hajela RK, et al. Characteristics of block by Pb2+ of function of human neuronal L-, N-, and R-type Ca2+ channels transiently expressed in human embryonic kidney 293 cells. Mol Pharmacol. 2002;62(6):1418–1430. doi: 10.1124/mol.62.6.1418. [DOI] [PubMed] [Google Scholar]
- Percy AK. Rett syndrome: exploring the autism link. Arch Neurol. 2011;68(8):985–989. doi: 10.1001/archneurol.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Cherednichenko G, et al. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacology & therapeutics. 2010;125(2):260–285. doi: 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Hansen LG, et al. Structure-activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1) Chemical research in toxicology. 2006;19(1):92–101. doi: 10.1021/tx050196m. [DOI] [PubMed] [Google Scholar]
- Phelan MC. Deletion 22q13.3 syndrome. Orphanet J Rare Dis. 2008;3:14. doi: 10.1186/1750-1172-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Pinelli M, Scala G, et al. Simulating gene-gene and gene-environment interactions in complex diseases: Gene-Environment iNteraction Simulator 2. BMC Bioinformatics. 2012;13(1):132. doi: 10.1186/1471-2105-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RL, Pearson BL, et al. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214(2):443–449. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poimenova A, Markaki E, et al. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience. 2010;167(3):741–749. doi: 10.1016/j.neuroscience.2010.02.051. [DOI] [PubMed] [Google Scholar]
- Porciuncula LO, Rocha JB, et al. Methylmercury inhibits glutamate uptake by synaptic vesicles from rat brain. Neuroreport. 2003;14(4):577–580. doi: 10.1097/00001756-200303240-00010. [DOI] [PubMed] [Google Scholar]
- Prasanth GK, Divya LM, et al. Bisphenol-A can bind to human glucocorticoid receptor as an agonist: an in silico study. J Appl Toxicol. 2010;30(8):769–774. doi: 10.1002/jat.1570. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, et al. Chlorpyrifos exposure during neurulation: cholinergic synaptic dysfunction and cellular alterations in brain regions at adolescence and adulthood. Brain Res Dev Brain Res. 2004;148(1):43–52. doi: 10.1016/j.devbrainres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, et al. Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environmental health perspectives. 2003;111(4):536–544. doi: 10.1289/ehp.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Entezam A, et al. A mouse model of the fragile X premutation: effects on behavior, dendrite morphology, and regional rates of cerebral protein synthesis. Neurobiol Dis. 2011;42(1):85–98. doi: 10.1016/j.nbd.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radyushkin K, Hammerschmidt K, et al. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8(4):416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- Ratra GS, Kamita SG, et al. Role of human GABA(A) receptor beta3 subunit in insecticide toxicity. Toxicology and applied pharmacology. 2001;172(3):233–240. doi: 10.1006/taap.2001.9154. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118(6):e1845–e1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray DE, Fry JR. A reassessment of the neurotoxicity of pyrethroid insecticides. Pharmacology & therapeutics. 2006;111(1):174–193. doi: 10.1016/j.pharmthera.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rett A. A cerebral atrophic syndrome in hypoammoniemia. Monatsschr Kinderheilkd. 1968;116(6):310–311. [PubMed] [Google Scholar]
- Ricceri L, Markina N, et al. Developmental exposure to chlorpyrifos alters reactivity to environmental and social cues in adolescent mice. Toxicology and applied pharmacology. 2003;191(3):189–201. doi: 10.1016/s0041-008x(03)00229-1. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Venerosi A, et al. Developmental neurotoxicity of organophosphorous pesticides: fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice. Toxicol Sci. 2006;93(1):105–113. doi: 10.1093/toxsci/kfl032. [DOI] [PubMed] [Google Scholar]
- Rice DC. Lead-induced behavioral impairment on a spatial discrimination reversal task in monkeys exposed during different periods of development. Toxicol Appl Pharmacol. 1990;106(2):327–333. doi: 10.1016/0041-008x(90)90251-o. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Miller GW. Acute exposure to aroclor 1016 or 1260 differentially affects dopamine transporter and vesicular monoamine transporter 2 levels. Toxicol Lett. 2004;148(1–2):29–40. doi: 10.1016/j.toxlet.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reproductive toxicology. 2007;24(2):199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropero AB, Alonso-Magdalena P, et al. Rapid endocrine disruption: environmental estrogen actions triggered outside the nucleus. The Journal of steroid biochemistry and molecular biology. 2006;102(1–5):163–169. doi: 10.1016/j.jsbmb.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Rosner D, Markowitz G. The politics of lead toxicology and the devastating consequences for children. Am J Ind Med. 2007;50(10):740–756. doi: 10.1002/ajim.20435. [DOI] [PubMed] [Google Scholar]
- Rossignol E. Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural Plast. 2011;2011 doi: 10.1155/2011/649325. 649325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotschafer SE, Trujillo MS, et al. Minocycline treatment reverses ultrasonic vocalization production deficit in a mouse model of Fragile X Syndrome. Brain Res. 2012;1439:7–14. doi: 10.1016/j.brainres.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Roullet FI, Crawley JN. Mouse models of autism: testing hypotheses about molecular mechanisms. Current topics in behavioral neurosciences. 2011;7:187–212. doi: 10.1007/7854_2010_113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge EJ, White R, et al. Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) alpha and ERbeta. The Journal of biological chemistry. 2000;275(46):35986–35993. doi: 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- Roy TS, Andrews JE, et al. Chlorpyrifos elicits mitotic abnormalities and apoptosis in neuroepithelium of cultured rat embryos. Teratology. 1998;58(2):62–68. doi: 10.1002/(SICI)1096-9926(199808)58:2<62::AID-TERA7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lenkowski JR, et al. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147(8):3681–3691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- Rusiecki JA, Baccarelli A, et al. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect. 2008;116(11):1547–1552. doi: 10.1289/ehp.11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas JA, Huff NC. Lead and conditioned fear to contextual and discrete cues. Neurotoxicol Teratol. 2002;24(4):541–550. doi: 10.1016/s0892-0362(02)00265-9. [DOI] [PubMed] [Google Scholar]
- Sargis RM, Johnson DN, et al. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity. 2010;18(7):1283–1288. doi: 10.1038/oby.2009.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, et al. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PloS one. 2008;3(8):e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, et al. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10(1):44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL. Developmental neurotoxicity of PCBs in humans: what do we know and where do we go from here? Neurotoxicol Teratol. 1996;18(3):217–227. doi: 10.1016/s0892-0362(96)90001-x. discussion 229–276. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Beg AA. Neuroscience: Angelman syndrome connections. Nature. 2010;468(7326):907–908. doi: 10.1038/468907a. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Baio J, et al. Risk for cognitive deficit in a population-based sample of U.S. children with autism spectrum disorders: variation by perinatal health factors. Disabil Health J. 2010;3(3):202–212. doi: 10.1016/j.dhjo.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Bush B, et al. Polychlorinated biphenyls induce regional changes in brain norepinephrine concentrations in adult rats. Neurotoxicology. 1985;6(3):13–23. [PubMed] [Google Scholar]
- Seegal RF, Bush B, et al. Sub-chronic exposure of the adult rat to Aroclor 1254 yields regionally-specific changes in central dopaminergic function. Neurotoxicology. 1991;12(1):55–65. [PubMed] [Google Scholar]
- Seegal RF, Bush B, et al. Lightly chlorinated ortho-substituted PCB congeners decrease dopamine in nonhuman primate brain and in tissue culture. Toxicol Appl Pharmacol. 1990;106(1):136–144. doi: 10.1016/0041-008x(90)90113-9. [DOI] [PubMed] [Google Scholar]
- Shafer TJ, Atchison WD. Block of 45Ca uptake into synaptosomes by methylmercury: Ca++- and Na+-dependence. J Pharmacol Exp Ther. 1989;248(2):696–702. [PubMed] [Google Scholar]
- Shafer TJ, Meyer DA, et al. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environmental health perspectives. 2005;113(2):123–136. doi: 10.1289/ehp.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim SH, Hwangbo Y, et al. Increased levels of plasma brain-derived neurotrophic factor (BDNF) in children with attention deficit-hyperactivity disorder (ADHD) Progress in neuro-psychopharmacology & biological psychiatry. 2008;32(8):1824–1828. doi: 10.1016/j.pnpbp.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Sidhu P, Nehru B. Relationship between lead-induced biochemical and behavioral changes with trace element concentrations in rat brain. Biol Trace Elem Res. 2003;92(3):245–256. doi: 10.1385/BTER:92:3:245. [DOI] [PubMed] [Google Scholar]
- Sidiropoulou E, Sachana M, et al. Fipronil interferes with the differentiation of mouse N2a neuroblastoma cells. Toxicol Lett. 2011;201(1):86–91. doi: 10.1016/j.toxlet.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Reidy JA, et al. Detection of phthalate metabolites in human amniotic fluid. Bulletin of environmental contamination and toxicology. 2004;72(6):1226–1231. doi: 10.1007/s00128-004-0374-4. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Smith DG, et al. Negative Allosteric Modulation of the mGluR5 Receptor Reduces Repetitive Behaviors and Rescues Social Deficits in Mouse Models of Autism. Sci Transl Med. 2012;4(131) doi: 10.1126/scitranslmed.3003501. 131ra151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, et al. Behavioural phenotyping assays for mouse models of autism. Nature reviews. Neuroscience. 2010;11(7):490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicology and applied pharmacology. 2004;198(2):132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Smialowicz RJ, Andrews JE, et al. Evaluation of the immunotoxicity of low level PCB exposure in the rat. Toxicology. 1989;56(2):197–211. doi: 10.1016/0300-483x(89)90133-9. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, et al. Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. Autism Res. 2011;4(1):40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Graham DF, et al. Social behavior in Fmr1 knockout mice carrying a human FMR1 transgene. Behav Neurosci. 2008;122(3):710–715. doi: 10.1037/0735-7044.122.3.710. [DOI] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119(1):19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Stehr CM, Linbo TL, et al. The developmental neurotoxicity of fipronil: notochord degeneration and locomotor defects in zebrafish embryos and larvae. Toxicol Sci. 2006;92(1):270–278. doi: 10.1093/toxsci/kfj185. [DOI] [PubMed] [Google Scholar]
- Svensson BG, Hallberg T, et al. Parameters of immunological competence in subjects with high consumption of fish contaminated with persistent organochlorine compounds. Int Arch Occup Environ Health. 1994;65(6):351–358. doi: 10.1007/BF00383243. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Paterson AD, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39(3):319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M. Epigenetics, DNA methylation, and chromatin modifying drugs. Annual review of pharmacology and toxicology. 2009;49:243–263. doi: 10.1146/annurev-pharmtox-061008-103102. [DOI] [PubMed] [Google Scholar]
- Ta TA, Koenig CM, et al. Bioaccumulation and behavioral effects of 2,2',4,4'-tetrabromodiphenyl ether (BDE-47) in perinatally exposed mice. Neurotoxicol Teratol. 33(3):393–404. doi: 10.1016/j.ntt.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi T. The neurobiology of mouse models syntenic to human chromosome 15q. J Neurodev Disord. 2011;3(3):270–281. doi: 10.1007/s11689-011-9088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talts U, Fredriksson A, et al. Changes in behavior and muscarinic receptor density after neonatal and adult exposure to bioallethrin. Neurobiol Aging. 1998;19(6):545–552. doi: 10.1016/s0197-4580(98)00093-1. [DOI] [PubMed] [Google Scholar]
- Tate P, Skarnes W, et al. The methyl-CpG binding protein MeCP2 is essential for embryonic development in the mouse. Nat Genet. 1996;12(2):205–208. doi: 10.1038/ng0296-205. [DOI] [PubMed] [Google Scholar]
- Tayebati SK, Di Tullio MA, et al. Influence of dermal exposure to the pyrethroid insecticide deltamethrin on rat brain microanatomy and cholinergic/dopaminergic neurochemistry. Brain Res. 2009;1301:180–188. doi: 10.1016/j.brainres.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Thomas AM, Bui N, et al. Group I metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for fragile X syndrome. Psychopharmacology (Berl) 2012;219(1):47–58. doi: 10.1007/s00213-011-2375-4. [DOI] [PubMed] [Google Scholar]
- Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. The Journal of steroid biochemistry and molecular biology. 2006;102(1–5):175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Tilson HA, Kodavanti PR. The neurotoxicity of polychlorinated biphenyls. Neurotoxicology. 1998;19(4–5):517–525. [PubMed] [Google Scholar]
- Toran-Allerand CD. On the genesis of sexual differentiation of the general nervous system: morphogenetic consequences of steroidal exposure and possible role of alpha-fetoprotein. Progress in brain research. 1984;61:63–98. doi: 10.1016/s0079-6123(08)64429-5. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Guilarte TR. Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev. 2005;49(3):529–554. doi: 10.1016/j.brainresrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Hashemzadeh-Gargari H, et al. Developmental Pb2+ exposure alters NMDAR subtypes and reduces CREB phosphorylation in the rat brain. Brain Res Dev Brain Res. 2002;139(2):217–226. doi: 10.1016/s0165-3806(02)00569-2. [DOI] [PubMed] [Google Scholar]
- Tran V, Hoffman N, et al. Bifenthrin inhibits neurite outgrowth in differentiating PC12 cells. Med Sci Monit. 2006;12(2):BR57–BR62. [PubMed] [Google Scholar]
- Turyk ME, Persky VW, et al. Hormone disruption by PBDEs in adult male sport fish consumers. Environmental health perspectives. 2008;116(12):1635–1641. doi: 10.1289/ehp.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino S, Wada H, et al. Direct interaction of post-synaptic density-95/Dlg/ZO-1 domain-containing synaptic molecule Shank3 with GluR1 alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor. J Neurochem. 2006;97(4):1203–1214. doi: 10.1111/j.1471-4159.2006.03831.x. [DOI] [PubMed] [Google Scholar]
- Utari A, Chonchaiya W, et al. Side effects of minocycline treatment in patients with fragile X syndrome and exploration of outcome measures. Am J Intellect Dev Disabil. 2010;115(5):433–443. doi: 10.1352/1944-7558-115.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Birgelen AP, Smit EA, et al. Subchronic effects of 2,3,7,8-TCDD or PCBs on thyroid hormone metabolism: use in risk assessment. Eur J Pharmacol. 1995;293(1):77–85. doi: 10.1016/0926-6917(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, et al. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Ciencia & saude coletiva. 2012;17(2):407–434. doi: 10.1590/s1413-81232012000200015. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, et al. Human exposure to bisphenol A (BPA) Reproductive toxicology. 2007;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Veenstra-Vanderweele J, Christian SL, et al. Autism as a paradigmatic complex genetic disorder. Annu Rev Genomics Hum Genet. 2004;5:379–405. doi: 10.1146/annurev.genom.5.061903.180050. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Verpelli C, Dvoretskova E, et al. Importance of Shank3 protein in regulating metabotropic glutamate receptor 5 (mGluR5) expression and signaling at synapses. The Journal of biological chemistry. 2011;286(40):34839–34850. doi: 10.1074/jbc.M111.258384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel SA. The politics of plastics: the making and unmaking of bisphenol a "safety". American journal of public health. 2009;99(Suppl 3):S559–S566. doi: 10.2105/AJPH.2008.159228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineagu I, Wang X, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474(7351):380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Hertz-Picciotto I, et al. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect. 2011;119(6):873–877. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstman JA, Staal WG, et al. Identification of novel autism candidate regions through analysis of reported cytogenetic abnormalities associated with autism. Mol Psychiatry. 2006;11(1):1, 18–28. doi: 10.1038/sj.mp.4001781. [DOI] [PubMed] [Google Scholar]
- Wahl M, Guenther R, et al. Polybrominated diphenyl ethers and arylhydrocarbon receptor agonists: Different toxicity and target gene expression. Toxicology letters. 2010;198(2):119–126. doi: 10.1016/j.toxlet.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, et al. Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T/+ tf/tf has severely reduced hippocampal commissure and absent corpus callosum. Brain Res. 2003;971(1):47–54. doi: 10.1016/s0006-8993(03)02354-0. [DOI] [PubMed] [Google Scholar]
- Walz NC. Parent report of stereotyped behaviors, social interaction, and developmental disturbances in individuals with Angelman syndrome. J Autism Dev Disord. 2007;37(5):940–947. doi: 10.1007/s10803-006-0233-8. [DOI] [PubMed] [Google Scholar]
- Wang X, McCoy PA, et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Human molecular genetics. 2011;20(15):3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CS, Bulayeva NN, et al. Signaling from the membrane via membrane estrogen receptor-alpha: estrogens, xenoestrogens, and phytoestrogens. Steroids. 2005;70(5–7):364–371. doi: 10.1016/j.steroids.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Weisglas-Kuperus N, Vreugdenhil HJ, et al. Immunological effects of environmental exposure to polychlorinated biphenyls and dioxins in Dutch school children. Toxicology letters. 2004;149(1–3):281–285. doi: 10.1016/j.toxlet.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Wiegers TC, Davis AP, et al. Text mining and manual curation of chemical-gene-disease networks for the comparative toxicogenomics database (CTD) BMC Bioinformatics. 2009;10:326. doi: 10.1186/1471-2105-10-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen R, Hoogeveen-Westerveld M, et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Human molecular genetics. 2003;12(9):949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]
- Winneke G, Walkowiak J, et al. PCB-induced neurodevelopmental toxicity in human infants and its potential mediation by endocrine dysfunction. Toxicology. 2002;181–182:161–165. doi: 10.1016/s0300-483x(02)00274-3. [DOI] [PubMed] [Google Scholar]
- Wohr M, Roullet FI, et al. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10(1):35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R, Vallero RO, et al. Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation. Hum Mol Genet. 2012;21(11):2399–2411. doi: 10.1093/hmg/dds046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Chen L, et al. Effects of decabrominated diphenyl ether (PBDE 209) exposure at different developmental periods on synaptic plasticity in the dentate gyrus of adult rats In vivo. Toxicol Sci. 2009;110(2):401–410. doi: 10.1093/toxsci/kfp114. [DOI] [PubMed] [Google Scholar]
- Yang M, Bozdagi O, et al. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci. 2012;32(19):6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Clarke AM, et al. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29(8):1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoi T, Itoh K, et al. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochemical and biophysical research communications. 2008;376(3):563–567. doi: 10.1016/j.bbrc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Ye H, Liu J, et al. Cell adhesion molecules and their involvement in autism spectrum disorder. Neuro-Signals. 2010;18(2):62–71. doi: 10.1159/000322543. [DOI] [PubMed] [Google Scholar]
- Yin Z, Milatovic D, et al. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res. 2007;1131(1):1–10. doi: 10.1016/j.brainres.2006.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Tsuda T, et al. Total mercury content in hair and neurologic signs: historic data from Minamata. Epidemiology. 2009;20(2):188–193. doi: 10.1097/EDE.0b013e318190e73f. [DOI] [PubMed] [Google Scholar]
- Young JG, Eskenazi B, et al. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26(2):199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Yu K, He Y, et al. DE-71-induced apoptosis involving intracellular calcium and the Bax-mitochondria-caspase protease pathway in human neuroblastoma cells in vitro. Toxicol Sci. 2008;104(2):341–351. doi: 10.1093/toxsci/kfn088. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Disruption by methylmercury of membrane excitability and synaptic transmission of CA1 neurons in hippocampal slices of the rat. Toxicol Appl Pharmacol. 1993;120(2):203–215. doi: 10.1006/taap.1993.1104. [DOI] [PubMed] [Google Scholar]
- Zhang C, Liu X, et al. Role of brominated diphenly ether-209 in the differentiation of neural stem cells in vitro. Int J Dev Neurosci. 2010;28(6):497–502. doi: 10.1016/j.ijdevneu.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Liu AP, et al. Effect of developmental lead exposure on the expression of specific NMDA receptor subunit mRNAs in the hippocampus of neonatal rats by digoxigenin-labeled in situ hybridization histochemistry. Neurotoxicol Teratol. 2002;24(2):149–160. doi: 10.1016/s0892-0362(01)00210-0. [DOI] [PubMed] [Google Scholar]
- Zoeller RT. Environmental chemicals as thyroid hormone analogues: new studies indicate that thyroid hormone receptors are targets of industrial chemicals? Molecular and cellular endocrinology. 2005;242(1–2):10–15. doi: 10.1016/j.mce.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Bansal R, et al. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146(2):607–612. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse? Science. 2003;302(5646):826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
- Zsarnovszky A, Le HH, et al. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology. 2005;146(12):5388–5396. doi: 10.1210/en.2005-0565. [DOI] [PubMed] [Google Scholar]