Abstract
The onset of neurodegenerations and nervous system injury both trigger cell signaling perturbations that lead to damage of neuronal circuits and synapic connections, as well as protective signaling that aims to halt disease onset. Here we review recent findings that support the role of the docosanoid mediator neuroprotectin D1 (NPD1) as an early response or sentinel during the initial phase of nervous system damage. NPD1 is derived from docosahexaenoic acid that is selectively concentrated and retained in the nervous system. The protein misfolding triggers the biosynthesis of NPD1 which in turn downregulates pathways that lead to cell death and changes the outcome to cell survival. Proteotoxic stress as a result of protein misfolding is a widespread event in many neurodegenerative diseases. Therefore, mechanisms and mediators such as NPD1 that curtail consequences of these events are of interest as leads in the search for novel preventive and or therapeutic approaches.
Keywords: Misfolding, Alzheimer’s disease, docosahexaenoic acid, ataxin-1, huntingtin, CAG repeats, APP, Retinal pigment epithelial cell
1. Introduction
Neurodegenerative diseases are genetically complex, progressive age-related disorders that often involve early proteotoxic stress due to protein misfolding [1, 2, 3, 4].
The selective endowment in omega-3 essential fatty acids (docosahexaenoyl – DHA- chains of membrane phospholipids, 22C and 6 double bonds) in synapses, photoreceptors and other membranes of the nervous system [5, 6, 7, 8, 9, 10, 11, 12] is being explored based on the bioactivity of the docosanoid synthesized from DHA [13, 14] Neuroprotectin D1 (NPD1,10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15E,19Z hexaenoic acid). Endogenous NPD1 biosynthesis is promptly induced in response to various forms of injury [14, 15], including protein misfolding and resulting proteotoxicity [16]. Interestingly, neurotrophins are agonists for the synthesis of this protective mediator, suggesting that the neurotrophins’ action may be one of the connections to evoke the formation of a defense response [17].
This cell survival cascade and the events that sustain neuronal network homeostatic integrity involve multiple checkpoints and signaling networks that include restoring proteostasis during protein misfolding and attenuation of misfolding-induced proteotoxicity. NPD1 regulation of upstream targets affects cell survival, neuroinflammatory signaling and transcription, which in turn promotes homeostatic regulation of synaptic and neural circuitry integrity. Highlighted here are recent insights on NPD1’s specific and potent bioactivity, including its role in proteotoxic and neuroinflammatory events. As this lipid mediator is biosynthesized on-demand during the early stages of neurodegenerative diseases and neural injury, NPD1 acts as a preliminary protective sentinel of cell homeostasis [5, 18].
2. NPD1 synthesis is triggered by protein misfolding
Pathological polyglutamine tracts impair homeostasis and protein folding, which often triggers cell damage and death [18]. These tracts are caused by errors in DNA replication, such as CAG repeats, which are responsible for subsets of many neurodegenerative disorders [19]. Other repeat errors include the expression of Ataxin-1 82Q mutants. Ataxin-1 82Q mutant-expression causes spinocerebellar ataxia type-1 (SCA1), but it also triggers the endogenous NPD1 synthesis (Fig. 1). NPD1 synthesis is also activated with the expression of huntingtin 72Q mutants, as revealed through LC MS/MS based lipidomics [16]. The addition of NPD1 to singular cultures was employed to test the hypothesis that mutant expression induced NPD1 synthesis was a protective responses. In conjunction, it was hypothesized that the impairment caused by the misfolded proteins was greater than the neuroprotection granted by endogenous NPD1. This avenue was explored by adding exogenous NPD1, which exerted significant antiapoptotic bioactivity at 50mM (Fig. 2). Increased apoptotic protection was recorded with the addition of the serpin family growth factor PEDF to 100mM DHA (Fig. 2). NPD1 is synthesized endogenously under these conditions, as in basal conditions [17].
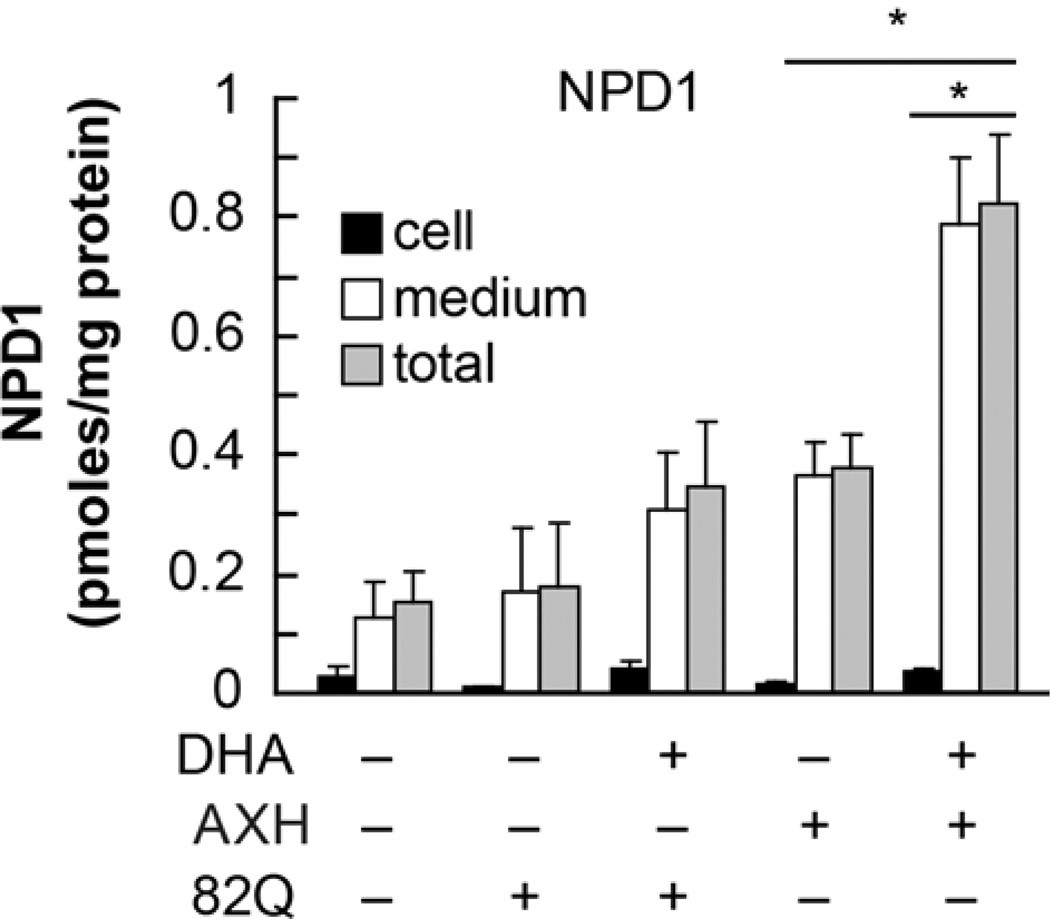
Figure 1. Endogenous NPD1 biosynthesis is enhanced upon expression of Ataxin-1 82Q in RPE cells.
Primary human RPE cells were transfected with 82Q. Cells (black bars), NPD1 content in media (white bars) and total (grey bars) was measured by LC MSMS with or without the addition of DHA. * p<0.005. (Figure modified and published with permission from Journal of Biological Chemistry (2012) 287(28):23726-39 “Ataxin 1 Poly(Q)-induced Proteotoxic Stress and Apoptosis are Attenuated in Neural Cells by Docosahexaenoic Acid-derived Neuroprotectin D1”; Calandria JM, et al.)
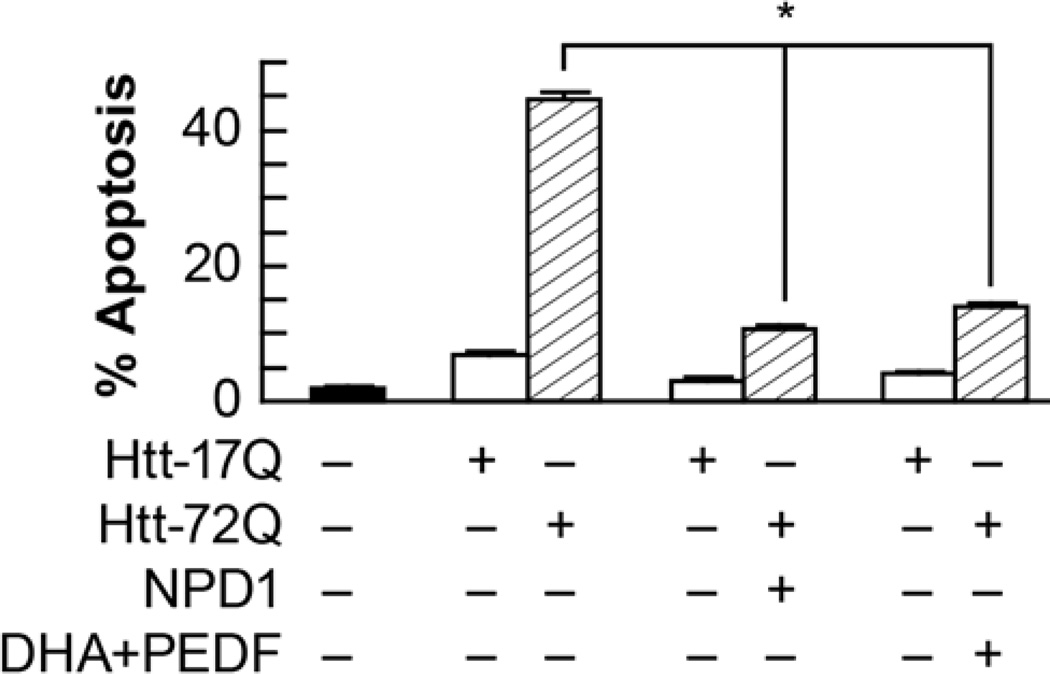
Figure 2. NPD1 prevents huntingtin-17Q-induced apoptosis in ARPE-19 cells.
ARPE-19 cells transfected with an expression construct containing ht-72Q were treated with 50 nM NPD1, or DHA (100 nM) along with PEDF (10 ng/mL). Apoptosis percentage was calculated by dividing pyknotic over the total count of cells. Results are averages ± SD. * p< 0.0005. (Figure modified and published with permission from Journal of Biological Chemistry (2012) 287(28):23726-39 “Ataxin-1 Poly(Q)-induced Proteotoxic Stress and Apoptosis are Attenuated in Neural Cells by Docosahexaenoic Acid-derived Neuroprotectin D1”; Calandria JM, et al.)
Previous findings indicate that NPD1 may work by modulating PP2A activity [16]. PP2A inhibition may be counteracted by this docosanoid, which would allow Ataxin 82Q to de-phosphorylate and be relocated into the spliceosome. This expanded Ataxin form is proposed to have a stronger interaction with Anp32, compared to the wild type Ataxin-1. This key difference makes this protein a potential candidate for NPD1 signaling. Accordingly, Ataxin-1 functionality is partially modulated by AXH, a selffolding domain present in Ataxin-1. AXH facilitates protein-protein interactions between Ataxin-1 and other transcription factors. The inactive counterparts of Ataxin-1 cause the sequestration of more complex partners, which in turn may be linked to neurodegenerative impairment. AXH domain-containing protein family-member brother of Ataxin-1 (Boat) is a case for the proposed observable loss of function. Boat is an in vivo binding partner of Ataxin-1 that is also affected by the malfunction of Ataxin-1 82Q. Malfunction of Ataxin-1 82Q also affects Boat, which is an in vivo binding partner of Ataxin-1. Accordingly, apoptosis increased in cells with only AXH expression. Moreover, Ataxin-1 82Q-induced cytotoxicity was increased by AXH expression. AHX expression is capable of increasing toxicity through upregulating the disassembly of complexes, which in turn deactivates its partners.
Because it promotes cell survival through gene modulation, NPD1 is capable of reversing the toxicity of misfolded and mutant proteins, such as Ataxin-1 82Q and Huntingtin 72Q [16]. The early phases of many neurodegenerative diseases are marked by few clinically measureable impairments, but protein misfolding and proteotoxic stress are present. We have explored the use of NPD1 as an agent for these events in culture models such as neuronal mixed cultures and human retinal pigment epithelial (RPE) cells.
3. NPD1 is reduced in Alzheimer’s disease brains
NPD1 levels are reduced in early stages of Alzheimer’s disease (AD), as is 15 lipoxygenase-1 (15-LOX-1) expression [20, 21]. 15-LOX-1 is crucial for NPD1 synthesis. This enzyme catalyzes conversion of arachidonic acid to several eicosanoids, and the addition of 12-HETE, 15-HETE and of protective lipoxin A4 fails to rescue 15-LOX-1-deficient human RPE cells from oxidative stress-induced cell death [22]. However, NPD1 is capable of selectively rescuing human RPE cells from oxidative stress-induced apoptosis. Therefore, we have explored the significance of NPD1 in cellular models that recapitulate part of AD pathology.
AD pathology includes double mutation of APPsw (Swedish) and amyloid-β that challenges neurons and astrocytes. NPD1 downregulates the processing of the amyloid-β precursor protein and shuts down pro-inflammatory gene expression (such as TNF-α, COX-2 and B-94-TNF-α inducible pro-inflammatory elements), which in turn promotes cell survival (Fig. 3). Moreover, anti-amyloidogenic processing by NPD1 targets α- and β-secretases and PPARγ receptor activation [15]. NPD1 positively modulates the levels of BCL-2 anti-apoptotic proteins, while simultaneously downregulating pro-apoptotic BCL-2 and microglia activation. This is achieved through modulation of S62-Bcl-xl, which is regulated by the protein phosphatase PP2A. In turn, Bcl-xl heterodimerizes with BAX, thus decreasing the availability of the pro-apoptotic BCL-2 protein and leading to positive cell survival outcomes [23]. Moreover, NPD1 attenuates oxidative stress consequences [24, 25]. The protective effects of NPD1 are seen in other studies displaying attenuation of choroidal neovascularization in a model of the wet form of agerelated macular degeneration (AMD) [26].
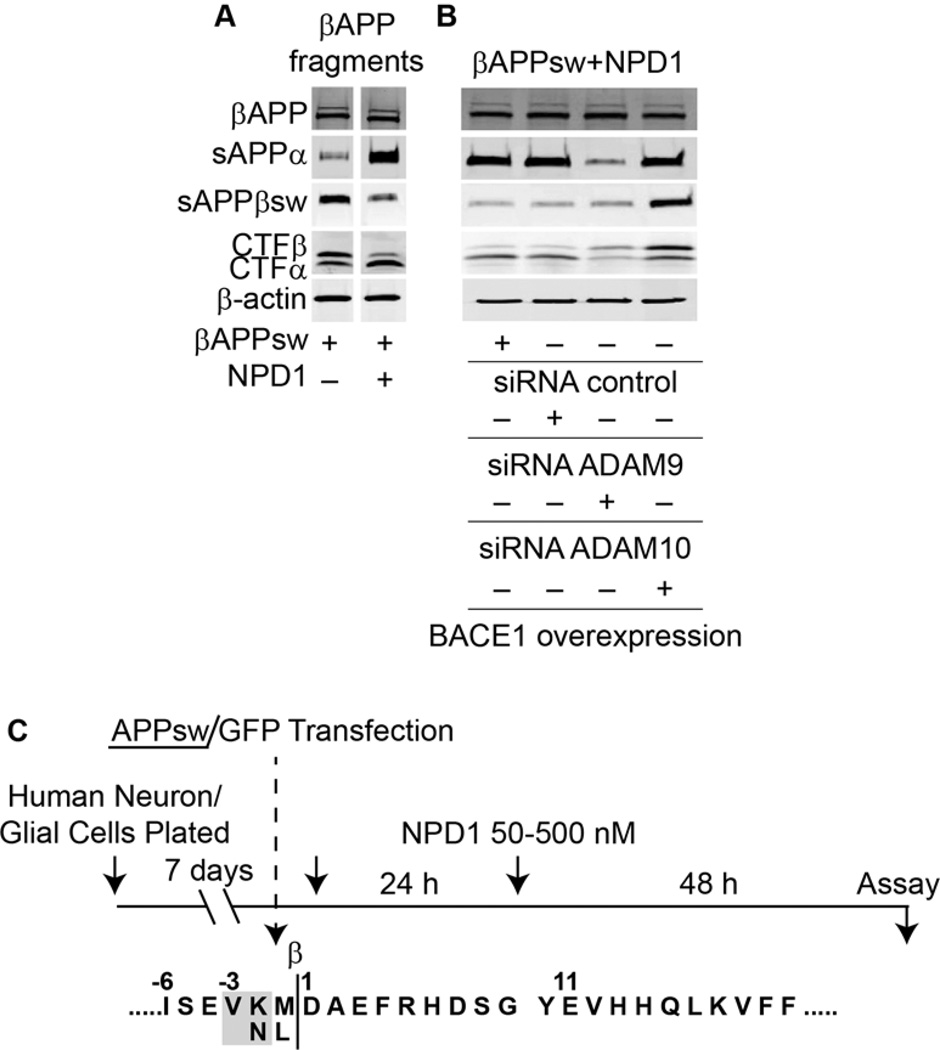
Figure 3. NPD1 shifts βAAP processing to a non-amyloidogenic pathway.
(A) Control or HNG cells over-expressing βAPPsw were treated with increasing concentrations (0, 50, 100, 500 nM) of NPD1 for 48 h and subjected to Western blot detection of holo-βAPP (βAPP holoenzyme), sAPPα, sAPPβsw, CTFα and CTFβ in comparison to β-actin levels in the same sample; (B) Quantification of gel bands in (A) analyzing βAPP fragments with increasing doses of NPD1. Results are means ± SEM (n=4); *p<0.01 vs. βAPPsw control.
The bioactivity of NPD1 attenuates neuronal circuit damage and its synthesis is one of the brain’s first responses to insult or disease. The wide range of nervous system damage that activates NPD1 synthesis is intriguing in that this docosanoid mediator seems to be a sentinel that may guard the integrity of the nervous system when confronted with adversity that tends to disrupt homeostasis. Understanding the myriad of NPD1-mediated mechanisms will help guide the development of therapeutic approaches. One outcome of these studies will be the design of preventative strategies and therapeutic approaches to slow down the initiation and early progression of neurodegenerative diseases such as Alzheimer’s, Parkinson’s and age-related macular degeneration.
Acknowledgments
This research has been supported by: NIH NINDS R01 NS046741, NEI R01 EY005121
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;6:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim D, Tsai LH. Bridging physiology and pathology in AD. Cell. 2009;6:997–1000. doi: 10.1016/j.cell.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s Disease. Neuron. 2009;3:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461:916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazan NG, Musto AE, Knott EJ. Endogenous signaling by omega-3 docosahexaenoic acid-derived mediators sustains homeostatic synaptic and circuitry integrity. Mol Neurobiol. 2011;2:216–222. doi: 10.1007/s12035-011-8200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HY, Spector AA, Xiong ZM. A synaptogenic amide N-docosahexaenoylethanolamide promotes hippocampal development. Prostaglandins Other Lipid Mediat. 2011;96:114–120. doi: 10.1016/j.prostaglandins.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapoport SI, Ramadan E, Basselin M. Docosahexaenoic acid (DHA) incorporation into the brain from plasma, as an in vivo biomarker of brain DHA metabolism and neurotransmission. Prostaglandins Other Lipid Mediat. 2011;96:109–113. doi: 10.1016/j.prostaglandins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto M, Hossain S. Neuroprotective and ameliorative actions of polyunsaturated fatty acids against neuronal diseases: beneficial effect of docosahexaenoic acid on cognitive decline in Alzheimer’s disease. J Pharmacol Sci. 2011;2:150–162. doi: 10.1254/jphs.10r33fm. [DOI] [PubMed] [Google Scholar]
- 9.Calon F. Omega-3 polyunsaturated fatty acid in Alzheimer’s disease: key questions and partial answers. Curr Alzheimer Res. 2011;5:470–478. doi: 10.2174/156720511796391881. [DOI] [PubMed] [Google Scholar]
- 10.Astarita G, Piomelli D. Towards a whole-body systems [multi-organ] lipidomics in Alzheimer’s disease. Prostaglandins Leukot Essent Fatty Acids. 2011;5:197–203. doi: 10.1016/j.plefa.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simopoulos AP. Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol Neurobiol. 2011;2:203–215. doi: 10.1007/s12035-010-8162-0. [DOI] [PubMed] [Google Scholar]
- 12.Cole GM, Ma QL, Frautschy SA. Dietary fatty acids and the aging brain. Nutr Rev. 2010;68:102–111. doi: 10.1111/j.1753-4887.2010.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;44:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, Bazan NG. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer's disease models. PLoS One. 2011;1:e15816. doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calandria JM, Mukherjee PK, de Rivero Vaccari JC, Zhu M, Petasis NA, Bazan NG. Ataxin-1 Poly(Q)-induced Proteotoxic Stress and Apoptosis are Attenuated in Neural Cells by Docosahexaenoic Acid-derived Neuroprotectin D1. J Biol Chem. 2012;28:23726–23739. doi: 10.1074/jbc.M111.287078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee PK, Marcheselli VL, de Rivero VaccariC JC, Gordon WC, Jackson FE, Bazan NG. Photoreceptor outer segment phagocytosis attenuates oxidative stress-induced apoptosis with concomitant neuroprotectin D1 synthesis. Proc Natl Acad Sci USA. 2007;32:13158–13163. doi: 10.1073/pnas.0705963104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bazan NG, Molina MF, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer's, and other neurodegenerative diseases. Annu Rev Nutr. 2011;31:321–351. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoghbi HY, Bear MF. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb Perspect Biol. 2012;3:a009886. doi: 10.1101/cshperspect.a009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoghbi HY, Orr HT. Pathogenic mechanisms of a polyglutamine-mediated neurodegenerative disease, spinocerebellar ataxia type-1. J Biol Chem. 2009;12:7425–7429. doi: 10.1074/jbc.R800041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukiw WJ, Cui JG, Marcheselli VL, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;10:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Astarita G, Jung KM, Berchtold NC, et al. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer's disease. PLoS One. 2010;9:e12538. doi: 10.1371/journal.pone.0012538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calandria JM, Bazan NG. Neuroprotectin D1 modulates the induction of pro-inflammatory signaling and promotes retinal pigment epithelial cell survival during oxidative stress. Adv Exp Med Biol. 2010;664:663–670. doi: 10.1007/978-1-4419-1399-9_76. [DOI] [PubMed] [Google Scholar]
- 24.Antony R, Lukiw WJ, Bazan NG. Neuroprotectin D1 induces dephosphorylation of Bcl-xL in a PP2A-dependent manner during oxidative stress and promotes retinal pigment epithelial cell survival. J Biol Chem. 2010;24:18301–18308. doi: 10.1074/jbc.M109.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive Decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheets KG, Zhou Y, Ertel MK, et al. Neuroprotectin D1 attenuates laser-induced choroidal neovascularization in mouse. Mol Vis. 2010;16:320–329. [PMC free article] [PubMed] [Google Scholar]