Abstract
Walking uphill and downhill can be challenging for community-dwelling old adults. We investigated the effects of age on leg muscle activity amplitudes and timing during level, uphill, and downhill walking. We hypothesized that old adults would exhibit smaller increases in ankle extensor muscle activities and greater increases in hip extensor muscle activities compared to young adults during uphill vs. level walking. We also hypothesized that, compared to level walking, antagonist leg muscle coactivation would be disproportionately greater in old vs. young adults during downhill walking. Ten old (72 ± 5 yrs) and ten young (25 ± 4 yrs) subjects walked at 1.25 m/s on a treadmill at seven grades (0, ±3, ±6, ±9°). We quantified the stance phase electromyographic activities of the gluteus maximus (GMAX), biceps femoris (BF), rectus femoris (RF), vastus medialis (VM), medial gastrocnemius (MG), soleus (SOL), and tibialis anterior (TA). Old adults exhibited smaller increases in MG activity with steeper uphill grade than young adults (e.g., +136% vs. +174% at 9°). A disproportionate recruitment of hip muscles led to GMAX activity approaching the maximum isometric capacity of these active old adults at steep uphill grades (e.g., old vs. young, 73% MVC vs. 33% MVC at +9°). Neither uphill nor downhill walking affected the greater coactivation of antagonist muscles in old vs. young adults. We conclude that the disproportionate recruitment of hip muscles with advanced age may have critical implications for maintaining independent mobility in old adults, particularly at steeper uphill grades.
Keywords: antagonist, cocontraction, elderly, electromyography, EMG, incline, decline
Introduction
Walking uphill and downhill can be challenging for community-dwelling old adults. During level walking, old adults alter their gait patterns; they often walk more slowly, take shorter, wider steps, and recruit leg muscles differently than young adults (e.g., [1–3]). However, little is known about how old adults walk up or down hills. Here, we recorded the electromyographic (EMG) activities of leg muscles to investigate how advanced age affects leg muscle recruitment during uphill and downhill walking. Advanced age may bring changes in muscle recruitment that uniquely compromise uphill or downhill walking ability in old adults.
Compared to young adults, even active and healthy old adults exhibit a distal to proximal redistribution of leg muscle recruitment during the stance phase of level walking [3–6]. Old adults rely less on ankle extensor muscles (e.g., gastrocnemius and soleus) and more on hip flexor (e.g., iliopsoas) and extensor (e.g., gluteus maximus and biceps femoris) muscles than young adults. It is possible that distal muscle weakness contributes to this greater reliance on hip muscles. Indeed, Christ et al. [7] showed that the ankle extensor muscles of women exhibited greater declines in isometric force production with advanced age than other muscle groups. For young adults, uphill walking involves a significantly greater recruitment of hip, knee, and ankle extensor muscles than level walking [8, 9]. Consequently, ankle extensor muscle weakness could especially limit uphill walking ability in old adults.
Alternatively, neural modifications with advanced age could beget hip muscle reliance, independent of muscle strength. Consistent with this premise, Schmitz et al. (2009) showed that old adults rely more on hip muscles during level walking at their preferred walking speed despite being able to significantly increase ankle extensor muscle activity at a modestly faster speed (mean increase, 0.26 m/s). It is not clear how much of the redistribution of leg muscle recruitment in old adults can be attributed to muscle weakness vs. neural mechanisms. However, even if due to neural changes alone, it is possible that hip muscle reliance in old adults is inadequate to meet the challenges posed by uphill walking. Thus, the markedly greater concentric demand placed on leg extensor muscles during uphill walking is most concerning for the maintenance of independent mobility in old adults.
Further, although not expected to limit their mobility, coactivation of antagonist muscle pairs may be disproportionately greater in old vs. young adults during uphill or downhill walking. Old adults demonstrate greater antagonist leg muscle coactivation than young adults during level walking [10–13]. Many suspect that antagonist coactivation increases leg joint stiffness and may thereby aid in responding to a trip or fall (e.g., [14]). Hortobagyi et al. (2011) found that modest uphill or downhill grades (± 3.4°; i.e., ± 6%) had little to no influence on the greater antagonist coactivation in old vs. young adults. However, such a modest grade may not reflect the muscular challenges posed by steeper grades encountered while walking in many communities. Even the Americans with Disabilities Act permits ramps of 4.8° (i.e, 8.3%). A fear of slipping and/or falling during downhill walking in particular may cause a disparate increase in the coactivation of antagonist leg muscle pairs in old adults [15, 16].
We investigated the effects of age on leg muscle activity during level, uphill, and downhill walking. We reasoned that due to the greater concentric demand on leg extensor muscles during the stance phase, uphill walking would exacerbate the disproportionate recruitment of hip muscles in old adults. Thus, we hypothesized that old adults would exhibit smaller increases in ankle extensor muscle activities and greater increases in hip extensor muscle activities compared to young adults during uphill vs. level walking. We also hypothesized that, compared to level walking, antagonist leg muscle coactivation would be disproportionately greater in old vs. young adults during downhill walking. We quantified the timing of leg muscle activity to complement our primary measures of amplitude and coactivation in evaluating these hypotheses.
Methods
Subjects
10 old adults (6F/4M, mean ± standard deviation, age: 72 ± 5 yrs, height: 1.70 ± 0.10 m, mass: 65.0 ± 13.3 kg) and 10 young adults (5F/5M, age: 25 ± 4 yrs, height: 1.73 ± 0.10 m, mass: 69.2 ± 13.1 kg) gave written informed consent as per the University of Colorado IRB. All subjects were healthy and exercised regularly. We recruited old adults from local senior mountain hiking and cycling groups. Subjects completed a health questionnaire based upon recommendations by the American College of Sports Medicine [17]. Exclusion criteria were: BMI≥30, sedentary lifestyle, coronary artery disease, cigarette smoking, high blood pressure, high cholesterol, diabetes or prediabetes, orthopedic or neurological condition, or medication that causes dizziness. All subjects scored at the highest possible mobility on the Short Physical Performance Battery (SPPB) and we found no difference in preferred level-ground walking speed in old vs. young adults (1.49 ± 0.14 m/s vs. 1.46 ± 0.14 m/s, p = 0.59).
Experimental Protocol
Subjects walked for 5 min at 1.25 m/s on a level, motorized treadmill (model 18–60, Quinton Instruments, Seattle, WA) with custom calibrated, digital electronic readouts for velocity and grade. Subjects then walked at 1.25 m/s for 1 min at each of seven grades (0°, ±3°, ±6°, ±9°; i.e., 5.2%, 10.5%, 15.8%) in randomized order. Finally, we collected a series of maximum voluntary contractions (MVCs), described in detail below.
Data Collection
After preparing the shaved skin with alcohol and fine sandpaper, we placed single differential electrodes with wireless pre-amplifiers (Trigno, Delsys, Inc., Boston, MA) over the following muscles of each subjects’ right leg according to Cram and Kasman [18]: gluteus maximus (GMAX), biceps femoris (BF), rectus femoris (RF), vastus medialis (VM), medial gastrocnemius (MG), soleus (SOL), and tibialis anterior (TA). We verified electrode positions and signal quality by visually inspecting the EMG signals, sampled at 2000Hz, while subjects contracted each instrumented muscle. Subjects also wore a pressure-sensitive insole in their right shoe (B&L Engineering, Tustin, CA) which we sampled at 1000 Hz and in synchrony with the EMG signals. We recorded all data during the final 15 s of each 1 min trial.
Subjects completed a series of MVCs to establish each muscle’s maximum isometric capacity against manual resistance as follows: GMAX (while lying prone and the leg extended), BF (while lying prone and the knee flexed to 90°), RF and VM, MG and SOL, and TA (all three while seated and the knee and ankle flexed to 90°) [18]. We verbally encouraged subjects to gradually reach a maximal effort over 3 seconds and to sustain that effort for 3 seconds. A 30 s rest period separated two repetitions for each MVC. For a subset of participants (9 old and 7 young), we recorded the maximum isometric force (N/kg) produced during the hip and knee extensor MVCs using a handheld dynamometer (Chatillon MSE-100, Ametek, Inc., Largo, FL). We did not measure isometric force during the ankle extensor MVC because, unlike hip and knee extension, handheld dynamometry provides unreliable measures of ankle extension strength [19]. Finally, we calculated subjects’ preferred walking speed as the average of two times taken to traverse the middle 10 m of a 30 m walkway at a normal, comfortable speed.
Data Analysis
A MATLAB script (Mathworks, Inc, Natick, MA) processed all data. The Delsys hardware bandpass filtered the EMG signals (20–450 Hz). We utilized the raw young adult data from our study published previously [8], which we reanalyzed together with the old adult data as follows. We defined a muscle’s baseline activity as a multiple of the standard deviation above the activity observed while subjects relaxed in a supine position. This multiple (mean, 1.5) ensured a muscle’s activity during the average level walking gait cycle reached an “off” state within an appropriate window established by the literature (e.g., [3]). Using the above criteria, we omitted GMAX data for two old adults. We full-wave rectified and normalized the EMG signals using two common methods: 1) to the mean amplitudes over a complete stride during level walking and 2) to the MVCs.
We synchronized EMG signals to the timing of heel-strikes and toe-offs identified from the insole voltage signal. A 5% of peak voltage threshold identified the timing of the stance phase, and provided each subject’s average stride frequency (SF) and stance time (tstance). We interpolated the EMG signals to obtain 1001 data points corresponding to the right leg gait cycle. Corresponding to the primary EMG bursts during stance, we computed subjects’ mean normalized EMG activity from heel-strike to midstance for GMAX, BF, VM, RF, and TA and from midstance to toe-off for MG and SOL. We also manually identified EMG activity onset and offset times to within 0.1% of the gait cycle from each muscle’s 10 Hz linear envelope.
We calculated coactivation indices (CI) for agonist-antagonist muscle pairs of the thigh (RF and BF, VM and BF) and lower leg (MG and TA, SOL and TA) from each muscles’ 10Hz linear envelope over an average gait cycle [13, 20]:
| (1) |
where min and max refer to the minimum and maximum of the two EMG linear envelopes, respectively, at each instant of the gait cycle.
Statistical Analysis
We calculated mean values of SF, tstance, and all EMG variables over seven consecutive strides per condition. An analysis of variance (ANOVA) for repeated measures tested for significant effects of age and grade with a p<0.05 criterion. Post-hoc pairwise comparisons evaluated significant main effects of grade with planned contrasts between level and all uphill and downhill grades. When a significant main effect of age was found, we performed independent-samples t-tests to determine at which grade(s) the differences occurred.
Results
EMG Amplitude and Timing
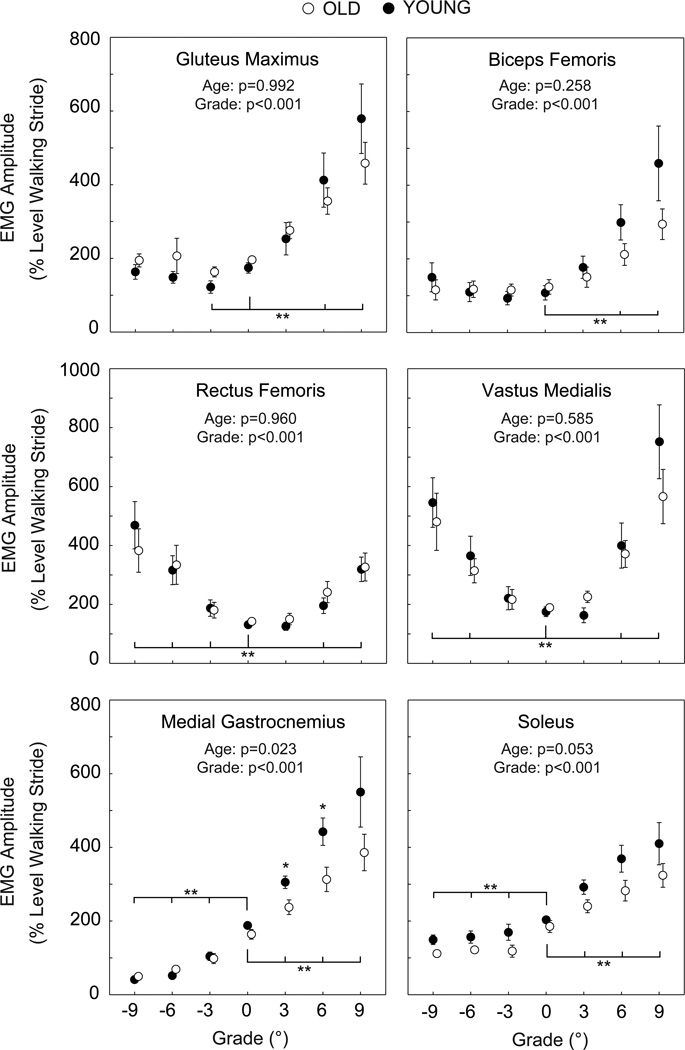
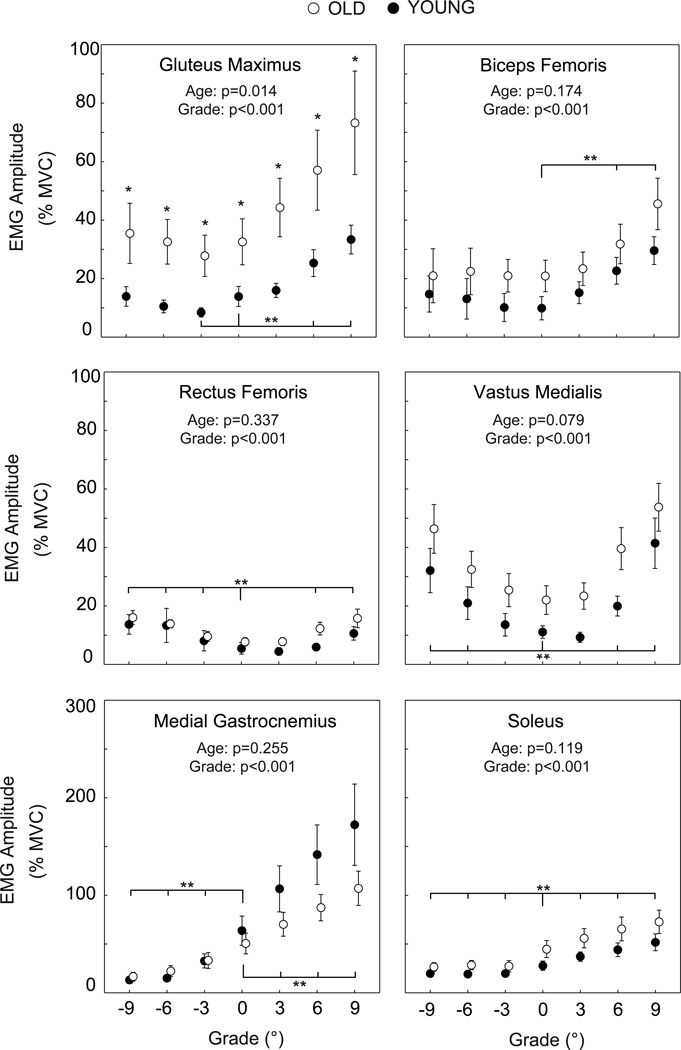
Independent of normalization method, stance phase hip, knee, and ankle extensor muscle activities increased significantly and progressively with steeper uphill grade for both old and young adults. However, old adults exhibited significantly smaller increases in MG activity with steeper uphill grade than young adults (Figure 1). Compared to level walking, MG activity increased by 136% to walk up 9° in old adults but by 174% in young adults. Old adults also exhibited greater than twice the GMAX activity of young adults at all grades, and approached their maximum GMAX isometric capacity at steep uphill grades (Figure 2).
Figure 1.
Mean (SE) stance phase EMG signals for old and young adults normalized to the mean amplitudes over a complete stride during level walking. We include p-values for the main effects of age and grade. TA activity exhibited no significant effects of age or grade, and thus is not shown. Single asterisks (*) indicate significant differences between old and young adults. Double asterisks (**) indicate significantly different from level walking. p<0.05 significant.
Figure 2.
Mean (SE) stance phase EMG signals for old and young adults normalized to each muscle’s maximum voluntary contractions. We include p-values for the main effects of age and grade. TA activity exhibited no significant effects of age or grade, and thus is not shown. Single asterisks (*) indicate significant differences between old and young adults. Double asterisks (**) indicate significantly different from level walking. p<0.05 significant.
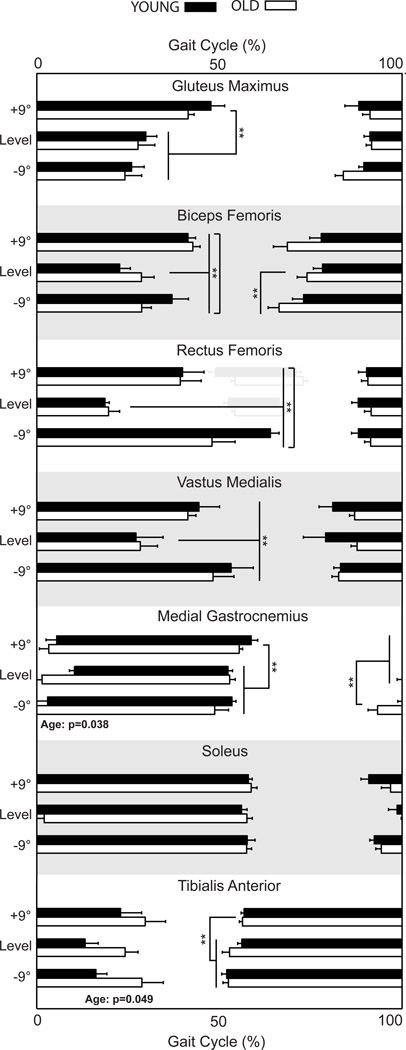
We observed no significant effect of age on leg muscle activity amplitudes during downhill walking. For both old and young adults, only knee extensor muscle activities increased significantly and progressively with steeper downhill grade, independent of normalization method (Figures 1–2). Old adults exhibited earlier MG activity onset and prolonged TA activity offset compared to young adults, but this was significant only during level walking (Figure 3).
Figure 3.
Horizontal bars indicate mean (SE) timing of muscle activities for old and young adults during level, uphill (+9°), and downhill (−9°) walking. A subset of subjects exhibited an RF peak near toe-off, which we show in gray and did not evaluate statistically. Old adults exhibited a significantly earlier MG onset and a delayed TA offset compared to young adults. Double asterisks (**) indicate significantly different from level walking. p<0.05 significant.
Antagonist Muscle Coactivation
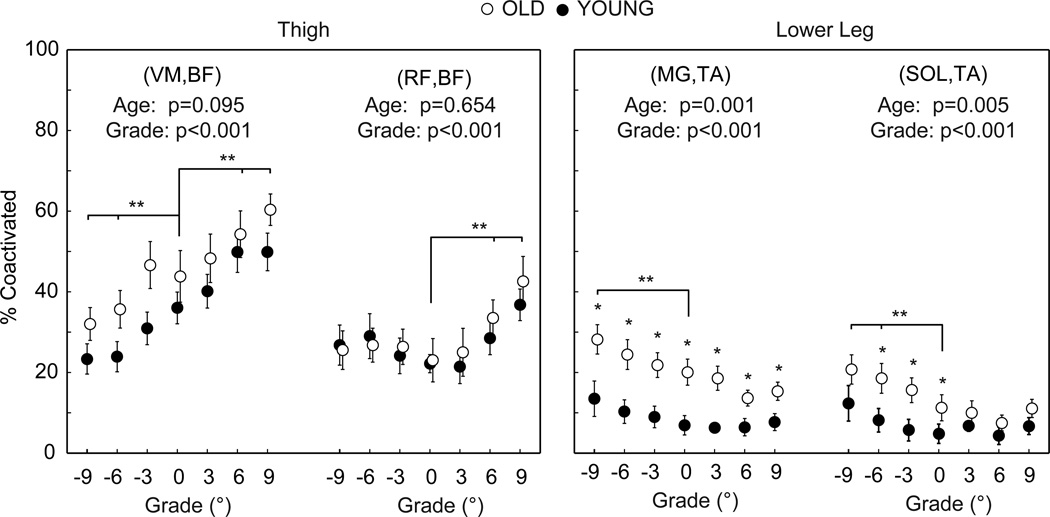
Old adults exhibited significantly greater coactivation of antagonist lower leg muscles compared to young adults (Figure 4). Antagonist lower leg muscle coactivation increased significantly and progressively with steeper downhill grade (e.g., +55% and +106% for CIMG,TA and CISOL,TA at −9°, respectively), but similarly in old vs. young adults (CIMG,TA: from 20.1% at 0° to 28.2% at −9° vs. from 6.9% at 0° to 13.5% at −9°; CISOL,TA: from 11.2% at 0° to 20.7% at −9° vs. from 4.8% at 0° to 12.3% at −9°). Antagonist thigh muscle coactivation did not differ between old and young adults, but increased significantly and progressively with steeper uphill grade (e.g., +38% and +75% for CIRF,BF and CIVM,BF at 9°, respectively) and decreased significantly and progressively with steeper downhill grade (e.g., −31% for CIVM,BF at −9°).
Figure 4.
Mean (SE) coactivation of antagonist thigh and lower leg muscles in old and young adults. We include p-values for the main effects of age and grade. Single asterisks (*) indicate significant differences between old and young adults. Double asterisks (**) indicate significantly different from level walking. p<0.05 significant.
Kinematics and Isometric Strength
Old adults took 11% faster strides than young adults during uphill walking (Table 1). Compared to level walking, both old and young adults took progressively faster strides and spent less time in stance on steeper downhill grades. Old adults demonstrated significantly weaker maximum isometric strength for the hip and knee extensor muscle groups compared to young adults (hip extensors: 1.49 ± 0.43 N/kg vs. 2.36 ± 0.50 N/kg, p=0.003; knee extensors: 3.11 ± 1.05 N/kg vs. 4.47 ± 1.34 N/kg, p=0.046).
Table 1.
Mean (SE) stride frequency and stance time.
| Downhill |
Uphill |
|||||||
|---|---|---|---|---|---|---|---|---|
| −9° | −6° | −3° | 0° | 3° | 6° | 9° | ||
| ** | ** | ** | ||||||
| SF (Hz) | Old | 1.07 (0.04) | 1.04 (0.04) | 1.03 (0.04) | 1.00 (0.03) | 1.01 (0.03)* | 1.00 (0.04)* | 1.03 (0.03)* |
| Young | 0.99 (0.01) | 0.98 (0.01) | 0.95 (0.01) | 0.93 (0.01) | 0.91 (0.01) | 0.91 (0.02) | 0.92 (0.01) | |
| ** | ||||||||
| tstance (s) | Old | 0.58 (0.03) | 0.59 (0.03) | 0.60 (0.02) | 0.61 (0.03) | 0.61 (0.03) | 0.61 (0.02) | 0.60 (0.03) |
| Young | 0.60 (0.04) | 0.62 (0.04) | 0.64 (0.04) | 0.64 (0.03) | 0.66 (0.04) | 0.65 (0.05) | 0.65 (0.04) | |
SF: stride frequency, tstance: stance time.
Asterisks (*) indicate significant differences between old and young adults.
Double Asterisks (**) indicate significantly different from level walking.
Discussion
In this study, we quantified leg muscle activities in old and young adults to investigate how advanced age affects leg muscle recruitment during uphill and downhill walking. Both old and young adults employed the same general muscle recruitment strategies during uphill walking (greater recruitment of hip, knee, and ankle extensor muscles) and downhill walking (only greater recruitment of knee extensor muscles). The durations of muscle activities largely mirrored these changes in amplitude. However, we discovered several notable and significant age-related differences in muscle recruitment that may reflect compromised walking ability in old adults.
As hypothesized, old adults exhibited smaller increases in MG activity and a strong trend toward smaller increases in SOL activity than young adults with steeper uphill grade. However, old adults did not compensate for these larger ankle muscle deficits during uphill walking by further exploiting hip muscles as expected. Instead, old adults uniformly demonstrated approximately twice the recruitment of GMAX at all grades compared to young adults. This is consistent with other studies [3, 4, 6] of level walking. However, GMAX activity during level walking (33% MVC) was far below the maximum isometric capacity of old adults. In contrast, GMAX activity approached the maximum isometric capacity of these exceptionally active old adults at steep uphill grades (e.g., 73% MVC at +9°). This is particularly significant when compared to young adults, for whom GMAX activity only reached 33% MVC at +9°. This finding suggests that the disproportionate recruitment of hip muscles may ultimately limit the uphill walking ability of old adults.
Compared to level walking, downhill walking is often associated with greater movement variability indicative of impaired balance and a greater fall risk [16, 21]. However, to our surprise, antagonist coactivation of lower leg muscles increased similarly in old and young adults during downhill vs. level walking. It is possible that the old adults in this study were not representative of those with compromised balance. But, our old adults did exhibit greater lower leg antagonist coactivation for all conditions compared to young adults, contributed to in part by an earlier MG onset and delayed TA offset. As an alternative explanation, despite a greater risk of falling, downhill walking may not be accompanied by greater antagonist coactivation in old vs. young adults. Consistent with this possibility, Finley et al. (2011) showed that antagonist lower leg muscle coactivation alone cannot adequately increase ankle joint stiffness to recover from an unexpected fall.
Despite several studies [3–6] demonstrating that old adults disproportionately recruit hip muscles during level walking, the mechanism(s) responsible for this apparent redistribution remain elusive. Despite their remarkably active lifestyles, old adults in our study exhibited ~30–37% less isometric leg strength than young adults. One might deduce that ankle muscle weakness contributes to greater hip muscle recruitment in old adults. However, we did not observe progressively greater recruitment of GMAX or BF in old vs. young adults to compensate for their reduced ankle muscle recruitment with steeper uphill grade. Further, compared to level walking, the old adults in this study were able to dramatically increase their ankle extensor muscle activities with steeper uphill grade (e.g., +136% and +74% for MG and SOL at +9°, respectively). We infer from these data that old adults have a considerable and possibly underutilized reserve available for recruiting these muscles during level walking. Thus, our findings corroborate the speculation of earlier studies [3, 4] that ankle muscle weakness contributes at most only marginally to the disproportionately greater recruitment of hip muscles with advanced age.
Rather than muscle weakness, we propose that modified neural control, from feedback (reactive and dependent upon afferent information) to feedforward (predictive and insensitive to afferent information), underlies both the greater antagonist coactivation and disproportionate recruitment of hip muscles in old adults. Consistent with impaired feedback control, advanced age is accompanied by a decline in sensory information, longer reflex latencies, and slower maximum rates of muscle force development [22–25]. The greater coactivation of antagonist muscles in old adults is often considered a feedforward strategy to increase joint stiffness and abate the effects of a trip or fall [12–14, 26, 27]. Although less intuitive, we also suspect a decline in feedback control may contribute to the disproportionate recruitment of hip muscles in old adults. Daley et al. (2007) found that muscle force regulation in the proximal leg muscles of birds is inherently under far greater feedforward control than distal muscles. In that study, an unexpected fall during running elicited a large feedback response only from the distal muscles, while proximal muscle output and hip kinematics remained the same as unperturbed running [28]. Studies in other animals have revealed that, unlike hip muscles, the magnitude and duration of ankle muscle activities largely depend upon afferent feedback [29–31]. This evidence suggests that a decline in feedback control is at least one factor affecting leg muscle recruitment in old adults.
There are several limitations of this study. First, our findings are limited to active and healthy old adults. Advanced sarcopenia and muscle weakness could more directly affect leg muscle activities and compromise uphill or downhill walking ability in sedentary and/or frail old adults. Second, we did not measure hip flexor muscle activity. Cofre et al. (2011) suggested that the hip flexors also compensate for reduced ankle extensor function in old adults. However, there is little net contribution from the hip flexors during the stance phase of uphill walking [32]. Third, the shorter steps taken by old vs. young adults during uphill walking imply subtle age differences in GMAX fiber length changes. However, GMAX activity normalized to isometric conditions remains essentially constant over a large range of fiber lengths [33]. Thus, these differences are unlikely to contribute to the significantly greater GMAX activity observed in old adults. Also, because handheld dynamometry only reliably measures hip and knee muscle strength, we cannot exclude a differential loss of ankle muscle strength. Finally, the mechanical behavior of muscle-tendon complexes cannot be directly inferred from EMG data alone, and can be particularly difficult to resolve for biarticular muscles. Age differences in the muscular contributions to uphill and downhill walking may be better characterized using musculoskeletal simulations.
In summary, we find that old adults walk uphill with diminished MG activity and GMAX activity that approaches their maximum isometric capacity. Our findings also suggest that neural mechanisms contribute notably more than muscle weakness to the reliance on hip muscles in old adults. Surprisingly, neither uphill nor downhill walking affected the greater coactivation of antagonist muscles in old vs. young adults. We conclude that the disproportionate recruitment of hip muscles with advanced age may have critical implications for maintaining independent mobility in old adults, particularly at steeper uphill grades.
Research Highlights.
Old adults exhibited smaller increases in medial gastrocnemius activity with steeper uphill grade than young adults.
Old adults uniformly demonstrated greater than twice the gluteus maximus activity at all grades compared to young adults.
Gluteus maximus activity approached the maximum isometric capacity of old adults at steep uphill grades.
Neither uphill nor downhill walking affected the greater coactivation of antagonist muscles in old vs. young adults.
Changes in muscle recruitment with age may negatively affect walking ability in old adults, particularly at steeper uphill grades.
Acknowledgements
We thank Dr. Alaa Ahmed for use of the EMG equipment, Dr. Cory Christiansen for use of the handheld dynamometer, and Alyse Kehler and Lauren MacDonald for aiding with data collection. This research was supported by a grant from NIH (5T32AG000279) and a student Grant-in-Aid Award from the American Society of Biomechanics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Prince F, Corriveau H, Hebert R, Winter DA. Gait in the elderly. Gait & Posture. 1997;5:128–135. [Google Scholar]
- 2.Oberg T, Karsznia A, Oberg K. Basic gait parameters: reference data for normal subjects, 10–79 years of age. J Rehabil Res Dev. 1993;30(2):210–223. [PubMed] [Google Scholar]
- 3.Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J Electromyogr Kinesiol. 2009;19:1085–1091. doi: 10.1016/j.jelekin.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol. 2000;88(5):1804–1811. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- 5.Cofre LE, Lythgo N, Morgan D, Galea MP. Aging modifies joint power and work when gait speeds are matched. Gait & Posture. 2011;33:484–489. doi: 10.1016/j.gaitpost.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 6.Savelburg HHCM, Verdijk L, Willems PJB, Meijer K. Robustness of age-related gait adaptations: can running counterbalance the consequences of ageing? Gait & Posture. 2007;25:259–266. doi: 10.1016/j.gaitpost.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Christ CB, Boileau RA, Slaughter MH, Stillman RJ, Cameron JA, Massey BH. Maximal voluntary isometric force production characteristics of six muscle groups in women aged 25 to 74 years. Am J Hum Biol. 1992;4:537–545. doi: 10.1002/ajhb.1310040413. [DOI] [PubMed] [Google Scholar]
- 8.Franz JR, Kram R. The effects of grade and speed on leg muscle activations during walking. Gait & Posture. 2012;35:143–147. doi: 10.1016/j.gaitpost.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lay AN, Hass CJ, Nichols RT, Gregor RJ. The effects of sloped surfaces on locomotion: an electromyographic analysis. J Biomech. 2007;40:1276–1285. doi: 10.1016/j.jbiomech.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Hortobagyi T, Solnik S, Gruber A, Rider P, Steinweg K, Helseth J, DeVita P. Interaction between age and gait velocity in the amplitude and timing of antagonist muscle coactivation. Gait & Posture. 2009;29:558–564. doi: 10.1016/j.gaitpost.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Hortobagyi T, Finch A, Solnik S, Rider P, DeVita P. Association between muscle activation and metabolic cost of walking in young and old adults. J Gerontol A Biol Sci Med Sci. 2011;66:541–547. doi: 10.1093/gerona/glr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol. 2006;186:127–139. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- 13.Peterson DS, Martin PE. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait & Posture. 2010;31:355–359. doi: 10.1016/j.gaitpost.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Finley JM, Dhaher YY, Perreault EJ. Contributions of feed-forward and feedback strategies at the human ankle during control of unstable loads. Exp Brain Res. 2012;217(1):53–66. doi: 10.1007/s00221-011-2972-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bentley TA, Haslam RA. Slip, trip and fall accidents occurring during the delivery of mail. Ergonomics. 1998;41(12):1859–1872. doi: 10.1080/001401398186027. [DOI] [PubMed] [Google Scholar]
- 16.Redfern MS, Cham R, Gielo-Perczak K, Gronqvist R, Hirvonen M, Lanshammar H, Marpet M, Pai CY, Powers C. Biomechanics of slips. Ergonomics. 2001;44(13):1138–1166. doi: 10.1080/00140130110085547. [DOI] [PubMed] [Google Scholar]
- 17.American College of Sports Medicine. Guidelines for Exercise Testing and Prescription. Philadelphia: Lippincott Williams & Wilkins; 2006. Participation, health screening and risk stratification; pp. 19–35. [Google Scholar]
- 18.Cram JR, Kasman GS. Introduction to surface electromyography. Gaithersburg: Aspen Publishers; 1998. [Google Scholar]
- 19.Arnold CM, Warkentin KD, Chilibeck PD, Magnus CR. The reliability and validity of handheld dynamometry for the measurement of lower-extremity muscle strength in older adults. J Strength Cond Res. 2010;24(3):815–824. doi: 10.1519/JSC.0b013e3181aa36b8. [DOI] [PubMed] [Google Scholar]
- 20.Falconer K, Winter DA. Quantitative assessment of co-contraction at the ankle joint in walking. Electromyogr Clin Neurophysiol. 1985;25(2–3):135–149. [PubMed] [Google Scholar]
- 21.Hunter LC, Hendrix EC, Dean JC. The cost of walking downhill: is the preferred gait energetically optimal? J Biomech. 2010;43:1910–1915. doi: 10.1016/j.jbiomech.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 22.Dorfman LJ, Bosley TM. Age-related changes in peripheral and central nerve conduction in man. Neurology. 1979;29(1):38–44. doi: 10.1212/wnl.29.1.38. [DOI] [PubMed] [Google Scholar]
- 23.Patel M, Magnusson M, Kristinsdottir E, Fransson PA. The contribution of mechanoreceptive sensation on stability and adaptation in the young and elderly. Eur J Appl Physiol. 2009;105(2):167–173. doi: 10.1007/s00421-008-0886-4. [DOI] [PubMed] [Google Scholar]
- 24.Thelen DG, Schultz AB, Alexander NB, Ashton-Miller JA. Effects of age on rapid ankle torque development. J Gerontol A Biol Sci Med Sci. 1996;51(5):M226–M232. doi: 10.1093/gerona/51a.5.m226. [DOI] [PubMed] [Google Scholar]
- 25.Tseng SC, Stanhope SJ, Morton SM. Impaired reactive stepping adjustments in older adults. J Gerontol A Biol Sci Med Sci. 2009;64:807–815. doi: 10.1093/gerona/glp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baudry S, Maerz AH, Enoka RM. Presynaptic modulation of Ia afferents in young and old adults when performing force and position control. J Neurophysiol. 2010;103:623–631. doi: 10.1152/jn.00839.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osu R, Morishige K, Miyamoto H, Kawato M. Feedforward impedance control efficiently reduce motor variability. Neurosci Res. 2009;65:6–10. doi: 10.1016/j.neures.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Daley MA, Felix G, Biewener AA. Run ning stability is enhanced by a proximo-distal gradient in joint neuromechanical control. J Exp Biol. 2007;210:383–394. doi: 10.1242/jeb.02668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson KG, Misiaszek JE, Fouad K. Enhancement and resetting of locomotor activity by muscle afferents. Ann N Y Acad Sci. 1998;860:203–215. doi: 10.1111/j.1749-6632.1998.tb09050.x. [DOI] [PubMed] [Google Scholar]
- 30.Whelan PJ. Control of locomotion in the decerebrate cat. Prog Neurobiol. 1996;49:481–515. doi: 10.1016/0301-0082(96)00028-7. [DOI] [PubMed] [Google Scholar]
- 31.Grillner S. Locomotion in vertebrates: central mechanisms and reflex interaction. Physiol Rev. 1975;55(2):247–304. doi: 10.1152/physrev.1975.55.2.247. [DOI] [PubMed] [Google Scholar]
- 32.Lay AN, Hass CJ, Gregor RJ. The effects of sloped surfaces on locomotion: a kinematic and kinetic analysis. J Biomech. 2006;39:1621–1628. doi: 10.1016/j.jbiomech.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Clark BC, Manini TM, Ploutz-Snyder LL. Fatigue-induced changes in phasic muscle activation patterns during dynamic trunk extension exercise. Am J Phys Med Rehabil. 2007;86(5):373–379. doi: 10.1097/PHM.0b013e3180321689. [DOI] [PubMed] [Google Scholar]