Abstract
Voltage gated calcium channels (VGCCs) are essential to neuronal excitation and signal transduction. They are multimeric in structure and comprised of an alpha subunit that functions as a calcium pore and two additional subunits: an alpha2delta subunit and a cytoplasmic beta subunit. To better understand the role of VGCCs in the retina we used immunohistochemical methods to determine the distribution of VGCC β subunits in normal and mutant mice. To verify the specificity of each antibody and to examine the potential for subunit redistribution when beta subunit expression is perturbed, we used 4 mutant mouse lines that each lack a specific β subunit isoform (β1–β4). We found the β1 subunit distributed on cell bodies in the inner nuclear layer (INL) and on processes within both the inner and outer limiting membrane; the β2 subunit localized to the outer plexiform layer (OPL) and inner plexiform layer (IPL); the β3 subunit was localized to three narrow and distinct bands within the IPL; the β4 subunit was localized to three diffuse bands within the IPL. Loss of one β subunit affected labeling intensity but not general distribution patterns of other β subunits. It is likely that VGCCs critical for retinal signal transmission are comprised of the β2 subunit in the OPL and any of the 4 β subunits in the IPL. Our results suggest that within the OPL the α1F subunit pairs predominantly with the β2 subunit while within the IPL it may pair with either any β subunit.
Keywords: Voltage gated calcium channel, L-type calcium channel, Retina, Congenital stationary night, blindness
1. Introduction
Neuronal voltage gated calcium channels (VGCCs) are heteromeric complexes composed of an α1 pore-forming subunit and usually regulatory subunits including α2δ, γ and β subunits (Birnbaumer et al., 1998; Lacinová, 2005; Zuccotti et al., 2011). These channels play integral roles in cell function throughout the body. For example, in the central nervous system (CNS), VGCCs are known to regulate neurotransmitter release and cell differentiation (Deisseroth et al., 2004; Nachman-Clewner et al., 1999; Vandael et al., 2010; Yasuda et al., 2003). Channel types are categorized as T, L, N, P, Q, and R types according to their sensitivity to pharmacological agents, kinetics and voltage dependence, which are determined by their subunit composition. In addition to the many α1 subunit isoforms, there are four β subunit genes that also produce many isoforms. The β subunits are thought to be required for proper channel formation and can alter channel kinetics (Dolphin, 2009; Gregg et al., 1996; Reichhart et al., 2010; Richards et al., 2004). β subunits had been thought to be critical for positioning of the α1 subunits on the cell surface. However, a study in the zebrafish mutant relaxed, which lacks the β1 subunit, showed the α1S subunit could be inserted into the skeletal muscle although it was not organized normally (Schredelseker et al., 2005, 2009). In mice, functional loss of any one of the β subunits results in severe abnormalities in channel function (Ball et al., 2002; Burgess et al., 1997; Cork et al., 2001; Fuller-Bicer et al., 2009; Gregg et al., 1996; Namkung et al., 1998; Neef et al., 2009). For example, loss of either the β1 or β2 subunit results in dysfunction of the excitation-contraction coupling mechanism affecting cardiac or respiratory function respectively (Burgess et al., 1997; Gregg et al., 1996).
The retina expresses many α1 subunits (Baumann et al., 2004; Berntson et al., 2003; Habermann et al., 2003; Logiudice et al., 2006; Wycisk et al., 2006a; Xu et al., 2003), and all four β subunits (Ball et al., 2002; Reichhart et al., 2010). Many details regarding the physiology, distribution and type of subunits in the retina remain unknown. However, analyses of mouse lines lacking each of the β subunits in retina revealed that only loss of β2 had dramatic effects on retinal function as determined by the electroretinogram (ERG). In mice lacking the β2 subunit, synaptic transmission between photoreceptors and bipolar cells is disrupted as indicated by the absence of the ERG b-wave. These mice also lack the α1F subunit, which is normally located at the photoreceptor terminals (Ball et al., 2002; Morgans, 2000; Morgans et al., 2005). A similar defect in synaptic transmission is seen when there is a loss of the α1F subunit in either humans (Bech-Hansen et al., 1998; Strom et al., 1998) or mice (Chang et al., 2006; Mansergh et al., 2005). Mutations in the α2/δ4 subunit also lead to loss of the ERG b-wave, disorganization of the OPL and degeneration of cells in the ONL (Wycisk et al., 2006a) and mutations in this gene in humans results in a cone dystrophy (Wycisk et al., 2006b). These observations suggest that the majority of VGCCs at photoreceptor terminals are composed of a combination of α1F/β2, and α2/δ4 subunits.
The α1 subunits subserve specific functions in neurons. In tottering mice, which lack the α1A subunit, hippocampal synapses that express both P/Q- and N-type channels, show increased presence of N-type channels (Qian and Noebels, 2000). This compensation would appear to occur only in cell types that express multiple α1 subunits. In contrast, if one β subunit is lost and others are expressed in the same cell, the remaining may be redistributed or “shuffled” so that there is no reduction in the number of VGCCs, although the amount of total current may be affected (Burgess et al., 1999; Qian and Noebels, 2000). Further, in several brain regions, deletion of one β subunit results in compensatory changes in another β subunit (Burgess et al., 1999; McEnery et al., 1998b). During normal development there also are changes in the percent of a particular α1/β combination (Vance et al., 1998). These subunit alterations suggest that loss of one β subunit in the retina could affect expression levels of the remaining subunits.
Given the critical role of subunit combinations in channel function we determined the distribution pattern of the β subunits in both WT and β subunit mutant mice. Our results show that each β subunit has a unique distribution and that the loss of one subunit does not change the general distribution pattern of the remaining subunits. However, some differences were noted in the intensity of label. Future identification of the α1 subunit distribution patterns will be useful in determining subunit pairings, and by inference specific functional properties of the VGCCs in retina.
2. Results
2.1. β subunit distribution patterns in WT retina
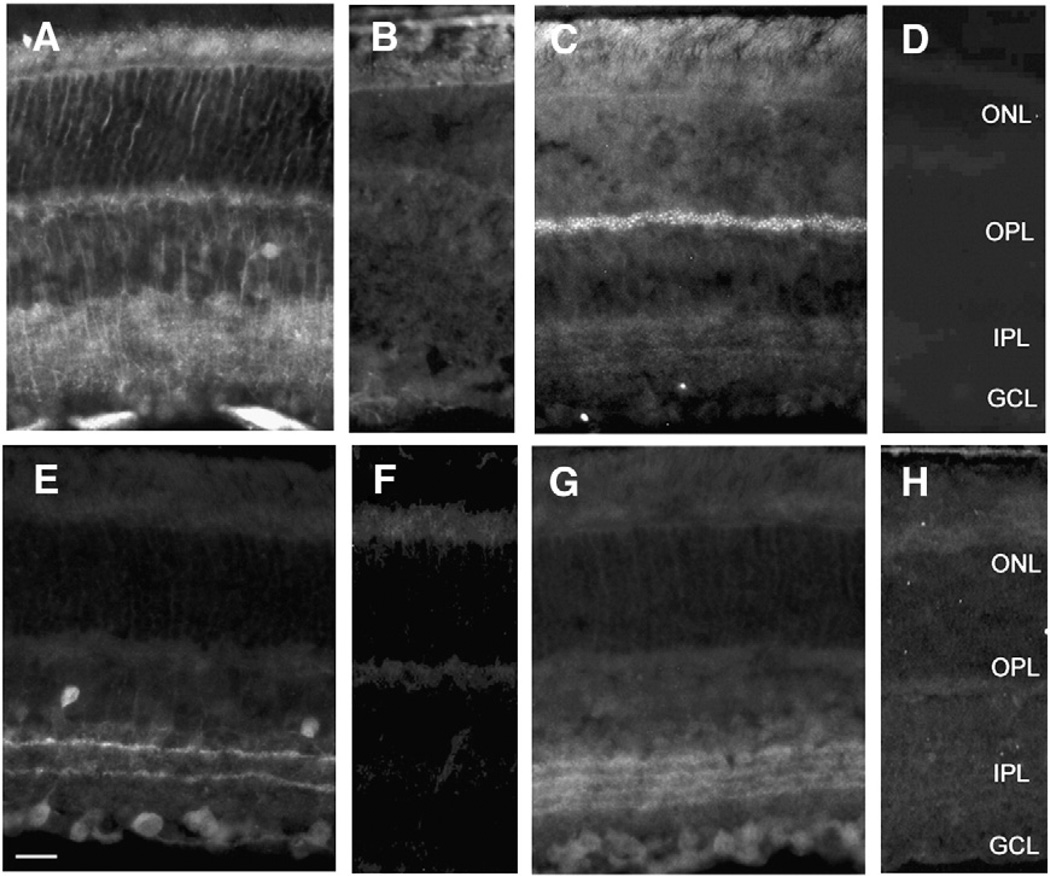
The distribution of each β subunit in WT retinas was determined by indirect immunofluorescence on sagittal sections; immunolabeling patterns for β subunits 1–4 are shown in Figs. 1A, C, E, and G, respectively. To confirm the specificity of each antibody we also labeled sections from the retinas of each of the corresponding β subunit null mice (Figs. 1B, D, F, & H). Figs. 1 (B, D, F, & H) confirm the specificity of each antibody. Labeling for each respective antibody was absent in the mouse line that lacked the corresponding β subunits. In WT retinal sections each antibody revealed a distinct labeling pattern (Figs. 1A, C, E, G), and we observed a uniform distribution pattern from central to peripheral regions of the retina (data not shown).
Fig. 1.
VGCC β subunit distribution in wild type and β subunit null mouse retina. A) β1 is present on cell membranes in the middle portions of the INL and processes extending into the ONL and IPL; lateral processes can be detected; B) no β1 is found in CNS β1 null retinas; C) β2 is expressed as punctate label in the OPL with weak but punctate label throughout the IPL; D) no β2 is present in CNS β2 null retinas; E) β3 is seen in two distinct narrow bands in the IPL, scattered cells throughout the most proximal row of the INL, and some cells in the GCL; The strongest immunoreactivity is observed in the outermost band of the IPL; F) no β3 is noted in β3 null retinas; G) β4 is distributed in 4 bands in the IPL H) no β4 is detected in β4 null retinas. Scale bar = 25 µm.
The β1 subunit distribution pattern in WT retina (Fig. 1A) closely resembled immunolabeling for Müller cells (Lavail and Reif-Lehrer, 1971) labeling cell bodies in the inner nuclear layer (INL) and processes within both the inner and outer limiting membrane. β1 labeling was also found along processes within the outer nuclear layer (ONL) and the IPL as well as on lateral projections in both the OPL and IPL. Labeling of β1 was also found on cell bodies in the middle portion of the INL where Müller cell bodies are located (Jeon et al., 1998).
The β2 subunit was expressed in the OPL where discrete puncta could be observed. There also was relative diffuse labeling in the IPL (Fig. 1C). This pattern was very similar to that seen for the α1F subunit (Morgans et al., 2001; Morgans, 2001; Chang et al., 2006). To examine the location of the β2 labeling in the OPL and IPL more closely we double labeled for PKC, a marker for rod bipolar cells (Fig. 2). β2 immunolabeling showed individual puncta within the OPL that did not overlap with PKC (Fig. 2A). Close inspection of these puncta revealed a horseshoe shaped pattern similar to that reported for several synaptic ribbon markers (Morgans, 2000). This was consistent with the fact that absence of the β2 subunit results in defective signaling to bipolar cells and a loss of the α1F subunit (Ball et al., 2002). The diffuse β2 labeling in the IPL (Fig. 2B) extended into the innermost layers ending proximal to rod bipolar cell terminals. The sparse nature of the β2 label suggests this subunit may have small or negligible contributions to synaptic function in the IPL.
Fig. 2.
Double labeling with β2 subunit and PKC antibodies. (A) High magnification view of the OPL immunolabeled for β2 and PKC, and (B) IPL immunolabeled for β2 and PKC. Scale bars = 10 µm.
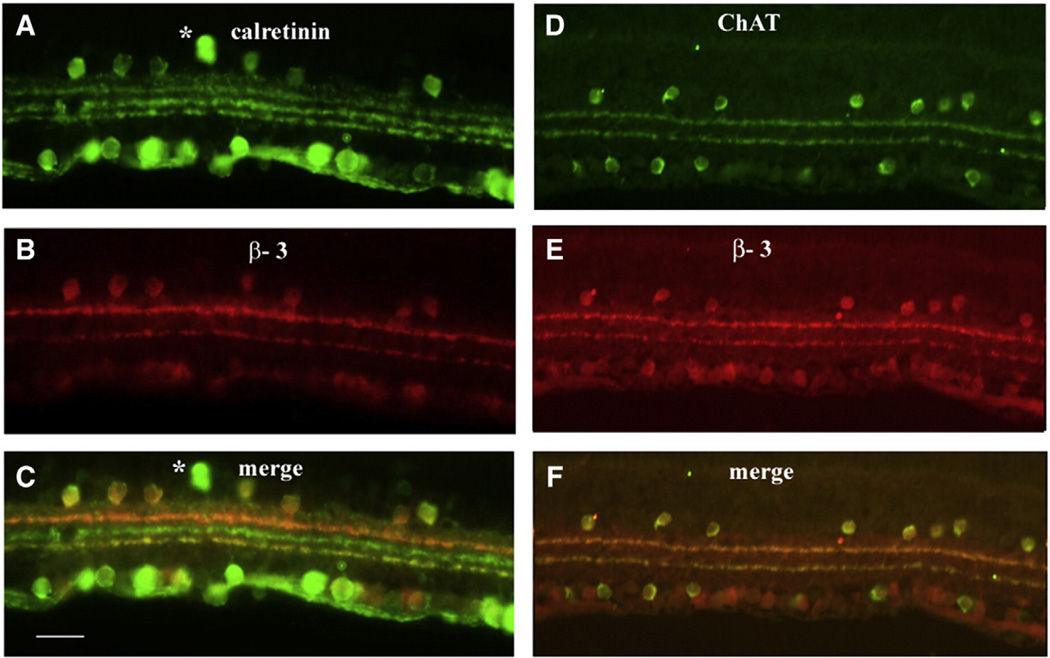
The β3 subunit was expressed in two narrow and distinct bands within the ON and OFF strata of the IPL (Fig. 1E). The level of labeling in the outermost β3 subunit band in the OFF sublaminae appeared brighter, suggesting more abundant expression. A small number of cell bodies were labeled in the most proximal row of cells of the INL as well as scattered cells throughout the ganglion cell layer (GCL). To more precisely identify the location of the β3 subunit within the IPL, double labeling studies were completed with 2 antibodies that clearly delineate IPL sublamina, calretinin (Figs. 3A–C) and choline acetyltransferase (Figs. 3D–F). Immunolabeling with the calretinin antibody reveals amacrine and displaced amacrine cells, RGCs, and three distinct bands in the IPL (Haverkamp and Wässle, 2004). The central calretinin immunolabeled band divides the IPL, into the ON and OFF sublamina. The β3 subunit co-localized with the inner and outer calretinin bands as well as most calretinin positive cell bodies. The ChAT antibody labels cholinergic amacrine cells that are located in the INL and the GCL, and their processes, revealing two distinct bands in the IPL. The β3 subunit co-localized with these two bands in the IPL (Fig. 3F). Immunolabeling of cell bodies for β3 also was seen in both the INL and the GCL. All ChAT positive amacrine cells in the IPL showed labeling of β3. However, numerous cells were labeled with β3 in the GCL and these labeled cells did not always overlap with the ChAT positive amacrine cells. Whether the labeling in the GCL was displaced amacrine cells or a specific sub-population of GCs must await further study.
Fig. 3.
Retinal sections double labeled for β3 and calretinin (A–C); (A) calretinin only, (B) β3 only and (C) merge of calretinin and β3; both β3 bands co-localize with calretinin. Most of the β3 labeled cells co-localize with calretinin in both the INL and the GCL while few label for calretinin only (*). Retinal sections double labeled for β3 and ChAT (D–F); (D) ChAT only, (E) β3 only, (F) merge of ChAT and β3 reveal that both β3 labeled bands co-localize with ChAT. Scale bar = 25 µm.
The β4 subunit was expressed in four bands within the IPL (Fig. 1G) spanning the ON and OFF sublamina. Of the four β4 subunit labeled bands, the two outer showed the most intense immunolabeling. Labeling patterns for the β4 subunit were consistent for each band and throughout central and peripheral regions.
2.2. β subunit distribution patterns in mutant retina
To determine whether loss of one β subunit was associated with shuffling of remaining subunits in the retina, we examined the distribution pattern of non-deleted β subunits. Although the general distribution patterns occurred in consistent layers among animals inconsistencies were noted among animals and between groups (data not shown). These differences may be attributed to differences in tissue quality.
3. Discussion
Mutations in the α1F, β2 and α2/δ4 subunit genes of VGCCs cause congenital stationary night blindness in mice and humans (Ball et al., 2002; Bech-Hansen et al., 1998; Chang et al., 2006; Mansergh et al., 2005; Strom et al., 1998; Wutz et al., 2002; Wycisk et al., 2006a,b). We have examined the distribution of VGCC β subunits as a prelude to understanding the most likely subunit combinations in the various retinal neurons. After confirming the specificity of antibodies for each VGCC β subunit we have examined the distribution of each β subunit within the retina. The findings that several are present in the OPL and IPL is not surprising given that VGCCs are required for neurotransmitter release. However, the observation that specific subunits are located in specific regions is a novel finding. In addition to extensive labeling of synaptic layers, the β3 subunits in particular, are located on the cell bodies of what are most likely amacrine cells. The distribution of β subunits in discrete strata in the IPL will confer unique properties on the VGCCs expressed by cells that make up those synapses. The presence of β3 on some amacrine cell bodies indicates that VGCCs on these cells may be important for transcription regulation, even in adult retina.
Comparisons with previously reported β subunit distributions of VGCC subunits suggest potential α–β pairings of these channels in the retina. We have previously shown that deletion of the β2 subunit results in the loss of α1F in the OPL (Ball et al., 2002). Here, we show that the β2 subunit distribution pattern is identical to that reported for the α1F subunit in the rat retina (Morgans, 2001; Morgans et al., 2001). β2 labeling in the OPL is strong and reveals the horseshoe-shaped structure that is characteristic of the synaptic ribbon and arciform densities on rod photoreceptor terminals and is present throughout the full extent of the OPL. In the IPL, relatively weak punctate label is found evenly dispersed throughout the entire layer. Given that glutamate release by photoreceptors is known to be modulated by VGCCs, these results strongly suggest that channels located on the rod photoreceptor terminals are comprised of a β2 subunit paired with the α1F subunit. This pairing is likely to occur to some extent in the IPL as well. However, other channel types are also present in each plexiform layer. For example, the α1D and α1C have been identified on photoreceptors terminals (Morgans et al., 2005; Wilkinson and Barnes, 1996; Taylor and Morgans, 1998) and T-type channels on bipolar cells terminals (Berntson et al., 2003).
Within the IPL, the distribution of the β2 and β1 subunit label was noted throughout both ON and OFF layers including the most proximal regions where rod ON bipolar cells project. In contrast, although label for β3 and β4 subunits was found in both ON and OFF layers of the IPL in specific strata, and the extreme proximal region was devoid of these two subunits. Thus, it is possible that rod ON bipolar cells utilize VGCCs composed of the α1F and β2 or β1 subunits but not β3 or β4 subunits. Previous studies have also identified the α1A–E subunit in both outer and inner plexiform and nuclear layers (Xu et al., 2002).
3.1. Colocalization with calretinin and ChAT
In mice, calretinin immunolabeling is found in some amacrine cell bodies in the INL, displaced amacrine cells and ganglion cells in the GCL as well as three strong bands in the IPL (Haverkamp and Wässle, 2004). The three calretinin bands enable clear distinction of ON and OFF sublaminae of the IPL. The distal and proximal calretinin bands are located within OFF and ON sublamina respectively, while the middle band marks the division between ON and OFF lamina (Haverkamp and Wässle, 2004). We found colocalization of the β3 subunit and the distal and proximal calretinin bands located in the OFF and ON sublamina of the IPL. In addition to the synaptic layers, calretinin labels a subset of cell bodies in both the INL and the GCLs. All calretinin labeled cells in the INL also express β3 while those in the GCL labeled primarily with calretinin only. The identity of these cells is unknown at this time. The ChAT/β3 double labeling shows complete colocalization of cholinergic amacrine cells; thus, it is likely that all calretinin/β3 cells are also cholinergic amacrine cells. Colocalization of high voltage-activated calcium channels subunits within cholinergic and dopaminergic cells in the retina has been previously reported in rat. The α1A subunit was detected primarily in the processes of cholinergic amacrine cells while α1E was found on both processes and cell bodies (Xu et al., 2002). Based on these data it is possible that cholinergic amacrine cells possess at least two types of VGCCs; perhaps used at the cell body and their processes innervating ON and OFF pathways.
3.2. Alternate subunit pairings
Although α–β pairings are crucial to structural and functional properties of Ca2+ channels, alternative pairing has been shown in response to developmental or compensatory situations (McEnery et al., 1998a), (Burgess et al., 1999). However, the abnormal signal transmission between photoreceptors and bipolar cells in the CNS β2-null mice indicates a lack of any compensatory mechanism to restore function. We also found that loss of one β subunit had minimal affect on distribution patterns of the other β subunits. Given the results from other neuronal systems where β subunit shuffling occurs it is possible that the expression in the retina is such that only a single β subunit is expressed in each cell type making shuffling impossible. However, if that were the case the fact that there is little overlap in the distribution patterns of the β subunits would indicate that some cells in the null mice would likely have altered synaptic transmission.
Mutations in VGCCs are responsible for a wide range of neurological disorders (see Ashcroft, 2000). The retina makes an excellent model system in which to study the composition and function of VGCCs as this system is easily accessible, well organized into discrete cell types and layers and appears to utilize many different types of calcium channels. In the brain, L-type channels are generally not responsible for neurotransmitter release but rather N- and P/Q type channels regulate this function (Ishikawa et al., 2005). However, the L-type channel characteristic of slow inactivation (de la Villa et al., 1998; Pan, 2000; Yagi and Macleish, 1994) makes it particularly suited as a modulator of glutamate release at the axon terminal of photoreceptors and bipolar cells, which use graded potentials and sustained transmitter release for signaling (Berntson et al., 2003; Nachman-Clewner et al., 1999; Rieke and Schwartz, 1996; Schmitz and Witkovsky, 1997). In spiking neurons, transmitter release is primarily regulated by N- and P/Q (Allen, 1999) type VGCCs which rapidly inactivate after opening (Barbour et al., 1994). The description of the location of the various VGCC subunits will begin to allow structure function relationships to be made in the many retinal neurons.
4. Experimental procedure
4.1. Mice
Mice studied here were generated from local breeding colonies and all procedures were approved by the institutional animal care and use committee at the Cleveland VA Medical Center. Because the loss of either the β1 or the β2 subunit in respiratory or cardiac muscle tissue is lethal the respective subunits were rescued outside of the CNS in the CNS β1 and β2 null mice (Ball et al., 2002). CNS β1, null mice were bred on a 129/Sv background and β2 null mice were bred on a C57Bl/6J background as described previously (Ball et al., 2002). The β3 null mice (Namkung et al., 1998) were on a 129/Sv background. The β4 null mice (B6EiC3H-a/A-Cacnb4lh; lethargic) were purchased from Jackson Laboratories (Bar Harbor, ME) and crossed onto the C57Bl/6J background to remove the rd1 mutation. For each line, matings were established that generated both homozygous mutant and heterozygous mice. The latter or C57Bl/6J mice were used as controls.
4.2. Immunohistochemistry
Retinas were collected from homozygous and WT mice (1–3 months of age) maintained under a 12:12 light/dark cycle. Animals were killed by anesthetic (sodium pentobarbital i.p. 100 mg/kg) overdose followed by cervical dislocation and the eyes were enucleated and immersion fixed in 4% paraformaldehyde in phosphate buffered saline (PBS, 0.1 M Sodium phosphate, pH 7.4, 0.9% NaCl) for approximately 30 min. The eyecups were cryoprotected for 1 h in 10% sucrose, 1 h in 20% and overnight in 30% sucrose. Eyecups were then embedded in OCT and sagittal sections were cut at 10 µm on a Leica cryostat. Sections through and near the optic nerve were collected on slides and stored at −70 °C. Frozen sections were rinsed in PBS, blocked in PBS containing 5% goat serum, 1% bovine serum albumin (BSA), and 0.1% Triton-X, before incubating in rabbit anti-β antibodies as listed in Table 1. Sections then were washed 3 times in PBS followed by incubation with goat anti-rabbit secondary antibodies conjugated to either Alexa 488 or 532 (Molecular Probes, Eugene, OR) in block solution for 1 h. After 3 washes in PBS, sections were coverslipped using Vectashield (Vector Laboratories, Burlingame, CA) and photographed using an RT Spot camera (Diagnostic Instruments, Sterling Heights, MI) attached to a Leica microscope with filters of appropriate wavelength. Double labeling studies were performed in series; after labeling with the first antibody, sections were rinsed and then treated in the same manner for labeling with a second antibody, either PKC, calretinin or choline acetyltransferase.
Table 1.
Antibody reaction conditions and number of animals examined.
| Antibody | Dilution | Condition | Control | β1−/− | β2−/− | β3−/− | β4−/− |
|---|---|---|---|---|---|---|---|
| β1 | 1:100 | Overnight at 4° | 5 | 5 | n.a. | n.a. | n.a. |
| β2 | 1:50 | Overnight at rt | 5 | 4 | 5 | 2 | 5 |
| β3 | 1:500 | overnight at 4° | 5 | 4 | 2 | 5 | 4 |
| β4 | 1:100 | overnight at 4° | 5 | 4 | 4 | 4 | 5 |
rt (room temperature); n.a. not applicable.
4.3. Antibodies
Specific anti-peptide polyclonal affinity purified antibodies to β subunits have been described previously (McEnery et al., 1998a; Pagani et al., 2004; Vance et al., 1998). Antibodies to PKC (P5704, Sigma, St. Louis), calretinin (AB1550, Chemicon, Temecula, CA) and choline acetyltransferase (ChAT, AB143, Chemicon, Temecula, CA) were used to label specific retinal features at 1:1000, 1:2000 and 1:3000, respectively.
Acknowledgments
This work was supported by a Medical Research grant from the Department of Veterans Affairs to SLB and by NIH R01 EY12354 to RGG and in part by a Research to Prevent Blindness Grant to Cleveland Clinic Lerner College of Medicine of Case Western Reserve University.
REFERENCES
- Allen TG. The role of N-, Q- and R-type Ca2+ channels in feedback inhibition of ACh release from rat basal forebrain neurones. J. Physiol. (Lond.) 1999;515(Pt 1):93–107. doi: 10.1111/j.1469-7793.1999.093ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM. Ion Channels and Disease. California: Academic Press; 2000. [Google Scholar]
- Ball SL, Powers PA, Shin H, Morgans CW, Peachey NS, Gregg RG. Role of the beta(2) subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest. Ophthalmol. Vis. Sci. 2002;43:1595–1603. [PubMed] [Google Scholar]
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Baumann L, Gerstner A, Zong X, Biel M, Wahl-Schott C. Functional characterization of the L-type Ca2+ channel Cav1.4alpha1 from mouse retina. Invest. Ophthalmol. Vis. Sci. 2004;45:708–713. doi: 10.1167/iovs.03-0937. [DOI] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat. Genet. 1998;19:264–267. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- Berntson A, Taylor WR, Morgans CW. Molecular identity, synaptic localization, and physiology of calcium channels in retinal bipolar cells. J. Neurosci. Res. 2003;71:146–151. doi: 10.1002/jnr.10459. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Qin N, Olcese R, Tareilus E, Platano D, Costantin J, Stefani E. Structures and functions of calcium channel beta subunits. J. Bioenerg. Biomembr. 1998;30:357–375. doi: 10.1023/a:1021989622656. [DOI] [PubMed] [Google Scholar]
- Burgess DL, Jones JM, Meisler MH, Noebels JL. Mutation of the Ca2+ channel beta subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell. 1997;88:385–392. doi: 10.1016/s0092-8674(00)81877-2. [DOI] [PubMed] [Google Scholar]
- Burgess DL, Biddlecome GH, McDonough SI, Diaz ME, Zilinski CA, Bean BP, Campbell KP, Noebels JL. beta subunit reshuffling modifies N- and P/Q-type Ca2+ channel subunit compositions in lethargic mouse brain. Mol. Cell. Neurosci. 1999;13:293–311. doi: 10.1006/mcne.1999.0748. [DOI] [PubMed] [Google Scholar]
- Chang B, Heckenlively JR, Bayley PR, Brecha NC, Davisson MT, Hawes NL, Hirano AA, Hurd RE, Ikeda A, Johnson BA, McCall MA, Morgans CW, Nusinowitz S, Peachey NS, Rice DS, Vessey KA, Gregg RG. The nob2 mouse, a null mutation in Cacna1f: anatomical and functional abnormalities in the outer retina and their consequences on ganglion cell visual responses. Vis. Neurosci. 2006;23:11–24. doi: 10.1017/S095252380623102X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork RJ, Namkung Y, Shin HS, Mize RR. Development of the visual pathway is disrupted in mice with a targeted disruption of the calcium channel beta(3)-subunit gene. J. Comp. Neurol. 2001;440:177–191. doi: 10.1002/cne.1378. [DOI] [PubMed] [Google Scholar]
- de la Villa P, Vaquero CF, Kaneko A. Two types of calcium currents of the mouse bipolar cells recorded in the retinal slice preparation. Eur. J. Neurosci. 1998;10:317–323. doi: 10.1046/j.1460-9568.1998.00051.x. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr. Opin. Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Fuller-Bicer GA, Varadi G, Koch SE, Ishii M, Bodi I, Kadeer N, Muth JN, Mikala G, Petrashevskaya NN, Jordan MA, Zhang S, Qin N, Flores CM, Isaacsohn I, Varadi M, Mori Y, Jones WK, Schwartz A. Targeted disruption of the voltage-dependent calcium channel alpha2/delta-1-subunit. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H117–H124. doi: 10.1152/ajpheart.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RG, Messing A, Strube C, Beurg M, Moss R, Behan M, Sukhareva M, Haynes S, Powell JA, Coronado R, Powers PA. Absence of the beta subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the alpha 1 subunit and eliminates excitation-contraction coupling. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13961–13966. doi: 10.1073/pnas.93.24.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann CJ, O’Brien BJ, Wässle H, Protti DA. AII amacrine cells express L-type calcium channels at their output synapses. J. Neurosci. 2003;23:6904–6913. doi: 10.1523/JNEUROSCI.23-17-06904.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Characterization of an amacrine cell type of the mammalian retina immunoreactive for vesicular glutamate transporter 3. J. Comp. Neurol. 2004;468:251–263. doi: 10.1002/cne.10962. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Kaneko M, Shin H, Takahashi T. Presynaptic N-type and P/Q-type Ca2+ channels mediating synaptic transmission at the calyx of held of mice. J. Physiol. (Lond.) 2005;568:199–209. doi: 10.1113/jphysiol.2005.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J. Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacinová L. Voltage-dependent calcium channels. Gen. Physiol. Biophys. 2005;24(Suppl 1):1–78. [PubMed] [Google Scholar]
- Lavail MM, Reif-Lehrer L. Glutamine synthetase in the normal and dystorphic mouse retina. J. Cell Biol. 1971;51:348–354. doi: 10.1083/jcb.51.1.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logiudice L, Henry D, Matthews G. Identification of calcium channel alpha1 subunit mRNA expressed in retinal bipolar neurons. Mol. Vis. 2006;12:184–189. [PMC free article] [PubMed] [Google Scholar]
- Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE, Bech-Hansen NT. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum. Mol. Genet. 2005;14:3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- McEnery MW, Copeland TD, Vance CL. Altered expression and assembly of N-type calcium channel alpha1B and beta subunits in epileptic lethargic (lh/lh) mouse. J. Biol. Chem. 1998a;273:21435–21438. doi: 10.1074/jbc.273.34.21435. [DOI] [PubMed] [Google Scholar]
- McEnery MW, Vance CL, Begg CM, Lee WL, Choi Y, Dubel SJ. Differential expression and association of calcium channel subunits in development and disease. J. Bioenerg. Biomembr. 1998b;30:409–418. doi: 10.1023/a:1021997924473. [DOI] [PubMed] [Google Scholar]
- Morgans CW. Presynaptic proteins of ribbon synapses in the retina. Microsc. Res. Tech. 2000;50:141–150. doi: 10.1002/1097-0029(20000715)50:2<141::AID-JEMT6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Morgans CW. Localization of the alpha(1F) calcium channel subunit in the rat retina. Invest. Ophthalmol. Vis. Sci. 2001;42:2414–2418. [PubMed] [Google Scholar]
- Morgans CW, Gaughwin P, Maleszka R. Expression of the alpha1F calcium channel subunit by photoreceptors in the rat retina. Mol. Vis. 2001;7:202–209. [PubMed] [Google Scholar]
- Morgans CW, Bayley PR, Oesch NW, Ren G, Akileswaran L, Taylor WR. Photoreceptor calcium channels: insight from night blindness. Vis. Neurosci. 2005;22:561–568. doi: 10.1017/S0952523805225038. [DOI] [PubMed] [Google Scholar]
- Nachman-Clewner M, St Jules R, Townes-Anderson E. L-type calcium channels in the photoreceptor ribbon synapse: localization and role in plasticity. J. Comp. Neurol. 1999;415:1–16. [PubMed] [Google Scholar]
- Namkung Y, Smith SM, Lee SB, Skrypnyk NV, Kim HL, Chin H, Scheller RH, Tsien RW, Shin HS. Targeted disruption of the Ca2+ channel beta3 subunit reduces N- and L-type Ca2+ channel activity and alters the voltage-dependent activation of P/Q-type Ca2+ channels in neurons. Proc. Natl. Acad. Sci. U.S.A. 1998;95:12010–12015. doi: 10.1073/pnas.95.20.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef J, Gehrt A, Bulankina AV, Meyer AC, Riedel D, Gregg RG, Strenzke N, Moser T. The Ca2+ channel subunit beta2 regulates Ca2+ channel abundance and function in inner hair cells and is required for hearing. J. Neurosci. 2009;29:10730–10740. doi: 10.1523/JNEUROSCI.1577-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani R, Song M, McEnery M, Qin N, Tsien RW, Toro L, Stefani E, Uchitel OD. Differential expression of alpha 1 and beta subunits of voltage dependent Ca2+ channel at the neuromuscular junction of normal and P/Q Ca2+ channel knockout mouse. Neuroscience. 2004;123:75–85. doi: 10.1016/j.neuroscience.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Pan ZH. Differential expression of high- and two types of low-voltage-activated calcium currents in rod and cone bipolar cells of the rat retina. J. Neurophysiol. 2000;83:513–527. doi: 10.1152/jn.2000.83.1.513. [DOI] [PubMed] [Google Scholar]
- Qian J, Noebels JL. Presynaptic Ca(2+) influx at a mouse central synapse with Ca(2+) channel subunit mutations. J. Neurosci. 2000;20:163–170. doi: 10.1523/JNEUROSCI.20-01-00163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichhart N, Milenkovic VM, Halsband C, Cordeiro S, Strauss O. Effect of bestrophin-1 on L-type Ca2+ channel activity depends on the Ca2+ channel beta-subunit. Exp. Eye Res. 2010;91:630–639. doi: 10.1016/j.exer.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Richards MW, Butcher AJ, Dolphin AC. Ca2+ channel beta-subunits: structural insights AID our understanding. Trends Pharmacol. Sci. 2004;25:626–632. doi: 10.1016/j.tips.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Rieke F, Schwartz EA. Asynchronous transmitter release: control of exocytosis and endocytosis at the salamander rod synapse. J. Physiol. (Lond.) 1996;493(Pt 1):1–8. doi: 10.1113/jphysiol.1996.sp021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz Y, Witkovsky P. Dependence of photoreceptor glutamate release on a dihydropyridine-sensitive calcium channel. Neuroscience. 1997;78:1209–1216. doi: 10.1016/s0306-4522(96)00678-1. [DOI] [PubMed] [Google Scholar]
- Schredelseker J, Di Biase V, Obermair GJ, Felder ET, Flucher BE, Franzini-Armstrong C, Grabner M. The beta 1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 2005;102:17219–17224. doi: 10.1073/pnas.0508710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredelseker J, Dayal A, Schwerte T, Franzini-Armstrong C, Grabner M. Proper restoration of excitation-contraction coupling in the dihydropyridine receptor beta1-null zebrafish relaxed is an exclusive function of the beta1a subunit. J. Biol. Chem. 2009;284:1242–1251. doi: 10.1074/jbc.M807767200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Rüther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A, Meindl A. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat. Genet. 1998;19:260–263. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Morgans C. Localization and properties of voltage-gated calcium channels in cone photoreceptors of Tupaia belangeri. Vis. Neurosci. 1998;15:541–552. doi: 10.1017/s0952523898153142. [DOI] [PubMed] [Google Scholar]
- Vance CL, Begg CM, Lee WL, Haase H, Copeland TD, McEnery MW. Differential expression and association of calcium channel alpha1B and beta subunits during rat brain ontogeny. J. Biol. Chem. 1998;273:14495–14502. doi: 10.1074/jbc.273.23.14495. [DOI] [PubMed] [Google Scholar]
- Vandael DH, Marcantoni A, Mahapatra S, Caro A, Ruth P, Zuccotti A, Knipper M, Carbone E. Ca(v)1.3 and BK channels for timing and regulating cell firing. Mol. Neurobiol. 2010;42:185–198. doi: 10.1007/s12035-010-8151-3. [DOI] [PubMed] [Google Scholar]
- Wilkinson MF, Barnes S. The dihydropyridine-sensitive calcium channel subtype in cone photoreceptors. J. Gen. Physiol. 1996;107:621–630. doi: 10.1085/jgp.107.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz K, Sauer C, Zrenner E, Lorenz B, Alitalo T, Broghammer M, Hergersberg M, de la Chapelle A, Weber BHF, Wissinger B, Meindl A, Pusch CM. Thirty distinct CACNA1F mutations in 33 families with incomplete type of XLCSNB and Cacna1f expression profiling in mouse retina. Eur. J. Hum. Genet. 2002;10:449–456. doi: 10.1038/sj.ejhg.5200828. [DOI] [PubMed] [Google Scholar]
- Wycisk KA, Budde B, Feil S, Skosyrski S, Buzzi F, Neidhardt J, Glaus E, Nürnberg P, Ruether K, Berger W. Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Invest. Ophthalmol. Vis. Sci. 2006a;47:3523–3530. doi: 10.1167/iovs.06-0271. [DOI] [PubMed] [Google Scholar]
- Wycisk KA, Zeitz C, Feil S, Wittmer M, Forster U, Neidhardt J, Wissinger B, Zrenner E, Wilke R, Kohl S, Berger W. Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy. Am. J. Hum. Genet. 2006b;79:973–977. doi: 10.1086/508944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhao J, Yang X. Expression of voltage-dependent calcium channel subunits in the rat retina. Neurosci. Lett. 2002;329:297–300. doi: 10.1016/s0304-3940(02)00688-2. [DOI] [PubMed] [Google Scholar]
- Xu HP, Zhao JW, Yang XL. Cholinergic and dopaminergic amacrine cells differentially express calcium channel subunits in the rat retina. Neuroscience. 2003;118:763–768. doi: 10.1016/s0306-4522(03)00022-8. [DOI] [PubMed] [Google Scholar]
- Yagi T, Macleish PR. Ionic conductances of monkey solitary cone inner segments. J. Neurophysiol. 1994;71:656–665. doi: 10.1152/jn.1994.71.2.656. [DOI] [PubMed] [Google Scholar]
- Yasuda R, Sabatini BL, Svoboda K. Plasticity of calcium channels in dendritic spines. Nat. Neurosci. 2003;6:948–955. doi: 10.1038/nn1112. [DOI] [PubMed] [Google Scholar]
- Zuccotti A, Clementi S, Reinbothe T, Torrente A, Vandael DH, Pirone A. Structural and functional differences between L-type calcium channels: crucial issues for future selective targeting. Trends Pharmacol. Sci. 2011;32:366–375. doi: 10.1016/j.tips.2011.02.012. [DOI] [PubMed] [Google Scholar]