Abstract
Stem cell engineering, the manipulation and control of cells, harnesses tremendous potential for diagnosis and therapy of disease; however, it is still challenging to impart multifunctionalization onto stem cells to achieve both. Here we describe a mesenchymal stem cell (MSC)-based multifunctional platform to target orthotopic glioblastoma by integrating the tumor targeted delivery of mesenchymal stem cells and the multimodal imaging advantage of mesoporous silica nanoparticles (MSNs). Rapid cellular uptake, long retention time and stability of particles exemplify the potential that the combination of MSNs and MSCs has as a stem cell-based multifunctional platform. Using such a platform, we verified tumor-targeted delivery of MSCs by in vivo multimodal imaging in an orthotopic U87MG glioblastoma model, displaying higher tumor uptake than particles without MSCs. As a proof-of-concept, this MSC platform opens a new vision for multifunctional applications of cell products by combining the superiority of stem cells and nanoparticles for actively targeted delivery.
Keywords: Mesenchymal stem cells (MSCs), mesoporous silica nanoparticles (MSNs), cell engineering, multimodal imaging, targeted delivery
1. Introduction
Over the past few decades, various nanomaterials have provided a versatile platform in biomedical applications, especially for the diagnosis and therapy of tumor. However, some nanoparticles (NPs) with outstanding properties for a powerful platform in tumor theranostics show poor delivery and tissue penetration into tumors due to their inappropriate size, shape and/or surface chemistry [1–3]. As such, the search for universal vehicles/carriers can enhance the potential diagnosis and therapy strategies. Previous investigations have demonstrated the unique tumor-tropic properties of mesenchymal stem cells (MSCs) as a vehicle/platform for targeted delivery of anticancer agents to tumor models, such as glioblastoma [4], breast cancer [5], colorectal cancer [6], and melanoma [7]. Genetic engineering is currently the major use of MSCs as carriers for imaging and therapeutic agents. However, transduction of MSCs, especially for viral transfection, can lead to unwanted transformation, significantly increasing the risk of secondary malignancies [8]. Recently, stem cell engineering using NPs allows the development of a simple and generalizable strategy for targeted delivery with low cytotoxicity, establishing a new direction for the modification of cell products [9–12]. In order to improve the quality and accuracy of disease management, the idea of multifunctionalization, the integration of complementary strengths from multiple imaging and therapeutic techniques, has recently gained popularity [13]. However, it is still challenging to impart multifunctionalization to stem cell products by direct chemical modification, which is limited by several major factors, such as the sensitivity of stem cells to the reaction environment and dynamic fluids of cellular components. In addition, the future success of stem cell-based cell therapy requires not only the well-controlled in vivo behavior of stem cells, but also the understanding of their in vivo dynamic fates [14]. Various imaging techniques have been developed for tracking the in vivo fates of stem cells, but each imaging modality has its own strengths and limitations [15–17]. Yet, until now, combining the targeted delivery of MSCs with multimodality imaging of NPs to construct a MSC-based multifunctional stem cell platform (MSC-platform) for in vivo systemic tumor-targeted delivery has not been examined.
In this report, we introduce a MSC-platform that combines the tumor tropism of stem cells and multimodality imaging of hyaluronic acid-based polymer (HA) coated MSNs (HA-MSNs) with FITC, NIR dye ZW800, Gd3+ and 64Cu imaging agents for optical, magnetic resonance (MR) and positron emission tomography (PET) imaging. To construct the MSC-platform, the multimodal HA-MSN nanoplatform was firstly established. Subsequently, MSCs were labeled with the nanoplatform, and the interactions of MSCs with particles were investigated, including the cellular uptake mechanism, the retention time, intracellular fates and cytotoxicity of particles. To further demonstrate the potential for in vivo applications, the orthotopic U87MG glioblastoma xenograft was used as a model to explore tumor-tropic ability of the MSC-platform.
2. Materials and Methods
2.1. Fabrication and characterization of dye-doped MSNs
60 nm dye-doped MSNs were synthesized by modifying previous methods [18, 19]. Briefly, APS-dyes were synthesized by labeling 3-aminopropyl triethoxysilane (APS) with active groups of dyes (Scheme S1). For example, APS (50 mg) was labeled with FITC (1 mg) and ZW800 (3 mg) in 200 μL of N, N-dimethylformamide (DMF) solution containing 2% diisopropylethyalamine (DIPEA), respectively. The reaction mixture was stirred at room temperature for 6 h. The resulting APS-FITC and APS-ZW800 were mixed for additional 2h. Separately, cetyltrimethylammonium bromide (CTAB) (0.27 mmol) was dissolved in 70 mL of deionized water and 14.29 mmol of NH3·H2O (28%–30%) was added with magnetic stirring for 10 min at room temperature. Half of tetroethyl orthosilicate (TEOS) (0.72 mmol) was then added with vigorous stirring for 30 min. 100 μL of APS-dyes was added, and the additional TEOS was added with vigorous stirring for 4 h. The resulting particles were collected by centrifugation and then washed three times with deionized water and ethanol, respectively. Unconjugated dyes were completely removed through centrifugation steps. The MSNs were obtained by removing CTAB in acidic ethanol (1 mL of concentrated HCl in 40 mL of ethanol) for 24 h. The particles were washed three times with deionized water and then stored at 4 °C. The resulting particles were observed by transmission electron microscopy (TEM). The UV-Vis and fluorescence spectrum of particles was recorded on a Genesys 10s UV-Vis spectrophotometer and an F-7000 fluorescence spectrophotometer (HiTachi, Japan), respectively.
2.2. Loading of Gd3+ and surface coating of dye-doped MSNs with HA-based polymer
For the loading of Gd3+ into porous channels, the dyes doped MSNs were incubated with GdCl3 overnight. Then, the particles were collected by centrifugation and then washed three times with deionized water.
The synthesis of HA-CA was reported in previous study [20]. In a typical reaction, a hydrophobic bile acid, 5β-cholanic acid was conjugated onto water soluble HA polymer via amide formation. First, 5β-cholanic acid was converted to aminoethyl 5β-cholanoamide (EtCA) and reacted via EDC and NHS chemistry with the carboxylic acids of hyaluronic acid. For the surface funtionalization of MSNs by HA-CA, a 10:1 weight ratio of MSNs to HA-CA was optimized. Briefly, 5 mg of MSNs were dispersed in 1 ml PBS containing 0.5 mg HA-CA. After 5 min’ standing, the particles were aggregated in solution. Then, the solutions were dispersed via probe sonication using a VCX-750 ultrasonic processor (Sonics & Materials, Newtown, CT). The probe was driven at 40% of the instrument’s maximum amplitude in an ice-bath. The particles were well dispersed in solution around 2 min sonication. After sonication, the solution was divided into 500 μL aliquots and purified by a disposable PD-10 desalting column (GE Healthcare).
2.3. 64Cu-labeling
For the 64Cu-labeling, DOTA-NH2 was firstly conjugated onto HA-MSNs through amide formation in the presence of EDC and HOBt. Subsequently, HA-MSN-DOTA were labeled with 64Cu. 64CuCl2 was converted to Cu(OAc)2 by adding 0.5 mL of 0.4 M ammonium acetate (NH4Ac, pH 5.5) to 20 μL 64CuCl2. Cu(OAc)2 (1 mCi) was added into a solution of HA-MSN-DOTA and incubated for 1 h with constant agitation. The labeled particles were purified with a PD-10 column to remove unreacted 64Cu molecules. The labeled efficiency was calculated based on the radiation dosimeter readings before and after purification. The radio-labeling yield was 50 to 60%.
2.4. Preparation of MSCs
Isolation and culture of MSCs were performed by following our previous protocol [21]. Briefly, balb/c mice were sacrificed by cervical dislocation. The marrow was harvested by inserting a syringe needle (27-gauge) into one end of the bone and flushing with PBS. The bone marrow cells were filtered through a70 mm nylon mesh filter. After collection by centrifugation, cells were dispersed into MesenCult® MSC Basal Medium (Mouse) (STEMCELL™ Technology), a standardized basal medium for the in vitro culture of mouse mesenchymal stem cells. Then, cells were cultured at 37 °C in a humidified atmosphere containing 95% air and 5% CO2. The adhered cells were split when they reached 80%–90% confluence. The MSC surface marker expression profile was confirmed by FACS prior to the use of MSCs. For the preparation of MSC-fluc, the cells were isolated from balb/c mice transfected luciferase. The culture of cells was same with MSCs.
2.5. Cellular uptake of particles
MSCs were plated 24 h before the start of the experiment in chamber slides at a density of 5×103 cell/cm2. After incubation with 100 μg/mL particles for 2 h, the MSCs were incubated by changing fresh medium for 2 h. The cells were fixed with Z-fix solution (Anatech, Battle Creek, MI) for 15 min. Then, the cells were incubated with 0.1% Triton X-100 in PBS at room temperature for 5 min and subsequently incubated with Alexa Fluor®568 phalloidin (Invitrogen) for staining F-actin for 20 min, followed by 1.5 mg/ml DAPI staining at room temperature. The slides were observed and 3D imaging was acquired with an Olympus confocal microscope (Olympus FV10i). As a control experiment, the cell labeling using CellTracker (Invitrogen) was performed by following the standard protocol.
Quantitative analysis of cellular uptake was performed by flow cytometry (FACS). 50 μg/mL particles were incubated with the MSCs for the indicated time points, and then removed by washing three times. Subsequently, the cells were collected by centrifugation and redispersed in PBS buffer. The fluorescence intensity of 10,000 cells was quantified by FACS.
For the cell retention study, the procedures were similar with cellular uptake of particles. Briefly, the particles were incubated with cells for 2 h and subsequently removed by PBS washing three times. The cells were incubated by changing fresh medium for indicated time points, and then were stained similar with the cellular uptake procedures. The images were acquired by the confocal microscopy.
Quantitative analysis of retention ability of particles was performed by flow cytometry (FACS). 50 μg/mL particles were incubated with the MSCs for 2 h, and then removed by washing three times. The cells were continued to culture by adding fresh medium for indicated time points. Subsequently, the cells were collected by centrifugation and redispersed in PBS buffer. The fluorescence intensity of 10, 000cells was quantified by FACS.
2.6. Intracellular fate
MSCs were incubated with 100μg/mL particles for the indicated time points. Cells were collected and the supernatant was removed. The cell pellets were fixed in a 0.1 M PBS solution containing 2% gluteraldehyde and 2.5% paraformaldehyde for 2 h. They were then rinsed with 0.1 M PBS, embedded in 2% agarose gel, postfixed in 4% osmium tetroxide solution for 1 h, rinsed with distilled water, stained with 0.5% uranyl acetate for 1 h, dehydrated in a graded series of ethanol (30, 60, 70, 90, and 100%), and embedded in epoxy resin. The resin was polymerized at 60 °C for 48 h. Ultrathin sections (50–70 nm) obtained with a ultramicrotome were stained with 5% aqueous uranyl acetate and 2% aqueous lead citrate and imaged under TEM.
For the co-localization of particles and lysosome, cells were incubated with 100μg/mL particles for the indicated time points. The cells were washed twice with PBS. Then, the lysosome of cells was stained with LysoTracker™ following the manufacturer’s protocol (Invitrogen/Molecular Probes). Briefly, the cells were incubated with 50 nM LysoTracker™ for 1 h. The cells were washed twice with PBS, and the fresh medium was added. The live cell imaging was observed with an Olympus confocal microscope (Olympus FV10i).
2.7. Cytotoxicity
The cytotoxicity of HA-MSNs was evaluated using the standard MTT assay protocol. Briefly, MSCs were incubated with a various concentrations of HA-MSNs (0.0625 to 1 mg/mL) for 24 h. The medium was replaced with 200 μL fresh media including 20 μL of MTT solution (5 mg/mL), and the incubation proceeded for 4 h. The media was then removed, and 150 μL dimethyl sulfoxide (DMSO) was added into each well to dissolve the internalized purple formazan crystals. An aliquot of 100 μL was taken from each well and transferred into a fresh 96-well plate. The absorption at 570 nm was measured using a microplate reader. The absorption from the control cells was set as 100% cell viability.
The luciferase was used as a model to study the effect of particles on protein expression. The MSCs transfected luciferase was were plated 24 h before the start of the experiment in 96 well plates at a density of 1×104 cell/well. MSCs were incubated with a various concentrations of HA-MSNs (0.0625 to 1 mg/mL) for 24 h. The medium was replaced with 100 μL fresh media. The cells in the plate were imaged using a Xenogen IVIS-100 system (Caliper Life Sciences, Hopkinton, MA, USA) after addition of the substrate D-luciferin (5 μL per well of 3 mg/mL stock).
2.8. Transwell assay
Cell migration was assayed using a modified protocol from the manufacturer (12 μm pore size, Corning Incorporated). After labeling with particles, MSCs were trypsinized, and resuspended in the medium. The cells were plated in the upper chambers at the density of 2×104 cell/well. The same numbers of U87MG cells were also seeded in the lower chamber. After incubation at 37 °C overnight, cells remaining at the upper surface of the membrane were removed using a swab, whereas the cells that migrated to the lower membrane surface were fixed with Z-fix solution and stained with 2% crystal violet. The number of cells migrating through the filter was counted and plotted as the number of migrating cells per optic field (×20).
2.9. Orthotopic glioblastoma model
The procedures for developing an orthotopic brain tumor model were performed according to a protocol approved by the National Institutes of Health Clinical Center Animal Care and Use Committee (NIH CC/ACUC). Briefly, female athymic nude mice (4–6 weeks) were intracranial injected with 1×105 U87MG cells in the right frontal lobe at coordinates 1.5 mm lateral from the bregma, 0.5 mm anterior, and 2.5 mm intraparenchymal. Tumor cells were allowed to engraft for 3 weeks. Successful tumor model was confirmed by MRI prior to the injection of MSCs.
2.10. Small animal imaging
The 3–5 generations of MSCs were prepared for mouse imaging after particle labeling. Briefly, MSCs were incubated with 50 μg/ml particles for 2 h. The cells subsequently were washed twice followed by adding fresh medium for further 2 h culture. After labeling with particles, MSCs were trypsinized, and resuspended in the PBS. Mice were anesthetized with isoflurane, and subcutaneously, 1×106 MSCs were injected by tail vein injection at s speed of 0.1 ml/min. The mice were imaged by PET, near-infrared fluorescence (NIRF) and MRI at the indicated time points.
Small animal MRI was performed based on our previous studies [3]. Three-dimensional gradient-echo scan (FLASH) images were acquired on a 7.05 T small-animal MR scanner (Bruker Biospin) before injection and at indicated time points post injection.
The details of small animal PET imaging and the region-of-interest (ROI) analysis have been reported in our previous studies [22]. PET scans and image analysis were performed using an Inveon microPET scanner (Siemens) at different post injection time points.
3. Results
3.1. Construction of MSC-platform
As shown in Figure 1A, the MSC-platform is composed of multi-stage layers: the inner layers are dyes (FITC and ZW800) doped into a silica matrix; HA molecules coated on the silica matrix make up the middle layer, which are internalized by MSCs as the outer layer. First, the multimodal imaging MSN probes were prepared and purified by modifying our previous methods [18, 23]. In order to understand the MSC-MSN interaction and the in vivo biodistribution of the platform, both FITC and ZW800 were doped into MSNs (Fig. 1B), and were used for in vitro and in vivo imaging of cells and/or tissues, respectively. Then, Gd3+ ions were loaded into pore channels of MSNs by mixing the particles with GdCl3 solution. After the loading of Gd3+, HA was coated onto the surface of MSNs by probe sonication. TEM images display the clear mesoporous structure of MSNs, which was not observed after HA-coating (Fig. 1C). Zeta potential of MSNs was changed after HA coating of MSNs from 23.6 ± 5.3 mV to −72.4 ± 9.4 mV (Supporting Information, Fig. S1), which matches the zeta potential of HA alone. These results show that HA molecules were successfully coated on the particles. Besides improving the interaction with a specific cell surface receptor CD44, a critical surface marker of MSCs [24], HA coating was also utilized to prevent leakage of Gd3+ loaded into the pores of MSNs. Finally, 64Cu was introduced on the surface of particles by the chelation of DOTA-NH2 conjugated with HA, and the labeling efficiency of 64Cu (t1/2 = 12.7 h) was 50–60%. Detailed information on the preparation of MSNs for multimodality imaging is provided in the Supporting Information. Next, to track the intracellular distribution of particles, three-dimensional (3-D) optical imaging was also implemented by co-staining of the actin and nucleus of MSCs. As seen in Fig. 1D and E, a large number of particles accumulate in the 3-D space of cytoplasm, indicating the excellent payload capacity of MSCs that could be used as universal carriers of NPs.
Figure 1.
Schematic illustration and characterization of a mesenchymal stem cell-based multifunctional cell platform (MSC-platform). (A) Schematic of the structure of the MSC-platform showing the internal and external layer. (B) The fluorescence properties of the NPs. (C) TEM images of particles before and after HA coating. (D) and (E) 3D co-localization imaging of MSC-platform by confocal microscopy. Actin (red) and nucleus (blue) were stained. The signal intensity (white line) of actin, particles and nucleus were quantified (E). Co-localization analysis of particles showed the distribution of cytoplasm in cells.
3.2. Uptake mechanism and intracellular fates of particles
To successfully construct an MSC-platform, multifunctional MSNs need to be tightly and securely combined with MSCs. Several important factors should be considered, including the stability of NPs, the interaction between NPs and cells, and the retention time of NPs in cells. We previously reported on the fluorescence stability of MSNs doped with dyes for long-term in vitro and in vivo imaging [19]. Therefore for the platform development, it is necessary to explore the interaction between NPs and MSCs and the particle retention time. First, we evaluated the uptake mechanisms and intracellular fates of particles to understand their interactions with MSCs. Numerous studies have shown that the uptake, either by specific or non-specific mechanisms, of NPs is dependent on the surface chemistry [25, 26]. As shown in Fig. 2A, phagocytosis and pinocytosis are the main courses of endocytosis for non-specific uptake of NPs. By surface modification of NPs, receptor-mediated endocytosis was highlighted to improve uptake rate and selectivity [27]. In this report, HA was utilized to coat MSNs because it specifically interacts with CD44 found on MSCs [20]. Therefore, the respective uptake mechanisms and intracellular fates of MSNs and HA-MSNs were hypothesized to follow the schematic illustration of Fig. 2A. After incubation with particles for 2 h, the cells were collected, and the uptake mechanism of MSNs and HA-MSNs were observed by TEM imaging. Fig. 2B demonstrates that the uptake of MSNs was mainly dependent on phagocytosis and pinocytosis. However, receptor-mediated endocytosis was also seen in the uptake process of some HA-MSNs. The uptake amount of MSNs and HA-MSNs were further quantified using flow cytometry (FACS) (Supporting Information, Fig. S2). Cellular uptake amount of HA-MSNs at 30 min was equivalent to the uptake of MSNs at 2 h, indicating the improved effect of HA coating on NP cellular uptake rates. Next, the intracellular fates of particles were explored by continuous culture. Similar intracellular fates were observed in both MSNs and HA-MSNs (Supporting Information, Fig. S3). After internalization, the particles formed endosomes and lysosomes in cells, leading to their eventual entry into the cytoplasm. Further studies found that the distribution of particles varied based on the NP retention time in cells. After 1 day, the particles were mainly localized in the endosome and lysosome; while, after 3 days, many particles were found in the cytoplasm (Fig. 2C). To ascertain these results, the lysosome of cells was stained with LysoTracker™ following the manufacturer’s protocol (Invitrogen/Molecular Probes). The co-localization of particles and lysosome (yellow) is shown in Fig. 2D. Co-localization was found at 1 day retention but decreased after 3 days, which demonstrates that the particles escape from lysosomes and enter into the cytoplasm, as implied in the TEM images.
Figure 2.
Uptake mechanism and intracellular fates of particles. (A) Schematic illustration of the uptake mechanism and intracellular fates of MSNs and HA-MSNs. (B) Representative TEM images of the cellular uptake mechanism of MSNs and HA-MSNs. The uptake of MSNs was mediated by phagocytosis and pinocytosis, while the HA-MSNs were dependent on receptor-mediated endocytosis. (C, D) TEM and fluorescence analysis of intracellular fates of particles. (C) TEM images showed the different localization of particles over time. (D) Fluorescence co-localization of lysosome and particles confirmed the particles escaped from lysosome and entered the cytoplasm over time.
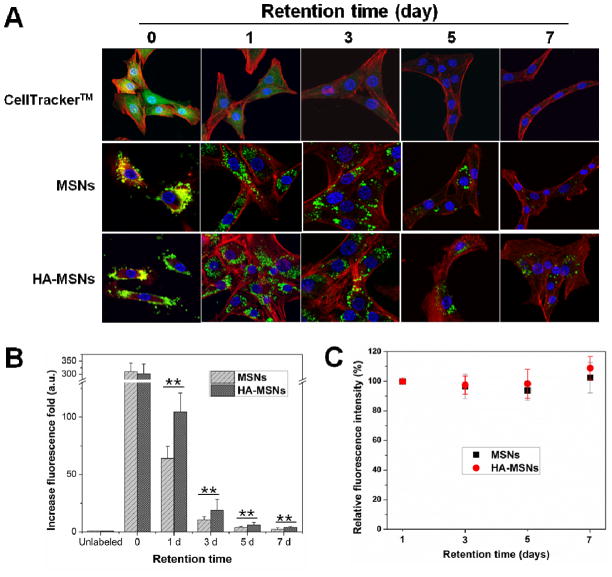
3.3. Retention capacity of particles in MSCs
The cell retention time of NPs is mainly determined by the dilution effect after cell division and efflux by exocytosis. We hypothesized that multifunctional MSNs would not be affected by exocytosis and therefore would maintain a long retention time in cells. To compare the retention time of MSNs with HA-MSNs, CellTracker™ green CMFDA (Invitrogen), a molecular probe for long-term tracking of living cells, was utilized. The cells were treated with CellTracker and either MSNs or HA-MSNs for 2 h (this retention time is indicated as 0 in Figure 3), and subsequently the cells were washed and cultured for up to a week. After staining actin (Alexa Fluor® 568 Phalloidin, Invitrogen) and nuclei (DAPI), the cell images were acquired on an Olympus confocal microscope (Olympus FV10i). As shown in Fig. 3A, the CellTrack™ signal significantly decreased over time, and no significant signal was observed after 3 days. In contrast, MSN and HA-MSN signals were maintained for at least 7 days. The particle number per cell was reduced due to the cell dilution, but the signal of each particle was not significantly changed. The obvious particle signal in cells was still observed after a retention time of 3 weeks (Supporting Information, Fig. S4). These results demonstrate the excellent stability of MSNs in cells. In terms of the retention capacity, a higher number of particles were observed in the HA-MSN group than the MSN group. To better compare the retention effect of MSNs and HA-MSNs, the fluorescence signal was quantified in MSCs by FACS (Fig. 3B, Supporting Information Fig. S5). MSCs treated with MSNs as well as HA-MSNs showed about a 300-fold increase in fluorescence signal after 2 h incubation compared with untreated cells. But, the signal of HA-MSNs was 1.6 ± 0.44-fold higher than MSNs after 1 day retention (p < 0.05). Similar results were shown in 3, 5 and 7 days after NP treatment. The improved retention effect seen in HA-MSNs could be related to the ability of the particles to interact with receptors found on the cell membrane. As seen in Figure 3A, many particles were localized on the cell surface 2 h after incubation. Yet, the particles on the cell surface could easily fall off after cell washing or during culture in fresh medium. For HA coated particles, on the other hand, the attachment ability can be improved by the interaction between HA and CD44 receptors expressed on the MSC surface. In addition, the signal of cells over different time points was analyzed by FACS, and was subsequently corrected according to the cell dilution fold. Cell fluorescence was essentially unchanged 1, 3, 5 and 7 days after treatment (Fig. 3B), implying that there was no cell efflux of particles. These cell studies demonstrate that HA-MSNs can be tightly and securely combined with MSCs and could be considered as components of MSCs as an MSC-platform.
Figure 3.
Long retention time of particles in MSCs. (A) The retention capacity of CellTracker™, MSNs and HA-MSNs over time by confocal imaging. (B) The retention effect of MSNs and HA-MSNs compared by FACS (**p<0.01). (C) The fluorescence intensity of MSN and HA-MSN labeled MSCs over time. The fluorescence was corrected based on the cell dilution folds.
3.4. Cytotoxicity of particles on MSCs
The cytotoxicity effect of HA-MSNs on MSCs was analyzed prior to the application of the MSC-platform. The cytotoxicity of HA-MSNs was evaluated using the standard MTT assay, and the results demonstrated low cytotoxicity of HA-MSNs on MSCs (Supporting Information, Fig. S6A). To further confirm the non-cytotoxicity of particles, we utilized MSCs that express firefly luciferase (MSC-fluc) as a model to study the effect of the particles on intracellular protein expression. The MSC-fluc were treated with the indicated concentration of particles for 24 h, and subsequently, images were acquired using an IVIS imaging system. The imaging and quantitative analysis of luciferase expression showed no significant effect by the particles on cell proliferation and intracellular protein expression (Supporting Information, Fig. S6B).
3.5. Tumor tropism of MSC-platform
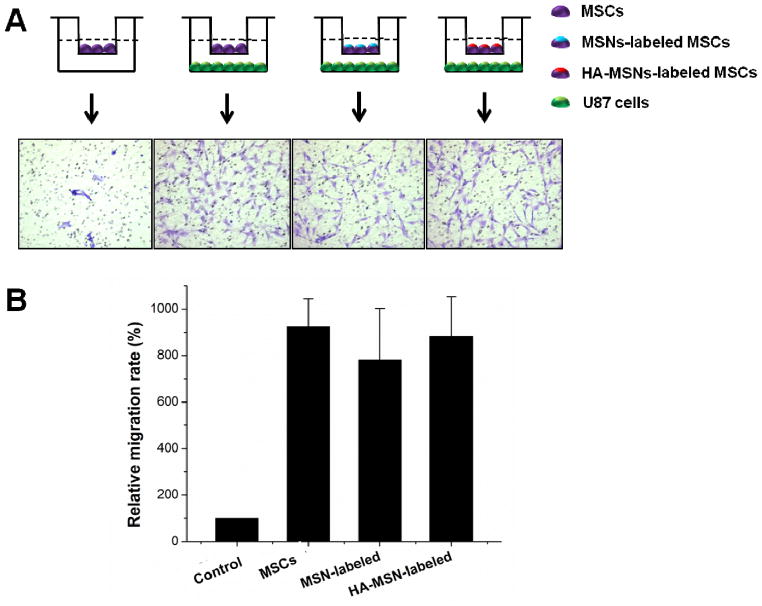
To examine the utility of the engineered MSC-platform for in vivo biomedical applications, we analyzed selective tumor delivery ability of the MSC-platform in an orthotopic U87MG glioblastoma-bearing mice model. Three main reasons to use an orthotopic U87MG glioblastoma-bearing mice model are: (i) Glioblastoma is the most common and most aggressive malignant primary brain tumor in humans, and it is very difficult to treat glioblastoma due to several complicating factors, such as the blood-brain barrier, drug resistance and limited repair capacity of brain [28, 29]; (ii) MSCs have shown the potential capacity of tumor tropism delivery for diagnosis and therapy of glioblastoma [4]; (iii) Poor tumor uptake of HA-based NPs was found in our previous studies (data not shown) due to the low CD44 expression of glioblastoma cells [30] and inappropriate size of NPs to overcome the blood-brain barrier [3]. We hypothesized that MSCs could improve the delivery of HA-MSNs to orthotopic glioblastoma. Prior to in vivo validation of the MSC-platform, the effect of particles on the tumor tropism capacity of MSCs was evaluated using a modified transwell assay protocol (Corning). The MSCs and U87MG cells were plated in the chamber following the scheme shown in Fig. 4A. Once the cells were in culture for 12 h, the cells in the upper surface of the membrane were removed, while the cells migrating to the lower membrane surface were fixed and stained with crystal violet. Compared with the absence of U87MG cells, the MSCs easily migrated to the lower membrane surface and showed a 9.2 ± 1.6 fold increase in migrated cell number in the presence of U87MG cells (Fig. 4B), demonstrating the tumor-tropic migration of MSCs. Moreover, the MSN- and HA-MSN- labeled MSCs still possessed the migration capacity toward U87MG cells, leading to a 7.6 ± 2.3 fold and 8.7 ± 1.9 fold increase in migrated cell number over the control, respectively. It is worth noting that the effect of HA-MSNs on migration capacity was less than naked MSNs. Combining the high cellular uptake properties with the retention capacity, HA-MSNs are undoubtedly better than MSNs for the construction of an MSC-platform.
Figure 4.
Impact of particle labeling on migration of MSCs. (A) Representative images of migrated MSCs through 12 μm pore sizes of membrane (Corning Incorporated). The migrated MSCs were stained by crystal violet. (B) Quantitative analysis of the migration of MSCs unlabeled and labeled particles.
3.6. In vivo tumor delivery of MSC-platform
Next, the tumor tropism of the MSC-platform was evaluated in an orthotopic U87MG glioblastoma-bearing mice model using three different imaging modalities: fluorescence, MR and PET imaging. To confirm the cell labeling of particles, different concentrations of the MSCs were imaged after labeling using a Maestro imaging system. As shown in Fig. 5A, the signal derived from ZW800 dyes is dependent on cell concentration. For in vivo imaging, each imaging modality has its own strengths and limitations [31]; therefore, using three different imaging modes will aid in the understanding of the MSC tumor homing effect. To better study the in vivo biodistribution of the MSC-platform, multimodal imaging was performed and a series of typical images are illustrated in Fig. 5. First, three-dimensional gradient-echo scan (FLASH) images of the glioblastoma were acquired with a 7.05 T small animal MR scanner. Compared with the pre-injection result, an obvious enhanced T1 signal in tumor (circle) was observed 24 h after injection (Fig. 5B), which demonstrates the successful targeted delivery of MSC-platform to the tumor. To further confirm the tumor homing of this developed MSC-platform, the in vivo tumor homing of MSCs labeled with HA-MSN-64Cu (MSC-HA-MSN-64Cu) was imaged by PET at 2 h and 24 h following intravenous injection of 1×106 cells to the orthotopic U87MG xenograft mouse model. As a control group, HA-MSN-64Cu with the same amount of radioactivity was also injected in order to compare the homing capability of NPs without MSCs. Indeed, there was a 5.2±1.3 fold increased accumulation in the glioblastoma by MSC-HA-MSN-64Cu within 24 h after injection compared with HA-MSN-64Cu alone (Fig. 5C, left). There was no obvious signal in the ventricle at 2 h postinjection, but a strong signal (arrow) was observed 24 h postinjection by MSC-HA-MSN-64Cu. On the other hand, no tumor uptake was detected 2 h and 24 h after injection of HA-MSN-64Cu (Fig. 5C, right). The results demonstrate that MSCs are responsible for the improved tumor delivery of HA-MSN-64Cu, implying the hypothesized tumor homing capacity of this MSC-platform. To verify this result, frozen tissue slices from the tumor were prepared and stained. As seen in Fig. 5D, an aggregated localization of fluorescence signal derived from FITC and ZW800 dyes was evidently observed by microscopy in the MSC-platform group in the tumor region. However, no significant signal was found in the control group, which was consistent with the in vivo results. These results demonstrate the successful tumor delivery and tracking ability of this MSC-platform, emphasizing the use of MSCs for tumor homing ability.
Figure 5.
In vivo multimodal imaging of the tumor homing of the MSC-platform to orthotopic U87MG glioblastoma. (A) The near-infrared fluorescence signal of MSCs labeled with MSN and HA-MSN particles varied with an increase in cell concentration. (B) MR imaging demonstrated the increased signal at the tumor sites after MSC-platform administration for 24 h compared with pre-injection. (C) PET imaging of the tumor targeting of the MSC-platform and HA-MSN-64Cu at the indicated time points. (D) Frozen tissue slices confirmed that the tumor uptake of the MSC-platform was significant higher than free HA-MSNs, implying homing of the MSC-platform to tumors.
4. Discussion
The integration of several components, each with different properties, into a single multifunctionalized platform allows the synergy necessary to engineer precise and fast diagnostics and therapeutics for diseases. Utilizing MSCs as one of these components can be extremely beneficial because of its reported tumor homing nature [17, 32]. In recent years, various kinds of multifunctional NPs have been reported that can integrate multiple imaging and therapeutic components [13, 33]. Therefore, the combination of stem cells with multifunctional NPs will open new discoveries in theranostics. As a proof-of concept, we herein designed a multifunctional MSC-platform by integrating the tumor-tropic properties of MSCs with multifunctionalized NPs. The studied interactions between MSCs and NPs show their compact combination towards the development of a single platform (Fig. 2 and 3). Current targeting strategies for functional NPs with specific targeting ligands, such as antibodies [34], peptides [35] or aptamers [36], have been shown to improve delivery efficacy. Generally, these approaches depend on the initial passive accumulation at tumor sites via the enhanced permeation and retention (EPR) effect. In contrast, cellular nanoparticle vehicles actively transmigrate the endothelial barrier and accumulate cell-attached cargo in tissues at more than two-log greater levels than systemically infused free particles [9]. We also confirmed that the MSC-platform actively targets orthotopic glioblastoma across the blood brain barrier, which significantly has 5.2±1.3 fold higher tumor uptake than the free NPs (Fig. 5). Each imaging modality has its own advantages and disadvantages. For example, MRI can provide three-dimensional tomography but is limited by low target sensitivity, whereas PET has good sensitivity but suffer from low spatial resolution or tissue penetration [31, 37]. In this report, we utilized MRI, PET and optical imaging to harness the strengths of different imaging methods. MRI can distinguish the distribution of MSCs in the tumor region, while PET imaging is used to understand the dynamics and quantify targeted delivery of the MSC-platform. Optical imaging is convenient [38] to monitor the interaction between MSNs and MSCs. As such, these complementary imaging techniques are undoubtedly helpful for improved tracking of the in vivo fates of the MSC-platform.
The current obstacle confronting all stem cell-based therapies is the manipulation of stem cell behaviors after the efficient targeted delivery for diagnostic and therapeutic outcomes. Some small molecular agents demonstrated potential by signal transduction on the tumor growth of stem cells [39, 40], showing the potential application in the treatment of tumor. Previous reports have used the universal capacity of MSNs to load small molecular drugs, including hydrophilic [41] and hydrophobic drugs [42]. To verify the delivery of small chemicals by our MSC-platform, Gd3+ could also be utilized as a model small chemical. As shown in Fig. 5B, the successful delivery of Gd3+ was found in an orthotopic U87MG glioblastoma-bearing mice model using MR imaging. Therefore, such a multifunctional MSC-platform combines the unique properties of stem cells with the superiority of NPs to provide a potentially clinical application towards disease diagnosis and therapies in the future.
5. Conclusion
In summary, we demonstrate stem cell-based multifunctional tools for in vivo tumor targeted delivery based on the tumor tropic properties of MSCs and the multifunctionalization of MSNs for optical, PET and MR imaging. We show that MSN-based multifunctional NPs can be stably integrated into MSCs without toxicity or interference with intrinsic cell functions, especially for the tropic capability to U87MG tumor cells. Long retention of the particles in the cells and the stability of the particles allow the integration of cells and particles into one platform, where the particles serve as a component of MSCs. In vivo multimodal imaging showed the feasibility of tumor tropism delivery of this multifunctional MSC-platform with improved tumor delivery of NPs compared with free particles. Given the wealth of available multifunctional NPs applied to different bio-applications, the range of particles that can be integrated to stem cells extends far beyond MSNs illustrated here.
Supplementary Material
Scheme S1. Synthesis schemes of a series of APS-dyes.
Supplemental Fig. S1. Zeta potential of MSNs and HA-MSNs
Supplemental Fig. S2. Quantitative analysis of cellular uptake of MSNs and HA-MSNs over time using FACS. *p<0.05
Supplemental Fig. S3. Localization of MSN and HA-MSN in MSCs. Similar intracellular fates were observed in both MSNs and HA-MSNs. After internalization, the particles formed endosomes and lysosomes in cells
Supplemental Fig. S4. Stability of particles in MSCs.
Supplemental Fig. S5. Quantitative analysis of retention ability of MSNs and HA-MSNs by FACS.
Supplemental Fig. S6. Effect of HA-MSNs on cell proliferation and protein expression. (A) Impact of different concentration of HA-MSNs on cell proliferation by MTT assay. (B) Effect of different concentration of HA-MSNs on luciferase expression of MSC-fluc. Left, imaging of luciferase expression using IVIS imaging system; Right, quantitative analysis of luficerase expression.
Supplemental Fig. S7. H&E staining of orthotopic glioblastoma model.
Acknowledgments
This work was supported in part, by National Basic Research Program of China (973 program) (No. 2013CB733802), the National Science Foundation of China (NSFC) (81201086, 81201129, 81100234, 81028009), the Chinese Academy of Sciences professorship for Senior International Scientists (2011T2J06), the Intramural Research Program (IRP) of the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH), and the Department of Defense in the Center for Neuroscience and Regenerative Medicine (CNRM),
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, Kamoun WS, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7(6):383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith BR, Kempen P, Bouley D, Xu A, Liu Z, Melosh N, et al. Shape matters: intravital microscopy reveals surprising geometrical dependence for nanoparticles in tumor models of extravasation. Nano Lett. 2012;12(7):3369–3377. doi: 10.1021/nl204175t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang F, Huang X, Zhu L, Guo N, Niu G, Swierczewska M, et al. Noninvasive monitoring of orthotopic glioblastoma therapy response using RGD-conjugated iron oxide nanoparticles. Biomaterials. 2012;33(21):5414–5422. doi: 10.1016/j.biomaterials.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci U S A. 2009;106(12):4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutova M, Najbauer J, Frank RT, Kendall SE, Gevorgyan A, Metz MZ, et al. Urokinase plasminogen activator and urokinase plasminogen activator receptor mediate human stem cell tropism to malignant solid tumors. Stem Cells. 2008;26(6):1406–1413. doi: 10.1634/stemcells.2008-0141. [DOI] [PubMed] [Google Scholar]
- 6.Chung TH, Hsiao JK, Hsu SC, Yao M, Chen YC, Wang SW, et al. Iron oxide nanoparticle-induced epidermal growth factor receptor expression in human stem cells for tumor therapy. ACS Nano. 2011;5(12):9807–9816. doi: 10.1021/nn2033902. [DOI] [PubMed] [Google Scholar]
- 7.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62(13):3603–3608. [PubMed] [Google Scholar]
- 8.Hu YL, Fu YH, Tabata Y, Gao JQ. Mesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapy. J Control Release. 2010;147(2):154–162. doi: 10.1016/j.jconrel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16(9):1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Guan Y, Liu H, Hao N, Liu T, Meng X, et al. Silica nanorattle-doxorubicin-anchored mesenchymal stem cells for tumor-tropic therapy. ACS Nano. 2011;5(9):7462–7470. doi: 10.1021/nn202399w. [DOI] [PubMed] [Google Scholar]
- 11.Cheng H, Kastrup CJ, Ramanathan R, Siegwart DJ, Ma M, Bogatyrev SR, et al. Nanoparticulate cellular patches for cell-mediated tumoritropic delivery. ACS Nano. 2010;4(2):625–631. doi: 10.1021/nn901319y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roger M, Clavreul A, Venier-Julienne MC, Passirani C, Sindji L, Schiller P, et al. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials. 2010;31(32):8393–8401. doi: 10.1016/j.biomaterials.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 13.Lin X, Xie J, Niu G, Zhang F, Gao H, Yang M, et al. Chimeric ferritin nanocages for multiple function loading and multimodal imaging. Nano Lett. 2011;11(2):814–819. doi: 10.1021/nl104141g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Law WC, Mahajan SD, Kopwitthaya A, Reynolds JL, Liu M, Liu X, et al. Gene silencing of human neuronal cells for drug addiction therapy using anisotropic nanocrystals. Theranostics. 2012;2(7):695–704. doi: 10.7150/thno.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruan J, Song H, Li C, Bao C, Fu H, Wang K, et al. DiR-labeled embryonic stem cells for targeted imaging of in vivo gastric cancer cells. Theranostics. 2012;2(6):618–628. doi: 10.7150/thno.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang NF, Okogbaa J, Babakhanyan A, Cooke JP. Bioluminescence imaging of stem cell-based therapeutics for vascular regeneration. Theranostics. 2012;2(4):346–354. doi: 10.7150/thno.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu E, Chen WY, Gu J, Burridge P, Wu JC. Molecular imaging of stem cells: tracking survival, biodistribution, tumorigenicity, and immunogenicity. Theranostics. 2012;2(4):335–345. doi: 10.7150/thno.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Swierczewska M, Choi KY, Zhu L, Bhirde A, Park J, et al. Multiplex imaging of an intracellular proteolytic cascade by using a broad-spectrum nanoquencher. Angew Chem Int Ed Engl. 2012;51(7):1625–1630. doi: 10.1002/anie.201107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X, Zhang F, Lee S, Swierczewska M, Kiesewetter DO, Lang L, et al. Long-term multimodal imaging of tumor draining sentinel lymph nodes using mesoporous silica-based nanoprobes. Biomaterials. 2012;33(17):4370–4378. doi: 10.1016/j.biomaterials.2012.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi KY, Chung H, Min KH, Yoon HY, Kim K, Park JH, et al. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials. 2010;31(1):106–114. doi: 10.1016/j.biomaterials.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Cao F, De A, Cao Y, Contag C, Gambhir SS, et al. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem Cells. 2009;27(7):1548–1558. doi: 10.1002/stem.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie J, Chen K, Huang J, Lee S, Wang J, Gao J, et al. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials. 2010;31(11):3016–3022. doi: 10.1016/j.biomaterials.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X, Li L, Liu T, Hao N, Liu H, Chen D, et al. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano. 2011;5(7):5390–5399. doi: 10.1021/nn200365a. [DOI] [PubMed] [Google Scholar]
- 24.Khaldoyanidi S. Directing stem cell homing. Cell Stem Cell. 2008;2(3):198–200. doi: 10.1016/j.stem.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Huang X, Teng X, Chen D, Tang F, He J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials. 2010;31(3):438–448. doi: 10.1016/j.biomaterials.2009.09.060. [DOI] [PubMed] [Google Scholar]
- 26.Mickler FM, Mockl L, Ruthardt N, Ogris M, Wagner E, Brauchle C. Tuning nanoparticle uptake: Live-cell imaging reveals two distinct endocytosis mechanisms mediated by natural and artificial EGFR targeting ligand. Nano Lett. 2012;12(7):3417–3423. doi: 10.1021/nl300395q. [DOI] [PubMed] [Google Scholar]
- 27.Vacha R, Martinez-Veracoechea FJ, Frenkel D. Receptor-mediated endocytosis of nanoparticles of various shapes. Nano Lett. 2011;11(12):5391–5395. doi: 10.1021/nl2030213. [DOI] [PubMed] [Google Scholar]
- 28.Fulci G, Chiocca EA. The status of gene therapy for brain tumors. Expert Opin Biol Ther. 2007;7(2):197–208. doi: 10.1517/14712598.7.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Liu H, Ren G, Kimura RH, Cochran JR, Cheng Z. PET imaging of integrin positive tumors using 18F labeled knottin peptides. Theranostics. 2011;1:403–412. doi: 10.7150/thno/v01p0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Upadhyay KK, Bhatt AN, Mishra AK, Dwarakanath BS, Jain S, Schatz C, et al. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly(gamma-benzyl L-glutamate)-b-hyaluronan polymersomes. Biomaterials. 2010;31(10):2882–2892. doi: 10.1016/j.biomaterials.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 31.Liu TW, Chen J, Burgess L, Cao W, Shi J, Wilson BC, et al. Multimodal bacteriochlorophyll theranostic agent. Theranostics. 2011;1:354–362. doi: 10.7150/thno/v01p0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87(9 Suppl):S42–45. doi: 10.1097/TP.0b013e3181a28533. [DOI] [PubMed] [Google Scholar]
- 33.Yu MK, Park J, Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2012;2(1):3–44. doi: 10.7150/thno.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bardhan R, Chen W, Bartels M, Perez-Torres C, Botero MF, McAninch RW, et al. Tracking of multimodal therapeutic nanocomplexes targeting breast cancer in vivo. Nano Lett. 2010;10 (12):4920–4928. doi: 10.1021/nl102889y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai W, Shin DW, Chen K, Gheysens O, Cao Q, Wang SX, et al. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6(4):669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 36.Guo J, Gao X, Su L, Xia H, Gu G, Pang Z, et al. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32(31):8010–8020. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Xie J, Liu G, Eden HS, Ai H, Chen X. Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Acc Chem Res. 2011;44(10):883–892. doi: 10.1021/ar200044b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang X, Lee S, Chen X. Design of “smart” probes for optical imaging of apoptosis. Am J Nucl Med Mol Imaging. 2011;1(1):3–17. [PMC free article] [PubMed] [Google Scholar]
- 39.Azarin SM, Lian X, Larson EA, Popelka HM, de Pablo JJ, Palecek SP. Modulation of Wnt/beta-catenin signaling in human embryonic stem cells using a 3-D microwell array. Biomaterials. 2012;33(7):2041–2049. doi: 10.1016/j.biomaterials.2011.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsiao WC, Sung SY, Liao CH, Wu HC, Hsieh CL. Vitamin D3-inducible mesenchymal stem cell-based delivery of conditionally replicating adenoviruses effectively targets renal cell carcinoma and inhibits tumor growth. Mol Pharm. 2012;9(5):1396–1408. doi: 10.1021/mp200649g. [DOI] [PubMed] [Google Scholar]
- 41.Liu H, Chen D, Li L, Liu T, Tan L, Wu X, et al. Multifunctional gold nanoshells on silica nanorattles: a platform for the combination of photothermal therapy and chemotherapy with low systemic toxicity. Angew Chem Int Ed Engl. 2011;50(4):891–895. doi: 10.1002/anie.201002820. [DOI] [PubMed] [Google Scholar]
- 42.Ferris DP, Lu J, Gothard C, Yanes R, Thomas CR, Olsen JC, et al. Synthesis of biomolecule-modified mesoporous silica nanoparticles for targeted hydrophobic drug delivery to cancer cells. Small. 2011;7(13):1816–1826. doi: 10.1002/smll.201002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scheme S1. Synthesis schemes of a series of APS-dyes.
Supplemental Fig. S1. Zeta potential of MSNs and HA-MSNs
Supplemental Fig. S2. Quantitative analysis of cellular uptake of MSNs and HA-MSNs over time using FACS. *p<0.05
Supplemental Fig. S3. Localization of MSN and HA-MSN in MSCs. Similar intracellular fates were observed in both MSNs and HA-MSNs. After internalization, the particles formed endosomes and lysosomes in cells
Supplemental Fig. S4. Stability of particles in MSCs.
Supplemental Fig. S5. Quantitative analysis of retention ability of MSNs and HA-MSNs by FACS.
Supplemental Fig. S6. Effect of HA-MSNs on cell proliferation and protein expression. (A) Impact of different concentration of HA-MSNs on cell proliferation by MTT assay. (B) Effect of different concentration of HA-MSNs on luciferase expression of MSC-fluc. Left, imaging of luciferase expression using IVIS imaging system; Right, quantitative analysis of luficerase expression.
Supplemental Fig. S7. H&E staining of orthotopic glioblastoma model.