Abstract
Breast cancers can be classified into those which express the estrogen (ER) and progesterone (PR) receptors, those with HER-2 amplification, and those without expression of ER, PR, or amplified HER-2 (referred to as triple-negative or basal-like breast cancer). Tumor Necrosis Factor Apoptosis Inducing Ligand (TRAIL) activates apoptosis upon binding to its receptors in many tumor types and the ligand and agonist antibodies are currently being studied in patients in clinical phase I and phase II trials. Cell line studies suggest that many breast cancer cell lines are very resistant to TRAIL-induced apoptosis. However, recent data suggest that a subset of triple-negative/basal-like breast cancer cells is sensitive to TRAIL as a single agent. In addition many studies have demonstrated that resistance to TRAIL-mediated apoptosis in breast cancer cells can be overcome by combinations of TRAIL with chemotherapy, radiation, and various targeted agents. This review will discuss the current understanding of the mechanisms which control TRAIL-mediated apoptosis in breast cancer cells. The preclinical data supporting the use of TRAIL ligands and agonistic antibodies alone and in combination in breast cancer will also be discussed.
I. INTRODUCTION
Breast cancer is the most frequently diagnosed cancer in women and one of the leading causes of cancer death for women. Worldwide, over 1.3 million cases of invasive breast cancer are diagnosed, and more than 450,000 women die from breast cancer annually (Garcia et al., 2007). In the US, approximately 180,000 cases of invasive breast cancer and 60,000 cases of in situ breast cancer are diagnosed annually, and more than 40,000 women die from breast cancer each year – second only to lung cancer (Jemal et al., 2008). The mortality due to breast cancer has been declining in the US since 1990. The death rate was 32.69 per 100,000 women in 1991 but fell to 25.19 per 100,000 women in 2003 (Jemal et al., 2007). The continuing decrease in mortality from breast cancer has been attributed to early detection due to screening, improved adjuvant therapy, and more recently to decreases in the incidence due to lowered rates of usage of hormone replacement therapy (Berry et al., 2005; Ravdin et al., 2007).
Breast cancer can be divided into several distinct subtypes that have prognostic and therapeutic implications. Clinically, breast cancer patients routinely have the expression of estrogen receptor (ER), progesterone receptor (PR), and amplification of HER-2 evaluated (Brenton et al., 2005). This allows the classification of breast cancer as hormone receptor positive tumors, HER-2 amplified tumors (which may or may not express hormone receptors), and those tumors which do not express ER, PR, and do not have HER-2 amplification. The latter group is referred to as triple-negative breast cancer based on the lack of these three molecular markers. Generally, hormone receptor expressing breast cancers have a more favorable prognosis than either those with HER-2 amplification or those that are triple-negative (Brenton et al., 2005). While all breast tumor types may be treated with chemotherapy, therapeutic options in both early and late stage breast cancer are affected significantly by the expression of these three markers. Tumors that express ER and PR are treated with agents that interfere with hormone production or action. Tumors that have amplified HER2 are treated with agents that inhibit HER-2. Triple-negative tumors are treated with predominantly chemotherapy (Brenton et al., 2005).
Recent expression profiling of human breast cancers has allowed classification of the tumors based on clustering and the similarity of expression patterns between normal breast cells and tumors (Perou et al., 2000; Sorlie et al., 2001). The hormone receptor expressing breast cancers resembled most closely the luminal cells of the breast ducts but could be further subdivided into several subgroups that have different prognoses and responses to hormonal therapy. The tumors with HER-2 amplification clustered together and were found to have a poorer prognosis than the luminal subtype. These data were compiled prior to the introduction of trastuzumab. The triple-negative tumors resembled most closely basal cells, cells found on the outside of the breast ducts, and had the worst prognosis (Perou et al., 2000; Sorlie et al., 2001). Subsequent analyses have suggested that the clinical triple-negative classification and the array based basal classification significantly overlap but are not identical (Rakha et al., 2009). Ongoing clinical trials are beginning to evaluate the use of these and other molecular classifications of breast cancer for making treatment decisions (Sotiriou and Pusztai, 2009). While yet to be applied to the routine care of breast cancer patients, array based molecular classification is likely to allow more individualized treatment in the future.
Despite the advances made in the detection and treatment of early breast cancer that have contributed to the declining mortality in the US, metastatic breast cancer remains an incurable disease. More efficacious treatments to prevent relapse in early stage patients and to treat metastatic disease are needed if a major impact is to be realized in the mortality of breast cancer. This review will focus on the potential use of Tumor Necrosis Factor Related Apoptosis Inducing Ligand (TRAIL) receptor agonists for the treatment of breast cancer.
II. TRAIL AND ITS RECEPTORS
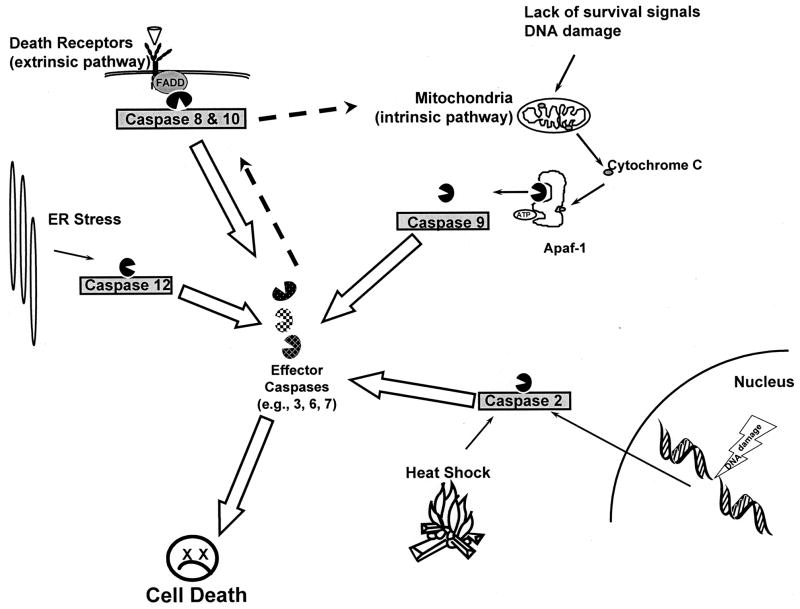
There are several fundamental apoptosis pathways in cells which are defined by where the initial caspase activation occurs (Fig. 1). One pathway, referred to as the extrinsic pathway, mediates activation of caspase 8 or caspase 10 by ligand binding to cell surface receptors (Ashkenazi, 2002; Danial and Korsmeyer, 2004). A second pathway, referred to as the intrinsic pathway, mediates caspase 9 activation by the mitochondrial release of proapoptotic proteins such as cytochrome c in response to a variety of stimuli such as the absence of growth factors, DNA damage, and viral infection (Danial and Korsmeyer, 2004). Cytosolic cytochrome c binds to apoptotic peptidase activating factor 1 (APAF-1) and activates caspase 9 in an ATP dependent reaction (Danial and Korsmeyer, 2004). Other pathways trigger the activation of caspase 2 in response to heat shock or DNA damage (Sidi et al., 2008; Tu et al., 2006) or caspase 12 in response to ER stress (Nakagawa et al., 2000). After the activation of the initiator caspases, the pathways converge on downstream caspases such as caspase 3, caspase 6 and caspase 7, so-called effector caspases (Danial and Korsmeyer, 2004). In addition, after the activation of the primary initiator caspases specific to each pathway, other initiator caspases can be activated downstream of these primary caspases (Fig. 1). For example, death receptor activated caspases 8 and 10 can cleave the BH3 only protein BID, leading to its translocation to the mitochondria where it activates the mitochondrial pathway leading to activation of caspase 9 (Ashkenazi, 2002; Suliman et al., 2001). Similarly, activated caspase 3 can directly activate the death receptor initiator caspase 8 (Slee et al., 1999; Sun et al., 1999). Thus, while each pathway is defined by the initiating stimuli and caspase that becomes activated, these pathways form an interconnected network within the cell.
Fig. 1.
Apoptosis pathways. Different stimuli and cell stresses result in activation of distinct initiator caspases (i.e., caspases 2, 8, 9, 10, and 12 shown in gray boxes) as discussed in the text. These in turn cleave and activate down stream effector caspases (e.g., caspases 3, 6 and 7). The primary initiator caspase can activate secondary initiator caspases as discussed in text (dashed arrows). The activation of effector caspases leads to apoptotic cell death.
The death receptors belong to the Tumor Necrosis Factor (TNF) family. The TNF family has more than 20 receptors of which six are death receptors (DR) and activate apoptosis in response to binding of their respective ligands (Ashkenazi, 2002). The six proteins are TNFR1 (a.k.a. Death Receptor 1 or DR1), FAS (a.k.a. CD95, DR2), DR3, TRAIL-R1, TRAIL-R2, and DR6. These receptors are activated by their respective ligand: TNF for TNFR1, CD95 Ligand for FAS (a.k.a FAS Ligand), TL1A for DR3, and TRAIL for TRAIL-R1 and TRAIL-R2 (Ashkenazi, 2002). A ligand for DR6 has not been identified. All of these receptors are homotrimeric proteins which activate apoptosis via a cytoplasmic domain known as the death domain (DD) (Fig. 2). These domains serve as protein dimerization motifs that, upon ligand binding, recruit the DD containing adaptor FAS-Associated via Death Domain (FADD) protein. FADD in turn recruits caspases 8 and/or 10 via a death effector domain (DED), a second protein dimerization motif. Recruitment of FADD and the caspases to the receptor forms the death inducing signaling complex (DISC) and results in activation of the initiator caspases (caspase 8 and caspase 10). The initiator caspases exist in the cell as inactive proenzymes which become activated upon dimerization at the DISC. They subsequently undergo auto-processing resulting in the release of a large subunit and a small subunit from the precursor. The processed caspases form a tetramer composed of two large subunits and two small subunits and have markedly increased activity compared to the unprocessed enzyme (Ashkenazi, 2002; Riedl and Shi, 2004). Once activated the initiator caspases can directly cleave and activate the downstream effector caspases. Also, activated caspase 8 and caspase 10 can cleave the BH3 only containing protein Bid, which then translocates to the mitochondrial membrane where it activates the intrinsic pathway (Fig. 2). Cellular FLICE inhibitory protein (cFLIP) is an important negative regulatory molecule in the death receptor pathway. cFLIP was identified by homology to viral FLIP proteins which inhibit apoptosis by binding to the DR/FADD complex via DED domains and preventing recruitment of caspases 8 or 10 (Irmler et al., 1997). cFLIP is similar in structure to the caspase 8 proenzyme, containing two N-terminal DED domains and caspase related domains in the C-terminal. However, the active site cysteine required for caspase activity is replaced by a tyrosine in cFLIP. Thus, cFLIP can be recruited to the DISC and prevent the initiator caspases from being recruited and activated (Fig. 2).
Fig. 2.
Death receptor pathway. TNF family death ligands (e.g., TNF, FAS, TRAIL) bind to their cognate receptors and initiate the formation of the death inducing signaling complex (DISC). All of the TNF family receptors which induce apoptosis contain a highly conserved death domain (DD) in their cytoplasmic tails. The adaptor protein FADD contains an N-terminal death effector domain (DED) and a C-terminal DD. FADD is recruited to the activated receptor by homotypic interations between the C-terminal DD of FADD and the DD of the receptor. Inactive caspase 8 and caspase 10 zymogens are recruited to the DISC by homotypic interactions between the N-terminal DED domains of the caspases and FADD. cFLIP can be recruited to the DISC and prevents recruitment of caspase 8 or 10. The recruitment of caspase 8 or caspase 10 to the DISC results in activation of the caspases and auto-processing into the active forms of the caspase (reviewed in Riedl and Shi, 2004). Activated caspase 8 or caspase 10 can directly activate effector caspases (e.g., caspases 3, 6, and 7). Activated caspase 8 or caspase 10 also can cleave the BH3 only protein Bid. Cleaved Bid (tBid) translocates to the mitochondria where it activates the extrinsic pathway.
Also, activation of DRs can result in signaling that does not induce apoptosis. For example, TNFR1 can, via recruitment of TRADD, regulate gene expression by activation of Nuclear Factor-kappa B (NF-κB) and AP1 transcription factors (Wilson et al., 2009). Similarly, FAS can activate proimflammatory responses in addition to apoptototic signaling (Wilson et al., 2009). Like TNFR1, TRAIL receptors can activate NF-κB. This appears to be mediated by the recruitment of RIP which leads to activation of the inhibitor of κB kinases, phosphorylation of the inhibitor of κB, and activation of NF-κB (Falschlehner et al., 2007). Also, TRAIL can activate AKT and MAPK, but the links to these pathways are unclear (Falschlehner et al., 2007). While the TRAIL receptors can signal to non-apoptotic pathways, this review will focus on the role of the TRAIL ligand and its DRs in inducing apoptosis in breast cancer cells.
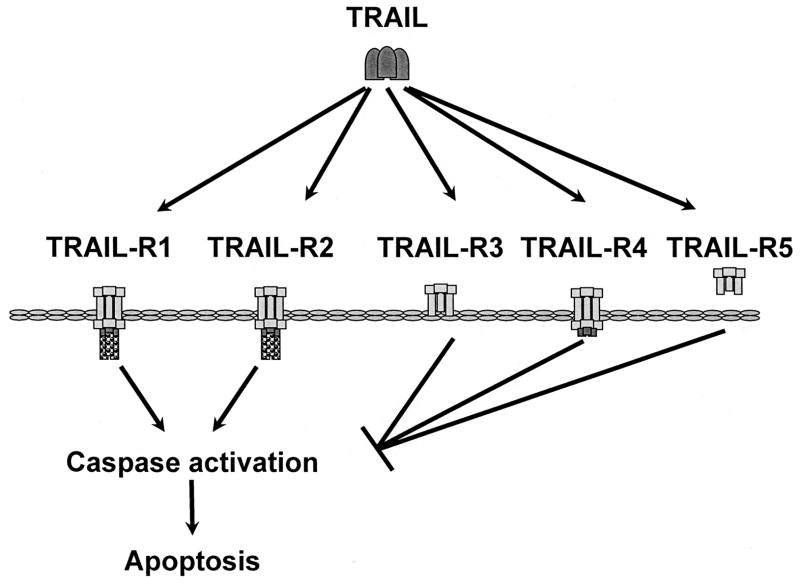
TRAIL (a.k.a. Apo2L) was initially identified and cloned based on homology searches of EST databases for cDNAs related to TNF and Fas ligand (Pitti et al., 1996; Wiley et al., 1995). These studies identified a ligand that is highly homologous to FAS and TNF and that is able to induce apoptosis in a diverse range of tumor cell lines. Also, the receptors for TRAIL were identified based on homology searches for ESTs that were similar to TNFR1 (Pan et al., 1997a; Pan et al., 1997b). In humans, there are two receptors for TRAIL that can induce apoptosis upon ligand binding, TRAIL-R1 (a.k.a. DR4) and TRAIL-R2 (a.k.a. DR5, TRICK2, and KILLER) (Fig. 3) (MacFarlane et al., 1997; Pan et al., 1997a; Pan et al., 1997b; Screaton et al., 1997; Sheridan et al., 1997; Walczak et al., 1997; Wu et al., 1997). There are three receptors, TRAIL-R3 (a.k.a. Decoy Receptor 1, TRID, and LIT), TRAIL-R4 (a.k.a. Decoy Receptor 2 and TRUNND), and TRAIL-R5 (a.k.a. osteoprotegerin) which have incomplete death domains or lack death domains (Degli-Esposti et al., 1997a; Degli-Esposti et al., 1997b; Emery et al., 1998; Marsters et al., 1997; Pan et al., 1997a; Pan et al., 1998; Schneider et al., 1997a; Sheridan et al., 1997). These three receptors act as inhibitors of TRAIL-induced apoptosis by binding the ligand and sequestering it from the death inducing receptors (Fig. 3). Expression of TRAIL and TRAIL receptors is found widely distributed throughout the organism (Spierings et al., 2004). Animal studies implicate TRAIL and its receptor as negative regulators of immune responses. TRAIL deficient mice have a defect in thymocyte apoptosis and a concomitant hypersensitivity to the development of autoimmune T-cell mediated responses in several experimental systems (Cretney et al., 2005; Lamhamedi-Cherradi et al., 2003). Also, there is evidence that TRAIL may enhance T-cell mediated neural cell death in an animal model of autoimmune encephalomyelitis (Aktas et al., 2005). Thus, TRAIL may both inhibit and promote autoimmune disease. Mice have a homologue of TRAIL-R2 but do not have a TRAIL-R1 (Kelley and Ashkenazi, 2004). Loss of TRAIL-R2 results in enhanced immune responses to CMV infection, consistent with a role as a negative regulator of innate immune responses (Diehl et al., 2004). Importantly, animal studies suggest that TRAIL plays a role in tumor surveillance. Neutralization or deletion of TRAIL in several animal models demonstrates that the loss of TRAIL activity promotes the growth and metastasis of tumors in both transplanted and spontaneous tumors (Cretney et al., 2002; Sedger et al., 2002; Takeda et al., 2001; Zerafa et al., 2005). The anti-tumor effects of TRAIL in these studies appear to be mediated by NK cells (Cretney et al., 2002; Takeda et al., 2001). In addition T-cell mediated graft versus tumor activity appears to be mediated at least in part by TRAIL as allogeneic hematopoietic-cell transplantation from TRAIL deficient animals resulted in less graft versus tumor activity (Schmaltz et al., 2002).
Fig. 3.
TRAIL receptors. TRAIL-R1 and TRAIL-R2 are type I transmembrane proteins that contain a DD. Ligand binding to these receptors results in activation of caspases. TRAIL-R3 is a glycophospholid-anchored cell surface protein, TRAIL-R4 is a transmembrane protein lacking an intact DD, and TRAIL-R5 is a secreted protein. These proteins bind TRAIL but are unable to activate caspases. These receptors act as decoy receptors and can inhibit TRAIL-mediated apoptosis by competing with TRAIL-R1 and TRAIL-R2 for the ligand.
The interest in the anti-tumor activity of TNF family ligands is based in part on work done by William Coley over 100 years ago which found that bacterial toxins could induce hemorrhagic necrosis of tumors and induce meaningful responses in patients with inoperable tumors such as sarcomas (Coley, 1893; Coley, 1906). The search for the biological mediators of these responses led to the identification of TNF and its receptor (Balkwill, 2009; Carswell et al., 1975). Inspired by the results reported by Coley, TNF was tested in patients with cancer but TNF causes severe toxicity and has little efficacy as systemic therapy for cancer (Balkwill, 2009). The second TNF family DR ligand, FAS ligand, has not been tested in clinical trials due to lethal hepatic apoptosis in animal studies (Ogasawara et al., 1993). TRAIL is currently in clinical trials and has generated much excitement as a potential systemic cancer therapy. Early in vitro experiments suggested that TRAIL could kill tumor cells in culture but was not toxic to nontransformed cells (Ashkenazi et al., 1999; Keane et al., 1999; Keane et al., 2000). Subsequent experiments with TRAIL in mice, cynomolgus monkeys, and chimpanzees confirmed that TRAIL is well tolerated by animals (Ashkenazi et al., 1999; Kelley et al., 2001; Lawrence et al., 2001). This has lead to phase I clinical trials of both TRAIL and agonistic TRAIL receptor antibodies which have demonstrated that these agents are well tolerated at doses that result in serum levels that are above the therapeutic concentrations that have been used in preclinical studies (Fig. 4) (Camidge et al., 2007; Hotte et al., 2008; Ling et al., 2006; Plummer et al., 2007; Tolcher et al., 2007). These agents are undergoing further testing in clinical trials as single agents and in combination with chemotherapy.
Fig. 4.
TRAIL agonists. Clinical trials testing TRAIL (a.k.a. rhApo2L), an agonistic TRAIL-R1 antibody (mapatumumab), and agonistic TRAIL-R2 antibodies (lexatumumab and apomab) are currently ongoing.
III. TRAIL-INDUCED APOPTOSIS IN BREAST CANCER CELLS
TNF and FAS agonists have been studied using in vitro models of breast cancer (e.g., (Jaattela et al., 1995; Keane et al., 1996). However, the toxicity and lack of efficacy of TNF in clinical trials and the toxicity of FAS ligands in preclinical studies has precluded further clinical development of these ligands (Balkwill, 2009; Ogasawara et al., 1993). Initial studies of TRAIL-mediated apoptosis in breast cancer cell lines demonstrated that while TRAIL could induce apoptosis in the MDA-MB-231 breast cancer cell line, the majority of cell lines tested were very resistant to TRAIL-mediated apoptosis (Ashkenazi et al., 1999; Buchsbaum et al., 2003; Keane et al., 1999; Keane et al., 2000; Singh et al., 2003). These studies were able to establish that TRAIL induced caspase mediated apoptosis in the sensitive cell line and that TRAIL activated caspases within minutes of addition to the cells (Keane et al., 1999; Keane et al., 2000). However, these studies did not systematically evaluate breast cancer cell lines with different phenotypes as defined above (e.g., hormone receptor positive, HER-2 amplified, or triple-negative cell lines). Recently, our laboratory reexamined TRAIL-sensitivity in breast cancer cells using a panel of cell lines that included multiple cell lines of each phenotype (Neve et al., 2006; Rahman et al., 2009). This study found that TRAIL sensitivity varied with the phenotype of the breast cancer cell lines (Fig. 5) (Rahman et al., 2009). Strikingly, eight of eleven triple-negative breast cancer cell lines were very sensitive to TRAIL-induced apoptosis with the IC50 ranging from 10–250 ng/ml (~0.2–5.8 nM). By contrast all five of the ER positive cell lines tested were resistant to TRAIL-induced apoptosis across a wide range of doses. Two of five cell lines with HER-2 amplification showed a modest sensitivity to TRAIL, only reaching an IC50 at approximately 1000 ng/ml (~20 nM). Other studies, although not designed to specifically look at TRAIL sensitivity based on the phenotype of the cell lines, found similar results. For example, Chinnaiyan et al. studied TRAIL sensitivity in 10 breast cancer cell lines (Chinnaiyan et al., 2000). They found that three of five triple-negative breast cancer cell lines were TRAIL-sensitive. Two HER-2 amplified breast cancer cell lines and three ER positive cell lines were TRAIL-resistant (Chinnaiyan et al., 2000). Similarly, Buchsbaum et al. found that an agonistic anti-TRAIL-R2 antibody induced apoptosis in one of two triple-negative breast cancer cell lines but not any of four HER-2 amplified breast cancer cell lines nor in an ER positive cell line (Buchsbaum et al., 2003). Together the data from these three studies demonstrated that 10 of 14 triple-negative breast cancer cell lines were sensitive to TRAIL-induced apoptosis while only two of eight HER-2 amplified cell lines, and none of seven ER positive lines were sensitive to TRAIL-induced apoptosis.
Fig. 5.
TRAIL selectively kills mesenchymal triple-negative breast cancer cell lines. (Top) Growth inhibition of breast cancer cells incubated with TRAIL. Black lines represent mesenchymal triple-negative cell lines, green lines represent epithelial triple-negative cell lines; blue lines represent HER-2 amplified cell lines, and red lines represent ER positive cell lines. (Bottom) Characterization of ER and HER-2 expression in the breast cell lines. This figure is reproduced with kind permission of Springer Science and Business Media from Fig. 1 in (Rahman et al., 2009).
In cancer cells, the mutation or absence of p53 renders cells resistant to chemotherapy or radiation therapy (Bunz et al., 1999; Lee and Bernstein, 1993; Lowe et al., 1994; Lowe et al., 1993). In breast cancer specifically, primary resistance to doxorubicin has been associated with p53 mutations (Aas et al., 1996). Interestingly, recent work has found that p53 absence or mutation is frequent in triple-negative/basal-like breast cancers (Brenton et al., 2005). The TRAIL-sensitive triple-negative breast cancer cell lines described above frequently have lost or mutated p53 (Neve et al., 2006; Rahman et al., 2009). The ability of TRAIL to kill tumors that are p53 mutant or deleted has been observed in cell lines from a wide variety of tumor types (Ashkenazi et al., 2008). This suggests that TRAIL ligands may be particularly useful as a therapeutic agent in tumors deficient in p53.
Recent work has classified a large number of breast cancer cell lines based on transcriptional profiling (Neve et al., 2006). Like the array based profiling of primary tumors described above (Perou et al., 2000; Sorlie et al., 2001), breast cancer cell lines could be classified into two main groups, luminal and basal. The triple-negative breast cancer cell lines were classified as basal by this analysis (Neve et al., 2006). The basal group was further subdivided into basal “A” and basal “B” groups. The Basal B cell lines were distinguished based on expression of mesenchymal markers such as the cytoskeletal protein vimentin. An independent group classified breast cancer cell lines by transcriptional profiling and similarly found that a subset of the triple-negative cell lines had mesenchymal features (Charafe-Jauffret et al., 2006). Interestingly, we found that all of the TRAIL-sensitive triple-negative cell lines we tested have mesenchymal features based on these analyses (Charafe-Jauffret et al., 2006; Neve et al., 2006; Rahman et al., 2009). In contrast, the TRAIL-resistant triple-negative cell lines in our study were ones that are classified as epithelial by these analyses (Charafe-Jauffret et al., 2006; Neve et al., 2006; Rahman et al., 2009). A number of studies using the mesenchymal triple-negative breast cancer cell line MDA-MB-231 have demonstrated the efficacy of TRAIL ligands or agonistic antibodies in xenograft studies, confirming the sensitivity of this cell line in vivo (Buchsbaum et al., 2003; Shankar et al., 2004; Singh et al., 2003; Thai le et al., 2006). Together these results suggest that triple-negative/basal-like breast cancers with mesenchymal features are more likely to be sensitive to TRAIL-induced apoptosis.
The mesenchymal characterization of the triple-negative breast cancer cell lines which are sensitive to TRAIL was initially identified by transcriptional profiling and confirmed by immunoblotting for vimentin, a mesenchymal marker protein (Charafe-Jauffret et al., 2006; Neve et al., 2006; Rahman et al., 2009). However, the mesenchymal subset of tumors was not identified in the early transcriptional profiling of primary breast cancer samples that defined the luminal and basal subsets of breast cancer (Sorlie et al., 2001). More recently, immunohistochemical studies of primary breast tumors have identified a subset of tumors in which the cancer cells express vimentin, consistent with the existence of mesenchymal tumors (Livasy et al., 2006; Umemura et al., 2005; Willipinski-Stapelfeldt et al., 2005). The largest study of more that 2500 primary breast tumors found that approximately 14% of all of the tumors and 35% of the ER negative tumors expressed vimentin (Willipinski-Stapelfeldt et al., 2005). In this study, approximately 7% of the ER positive tumors expressed vimentin. The enrichment of vimentin positive tumors within the ER negative samples is consistent with an enrichment within the triple-negative samples, but this study did not simultaneously evaluate HER-2 amplification so that vimentin positive tumors cannot be classified as triple-negative (Willipinski-Stapelfeldt et al., 2005). The other two studies identified vimentin expression in 17 of 18 and 4 of 11 triple-negative breast cancer samples (Livasy et al., 2006; Umemura et al., 2005). In the study by Livasy et al., the tumors were catergorized as luminal, basal, or HER-2 amplified by cDNA microarray expression profiling (Livasy et al., 2006). This study found 17 of 18 triple-negative/basal-like tumors had strong and diffuse vimentin staining in the tumor cells (Livasy et al., 2006). Only 1 of 16 ER positive/luminal cancers and 1 of 12 HER-2 amplified tumors expressed vimentin in the tumor cells. These studies suggest that a subset of triple-negative breast cancers have mesenchymal features.
As described above, in humans there are two receptors for TRAIL that induce apoptosis, TRAIL-R1 and TRAIL-R2. Previous work using mutants of TRAIL that bind selectively to either TRAIL-R1 or TRAIL-R2 has demonstrated that TRAIL induces apoptosis predominantly via TRAIL-R1 in some tumor types and via TRAIL-R2 in others (Kelley et al., 2005; MacFarlane et al., 2005; van der Sloot et al., 2006). Both receptors are expressed at the mRNA and protein levels in the TRAIL-sensitive breast cancer cells (Keane et al., 1999; Rahman et al., 2009). A study using agonist antibodies to either TRAIL-R1 or TRAIL-R2 has shown that both can induce apoptosis in the MDA-MB-231 cell line (Zhang and Zhang, 2008). Interestingly, despite expression of both receptors on the breast cancer cells and the ability of the agonist anti-TRAIL-R1 antibody to induce apoptosis, experiments using RNA interference or receptor selective mutants of TRAIL indicate that TRAIL-R2 is the predominant mediator of apoptosis in the breast cancer cells exposed to TRAIL (Kelley et al., 2005; Rahman et al., 2009). The basis for the selective activity of TRAIL-R2 in the breast cancer cells is not clear. One possibility is that the absolute level of TRAIL-R2 at the cell surface is significantly greater than that of TRAIL-R1, but this has not been demonstrated. Alternatively, binding studies suggest that TRAIL-R2 has a significantly higher affinity (Kd ≤ 2 nM) for TRAIL than TRAIL-R1 (Kd = 70 nM) when the binding studies are carried out at 37°C (Truneh et al., 2000). This observation could explain the discordant results described above, where the agonist anti-TRAIL-R1 antibody can induce apoptosis in MDA-MB-231 cells but the ligand utilizes preferentially TRAIL-R2.
Overall, these observations about breast cancer subtype and receptor selectivity will be important in planning clinical trials of TRAIL ligands or agonistic antibodies in breast cancer patients.
IV. MECHANISMS DETERMINING TRAIL SENSITIVITY IN BREAST CANCER CELLS
The underlying determinants of TRAIL sensitivity in the breast cancer cell lines have not been clearly established. While the experiments described above suggest a subset of breast cancer cells are intrinsically more sensitive to TRAIL (i.e., triple-negative breast cancer cells with mesenchymal features), no clear mechanistic basis for this was determined (Rahman et al., 2009).
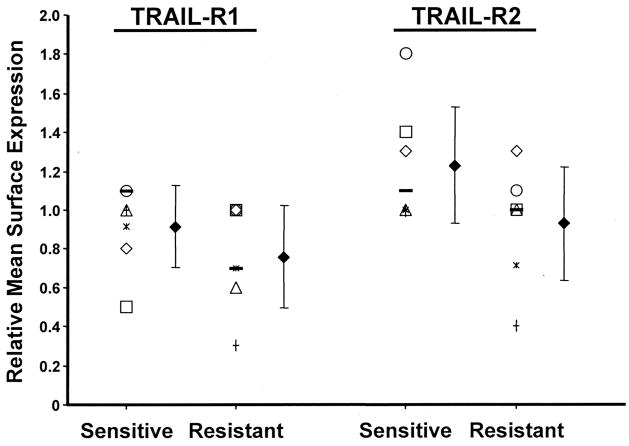
Of the five receptors for TRAIL two receptors, TRAIL-R1 and TRAIL-R2, induce caspase activation and apoptosis upon ligand binding (Fig. 3) (MacFarlane et al., 1997; Pan et al., 1997a; Pan et al., 1997b; Screaton et al., 1997; Sheridan et al., 1997; Walczak et al., 1997; Wu et al., 1997). In some tumor types, such as neuroblastoma, lack of surface expression of TRAIL-R1 or TRAIL-R2 has been found to correlate with the lack of TRAIL sensitivity (Yang et al., 2003). Expression of TRAIL receptors on breast cancer cells has been examined in a number of studies. The levels of receptor either by mRNA, total protein levels, or surface expression are not predictive of TRAIL-sensitivity (Buchsbaum et al., 2003; Keane et al., 1999; Rahman et al., 2009). For example, we determined the surface expression of both TRAIL-R1 and TRAIL-R2 in seven sensitive and seven resistant breast cancer cell lines (Fig. 6). The expression of TRAIL-R1 overlapped significantly between the sensitive and resistant cell lines and did not allow discrimination of the sensitive and resistance cells. However, as described above, studies suggest that TRAIL-R1 may not contribute significantly to the induction of apoptosis by TRAIL in breast cancer cells (Kelley et al., 2005; Rahman et al., 2009). While the expression of TRAIL-R2 is generally higher on TRAIL-sensitive cells than on TRAIL-resistant cells there was again significant overlap between the surface level on sensitive and resistant cells (Fig. 6). In addition, many of the TRAIL-resistant cells expressed only marginally less surface TRAIL-R2 than the sensitive cells (Fig. 6). Three receptors, TRAIL-R3, TRAIL-R4, and TRAIL-R5, can bind TRAIL but do not have a functional death domain (Fig. 3) (Degli-Esposti et al., 1997a; Degli-Esposti et al., 1997b; Emery et al., 1998; Marsters et al., 1997; Pan et al., 1997a; Pan et al., 1998; Schneider et al., 1997a; Sheridan et al., 1997). These receptors have been shown to inhibit TRAIL-induced apoptosis when overexpressed and have been called decoy receptors. However, the expression of these decoy receptors has not been found to correlate with resistance to TRAIL-induced apoptosis in breast cancer cell lines or in other tumors (Griffith et al., 1998; Griffith et al., 1999; Keane et al., 1999; Rahman et al., 2009). Thus expression levels of TRAIL receptors do not appear to be predictive of TRAIL sensitivity.
Fig. 6.
Cell surface expression of TRAIL-R1 and TRAIL-R2 do not correlate with TRAIL-senstivity. Mean cell surface expression of TRAIL-R1 and TRAIL-R2 in seven TRAIL-sensitive and seven TRAIL-resistant breast cancer cells was measured by flow cytometry (see text for discussion).
Examination for evidence of mutation in the death inducing TRAIL receptors (TRAIL-R1 and TRAIL-R2) in breast cancer cells has given contradictory results. One study found that 3 of 57 (5.3%) primary breast cancers had mutations in the death domain of TRAIL-R1 and 4 of 57 (7.0%) had mutations in the death domain of TRAIL-R2 (Shin et al., 2001). Interestingly, all of these mutations were found in the group of 34 breast cancers that were metastatic to the regional lymph nodes and none were found in the 23 samples from tumors that had not spread to regional nodes. In addition, these mutant receptors were impaired in their ability to induce apoptosis compared to wild type receptors (Shin et al., 2001). A second study looked at a series of primary breast cancers and found that 2 out of 50 had sequence variants in TRAIL-R1 and 11 of 95 tumors had sequence variants in TRAIL-R2. However, all of the sequence variants were found in matched normal tissue leading to the conclusion that they represented polymorphisms and not cancer specific mutations. None of these sequence variants were in the death domain but no functional studies were undertaken for these sequence variants. Overall, the second study concluded that there was no relationship between these polymorphisms and breast cancer. While no systematic sequence data for the cell lines has been reported, one study has sequenced TRAIL-R1 and TRAIL-R2 from seven breast cancer cell lines but found no correlation of sequence variants with TRAIL sensitivity (Zhang and Zhang, 2008). Similarly, we have examined the mRNA sequence for TRAIL-R1 and TRAIL-R2 from a number of the TRAIL-sensitive and resistant cells and have not found evidence for mutation or sequence variation that correlates with TRAIL sensitivity or resistance (unpublished data).
In general, studies have not identified individual components of either the TRAIL pathway (e.g., TRAIL receptors, FADD, caspase 8) or apoptosis modulators (e.g., cFLIP, IAPs, or Bcl-2 family members) whose expression is predictive of TRAIL sensitivity or resistance (Keane et al., 1999; Rahman et al., 2009). A number of studies have found that altering the levels or activity of antiapoptotic proteins such as Bcl-2 or Bcl-XL, FLIP, NFκB or Survivin can alter the sensitivity of cells to TRAIL (Fulda and Debatin, 2004; Fulda et al., 2002; Guseva et al., 2008; Keane et al., 2000; Kim et al., 2003; Palacios et al., 2006). However, these studies do not demonstrate that the levels or activity of these proteins are the primary reason for TRAIL resistance in the breast cancer cells.
Recent analysis in pancreatic cancer, colorectal cancer, non-small-cell lung cancer, and melanoma cell lines has identified low expression of O-glycosylation genes as a potential mechanism of TRAIL-resistance (Wagner et al., 2007). This study found that O-glycosylation of the TRAIL receptors promoted ligand induced clustering of the receptors and subsequent recruitment and activation of initiator caspase 8. However, gene expression analysis in the breast cancer cell lines did not find a correlation between the genes which regulate O-glycosylation and TRAIL sensitivity (Rahman et al., 2009). Further experiments with inhibitors of O-glycosylation or overexpression of genes which mediate O-glycosylation did not affect TRAIL sensitivity in the breast cancer cell lines (unpublished observations). Thus, this mechanism does not appear to determine TRAIL sensitivity in breast cancer cells.
An intriguing observation is that the TRAIL-sensitive MDA-MB-231 breast cancer cell line (a triple-negative breast cancer cell line with basal and mesenchymal features) has low expression of the small heat shock protein, αB-crystallin, while several TRAIL-resistant cell lines (including a TRAIL-resistant triple-negative and an ER positive cell line) have high expression of αB-crystallin (Kamradt et al., 2005). Overexpression of αB-crystallin in MDA-MB-231 decreases the sensitivity to TRAIL and RNAi mediated knockdown of αB-crystallin in one cell line increased sensitivity to TRAIL (Kamradt et al., 2005). No systematic evaluation of αB-crystallin and TRAIL sensitivity in a more extensive panel of breast cancer cells representing the different subtypes has been undertaken. Paradoxically, studies of primary breast cancer samples have demonstrated that αB-crystallin is expressed predominantly in triple-negative/basal-like breast cancers and not in ER positive or HER-2 positive tumors (Moyano et al., 2006; Sitterding et al., 2008). These results are at odds with the cell line data described above which showed low expression in one triple-negative/basal-like breast cancer cell line (MDA-MB-231) but high expression in one triple-negative/basal-like and one ER positive cell line (MDA-MB-468 and MCF 7 respectively) (Kamradt et al., 2005). Thus, further work will be needed to determine if αB-crystallin is predictive of TRAIL sensitivity in a wider sample of breast cancer cells.
Like many cell surface receptors, death receptors undergo activation induced internalization via the endocytic pathway (Austin et al., 2006; Higuchi and Aggarwal, 1994; Kohlhaas et al., 2007; Lee et al., 2006; Schneider-Brachert et al., 2004; Siegel et al., 2004). Internalization appears required for optimal induction of apoptosis by TNFR and FAS (Lee et al., 2006; Schneider-Brachert et al., 2004). Studies with TRAIL receptors have shown that TRAIL-R1 and TRAIL-R2 undergo clathrin dependent endocytosis upon ligand activation (Austin et al., 2006; Kohlhaas et al., 2007; Zhang et al., 2008). In contrast to TNFR and FAS, internalization is not required for effective apoptotic signaling by TRAIL receptors (Austin et al., 2006; Kohlhaas et al., 2007). Interestingly, activation of TRAIL-R2 results in caspase dependent cleavage of clathrin and this attenuates internalization (Austin et al., 2006). These studies further suggest that endocytosis negatively regulates apoptotic signaling and that blocking endocytosis (e.g., by expression of a dominant negative dynamin mutant or by inhibition with chlorpromazine) potentiates TRAIL-induced apoptosis (Austin et al., 2006; Zhang et al., 2008). One study has found that the TRAIL receptors are localized predominantly in the cytosol in TRAIL-resistant breast cancer cells while they are localized on the plasma membrane on TRAIL-sensitive cells (Zhang and Zhang, 2008). Inhibition of clathrin mediated endocytosis increased the cell surface expression of TRAIL receptors and the sensitivity to TRAIL-mediated apoptosis in these resistant breast cancer cells (Zhang and Zhang, 2008). This suggests that a defect in proper trafficking of the TRAIL receptors could account for TRAIL resistance. No mechanism was described to account for the preferential localization of the TRAIL receptors in the cytosol of resistant cells.
V. OVERCOMING TRAIL RESISTANCE
Many studies in the literature have investigated the combination of a wide range of drugs with TRAIL in order to potentiate cell death and/or overcome resistance. This has also been investigated in breast cancer cells and, as will be outlined below, the results of many studies suggest that TRAIL may have the widest use in treating breast cancer when used in combination with other agents.
Combinations of chemotherapy with TRAIL have been extensively studied in many cancer cell types (Ashkenazi et al., 2008). In breast cancer cells, the combination of TRAIL with chemotherapeutic drugs commonly used in the treatment of breast cancer can enhance the induction of apoptosis in the cancer cells (Buchsbaum et al., 2003; Keane et al., 1999; Singh et al., 2003). A wide range of drugs has been tested in these studies including camptothecin, doxorubicin, etoposide, 5-flourouracil, melphalan, methotrexate, paclitaxel, vincristin, and vinblastin. While each of the drugs can enhance TRAIL-mediated apoptosis in some of the breast cancer cells tested, the most consistent finding across the three studies is that doxorubicin synergistically enhances TRAIL-mediated apoptosis (Buchsbaum et al., 2003; Keane et al., 1999; Singh et al., 2003). Importantly, the combination of TRAIL with chemotherapeutic drugs can overcome the intrinsic resistance to TRAIL in breast cancer cell lines (Buchsbaum et al., 2003; Keane et al., 1999; Singh et al., 2003). Similarly, tumor xenograft studies using the TRAIL-sensitive MDA-MB-231 cell line have shown that the combination of TRAIL and chemotherapeutic drugs more effectively inhibits the growth of tumors than either alone (Buchsbaum et al., 2003; Singh et al., 2003). Several mechanisms have been proposed by which chemotherapeutic drugs enhance TRAIL-mediated apoptosis in the resistant breast cancer cells. In one study, concurrent administration of TRAIL and the chemotherapeutic agent caused markedly increased caspase activation. Interestingly, drugs that themselves activated caspases interacted synergistically with TRAIL while those that did not activate caspases did not enhance TRAIL-mediated apoptosis (Keane et al., 1999). Caspase inhibition using the pancaspase inhibitor ZVAD-FMK blocked the cell death induced by TRAIL, the caspase activating chemotherapeutic drugs, and the combination of the two. In this study, using simultaneous treatment with TRAIL and chemotherapeutic drugs, no consistent change in mRNA for TRAIL receptors or other apoptosis regulators was identified. This suggested that the independent activation of caspases by TRAIL and the chemotherapeutic drug accounted for the synergism. A second study found that a 24 hour pretreatment of breast cancer cells with chemotherapeutic drugs resulted in an up regulation of TRAIL-R1 and TRAIL-R2 mRNA and protein and that this correlated with the increased sensitivity of the cells to TRAIL (Singh et al., 2003). This study found that simultaneous treatment of cells with TRAIL and chemotherapeutic drug or pretreatment with TRAIL followed by the chemotherapeutic drug was not as effective as preincubation of the cells with the chemotherapeutic drug. In addition, up regulation of the mRNA for proapoptotic Bcl2 family members (e.g., BAX and BAD) was observed in cells pretreated with chemotherapeutic drugs. Consistent with the first study, this second study also found that the chemotherapeutic drugs activated caspases and that caspase inhibition blocked the toxicity of the drugs, TRAIL, and the combination (Singh et al., 2003). As in cell lines from other tumor types, the interaction of TRAIL and chemotherpuetic drugs appears independent of p53 status in the cell lines studied (Ashkenazi et al., 2008; Keane et al., 1999; Singh et al., 2003). The ability of chemotherapeutic drugs to enhance TRAIL-mediated apoptosis and overcome the intrinsic resistance of breast cancer cells to TRAIL provides a rationale for combining TRAIL with chemotherapeutic drugs in clinical trials.
Radiation also enhances TRAIL-mediated apoptosis in cell lines and in tumor xenografts (Buchsbaum et al., 2003; Chinnaiyan et al., 2000). Mechanistic studies suggest that radiation results in up regulation of TRAIL-R2 in a p53 dependent fashion (Chinnaiyan et al., 2000). Interestingly, TRAIL-R2 was independently identified as a p53 regulated gene induced by DNA damage (Wu et al., 1997). However, while the combination of radiation and TRAIL appears to be synergistic, the utility of this approach to treating breast cancer is limited by the systemic nature of the disease.
While chemotherapeutic agents have shown promise in combination with TRAIL, they also may increase toxicity. For example, while we found that chemotherapeutic drugs could enhance TRAIL-mediated apoptosis in resistant breast cancer cell lines, these combinations also resulted in increased death of normal mammary epithelial cells (Keane et al., 1999). This has lead to an interest in combining TRAIL with targeted agents in breast cancer and in other tumor types in an effort to identify combinations that may potentiate the death of tumor cells without increasing toxicity (Ashkenazi et al., 2008).
HER-2 is a member of the EGFR family that is amplified and overexpressed in 15–30% of breast and ovarian cancers (Slamon et al., 1987; Slamon et al., 1989; Tyson et al., 1991; Zhang et al., 1989). The majority of breast cancer cell lines with HER-2 amplification are resistant to TRAIL-mediated apoptosis (Buchsbaum et al., 2003; Chinnaiyan et al., 2000; Rahman et al., 2009). The humanized anti-HER-2 antibody, trastuzumab (Herceptin®), has clinical activity alone and in combination with chemotherapy in metastatic breast cancer, but only when HER-2 is amplified (Pegram et al., 1998; Pegram and Slamon, 1999; Slamon et al., 2001; Vogel et al., 2002). TRAIL-induced apoptosis could be enhanced in some resistant breast and ovarian cancer cell lines with HER-2 amplification (e.g., SKBR3, MDA-MB453, and SKOV3) when the cells were pretreated with trastuzumab (Cuello et al., 2001; Dubska et al., 2005). There was no interaction between TRAIL and trastuzumab in cells without HER-2 amplification. Mechanistic studies demonstrated that trastuzumab induces down-regulation of the HER-2 protein and that this results in inhibition of AKT kinase activity (Cuello et al., 2001). Also, AKT inhibition resulted in increased TRAIL sensitivity in these cells and expression of constitutively active AKT inhibited both TRAIL-mediated apoptosis and its potentiation by trastuzumab. In contrast to these results, investigation of another cell line (BT474) with HER-2 amplification showed that trastuzumab decreased TRAIL-mediated apoptosis (Dubska et al., 2005). As in the report by Cuello et al., this study found that incubating cells with trastuzumab resulted in decreased AKT kinase activity in the BT474 cells. However, in these cells this lead to a decrease in the surface expression of TRAIL-R1 and TRAIL-R2 and resistance to TRAIL-induced apoptosis. How a decrease in AKT activity can lead to such different results is unclear. Recent studies have demonstrated that different isoforms of AKT have different biological roles and different affects on apoptosis (Irie et al., 2005; Kim et al., 2009; Maroulakou et al., 2007). Thus, it is possible that the opposing effects of trastuzumab on TRAIL-induced apoptosis are mediated by different AKT isoforms. Interestingly, the BT474 cell line expresses ER in addition to amplified HER-2 (Neve et al., 2006; Rahman et al., 2009). This result suggests that within HER-2 amplified breast cancers, there may be distinct subgroups that will have very different outcomes if TRAIL and trastuzumab are combined. Thus, tumors with HER-2 amplification may benefit from combined molecularly targeted therapies of TRAIL and trastuzumab. However, more studies will be needed to identify which tumors do and which do not benefit.
Epidermal growth factor receptor (EGFR) activity can attenuate death receptor mediated apoptosis and EGFR inhibition can increase sensitivity of cancer cells to TRAIL (Bremer et al., 2005; Gibson et al., 2002; Gibson et al., 1999; Park and Seol, 2002; Shrader et al., 2007; Teraishi et al., 2005). High levels of EGFR expression are frequently seen in triple-negative/basal-like breast cancer cells (Korsching et al., 2002; Livasy et al., 2006; Neve et al., 2006; Rahman et al., 2009). EGFR inhibition can enhance TRAIL-mediated apoptosis in EGFR-expressing breast cancer cells that are already sensitive to TRAIL (i.e., the mesenchymal triple-negative/basal-like breast cancer cell lines) but not in TRAIL-resistant cancer cell lines (Rahman et al., 2009). This suggests that the expression of EGFR in breast cancer cells can modulate TRAIL sensitivity but that it is not the primary reason for resistance to TRAIL-mediated apoptosis. The mechanisms by which EGFR inhibition enhances TRAIL-induced apoptosis in breast cancer cells are not known, but studies in other tumor cell types implicate the inhibition of AKT in mediating these effects (Bremer et al., 2005; Gibson et al., 2002; Gibson et al., 1999; Henson et al., 2003; Shrader et al., 2007; Teraishi et al., 2005). A variety of possible mechanisms downstream of AKT inhibition have been suggested by these studies, including decreased cFLIP expression, decreased XIAP expression, inactivation of Bcl-xL, and decreased Mcl-1 expression. Overall, these data support the investigation of EGFR inhibitors in combination with TRAIL.
ER expressing cell lines have all been resistant to the induction of apoptosis by TRAIL alone (Buchsbaum et al., 2003; Keane et al., 1999; Singh et al., 2003). These studies have shown synergistic interactions between TRAIL and chemotherapeutic drugs demonstrating that the TRAIL resistance can be overcome in ER positive cells. ER positive breast cancers are treated with agents that inhibit the activity of the ER including the selective ER modulators tamoxifen (Jordan and Brodie, 2007). A recent study demonstrated that tamoxifen can enhance TRAIL-induced apoptosis in breast cancer cell lines in vitro and in vivo (Lagadec et al., 2008). Surprisingly, tamoxifen enhanced TRAIL-mediated apoptosis in both ER positive (MCF 7 and T47D) and ER negative (MDA-MB-231 and BT20) breast cancer cell lines. Tamoxifen has been shown to induce apoptosis in an ER independent fashion (Mandlekar and Kong, 2001). Possible mechanisms include activation of Jun N-terminal kinase signaling, activation of p38 signaling, induction of oxidative stress, and induction of ceramide production (Mandlekar and Kong, 2001). Further work is necessary to understand the enhancement of TRAIL-induced apoptosis by tamoxifen.
TRAIL receptors can activate the anti-apoptotic transcription factor NF-κB (Chaudhary et al., 1997; Degli-Esposti et al., 1997a; Schneider et al., 1997b). NF-κB can protect cells from a variety of apoptotic stimuli by increasing expression of anti-apoptotic proteins (Baeuerle and Baltimore, 1996; Beg and Baltimore, 1996; Mayo et al., 1997; Ravi et al., 2001; Van Antwerp et al., 1996; Wang et al., 1998). Both TRAIL-sensitive and TRAIL-resistant breast cancer cells have detectable NF-κB activity in nuclear extracts prior to treatment with TRAIL which increases upon TRAIL treatment (Keane et al., 2000). In TRAIL-sensitive breast cancer cells, the activation of caspases occurs within minutes of ligand addition to the cells so that the induced NF-κB activity is not likely to have time via transcriptional activation to inhibit the apoptosis (Keane et al., 2000). In TRAIL-resistant breast cancer cell lines, inhibition of NF-κB by overexpression of a genetic inhibitor increased TRAIL-mediated apoptosis (Keane et al., 2000). Similar enhancement of TRAIL-mediated apoptosis has been seen in other tumor types (Jeremias and Debatin, 1998; Jeremias et al., 1998). A recent paper, using the MDA-MB-435 cell line has demonstrated that aspirin can enhance TRAIL-mediated apoptosis in vitro and in xenografts (Lu et al., 2008). They found that aspirin treatment resulted in proteasomal degradation of survivin and similarly that RNAi based down regulation of survivin enhanced TRAIL-mediated apoptosis (Lu et al., 2008). Interestingly, salycilates have been shown to inhibit NF-κB at the concentrations used in this study (Yin et al., 1998). However, the study by Lu et al. did not investigate whether inhibition of NF-κB contributed to the enhancement of TRAIL-mediated apoptosis (Lu et al., 2008). One caveat in this work is that while the MDA-MB-435 cell line was originally derived from a patient with breast cancer, there is controversy in the literature as to whether the cell line in use by investigators originated from a breast cancer or melanoma (Chambers, 2009; Lacroix, 2009). None-the-less, together these results suggest that small molecule inhibitors of NF-κB could be a means to enhance TRAIL-mediated apoptosis in breast cancer cells.
Histone deacetylase (HDAC) inhibitors are currently under investigation for the treatment of cancer (Carew et al., 2008). HDACs mediate deacetylation of histones, generally leading to chromatin compaction in histones and transcriptional repression. By altering the epigenetic regulation of gene transcription, HDAC inhibitors have been shown to induce cell-cycle arrest, promote differentiation, and cause tumor cell death (Carew et al., 2008). One HDAC inhibitor, vorinostat, has been approved for the treatment of cutaneous T-cell lymphoma (Duvic and Vu, 2007). Preclinical studies combining HDAC inhibitors with TRAIL have shown synergistic induction of apoptosis in tumor cells (Fulda, 2008). Multiple mechanisms have been demonstrated for the enhancement of TRAIL in tumor cells by HDAC inhibitors including up regulation of TRAIL receptors, redistribution of TRAIL receptors to membrane lipid rafts, increased activation of the mitochondrial pathway, down-regulation of antiapoptotic proteins (e.g., cFLIP, antiapoptotic Bcl-2 family members, and survivin), and up regulation of proapoptotic Bcl-2 family members (Fulda, 2008). In breast cancer cells, several HDAC inhibitors have been shown to enhance TRAIL-mediated apoptosis (Chopin et al., 2004; Singh et al., 2005). One study found that the synergistic enhancement of TRAIL-mediated apoptosis in several TRAIL-resistant cell lines by HDAC inhibition was secondary to p21 expression (Chopin et al., 2004). The second study described enhanced TRAIL-mediated apoptosis in both TRAIL-sensitive and resistant cell lines by HDAC inhibitors (Singh et al., 2005). This study found that HDAC inhibition resulted in up regulation of TRAIL-R1, TRAIL-R2, and proapoptotic Bcl-2 family members. Interestingly, the up regulation of TRAIL-R1 and TRAIL-R2 by HDAC inhibitors is mediated by NF-κB (Shetty et al., 2005; Singh et al., 2005). This latter result suggests that NF-κB inhibition (described above) may either enhance or inhibit TRAIL-mediated apoptosis depending on the context.
Triterpenoids are naturally occurring compounds synthesized by many plants and two of the naturally occurring triterpenoids, oleanolic acid and ursolic acid, have weak anti-inflammatory and anti-tumor effects in vivo (Liby et al., 2007). More potent synthetic derivatives 2-cyano-3,12-dioxooleana-1, 9(11)-diene-28-oic acid (CDDO) and its derivative 1-(2-cyano-3,12-dioxooleana-1, 9(11)-diene-28-oyl) imidazole (CDDO-Im) have been shown to enhance TRAIL-mediated apoptosis in TRAIL-resistant breast cancer cells both in vitro and in vivo (Hyer et al., 2005). These studies found that CDDO and CDDO-Im enhance apoptosis induced by TRAIL and by agonistic antibodies to either TRAIL-R1 or TRAIL-R2. These studies further showed that CDDO and CDDO-Im down-regulate the expression of the antiapoptotic protein cFLIP and upregulate the mRNA and protein expression of TRAIL-R1 and TRAIL-R2. The mechanism by which these compounds regulate the expression of these proteins is unclear (Hyer et al., 2005).
VI. FUTURE DIRECTIONS
The data discussed above provide evidence that TRAIL or agonist antibodies directed at TRAIL-R2 could have clinical activity in the treatment of breast cancer. The underlying mechanisms that control TRAIL sensitivity in breast cancer cells have not been clearly defined. The phenotypic markers such as triple-negative/basal-like features and mesenchymal gene expression (e.g., vimentin) may act as surrogate biomarkers to predict the patients most likely to benefit from TRAIL treatment. Clinical trials aimed at these patients would be a logical first step in the clinic. However, further work is necessary to identify the true determinants of TRAIL sensitivity or resistance in breast cancer cells as these are more likely to be robust biomarkers. In addition, a large body of evidence suggests that resistance to TRAIL may be overcome in the other types of breast cancer by combinations of TRAIL ligands with various agents including chemotherapy and targeted therapies. Again, understanding the underlying molecular mechanisms that determine resistance may ultimately lead to more efficacious agents to combine with TRAIL in clinical studies.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. We thank Marion Nau for critical reading of this manuscript.
References
- Aas T, Borresen AL, Geisler S, Smith-Sorensen B, Johnsen H, Varhaug JE, Akslen LA, Lonning PE. Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat Med. 1996;2:811–4. doi: 10.1038/nm0796-811. [DOI] [PubMed] [Google Scholar]
- Aktas O, Smorodchenko A, Brocke S, Infante-Duarte C, Schulze Topphoff U, Vogt J, Prozorovski T, Meier S, Osmanova V, Pohl E, Bechmann I, Nitsch R, Zipp F. Neuronal damage in autoimmune neuroinflammation mediated by the death ligand TRAIL. Neuron. 2005;46:421–32. doi: 10.1016/j.neuron.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–30. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26:3621–30. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CD, Lawrence DA, Peden AA, Varfolomeev EE, Totpal K, De Maziere AM, Klumperman J, Arnott D, Pham V, Scheller RH, Ashkenazi A. Death-receptor activation halts clathrin-dependent endocytosis. Proc Natl Acad Sci U S A. 2006;103:10283–8. doi: 10.1073/pnas.0604044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–71. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–92. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- Bremer E, Samplonius DF, van Genne L, Dijkstra MH, Kroesen BJ, de Leij LF, Helfrich W. Simultaneous inhibition of epidermal growth factor receptor (EGFR) signaling and enhanced activation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor-mediated apoptosis induction by an scFv:sTRAIL fusion protein with specificity for human EGFR. J Biol Chem. 2005;280:10025–33. doi: 10.1074/jbc.M413673200. [DOI] [PubMed] [Google Scholar]
- Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- Buchsbaum DJ, Zhou T, Grizzle WE, Oliver PG, Hammond CJ, Zhang S, Carpenter M, LoBuglio AF. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin Cancer Res. 2003;9:3731–41. [PubMed] [Google Scholar]
- Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW, Vogelstein B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–9. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camidge D, Herbst RS, Gordon M, Eckhardt S, Kurzroc R, Durbin B, Ing J, Ling J, Sager J, Mendelson D. A phase I safety and pharmacokinetic study of apomab, a human DR5 agonist antibody, in patients with advanced cancer. J Clin Oncol. 2007;25:3582. [Google Scholar]
- Carew JS, Giles FJ, Nawrocki ST. Histone deacetylase inhibitors: mechanisms of cell death and promise in combination cancer therapy. Cancer Lett. 2008;269:7–17. doi: 10.1016/j.canlet.2008.03.037. [DOI] [PubMed] [Google Scholar]
- Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–70. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AF. MDA-MB-435 and M14 Cell Lines: Identical but not M14 Melanoma? Cancer Res. 2009;69:5292–5293. doi: 10.1158/0008-5472.CAN-09-1528. [DOI] [PubMed] [Google Scholar]
- Charafe-Jauffret E, Ginestier C, Monville F, Finetti P, Adelaide J, Cervera N, Fekairi S, Xerri L, Jacquemier J, Birnbaum D, Bertucci F. Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene. 2006;25:2273–84. doi: 10.1038/sj.onc.1209254. [DOI] [PubMed] [Google Scholar]
- Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity. 1997;7:821–830. doi: 10.1016/s1074-7613(00)80400-8. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan A, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, Ross BD, Rehemtulla A. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA. 2000;97:1754–1759. doi: 10.1073/pnas.030545097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin V, Slomianny C, Hondermarck H, Le Bourhis X. Synergistic induction of apoptosis in breast cancer cells by cotreatment with butyrate and TNF-alpha, TRAIL, or anti-Fas agonist antibody involves enhancement of death receptors’ signaling and requires P21(waf1) Exp Cell Res. 2004;298:560–73. doi: 10.1016/j.yexcr.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am J Med Sci. 1893;105:487–511. [PubMed] [Google Scholar]
- Coley WB. Late results of the treatment of inoperable sarcoma by the mixed toxins of erysipelas nd Bacillus prodigiosus. Am J Med Sci. 1906;131:375–430. [Google Scholar]
- Cretney E, McQualter JL, Kayagaki N, Yagita H, Bernard CC, Grewal IS, Ashkenazi A, Smyth MJ. TNF-related apoptosis-inducing ligand (TRAIL)/Apo2L suppresses experimental autoimmune encephalomyelitis in mice. Immunol Cell Biol. 2005;83:511–9. doi: 10.1111/j.1440-1711.2005.01358.x. [DOI] [PubMed] [Google Scholar]
- Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–61. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- Cuello M, Ettenberg SA, Clark AS, Keane MM, Posner RH, Nau MM, Dennis PA, Lipkowitz S. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res. 2001;61:4892–4900. [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997a;7:813–820. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA. Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med. 1997b;186:1165–1170. doi: 10.1084/jem.186.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, Lenz LL, Cado D, Riley LW, Winoto A. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877–89. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Dubska L, Andera L, Sheard MA. HER2 signaling downregulation by trastuzumab and suppression of the PI3K/Akt pathway: an unexpected effect on TRAIL-induced apoptosis. FEBS Lett. 2005;579:4149–58. doi: 10.1016/j.febslet.2005.06.047. [DOI] [PubMed] [Google Scholar]
- Duvic M, Vu J. Vorinostat: a new oral histone deacetylase inhibitor approved for cutaneous T-cell lymphoma. Expert Opin Investig Drugs. 2007;16:1111–20. doi: 10.1517/13543784.16.7.1111. [DOI] [PubMed] [Google Scholar]
- Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC, Young PR. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–7. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- Falschlehner C, Emmerich CH, Gerlach B, Walczak H. TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol. 2007;39:1462–75. doi: 10.1016/j.biocel.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Fulda S. Modulation of TRAIL-induced apoptosis by HDAC inhibitors. Curr Cancer Drug Targets. 2008;8:132–40. doi: 10.2174/156800908783769355. [DOI] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 2004;64:337–346. doi: 10.1158/0008-5472.can-03-1656. [DOI] [PubMed] [Google Scholar]
- Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002;21:2283–94. doi: 10.1038/sj.onc.1205258. [DOI] [PubMed] [Google Scholar]
- Garcia M, Jemal A, Ward EM, Center MM, Hao Y, Siegel RL, Thun MJ. Global Cancer Facts & Figures 2007. American Cancer Society; 2007. pp. 1–52. [Google Scholar]
- Gibson EM, Henson ES, Haney N, Villanueva J, Gibson SB. Epidermal growth factor protects epithelial-derived cells from tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by inhibiting cytochrome c release. Cancer Res. 2002;62:488–496. [PubMed] [Google Scholar]
- Gibson S, Tu S, Oyer R, Anderson SM, Johnson GL. Epidermal growth factor protects epithelial cells against Fas-induced apoptosis. Requirement for Akt activation. J Biol Chem. 1999;274:17612–17618. doi: 10.1074/jbc.274.25.17612. [DOI] [PubMed] [Google Scholar]
- Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998;161:2833–2840. [PubMed] [Google Scholar]
- Griffith TS, Rauch CT, Smolak PJ, Waugh JY, Boiani N, Lynch DH, Smith CA, Goodwin RG, Kubin MZ. Functional analysis of TRAIL receptors using monoclonal antibodies. J Immunol. 1999;162:2597–2605. [PubMed] [Google Scholar]
- Guseva NV, Rokhlin OW, Taghiyev AF, Cohen MB. Unique resistance of breast carcinoma cell line T47D to TRAIL but not anti-Fas is linked to p43cFLIP(L) Breast Cancer Res Treat. 2008;107:349–57. doi: 10.1007/s10549-007-9563-2. [DOI] [PubMed] [Google Scholar]
- Henson ES, Gibson EM, Villanueva J, Bristow NA, Haney N, Gibson SB. Increased expression of Mcl-1 is responsible for the blockage of TRAIL-induced apoptosis mediated by EGF/ErbB1 signaling pathway. J Cell Biochem. 2003;89:1177–92. doi: 10.1002/jcb.10597. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Aggarwal BB. TNF induces internalization of the p60 receptor and shedding of the p80 receptor. J Immunol. 1994;152:3550–8. [PubMed] [Google Scholar]
- Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A, Iacobucci A, MacLean M, Lo L, Fox NL, Oza AM. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:3450–5. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- Hyer ML, Croxton R, Krajewska M, Krajewski S, Kress CL, Lu M, Suh N, Sporn MB, Cryns VL, Zapata JM, Reed JC. Synthetic triterpenoids cooperate with tumor necrosis factor-related apoptosis-inducing ligand to induce apoptosis of breast cancer cells. Cancer Res. 2005;65:4799–808. doi: 10.1158/0008-5472.CAN-04-3319. [DOI] [PubMed] [Google Scholar]
- Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–34. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–5. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Jaattela M, Benedict M, Tewari M, Shayman JA, Dixit VM. Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene. 1995;10:2297–2305. [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Jeremias I, Debatin KM. TRAIL induces apoptosis and activation of NFkappaB. Eur Cytokine Netw. 1998;9:687–678. [PubMed] [Google Scholar]
- Jeremias I, Kupatt C, Baumann B, Herr I, Wirth T, Debatin KM. Inhibition of nuclear factor kappaB activation attenuates apoptosis resistance in lymphoid cells. Blood. 1998;91:4624–4631. [PubMed] [Google Scholar]
- Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamradt MC, Lu M, Werner ME, Kwan T, Chen F, Strohecker A, Oshita S, Wilkinson JC, Yu C, Oliver PG, Duckett CS, Buchsbaum DJ, LoBuglio AF, Jordan VC, Cryns VL. The small heat shock protein alpha B-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 2005;280:11059–66. doi: 10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- Keane MM, Ettenberg SA, Lowrey GA, Russell EK, Lipkowitz S. Fas expression and function in normal and malignant breast cell lines. Cancer Res. 1996;56:4791–4798. [PubMed] [Google Scholar]
- Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999;59:734–741. [PubMed] [Google Scholar]
- Keane MM, Rubinstein Y, Cuello M, Ettenberg SA, Banerjee P, Nau MM, Lipkowitz S. Inhibition of NF-kappaB activity enhances TRAIL mediated apoptosis in breast cancer cell lines. Breast Cancer Res Treat. 2000;64:211–219. doi: 10.1023/a:1006458407515. [DOI] [PubMed] [Google Scholar]
- Kelley RF, Totpal K, Lindstrom SH, Mathieu M, Billeci K, Deforge L, Pai R, Hymowitz SG, Ashkenazi A. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem. 2005;280:2205–12. doi: 10.1074/jbc.M410660200. [DOI] [PubMed] [Google Scholar]
- Kelley SK, Ashkenazi A. Targeting death receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol. 2004;4:333–9. doi: 10.1016/j.coph.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther. 2001;299:31–8. [PubMed] [Google Scholar]
- Kim IK, Jung YK, Noh DY, Song YS, Choi CH, Oh BH, Masuda ES, Jung YK. Functional screening of genes suppressing TRAIL-induced apoptosis: distinct inhibitory activities of Bcl-XL and Bcl-2. Br J Cancer. 2003;88:910–7. doi: 10.1038/sj.bjc.6600795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MA, Kim HJ, Jee HJ, Kim AJ, Bae YS, Bae SS, Yun J. Akt2, but not Akt1, is required for cell survival by inhibiting activation of JNK and p38 after UV irradiation. Oncogene. 2009;28:1241–7. doi: 10.1038/onc.2008.487. [DOI] [PubMed] [Google Scholar]
- Kohlhaas SL, Craxton A, Sun XM, Pinkoski MJ, Cohen GM. Receptor-mediated endocytosis is not required for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. J Biol Chem. 2007;282:12831–41. doi: 10.1074/jbc.M700438200. [DOI] [PubMed] [Google Scholar]
- Korsching E, Packeisen J, Agelopoulos K, Eisenacher M, Voss R, Isola J, van Diest PJ, Brandt B, Boecker W, Buerger H. Cytogenetic alterations and cytokeratin expression patterns in breast cancer: integrating a new model of breast differentiation into cytogenetic pathways of breast carcinogenesis. Lab Invest. 2002;82:1525–33. doi: 10.1097/01.lab.0000038508.86221.b3. [DOI] [PubMed] [Google Scholar]
- Lacroix M. MDA-MB-435 cells are from melanoma, not from breast cancer. Cancer Chemother Pharmacol. 2009;63:567. doi: 10.1007/s00280-008-0776-9. [DOI] [PubMed] [Google Scholar]
- Lagadec C, Adriaenssens E, Toillon RA, Chopin V, Romon R, Van Coppenolle F, Hondermarck H, Le Bourhis X. Tamoxifen and TRAIL synergistically induce apoptosis in breast cancer cells. Oncogene. 2008;27:1472–7. doi: 10.1038/sj.onc.1210749. [DOI] [PubMed] [Google Scholar]
- Lamhamedi-Cherradi SE, Zheng SJ, Maguschak KA, Peschon J, Chen YH. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL−/− mice. Nat Immunol. 2003;4:255–60. doi: 10.1038/ni894. [DOI] [PubMed] [Google Scholar]
- Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, Hillan K, Totpal K, DeForge L, Schow P, Hooley J, Sherwood S, Pai R, Leung S, Khan L, Gliniak B, Bussiere J, Smith CA, Strom SS, Kelley S, Fox JA, Thomas D, Ashkenazi A. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- Lee JM, Bernstein A. p53 mutations increase resistance to ionizing radiation. Proc Natl Acad Sci U S A. 1993;90:5742–6. doi: 10.1073/pnas.90.12.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Feig C, Tchikov V, Schickel R, Hallas C, Schutze S, Peter ME, Chan AC. The role of receptor internalization in CD95 signaling. Embo J. 2006;25:1009–23. doi: 10.1038/sj.emboj.7601016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–69. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- Ling J, Herbst RS, Mendelson DS, Eckhardt EG, O’Dwyer P, Ebbinghaus S, Osborne R, Cheu G, Lieberman B, Lum BL. Apo2L/TRAIL pharmacokinetics in a phase 1a trial in advanced cancer and lymphoma. J Clin Oncol. 2006;24:3047. [Google Scholar]
- Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, Perou CM. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264–71. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE, Housman DE, Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994;266:807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Lu M, Strohecker A, Chen F, Kwan T, Bosman J, Jordan VC, Cryns VL. Aspirin sensitizes cancer cells to TRAIL-induced apoptosis by reducing survivin levels. Clin Cancer Res. 2008;14:3168–76. doi: 10.1158/1078-0432.CCR-07-4362. [DOI] [PubMed] [Google Scholar]
- MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem. 1997;272:25417–20. doi: 10.1074/jbc.272.41.25417. [DOI] [PubMed] [Google Scholar]
- MacFarlane M, Kohlhaas SL, Sutcliffe MJ, Dyer MJ, Cohen GM. TRAIL receptor-selective mutants signal to apoptosis via TRAIL-R1 in primary lymphoid malignancies. Cancer Res. 2005;65:11265–70. doi: 10.1158/0008-5472.CAN-05-2801. [DOI] [PubMed] [Google Scholar]
- Mandlekar S, Kong AN. Mechanisms of tamoxifen-induced apoptosis. Apoptosis. 2001;6:469–77. doi: 10.1023/a:1012437607881. [DOI] [PubMed] [Google Scholar]
- Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67:167–77. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P, Ashkenazi A. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol. 1997;7:1003–6. doi: 10.1016/s0960-9822(06)00422-2. [DOI] [PubMed] [Google Scholar]
- Mayo MW, Wang CY, Cogswell PC, Rogers-Graham KS, Lowe SW, Der CJ, Baldwin AS., Jr Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- Moyano JV, Evans JR, Chen F, Lu M, Werner ME, Yehiely F, Diaz LK, Turbin D, Karaca G, Wiley E, Nielsen TO, Perou CM, Cryns VL. AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006;116:261–70. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara J, Watanabe FR, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Palacios C, Yerbes R, Lopez-Rivas A. Flavopiridol induces cellular FLICE-inhibitory protein degradation by the proteasome and promotes TRAIL-induced early signaling and apoptosis in breast tumor cells. Cancer Res. 2006;66:8858–69. doi: 10.1158/0008-5472.CAN-06-0808. [DOI] [PubMed] [Google Scholar]
- Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997a;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- Pan G, Ni J, Yu G, Wei YF, Dixit VM. TRUNDD, a new member of the TRAIL receptor family that antagonizes TRAIL signalling. FEBS Lett. 1998;424:41–5. doi: 10.1016/s0014-5793(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997b;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- Park SY, Seol DW. Regulation of Akt by EGF-R inhibitors, a possible mechanism of EGF-R inhibitor-enhanced TRAIL-induced apoptosis. Biochem Biophys Res Commun. 2002;295:515–518. doi: 10.1016/s0006-291x(02)00719-2. [DOI] [PubMed] [Google Scholar]
- Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA, Slamon DJ. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- Pegram MD, Slamon DJ. Combination therapy with trastuzumab (Herceptin) and cisplatin for chemoresistant metastatic breast cancer: evidence for receptor-enhanced chemosensitivity. Semin Oncol. 1999;26:89–95. [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–90. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, Barrett M, Judson I, Kaye S, Fox NL, Halpern W, Corey A, Calvert H, de Bono J. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–94. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- Rahman M, Davis SR, Pumphrey JG, Bao J, Nau MM, Meltzer PS, Lipkowitz S. TRAIL induces apoptosis in triple-negative breast cancer cells with a mesenchymal phenotype. Breast Cancer Res Treat. 2009;113:217–30. doi: 10.1007/s10549-008-9924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakha EA, Elsheikh SE, Aleskandarany MA, Habashi HO, Green AR, Powe DG, El-Sayed ME, Benhasouna A, Brunet JS, Akslen LA, Evans AJ, Blamey R, Reis-Filho JS, Foulkes WD, Ellis IO. Triple-negative breast cancer: distinguishing between basal and nonbasal subtypes. Clin Cancer Res. 2009;15:2302–10. doi: 10.1158/1078-0432.CCR-08-2132. [DOI] [PubMed] [Google Scholar]
- Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, Edwards BK, Berry DA. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–4. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- Ravi R, Bedi GC, Engstrom LW, Zeng Q, Mookerjee B, Gelinas C, Fuchs EJ, Bedi A. Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NF-kappaB. Nat Cell Biol. 2001;3:409–416. doi: 10.1038/35070096. [DOI] [PubMed] [Google Scholar]
- Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- Schmaltz C, Alpdogan O, Kappel BJ, Muriglan SJ, Rotolo JA, Ongchin J, Willis LM, Greenberg AS, Eng JM, Crawford JM, Murphy GF, Yagita H, Walczak H, Peschon JJ, van den Brink MR. T cells require TRAIL for optimal graft-versus-tumor activity. Nat Med. 2002;8:1433–7. doi: 10.1038/nm1202-797. [DOI] [PubMed] [Google Scholar]
- Schneider-Brachert W, Tchikov V, Neumeyer J, Jakob M, Winoto-Morbach S, Held-Feindt J, Heinrich M, Merkel O, Ehrenschwender M, Adam D, Mentlein R, Kabelitz D, Schutze S. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–28. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Schneider P, Bodmer JL, Thome M, Hofmann K, Holler N, Tschopp J. Characterization of two receptors for TRAIL. FEBS Lett. 1997a;416:329–334. doi: 10.1016/s0014-5793(97)01231-3. [DOI] [PubMed] [Google Scholar]
- Schneider P, Thome M, Burns K, Bodmer JL, Hofmann K, Kataoka T, Holler N, Tschopp J. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity. 1997b;7:831–836. doi: 10.1016/s1074-7613(00)80401-x. [DOI] [PubMed] [Google Scholar]
- Screaton GR, Mongkolsapaya J, Xu XN, Cowper AE, McMichael AJ, Bell JI. TRICK2, a new alternatively spliced receptor that transduces the cytotoxic signal from TRAIL. Curr Biol. 1997;7:693–696. doi: 10.1016/s0960-9822(06)00297-1. [DOI] [PubMed] [Google Scholar]
- Sedger LM, Glaccum MB, Schuh JC, Kanaly ST, Williamson E, Kayagaki N, Yun T, Smolak P, Le T, Goodwin R, Gliniak B. Characterization of the in vivo function of TNF-alpha-related apoptosis-inducing ligand, TRAIL/Apo2L, using TRAIL/Apo2L gene-deficient mice. Eur J Immunol. 2002;32:2246–54. doi: 10.1002/1521-4141(200208)32:8<2246::AID-IMMU2246>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Shankar S, Singh TR, Chen X, Thakkar H, Firnin J, Srivastava RK. The sequential treatment with ionizing radiation followed by TRAIL/Apo-2L reduces tumor growth and induces apoptosis of breast tumor xenografts in nude mice. Int J Oncol. 2004;24:1133–40. [PubMed] [Google Scholar]
- Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- Shetty S, Graham BA, Brown JG, Hu X, Vegh-Yarema N, Harding G, Paul JT, Gibson SB. Transcription factor NF-kappaB differentially regulates death receptor 5 expression involving histone deacetylase 1. Mol Cell Biol. 2005;25:5404–16. doi: 10.1128/MCB.25.13.5404-5416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MS, Kim HS, Lee SH, Park WS, Kim SY, Park JY, Lee JH, Lee SK, Lee SN, Jung SS, Han JY, Kim H, Lee JY, Yoo NJ. Mutations of tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1) and receptor 2 (TRAIL-R2) genes in metastatic breast cancers. Cancer Res. 2001;61:4942–6. [PubMed] [Google Scholar]
- Shrader M, Pino MS, Lashinger L, Bar-Eli M, Adam L, Dinney CP, McConkey DJ. Gefitinib reverses TRAIL resistance in human bladder cancer cell lines via inhibition of AKT-mediated X-linked inhibitor of apoptosis protein expression. Cancer Res. 2007;67:1430–5. doi: 10.1158/0008-5472.CAN-06-1224. [DOI] [PubMed] [Google Scholar]
- Sidi S, Sanda T, Kennedy RD, Hagen AT, Jette CA, Hoffmans R, Pascual J, Imamura S, Kishi S, Amatruda JF, Kanki JP, Green DR, D’Andrea AA, Look AT. Chk1 suppresses a caspase-2 apoptotic response to DNA damage that bypasses p53, Bcl-2, and caspase-3. Cell. 2008;133:864–77. doi: 10.1016/j.cell.2008.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RM, Muppidi JR, Sarker M, Lobito A, Jen M, Martin D, Straus SE, Lenardo MJ. SPOTS: signaling protein oligomeric transduction structures are early mediators of death receptor-induced apoptosis at the plasma membrane. J Cell Biol. 2004;167:735–44. doi: 10.1083/jcb.200406101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh TR, Shankar S, Chen X, Asim M, Srivastava RK. Synergistic interactions of chemotherapeutic drugs and tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand on apoptosis and on regression of breast carcinoma in vivo. Cancer Res. 2003;63:5390–400. [PubMed] [Google Scholar]
- Singh TR, Shankar S, Srivastava RK. HDAC inhibitors enhance the apoptosis-inducing potential of TRAIL in breast carcinoma. Oncogene. 2005;24:4609–23. doi: 10.1038/sj.onc.1208585. [DOI] [PubMed] [Google Scholar]
- Sitterding SM, Wiseman WR, Schiller CL, Luan C, Chen F, Moyano JV, Watkin WG, Wiley EL, Cryns VL, Diaz LK. AlphaB-crystallin: a novel marker of invasive basal-like and metaplastic breast carcinomas. Ann Diagn Pathol. 2008;12:33–40. doi: 10.1016/j.anndiagpath.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, Press MF. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- Spierings DC, de Vries EG, Vellenga E, van den Heuvel FA, Koornstra JJ, Wesseling J, Hollema H, de Jong S. Tissue distribution of the death ligand TRAIL and its receptors. J Histochem Cytochem. 2004;52:821–831. doi: 10.1369/jhc.3A6112.2004. [DOI] [PubMed] [Google Scholar]
- Suliman A, Lam A, Datta R, Srivastava RK. Intracellular mechanisms of TRAIL: apoptosis through mitochondrial-dependent and -independent pathways. Oncogene. 2001;20:2122–2133. doi: 10.1038/sj.onc.1204282. [DOI] [PubMed] [Google Scholar]
- Sun XM, MacFarlane M, Zhuang J, Wolf BB, Green DR, Cohen GM. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem. 1999;274:5053–5060. doi: 10.1074/jbc.274.8.5053. [DOI] [PubMed] [Google Scholar]
- Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- Teraishi F, Kagawa S, Watanabe T, Tango Y, Kawashima T, Umeoka T, Nisizaki M, Tanaka N, Fujiwara T. ZD1839 (Gefitinib, ‘Iressa’), an epidermal growth factor receptor-tyrosine kinase inhibitor, enhances the anti-cancer effects of TRAIL in human esophageal squamous cell carcinoma. FEBS Lett. 2005;579:4069–75. doi: 10.1016/j.febslet.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Thaile M, Labrinidis A, Hay S, Liapis V, Bouralexis S, Welldon K, Coventry BJ, Findlay DM, Evdokiou A. Apo2l/Tumor necrosis factor-related apoptosis-inducing ligand prevents breast cancer-induced bone destruction in a mouse model. Cancer Res. 2006;66:5363–70. doi: 10.1158/0008-5472.CAN-05-4386. [DOI] [PubMed] [Google Scholar]
- Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, Hill M, Mays T, McCoy T, Fox NL, Halpern W, Corey A, Cohen RB. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25:1390–5. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- Truneh A, Sharma S, Silverman C, Khandekar S, Reddy MP, Deen KC, McLaughlin MM, Srinivasula SM, Livi GP, Marshall LA, Alnemri ES, Williams WV, Doyle ML. Temperature-sensitive differential affinity of TRAIL for its receptors. DR5 is the highest affinity receptor. J Biol Chem. 2000;275:23319–25. doi: 10.1074/jbc.M910438199. [DOI] [PubMed] [Google Scholar]
- Tu S, McStay GP, Boucher LM, Mak T, Beere HM, Green DR. In situ trapping of activated initiator caspases reveals a role for caspase-2 in heat shock-induced apoptosis. Nat Cell Biol. 2006;8:72–7. doi: 10.1038/ncb1340. [DOI] [PubMed] [Google Scholar]
- Tyson FL, Boyer CM, Kaufman R, O’Briant K, Cram G, Crews JR, Soper JT, Daly L, Fowler WC, Jr, Haskill JS, et al. Expression and amplification of the HER-2/neu (c-erbB-2) protooncogene in epithelial ovarian tumors and cell lines. Am J Obstet Gynecol. 1991;165:640–646. doi: 10.1016/0002-9378(91)90300-g. [DOI] [PubMed] [Google Scholar]
- Umemura S, Takekoshi S, Suzuki Y, Saitoh Y, Tokuda Y, Osamura RY. Estrogen receptor-negative and human epidermal growth factor receptor 2-negative breast cancer tissue have the highest Ki-67 labeling index and EGFR expression: gene amplification does not contribute to EGFR expression. Oncol Rep. 2005;14:337–43. [PubMed] [Google Scholar]
- Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- van der Sloot AM, Tur V, Szegezdi E, Mullally MM, Cool RH, Samali A, Serrano L, Quax WJ. Designed tumor necrosis factor-related apoptosis-inducing ligand variants initiating apoptosis exclusively via the DR5 receptor. Proc Natl Acad Sci U S A. 2006;103:8634–9. doi: 10.1073/pnas.0510187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K, Huw L, Katta V, Cavet G, Hymowitz SG, Amler L, Ashkenazi A. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, Goodwin RG, Rauch CT. TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. Embo J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Willipinski-Stapelfeldt B, Riethdorf S, Assmann V, Woelfle U, Rau T, Sauter G, Heukeshoven J, Pantel K. Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res. 2005;11:8006–14. doi: 10.1158/1078-0432.CCR-05-0632. [DOI] [PubMed] [Google Scholar]
- Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol. 2009;10:348–55. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- Wu GS, Burns TF, McDonald ER, 3rd, Jiang W, Meng R, Krantz ID, Kao G, Gan DD, Zhou JY, Muschel R, Hamilton SR, Spinner NB, Markowitz S, Wu G, el-Deiry WS. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat Genet. 1997;17:141–143. doi: 10.1038/ng1097-141. [DOI] [PubMed] [Google Scholar]
- Yang X, Merchant MS, Romero ME, Tsokos M, Wexler LH, Kontny U, Mackall CL, Thiele CJ. Induction of caspase 8 by interferon gamma renders some neuroblastoma (NB) cells sensitive to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) but reveals that a lack of membrane TR1/TR2 also contributes to TRAIL resistance in NB. Cancer Res. 2003;63:1122–9. [PubMed] [Google Scholar]
- Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- Zerafa N, Westwood JA, Cretney E, Mitchell S, Waring P, Iezzi M, Smyth MJ. Cutting edge: TRAIL deficiency accelerates hematological malignancies. J Immunol. 2005;175:5586–90. doi: 10.4049/jimmunol.175.9.5586. [DOI] [PubMed] [Google Scholar]
- Zhang X, Silva E, Gershenson D, Hung MC. Amplification and rearrangement of c-erb B proto- oncogenes in cancer of human female genital tract. Oncogene. 1989;4:985–989. [PubMed] [Google Scholar]
- Zhang Y, Yoshida T, Zhang B. TRAIL induces endocytosis of its death receptors in MDA-MB-231 breast cancer cells. Cancer Biol Ther. 2008;8:917–922. doi: 10.4161/cbt.8.10.8141. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang B. TRAIL resistance of breast cancer cells is associated with constitutive endocytosis of death receptors 4 and 5. Mol Cancer Res. 2008;6:1861–71. doi: 10.1158/1541-7786.MCR-08-0313. [DOI] [PubMed] [Google Scholar]