Abstract
Background
Beat-to-beat alternation of the cardiac action potential (Vm-Alt) attributable to fluctuations in cellular calcium cycling (Ca-Alt) is a recognized mechanism of ventricular fibrillation (VF). Recently, we reported that SERCA2a, the pump responsible for re-uptake of cytosolic calcium during diastole, plays a central role in the molecular mechanism of cardiac alternans. Heart failure (HF) is associated with impaired myocardial calcium handling, deficient SERCA2a, and increased susceptibility to cardiac alternans. Therefore, we hypothesized that restoring deficient SERCA2a by gene transfer will significantly reduce arrhythmogenic cardiac alternans in the failing heart.
Methods and Results
Adult guinea pigs that were divided into 3 groups: 1) Control, 2) HF by thoracic aorta banding, and 3) HF + SERCA2a gene transfer using a cardiotropic vector (AAV9.SERCA2a) delivered by intra-coronary methods 6±2 months after aortic banding but 6 weeks prior to all measurements. Electrophysiological studies were performed in isolated myocytes and Langendorff-perfused hearts using standard patch clamp and optical mapping techniques, respectively. HF resulted in a decrease in LV fractional shortening (25±2%) compared to controls (50±2%, p<0.001). As expected, isolated HF myocytes demonstrated slower SR calcium uptake (361±30 ms vs 243±21 ms, p=0.05), decreased Ca2+ release (182±20 nM vs 368±73 nM, p< 0.05) and increase diastolic Ca2+ (333±37 nM vs 229±26 nM, p<0.05) compared to controls. Moreover, SERCA2a, RyR2 and NCX protein expression were decreased in HF when compared to control (p<0.05). As predicted, HF increased susceptibility to cardiac alternans as evidenced by decreased heart rate thresholds for both Vm-ALT (290±12 bpm vs. 388±8 bpm, p<0.01) and Ca-ALT (230±15 bpm vs 370±13 bpm, p<0.01) compared to controls. In vivo gene transfer of AAV9.SERCA2a increased LV fractional shortening and SERCA2a protein expression compared to HF alone (p<0.01). Importantly, targeted restoration of SERCA2a overexpression in HF reduced cardiac alternans (APD-ALT threshold – HF + SERCA2a: 430 bpm, vs HF: 290 bpm, and Control: 393 bpm; p<0.01) and susceptibility to inducible ventricular arrhythmias (50% vs 100% and 100%, respectively; p=0.05) compared to both HF alone and control.
Conclusions
These data provide new evidence that in HF reduced expression of a single calcium handling protein, SERCA2a modulates susceptibility to arrhythmogenic cardiac alternans. Moreover, therapies targeting SERCA2a can improve contractile dysfunction and restore electrical stability in HF.
Keywords: alternans; action potentials; intracellular calcium; adenoviral gene transfer, repolarization, arrhythmia
INTRODUCTION
Sudden arrhythmic death is one of the most devastating manifestations of heart failure (HF) yet, the complex sequence of events responsible for the development of fatal ventricular arrhythmias in HF is poorly understood. The majority of sudden arrhythmic death occurs in the setting of contractile dysfunction, yet the precise mechanistic relationship linking mechanical cardiac failure and electrical instability remains elusive. Consequently, current anti-arrhythmic strategies are either palliative 1, 2, temporizing3, or completing ineffective 4, in part because they fail to target specific arrhythmia mechanisms. One postulated mechanisms for arrhythmogenesis in HF is repolarization alternans. There is compelling evidence that subtle beat-to-beat alternation of repolarization (i.e. T wave alternans) is an important marker of arrhythmic risk in HF 5. Moreover, absence of T wave alternans in patients with HF appears to protect against ventricular arrhythmias6. At the tissue level, repolarization alternans serves to amplify heterogeneities of repolarization required for conduction block and VF 7. At the cellular level, T wave alternans arises from alternation of action potential duration (APD-ALT) secondary to alternation in cellular calcium cycling (Ca-Alt)8-10.
It is postulated that abnormal myocardial calcium cycling is a common mechanism linking contractile and electrophysiological dysfunction. For example, HF is associated with significant blunting of sarcoplasmic reticulum (SR) Ca2+ reuptake secondary to decreased SERCA2a expression/function. Our laboratory and others have shown that SERCA2a plays a central role in the molecular mechanism of cardiac alternans in the normal heart11, 12. Moreover, we recently demonstrated that impaired SR Ca2+ reuptake in a rapid pacing model of HF was associated with enhanced susceptibility to cardiac alternans13. Therefore, it would be predicted that therapies targeting improved SERCA2a expression/function could improve both mechanical and electrophysiological function in HF. This possibility is particularly attractive because current therapies designed to enhance contractile function have been associated with increased arrhythmogenesis.
There is accumulating evidence that gene therapy targeting increased expression of SERCA2a improves mechanical function in a variety of animal models of HF 14-16 and more recently in human clinical trials 17. Moreover, we recently demonstrated that targeted SERCA2a gene transfer in the normal heart reduces cardiac alternans and alternans-mediated arrhythmogenesis 11. However, the effect of restoring SERCA2a expression to normal levels on cardiac alternans in the setting of HF is unknown. Therefore, in the present study we tested the hypothesis that in vivo gene therapy targeting deficient SERCA2a will improve contractile function and significantly reduce arrhythmogenic cardiac alternans in the failing heart.
MATERIALS AND METHODS
Pressure-Overload Model of HF in the Guinea Pig
Experiments were carried out in accordance with the United States Public Health Service guidelines for the care and use of laboratory animals. Chronic pressure overload was produced in young male guinea pigs weighing 225-275g by subtotal descending thoracic aortic banding as previously described18, 19. Briefly, animals were anesthetized (Ketamine, Xylazine, Acepromazine and Atropine) and mechanically ventilated (2.0 cc tidal volume at 50 cycles per minute) via a tracheostomy (18 g tube). Lateral thoracotomy was performed and the descending thoracic aorta was isolated. Constriction around the descending thoracic aorta was produced by tying 2-0 surgical silk ligature tightly around a 6-mm length of hypodermic tubing having an external diameter of 1.24 mm. The tubing was withdrawn from the ligature, the chest closed and intrathoracic air evacuated. Animals were extubated upon spontaneous breathing and closely observed until fully awake. In a subset of animals sham operations were performed using a similar procedure with the exception of ligature placement. Aortic-banded and shamo-perated animals were housed and fed under identical conditions for an average of 27±2 months. Echocardiography was performed using a Phillips iE33 Ultrasound System with a S12-4 sector array ultrasound probe immediately prior to terminal electrophysiological studies. Incidence of sudden death, defined as any death that occurred suddenly (i.e. <12 hrs since last observed alive and in no distress) greater than 2 wks post-op, was monitored in all animals.
In vivo gene delivery
Recombinant adeno-associated virus (serotype 9) vectors carrying SERCA2a cDNA with a CMV promoter (AAV9.SERCA2a) were used. In vivo gene transfer was performed using a catheter based procedure. In particular, animals were anesthetized (Ketamine, Xylazine, Acepromazine and Atropine) and mechanically ventilated (2.0 cc tidal volume at 50 cycles per minute) via a tracheostomy (18 g tube). A small anterior thoracotomy was performed and the exposing the left ventricular apex. A 27 gauge catheter was advanced from the apex to the aortic root. Subsequently, the virus solution (5 × 1011 viral genomes/animal of AAV9.SERCA2a diluted to a total volume of 1 mL) was slowly injected. The animals were placed on a heating pad (42°C), the chest closed and intrathoracic air evacuated. Animals were extubated upon spontaneous breathing and closely observed until fully awake. Terminal electrophysiological studies were performed six weeks following in vivo gene transfer.
Protein expression
Tissue was obtained from the left ventricular free wall from control, HF and HF + AAV9.SERCA2a (SERCA2a expression only). Western blotting was performed to determine the relative expression levels of cardiac ryanodine receptor (RyR2), sarcoplasmic reticulum Ca2+ ATPase (SERCA2), sarcolemmal Na/Ca exchanger (NCX), phospholamban (PLB) and L-type Ca2+ channel (LTCC). Cardiac homogenates were separated on SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. Blots were probed with the following primary antibodies, respectively: mouse anti-RyR2, mouse anti-NCX, and mouse anti-PLB (all three from Affinity Bioreagents Inc.), mouse anti-Cav1.2 Ca2+ channel clone L57-46(UC Davis/NIH NeuroMab Facility), rabbit anti-SERCA (Dr. Periasamy, Ohio State University) and polyclonal anti-rabbit RyR-PS2814 (Dr. Wehrens, Baylor College of Medicine). They were then treated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (Amersham). Protein bands were quantitated using ImageQuant software.
Isolated myocyte studies
In a subset of HF and control animals isolated myocyte studies were performed. The amphotericin perforated patch technique was used to obtain whole-cell recordings of membrane voltage under current clamp conditions and intracellular Ca2+ transients were simultaneously measured using the fluorescent Ca2+ indicator indo-1AM, as described previously 11, 20, 21.
Whole heart studies
Whole heart electrophysiological studies were performed in control, HF alone and HF + AAV9.SERCA2a hearts. Isolated hearts were Langendorff perfused with oxygenated (95% O2, 5% CO2) Tyrode’s solution (in mmol/L: NaCl 130, NaHCO3 25.0, MgSO4 1.2, KCl 4.75, dextrose 5.0, CaCl2 1.25, pH 7.40, 32°C. Dual calcium and voltage mapping was performed using high resolution optical mapping. Specifically, optical action potentials and calcium transients were simultaneously measured from the anterior surface of the left ventricle using the voltage sensitive dye di-4-ANEPPS (15 μmol/L) and the calcium sensitive dye Indo-1AM, as previously described10. Cardiac alternans was induced by stepwise decrements (10 ms) in pacing cycle length (CL) but was not measured until 30 seconds after the decrement in rate to assure its stability. CL was decremented until failure to capture the preparation or the development of a ventricular arrhythmia. Spontaneous diastolic SR Ca2+ release (a marker of RyR Po) in the whole heart22, 23 was measured as the maximum derivative of the optical Ca2+ tracing (dCa/dt max) using computer software. These measurements were made during diastole, immediately following the last paced beat (CL 130-150 ms). For each heart studied, the slope of the optical Ca2+ tracing was calculated from 256 separate myocyte clusters.
Data analysis
Isolated myocytes
Action potential duration (APD) was measured at 90% repolarization (APD90). Ca2+ transient parameters were defined as described previously 24, 25: Diastolic Ca2+ was defined as cytosolic Ca2+ level just prior to the onset of the Ca2+ transient or just prior to the action potential upstroke in the cases where there was no obvious Ca2+ transient. Amplitude of intracellular Ca2+ transient was calculated from the difference between peak and diastolic Ca2+. The rate of reuptake of intracellular Ca2+, the decay portion of the Ca2+ transient (from 30% to 100% of decline phase) was fit to a single exponential function whose time constant, τ, was used to measure Ca2+ decay.
Whole heart
APD alternans (APD-ALT) was defined as the difference in APD between two consecutive beats. Similarly, Ca alternans (Ca-ALT) was defined as the difference in Ca2+ transient amplitude between two consecutive beats. The alternans-HR relation was plotted as the magnitude of APD-ALT or Ca-ALT as a function of HR. A leftward or rightward shift in this relation (i.e. development of alternans at lower or higher HRs) indicates greater or reduced susceptibility to alternans, respectively. The slowest HR that induces stable APD alternans of >10 ms and stable Ca2+ alternans of >10% were defined as the threshold HR’s for alternans 10, 26. Activation time, repolarization time, APD and Ca2+ transient amplitude were measured from optically recorded action potentials and Ca2+ transients using methods that have been validated extensively. Arrhythmia susceptibility was determined using a ramp pacing protocol starting at 300 ms (200 bpm) with stepwise 10 ms decrements in pacing CL until failure of 1/1 capture or the induction of a ventricular arrhythmia. This arrhythmia induction protocol has previously been validated in our laboratory to assess alternans mediated arrhythmogenesis and has similarities to the clinical test for alternans (T-wave alternans test) which increases heart rate in a ramp like fashion by exercise and is, thus, a good correlate to our ramp pacing protocol. Pacing was performed at each CL for 1-2 min to ensure steady state. Arrhythmias were defined as a tachyarrhythmia that is sustained for greater than 30 sec after pacing was halted. The incidence of sudden death was monitor throughout the duration of the study. Sudden death was defined as an unexpected death of an animal that had been observed to be clinically stable within 12 hours being found dead. As continuous telemetry monitoring was not performed it is not possible to define the exact mode of death (i.e. tachyarrhythmia) in these animals.
Statistical analysis
Statistical analysis of data was performed using Sigmastat (SPSS Inc., Chicago, Illinois, U.S.A.). Normality was tested using the Shapiro Wilks test and by visual inspection of the distribution of all data sets. Homogeneity of variance was assessed using the Levene’s test. When data were found to have failed the homogeneity of variance assumption, the Wilcoxon rank sum test was performed in place of the Student t-test or the Kruskal-Wallis test in place of the ANOVA. Statistical differences were assessed with ANOVA, Student T-test, Wilcoxon Rank Sum test, Kruskal-Wallis test and Chi Square test as appropriate. All data are expressed as mean ± standard error of the mean (SEM).
RESULTS
Guinea pig model of pressure-overload heart failure
Model Characteristics
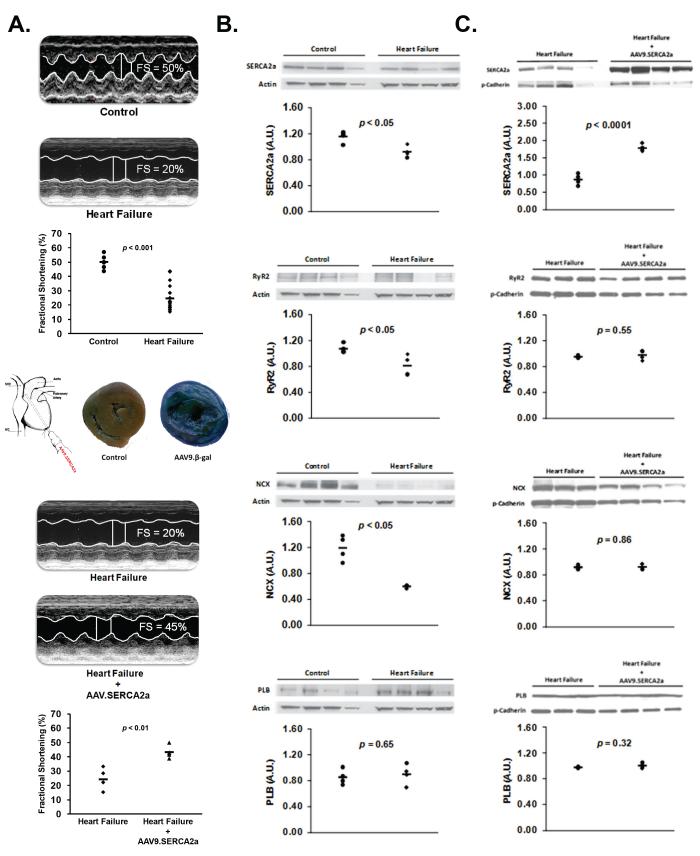
Table 1 demonstrates characteristics of the guinea pig thoracic aortic banded model of heart failure. Consistent with the development of systolic HF, aortic-banding (n=12) increased heart weight, body/heart weight ratio, and end-diastolic diameter compared to sham controls (n=7) (p<0.01). Also, action potential duration (APD) in the intact heart was prolonged in heart failure compared to sham control (p<0.05). Figure 1a, top panel illustrates representative M-mode echocardiographic tracings comparing sham control and aortic banded animals. Fractional shortening decreased 27±2 weeks following aortic banding compared to sham controls (p<0.001), reaffirming the development of HF.
Table 1.
Pressure-overload heart failure – Model characteristics. Data presented as mean ± SEM.
| Control (n = 7) |
Heart Failure (n = 12) |
p value | |
|---|---|---|---|
| Males (%) | 100 | 100 | NS |
| Body Weight (gm) | 1121 ± 40 | 996 ± 36 | 0.02 |
| Heart Weight (gm) | 4.1 ± 0.1 | 6.3 ± 0.4 | 0.002 |
| Body/Heart Weight (mg) | 3.7 ± 0.2 | 6.4 ± 0.4 | <0.001 |
| Lung Weight (gm) | 4.6 ± 0.3 | 4.6 ± 0.3 | NS |
| End-Diastolic Diameter (mm) | 6.6 ± 0.4 | 9.8 ± 0.4 | 0.002 |
| End-Systolic Diameter (mm) | 3.3 ± 0.3 | 7.6 ± 0.4 | <0.0001 |
| Fractional Shortening (%) | 50 ± 2 | 24 ± 2 | <0.001 |
| Action Potential Duration (ms) | 176 ± 4 | 188 ± 4 | 0.04 |
Figure 1. Pressure-overload heart failure – Functional characteristics.
Panel A (Top): Representative M-mode echocardiographic tracings comparing sham control (n=7) and aortic banded (n=12) animals. (Middle) Example of X-gal–stained cross section of guinea pig ventricles excised 6 weeks after AAV9.β-gal exposure showing relatively homogenous gene delivery. (Bottom) Representative M-mode echocardiographic tracings comparing left ventricular fractional shortening before and 6 weeks after AAV9.SERCA2a gene transfer in heart failure (n=4). Panel B: Example of protein expression of key Ca2+ cycling proteins in heart failure (n=4) and control (n=4). p values derived from Student t-test (SERCA2a and PLB) and Wilcoxon Rank Sum test (RyR2 and NCX). Panel C: Example of protein expression of key Ca2+ cycling proteins in heart failure (n=4) and heart failure + AAV9.SERCA2a gene transfer (n=4). p values derived from Student t-test (SERCA2a, NCX and PLB) and Wilcoxon Rank Sum test (RyR2).
Isolated Myocytes
Heart failure was further confirmed in isolated myocytes measuring intracellular Ca2+ transients. As expected, isolated HF myocytes (n=5) demonstrated slower SR calcium uptake (361±30 ms vs 243±21 ms, p=0.05), decreased Ca2+ release (182±20 nM vs 368±73 nM, p<0.05) and increase diastolic Ca2+ (333±37 nM vs 229±26 nM, p<0.01) compared to controls (n=5).
Protein Expression
Figure 1b, shows western blots of key Ca2+ handling proteins from aortic-banded (n=4) and sham control (n=4) hearts. As shown, HF was associated with decreased SERCA2a, LTCC (data not shown), RyR2 and NCX protein expression compared to control (p<0.02). PLB protein expression was unchanged by HF (p=0.65).
Effect of SERCA2a gene transfer on contractile function and Ca2+ cycling protein proteins in pressure-overload heart failure
In vivo gene transfer of AAV9.SERCA was performed using an intracoronary delivery method and produced relatively homogenous whole heart gene transfer. For example, Figure 1a, middle panel shows a X-gal stained cross section of guinea pig ventricles excised 6 weeks after AAV9.β-gal exposure compared to an X-gal stained untreated, control heart. Figure 1a, bottom panel illustrates that AAV9.SERCA2a gene transfer in HF improved left ventricular fractional shortening compared to HF prior to undergoing in vivo gene transfer (n=4; p<0.01). Also, Figure 1c shows protein expression of key Ca2+ cycling proteins, 6 weeks following in vivo gene transfer of AAV9.SERCA2a in HF animals and HF animals that did not undergo in vivo gene transfer. Our results demonstrate that AAV9.SERCA2a (n=4) gene transfer in HF significantly increased SERCA2a protein expression but did not change expression of NCX, RyR2, PLB or LTCC (data not shown) in the left ventricular free wall, compared to HF alone (n=4).
Effect of SERCA2a gene transfer on susceptibility to cellular alternans in pressure-overload heart failure
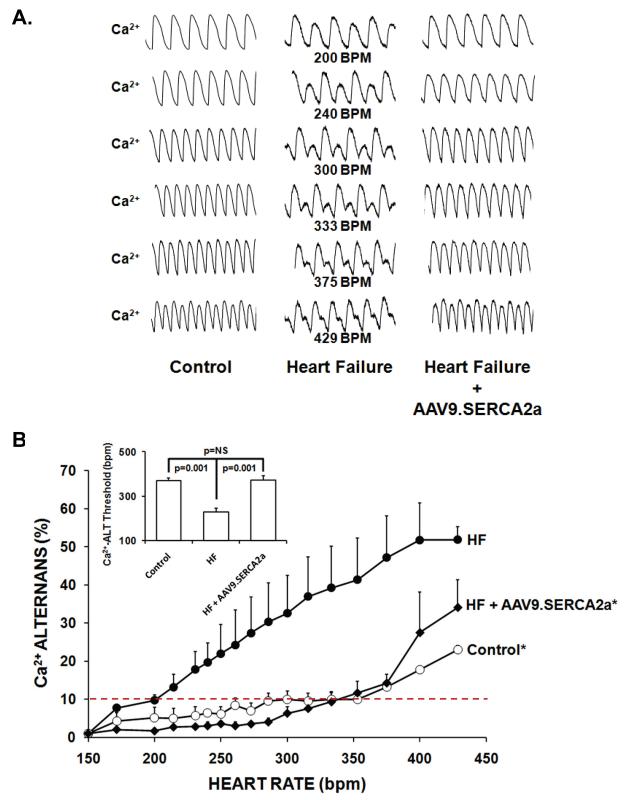
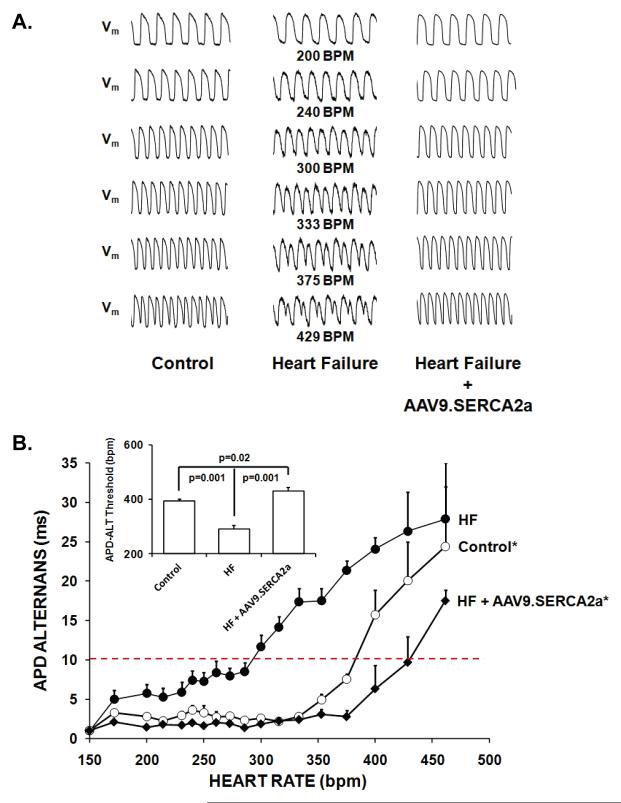
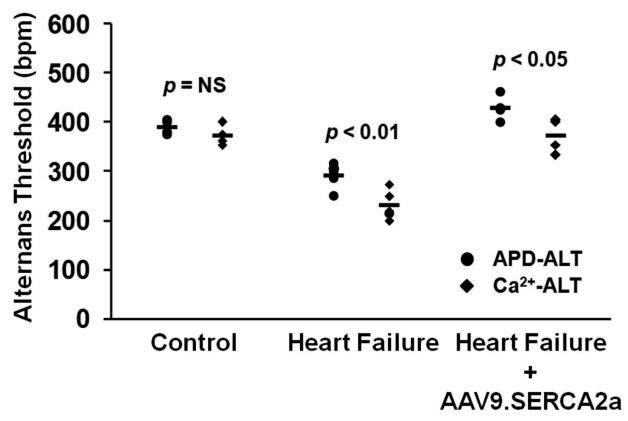
Ca2+ transient alternans (Ca-ALT) and action potential duration alternans (APD-ALT) were measured as pacing rate was progressively increased in the Langendorff perfused whole heart. Figures 2a and 3a show a representative example of optical calcium transients and action potential tracings from control, HF and HF + AAV9.SERCA2a hearts over a range of pacing rates. As illustrated, HF substantially enhanced susceptibility to alternans as the Ca-ALT and APD-ALT appeared at slower heart rates in HF compared to sham controls. Moreover, treatment with SERCA2a overexpression in the failing heart markedly reduced both Ca-ALT and APD-ALT compared to heart failure and was similar or even slightly improved compared to control hearts. Also, plots of pacing rate versus magnitude of Ca-ALT (Figure 2b) and APD-ALT (Figure 3b) shows that alternans magnitude increased as pacing rate increased, and the magnitude of APD-ALT and Ca-ALT was consistently greater in HF (n=5) compared with sham control hearts (n=6). Importantly, SERCA2a overexpression in HF markedly increased the HR threshold (Figures 2b and 3b, inset) APD-ALT and Ca-ALT compared to HF alone (p=0.001). Interestingly, Ca-ALT susceptibility in HF with AAV9.SERCA2a treatment was similar to control yet, APD-ALT susceptibility was modestly decreased even compared to non-failing control hearts (p=0.02). Surprisingly, in control hearts the heart rate threshold for the onset of Ca-ALT and APD-ALT were similar, however in HF hearts Ca-ALT developed at a significantly slower HR than APD-ALT developed (Figure 4; p<0.01) and this disparity persisted even after AAV9.SERCA2a treatment (p<0.05).
Figure 2. AAV9.SERCA2a gene transfer reverses heart failure-induced increases in Ca alternans.
Panel A: Representative optical action potentials over a range of pacing rates from a sham control, heart failure and heart failure + AAV9.SERCA2a. Panel B: Plot of pacing rate versus magnitude of Ca-ALT shows that alternans magnitude increased as pacing rate increased, and the magnitude of APD-ALT and Ca-ALT was consistently greater in HF (n=5) compared with sham control hearts (n=6). SERCA2a overexpression in HF markedly increased the HR threshold (See inset) and decreased the magnitude Ca-ALT compared to HF alone and was similar to control. *p<0.01 by ANOVA.
Figure 3. AAV9.SERCA2a gene transfer reverses heart failure-induced increases in APD alternans.
Panel A: Representative optical action potentials over a range of pacing rates from a control and heart failure and heart failure + AAV9.SERCA2a. Panel B: Plot of pacing rate versus magnitude of APD-ALT shows that alternans magnitude increased as pacing rate increased, and the magnitude of APD-ALT was consistently greater in HF (n=5) compared with sham control hearts (n=6). SERCA2a overexpression in HF markedly increased the HR threshold (See inset) and decreased the magnitude APD-ALT compared to HF alone and was modestly decreased even compared to non-failing control hearts. *p<0.01 by ANOVA
Figure 4. Relationship of Ca-ALT and APD-ALT thresholds.
In control hearts (n=6) the HR threshold for Ca-ALT and APD-ALT were similar. In contrast, Ca-ALT developed at a significantly slower heart rate than APD-ALT in the failing heart (n=5). This disparity between the onset of Ca-ALT and APD-ALT persist in the failing heart following treatment with AAV9.SERCA2a (n=4). p values derived from Student t-test.
Modulation of alternans-mediated arrhythmogenesis by SERCA2a gene transfer
Previously, we showed that SERCA2a gene transfer in young healthy guinea pigs can suppress cardiac alternans and reduce alternans-mediated arrhythmogenesis 11. In that study, we reported a 100% incidence of pacing-induced ventricular arrhythmias in healthy control hearts. In the current study all control and HF hearts developed pacing-induced ventricular arrhythmias and there was no clear difference in the pacing rate in which these arrhythmias developed. This is likely in part, secondary to the aggressiveness of the ramp pacing protocol utilized in these studies. However, there was a 22.7% incidence of sudden death in HF animals compared to 0% in control and HF + AAV9.SERCA2a animals (p=0.05), suggesting an increase in arrhythmia susceptibility in heart failure animals.
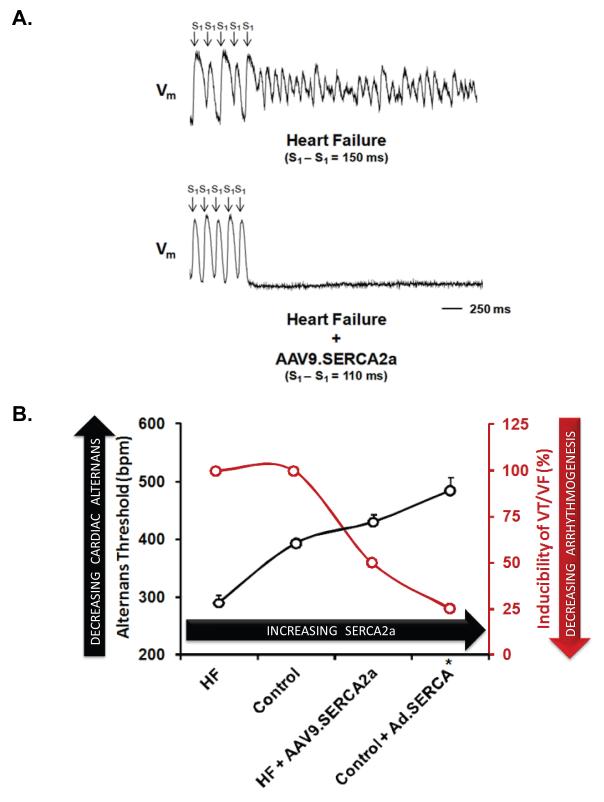
Figure 5a demonstrates the effect of SERCA2a gene transfer on arrhythmogenesis in the failing heart. Demonstrated is an optically recorded action potential tracing from a HF heart paced at a CL of 150 ms. Notice the marked APD alternans immediately preceding the onset of ventricular fibrillation. In contrast, in a failing heart treated with SERCA2a gene transfer paced at an even faster cycle length (110 ms) there was negligible APD alternans and failure to induce an arrhythmia. On average, SERCA2a overexpression in the failing heart produced a 50% reduction in alternans-mediated ventricular arrhythmias (p<0.05).
Figure 5. Modulation of alternans-mediated arrhythmogenesis by SERCA2a gene transfer.
Panel A: The effect of SERCA2a gene transfer on arrhythmogenesis in the failing heart. Demonstrated is an optically recorded action potential tracing from a HF heart paced at a CL of 150 ms. Notice the marked APD alternans immediately preceding the onset of ventricular fibrillation. In contrast, in a failing heart treated with SERCA2a gene transfer paced at an even faster cycle length (110 ms) there was negligible APD alternans and failure to induce an arrhythmia. Panel B: Plotted is alternans susceptibility as measured by alternans thresholds from 4 experimental groups: HF (n=6), Non HF control (n=10, HF + SERCA2a (n=4) and healthy control + SERCA2a (n=4; previously reported11) hearts, plotted in order of increasing SERCA2a expression. Increasing SERCA2a results in a progressive reduction in cardiac alternans as demonstrated by an increased alternans HR threshold (p<0.01 by ANOVA). Moreover, as alternans HR threshold increases there is a concomitant decrease in arrhythmia susceptibility(p=0.05 by Kruskal-Wallis test).
Figure 5b demonstrates the central role of SERCA2a in modulating alternans-mediated arrhythmogenesis. Plotted is alternans susceptibility as measured by alternans thresholds from 4 experimental groups: HF, Non HF control, HF + SERCA2a and healthy control + SERCA2a (previously reported11) hearts, plotted in order of increasing SERCA2a expression. It is evident, that with increasing SERCA2a there is a progressive reduction in cardiac alternans as demonstrated by an increased alternans HR threshold. Moreover, as alternans HR threshold increases with increasing SERCA2a there is a concomitant decrease in arrhythmia susceptibility, highlighting the role of SERCA2a in modulating arrhythmogenic cardiac alternans.
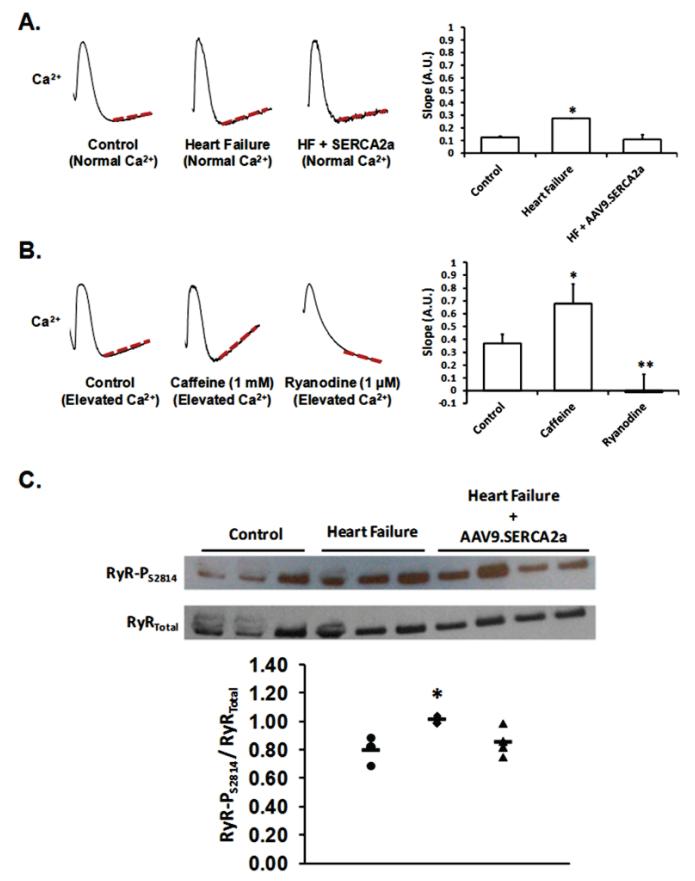
It is possible, that SERCA2a gene delivery has an influence on calcium-mediated arrhythmia substrates other than decreased cardiac alternans. Therefore, we assessed the effect of SERCA2a gene transfer on spontaneous diastolic SR Ca2+ release (a marker of RyR Po) in the whole heart22, 23. Specifically, we measured the slope of optically-recorded Ca2+ tracings during diastole, immediately following the last paced beat (CL 130-150 ms). As predicted, we found increased spontaneous diastolic SR Ca2+ release (increased RyR Po) in heart failure when compared to control (Figure 6a, p<0.01). Interestingly, SERCA2a gene transfer in HF decreased spontaneous diastolic SR Ca2+ release when compared to HF alone (Figure 6a, p<0.01) and was similar to control. For comparison, in normal hearts we observed a similar increase and decrease in spontaneous diastolic SR Ca2+ in response to treatment with caffeine and ryanodine, respectively (Figure 6b, p<0.01). Finally, CaMKII mediated RyR phosphorylation (RyR-PS2814) was found to be increased in HF when compared to control and SERCA2a gene transfer in HF reduced RyR-PS2814 to near control levels. These data suggest that SERCA2a gene transfer decreased RyR Po secondary to a reduction in RyR-PS2814.
Figure 6. AAV9.SERCA2a gene transfer reverses increased RyR2 Po in heart failure.
Panel A. Measurement of the slope of optically-recorded Ca2+ tracings during diastole, immediately following the last paced beat (CL 130-150 ms) as a marker of spontaneous diastolic SR Ca2+ release (i.e. RyR Po). As demonstrated, spontaneous diastolic SR Ca2+ release increased (i.e. increased RyR Po) in heart failure when compared to control. SERCA2a gene transfer in HF decreased spontaneous diastolic SR Ca2+ release when compared to HF alone and was similar to control. Panel B: In normal hearts, we observed a similar increase and decrease in spontaneous diastolic SR Ca2+ in response to treatment with caffeine (increasing RyR Po) and ryanodine (decreasing RyR2 Po), respectively. *p<0.01 by ANOVA. Panel C: CaMKII RyR phosphorylation (RyR-PS2814) in control (n=3), heart failure (n=3) and heart failure + AAV9.SERCA2a gene transfer (n=4). As shown, RyR-PS2814 increased in HF when compared to control and AAV9.SERCA2a gene transfer in the failing heart decreased RyR-PS2814 (p<0.05 by ANOVA).
DISCUSSION
Our studies provide convincing evidence that dysfunctional SR Ca2+ cycling is a common mechanism linking contractile and electrophysiological dysfunction in the failing heart and that therapy targeting key SR Ca2+ cycling proteins (i.e. SERCA2a) can restore both contractile function and electrical stability. We show that SERCA2a gene transfer in the failing heart not only improves contractile function but it directly restores electrical stability through the amelioration of key arrhythmogenic substrate (i.e. cardiac alternans) and triggers (i.e. SR Ca2+ leak). More specifically, the mitigation of cardiac alternans by selective SERCA2a gene transfer indicates that SERCA2a is linked to this key “mechanism” of electrical instability in the failing heart. Additionally, we found that SERCA2a gene transfer reduced RyR2 Po (i.e. SR Ca2+ leak) secondary to reduced RyR-PS2814 thus, suppressing a key trigger of ventricular arrhythmias in HF. The fact that we demonstrated complete recovery of susceptibility to alternans and SR Ca2+ leak to pre-HF levels with a concomitant reduction in arrhythmias following restoration of SERCA2a illustrates that regardless of HFs numerous impacts on various arrhythmia processes, SERCA2a gene delivery is a viable approach for electrically stabilizing the failing heart. Finally, this study also highlights the attractiveness of therapies targeting SERCA2a in HF because of the dual effect of enhanced SERCA2a on both contractile and electrical function.
SERCA2a underlies mechanism of cardiac alternans in heart failure
In the present study we show that abnormal SR Ca2+ cycling and more specifically, that impaired SERCA2a is a key mechanism of cardiac alternans in HF. This is consistent with the calcium cycling hypothesis, which states that alternans occurs when HR exceeds the capacity of the myocyte to cycle calcium10, 25, 27, 28. Based on the findings of the present study it is postulated that reduced SERCA2a protein expression in HF limits the ability of the SR to cycle Ca2+ thus, preventing complete SR reclamation of cytosolic Ca2+ above a critical HR threshold. Hence, cytosolic Ca2+ is reclaimed on an every-other-beat basis resulting in alternating Ca2+ transients. In the present study, this hypothesis is supported by the observation that selective enhancement of SERCA2a expression in HF reduced susceptibility to alternans and are consistent with earlier findings that SERCA2a is a key molecular mechanism in the development of cardiac alternans in cardiac myocyte monolayers and the intact healthy heart 11, 12.
Recently, it was demonstrated that decreased Ca2+ transient restitution in the spontaneously hypertensive rats model of heart failure when compared to control29, 30. It was postulated that this change in Ca2+ transient restitution is an important mechanism in increasing Ca-ALT in HF. Since the rate of recovery of SR Ca2+ release is a primary determinant of Ca2+ transient restitution; it is predicted that enhanced SERCA2a expression/function would increase Ca2+ transient restitution and therefore reduce Ca-ALT. Therefore, it is possible that the reduction in Ca-ALT susceptibility reported in the present study following SERCA2a gene transfer is secondary to the effect of increased SERCA2a on Ca2+ transient restitution. Further studies are needed to investigate this postulate.
Though we demonstrate with a high degree of specificity that SERCA2a directly affects susceptibility to cardiac alternans in HF, these data do not rule out other synergistic or complementary molecular mechanisms. For example, as demonstrated in the present study, HF is associated with remodeling of multiple Ca2+ cycling proteins.31-33 It has previously been demonstrated that instabilities of SR Ca2+ release can also lead to Ca-ALT. For example, Huser et al.31 demonstrated that refractory-like properties of RyR can produce alternating RyR Po and therefore Ca-ALT, irrespective of SR Ca load. Interestingly, our studies demonstrate that SERCA2a gene transfer in HF decreased RyR2 Po secondary to reduced RyR-PS2814 without altering RyR2 expression. This implies that in addition to improving SR Ca2+ reuptake, SERCA2a improves the stability of RyR2, suggesting a dual mechanism by which SERCA2a gene transfer ameliorates Ca-ALT in the failing heart.
It has been suggested that Ca2+ transient amplitude directly affects APD via Ca2+-sensitive currents such as NCX and L-type Ca2+ current. Thus, it is predicted that a reduction in NCX will decrease the Ca2+ to APD gain resulting a disparity between the magnitudes of Ca- and APD-ALT. In the present study, NCX protein expression was consistently reduced in HF and in HF + SERCA2a gene transfer and there was an accompanying disparity between the onset of Ca-ALT and APD-ALT in HF and HF + SERCA2a hearts that was not present in control hearts. For example, in control hearts the HR thresholds for Ca-ALT and APD-ALT were similar (i.e. 370±13 vs 388±8 bpm; p = NS). In contrast, in HF and HF + SERCA2a there was a disparity between the HR thresholds Ca-ALT and APD-ALT (i.e. 230±12 vs 290±12 bpm and 372±20 vs 430±13 bpm; p < 0.05) such that Ca-ALT developed on average ~60 bpm earlier than APD-ALT. Our data support that NCX current is an important mechanism linking alternans of intracellular calcium cycling and APD. To our knowledge, this is the first experimental evidence supporting a direct mechanistic role for NCX in governing Ca-ALT to APD-ALT gain.
ICaL is also postulated to be mechanistically involved in governing Ca-ALT to APD-ALT gain. The present study showed a reduction in expression of LTCC and it has been reported that pressure-overload HF model in the guinea pig is associated with decreased ICaL 34. Also, we showed that SERCA2a gene transfer in heart failure did not alter LTCC expression compared to HF alone and prior studies in control hearts (rabbit) showed no change in ICaL following SERCA2a gene transfer but may altered other inward sarcolemmal Ca2+ currents35. Thus, it is possible that the reduction in Ca-ALT to APD-ALT gain seen in the present study could be in part secondary to changes in ICaL.
SERCA2a as a therapeutic target in heart failure
Heart failure is associated with both mechanical and electrophysiological dysfunction. In the present study we show that impaired SR Ca2+ cycling is associated with contractile dysfunction and increased susceptibility to a known arrhythmia substrate, cardiac alternans. Interestingly, we demonstrate that selective SERCA2a gene transfer in HF, improving SR Ca2+ uptake and stabilizing SR Ca2+ release, can improve LV contractile function and ameliorate key arrhythmia substrate. These data support that hypothesis that impaired SR Ca2+ cycling is a common mechanism for both mechanical and electrophysiological dysfunction in HF. Our findings are consistent with a growing body of literature demonstrating a favorable effect of SERCA2a gene transfer on contractile function14-16. Moreover, previous studies have suggested that targeted enhancement of SERCA2a has anti-arrhythmic properties but the mechanisms of these effects were not clear36, 37.
The present investigation provides important mechanistic insights into the anti-arrhythmic properties of therapies targeting SERCA2a. First, as demonstrated in Figure 5 SERCA2a gene transfer modulates susceptibility to cardiac alternans and correspondingly reduces alternans-mediated ventricular arrhythmias. Additionally, we found that SERCA2a gene transfer may decrease Ca2+ mediated triggers of arrhythmias as evidenced by decreased spontaneous diastolic Ca2+ release (i.e. RyR Po) resulting from reduced RyR-PS2814. This is consistent with the findings of Lyons et al.38 demonstrating reduced SR Ca2+ leak and RyR2 phosphorylation in isolated myocytes. Our studies and Lyons et al. clearly demonstrate an effect of SERCA2a gene delivery on an important arrhythmic trigger (i.e. SR Ca2+ leak and DADs) and an important substrate for ventricular arrhythmias (i.e. alternans).
It is possible that therapies that enhance SERCA2a in HF could be arrhythmogenic as enhanced SR calcium load could increase susceptibility to spontaneous SR calcium release and DAD-mediated arrhythmias. However, in the present study and in our prior investigation of SERCA2a overexpression in healthy hearts, we saw no evidence of DADs or DAD-mediated arrhythmias. Also, in a recent study by Lyon et al. 38 SERCA2a overexpression in a chronic MI model of heart failure actually reduced spontaneous catecholamine-induced ventricular arrhythmias. More compelling is the fact that in recent clinical trials there were was no reported increase in arrhythmias in patients with severe heart failure who received AAV.SERCA2a17.
Clinical Implications
Our data have important clinical implications as they highlight the attractiveness of therapies targeting SERCA2a in HF because of the dual effect of enhanced SERCA2a on contractile function and both arrhythmia triggers and substrate. There are clear limitations to inotropic therapies in HF as they increase mortality and have been linked to increased arrhythmogenesis. Furthermore, current anti-arrhythmic therapies are palliative, temporizing, or increase sudden death mortality. Therefore, therapies targeting SERCA2a to improve contractile function while altering key arrhythmia mechanisms have the potential to change the paradigm of HF management by offering the opportunity to genetically induce a heart that is resistance to ventricular fibrillation.
Clinical Perspective.
Sudden arrhythmic death is a major risk in heart failure (HF) yet, the mechanisms are incompletely understood. T-wave alternans arises from beat to beat alternans of cellular repolarization, is a consistent precursor to ventricular fibrillation in experimental animals, and marker of electrical instability in patients. Previously, we reported that SERCA2a, the pump responsible for re-uptake of cytosolic calcium during diastole, plays a central role in the molecular mechanism of cardiac alternans. In the present study SERCA2a gene transfer in the failing heart not only improved contractile function, but restored electrical stability through the amelioration cardiac alternans and SR Ca2+ leak. It provides evidence that dysfunctional sarcoplasmic reticulum Ca2+ cycling links contractile and electrophysiological dysfunction in the failing heart, and that therapy targeting key SR Ca2+ cycling proteins (i.e. SERCA2a) can restore both contractile function and electrical stability. Thus therapies targeting SERCA2a warrant further evaluation in heart failure.
Acknowledgments
Funding Sources This study was supported by National Institutes of Health grant R01-HL54807 (DSR) and Fellowship Awards from the Heart Rhythm Society and NIH National Research Service Award and Physician Scientist Training Pathway – MetroHealth Campus, Case Western Reserve University (MJC).
Footnotes
Disclosures Dr. Hajjar has significant (>$10K) ownership interest in both Celladon and Nanocr.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 2.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter unsustained tachycardia trial investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 3.Mallidi J, Nadkarni GN, Berger RD, Calkins H, Nazarian S. Meta-analysis of catheter ablation as an adjunct to medical therapy for treatment of ventricular tachycardia in patients with structural heart disease. Heart Rhythm. 2011;8:503–510. doi: 10.1016/j.hrthm.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The cardiac arrhythmia suppression trial (cast) investigators. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235–241. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 6.Bloomfield DM, Steinman RC, Namerow PB, Parides M, Davidenko J, Kaufman ES, Shinn T, Curtis A, Fontaine J, Holmes D, Russo A, Tang C, Bigger JT., Jr Microvolt t-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: A solution to the multicenter automatic defibrillator implantation trial (madit) ii conundrum. Circulation. 2004;110:1885–1889. doi: 10.1161/01.CIR.0000143160.14610.53. [DOI] [PubMed] [Google Scholar]
- 7.Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking t-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- 8.Diaz ME, O’Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res. 2004;94:650–656. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- 9.Goldhaber JI, Xie LH, Duong T, Motter C, Khuu K, Weiss JN. Action potential duration restitution and alternans in rabbit ventricular myocytes: The key role of intracellular calcium cycling. Circ Res. 2005;96:459–466. doi: 10.1161/01.RES.0000156891.66893.83. [DOI] [PubMed] [Google Scholar]
- 10.Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004;94:1083–1090. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 11.Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS. Targeted serca2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythm Electrophysiol. 2009;2:686–694. doi: 10.1161/CIRCEP.109.863118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie LH, Sato D, Garfinkel A, Qu Z, Weiss JN. Intracellular ca alternans: Coordinated regulation by sarcoplasmic reticulum release, uptake, and leak. Biophys J. 2008;95:3100–3110. doi: 10.1529/biophysj.108.130955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson LD, Jeyaraj D, Wan X, Hoeker GS, Said TH, Gittinger M, Laurita KR, Rosenbaum DS. Heart failure enhances susceptibility to arrhythmogenic cardiac alternans. Heart Rhythm. 2009;6:251–259. doi: 10.1016/j.hrthm.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.del Monte F, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of serca2a. Circulation. 1999;100:2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, Hadri L, Yoneyama R, Hoshino K, Takewa Y, Sakata S, Peluso R, Zsebo K, Gwathmey JK, Tardif JC, Tanguay JF, Hajjar RJ. Reversal of cardiac dysfunction after long-term expression of serca2a by gene transfer in a preclinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T, Guerrero JL, Gwathmey JK, Rosenzweig A, Hajjar RJ. Adenoviral gene transfer of serca2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci USA. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, Yaroshinsky A, Zsebo KM, Dittrich H, Hajjar RJ. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (cupid): A phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum ca2+-atpase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmmed GU, Dong PH, Song G, Ball NA, Xu Y, Walsh RA, Chiamvimonvat N. Changes in ca(2+) cycling proteins underlie cardiac action potential prolongation in a pressure-overloaded guinea pig model with cardiac hypertrophy and failure. Circ Res. 2000;86:558–570. doi: 10.1161/01.res.86.5.558. [DOI] [PubMed] [Google Scholar]
- 19.Kiss E, Ball NA, Kranias EG, Walsh RA. Differential changes in cardiac phospholamban and sarcoplasmic reticular ca(2+)-atpase protein levels. Effects on ca2+ transport and mechanics in compensated pressure-overload hypertrophy and congestive heart failure. Circ Res. 1995;77:759–764. doi: 10.1161/01.res.77.4.759. [DOI] [PubMed] [Google Scholar]
- 20.Chudin E, Goldhaber J, Garfinkel A, Weiss J, Kogan B. Intracellular ca(2+) dynamics and the stability of ventricular tachycardia. Biophys J. 1999;77:2930–2941. doi: 10.1016/S0006-3495(99)77126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konta T, Ikeda K, Yamaki M, Nakamura K, Honma K, Kubota I, Yasui S. Significance of discordant st alternans in ventricular fibrillation. Circulation. 1990;82:2185–2189. doi: 10.1161/01.cir.82.6.2185. [DOI] [PubMed] [Google Scholar]
- 22.Plummer BN, Cutler MJ, Wan X, Laurita KR. Spontaneous calcium oscillations during diastole in the whole heart: The influence of ryanodine reception function and gap junction coupling. Am J Physiol Heart Circ Physiol. 2011;300:H1822–1828. doi: 10.1152/ajpheart.00766.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoeker GS, Katra RP, Wilson LD, Plummer BN, Laurita KR. Spontaneous calcium release in tissue from the failing canine heart. Am J Physiol Heart Circ Physiol. 2009;297:H1235–1242. doi: 10.1152/ajpheart.01320.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laurita KR, Katra R, Wible B, Wan X, Koo MH. Transmural heterogeneity of calcium handling in canine. Circ Res. 2003;92:668–675. doi: 10.1161/01.RES.0000062468.25308.27. [DOI] [PubMed] [Google Scholar]
- 25.Wan X, Laurita KR, Pruvot EJ, Rosenbaum DS. Molecular correlates of repolarization alternans in cardiac myocytes. J Mol Cell Cardiol. 2005;39:419–428. doi: 10.1016/j.yjmcc.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Pastore JM, Rosenbaum DS. Role of structural barriers in the mechanism of alternans-induced reentry. Circ Res. 2000;87:1157–1163. doi: 10.1161/01.res.87.12.1157. [DOI] [PubMed] [Google Scholar]
- 27.Cutler MJ, Rosenbaum DS. Explaining the clinical manifestations of t wave alternans in patients at risk for sudden cardiac death. Heart Rhythm. 2009;6:S22–28. doi: 10.1016/j.hrthm.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: The saga of cardiac alternans. Circ Res. 2006;98:1244–1253. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 29.Wasserstrom JA, Sharma R, Kapur S, Kelly JE, Kadish AH, Balke CW, Aistrup GL. Multiple defects in intracellular calcium cycling in whole failing rat heart. Circ Heart Fail. 2009;2:223–232. doi: 10.1161/CIRCHEARTFAILURE.108.811539. [DOI] [PubMed] [Google Scholar]
- 30.Kapur S, Aistrup GL, Sharma R, Kelly JE, Arora R, Zheng J, Veramasuneni M, Kadish AH, Balke CW, Wasserstrom JA. Early development of intracellular calcium cycling defects in intact hearts of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299:H1843–1853. doi: 10.1152/ajpheart.00623.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huser J, Wang YG, Sheehan KA, Cifuentes F, Lipsius SL, Blatter LA. Functional coupling between glycolysis and excitation-contraction coupling underlies alternans in cat heart cells. J Physiol. 2000;524(Pt 3):795–806. doi: 10.1111/j.1469-7793.2000.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picht E, DeSantiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res. 2006;99:740–748. doi: 10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- 33.Lehnart SE, Terrenoire C, Reiken S, Wehrens XH, Song LS, Tillman EJ, Mancarella S, Coromilas J, Lederer WJ, Kass RS, Marks AR. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc Natl Acad Sci USA. 2006;103:7906–7910. doi: 10.1073/pnas.0602133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ming Z, Nordin C, Siri F, Aronson RS. Reduced calcium current density in single myocytes isolated from hypertrophied failing guinea pig hearts. J Mol Cell Cardiol. 1994;26:1133–1143. doi: 10.1006/jmcc.1994.1132. [DOI] [PubMed] [Google Scholar]
- 35.Terracciano CM, Hajjar RJ, Harding SE. Overexpression of serca2a accelerates repolarisation in rabbit ventricular myocytes. Cell Calcium. 2002;31:299–305. doi: 10.1016/s0143-4160(02)00058-1. [DOI] [PubMed] [Google Scholar]
- 36.del Monte F, Lebeche D, Guerrero JL, Tsuji T, Doye AA, Gwathmey JK, Hajjar RJ. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci USA. 2004;101:5622–5627. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prunier F, Kawase Y, Gianni D, Scapin C, Danik SB, Ellinor PT, Hajjar RJ, Del Monte F. Prevention of ventricular arrhythmias with sarcoplasmic reticulum ca2+ atpase pump overexpression in a porcine model of ischemia reperfusion. Circulation. 2008;118:614–624. doi: 10.1161/CIRCULATIONAHA.108.770883. [DOI] [PubMed] [Google Scholar]
- 38.Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, Garcia E, O’Gara P, Liang L, Kohlbrenner E, Hajjar RJ, Peters NS, Poole-Wilson PA, Macleod KT, Harding SE. Serca2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol. 2011;4:362–372. doi: 10.1161/CIRCEP.110.961615. [DOI] [PMC free article] [PubMed] [Google Scholar]