Abstract
Sulforaphane (SFN), is an effective in vitro antagonist of ligand activation of the human pregnane and xenobiotic receptor (PXR). PXR mediated CYP3A4 up-regulation is implicated in adverse drug-drug interactions making identification of small molecule antagonists a desirable therapeutic goal. SFN is not an antagonist to mouse or rat PXR in vitro; thus, normal rodent species are not suitable as in vivo models for human response. To evaluate whether SFN can effectively antagonize ligand activation of human PXR in vivo, a three-armed, randomized, crossover trial was conducted with 24 healthy adults. The potent PXR ligand – rifampicin (300 mg/d) was given alone for 7 days in arm 1, or in daily combination with 450 µmoles SFN (Broccoli Sprout extract) in arm 2; SFN was given alone in arm 3. Midazolam as an in vivo phenotype marker of CYP3A was administered before and after each treatment arm. Rifampicin alone decreased midazolam AUC by 70%, indicative of the expected increase in CYP3A4 activity. Co-treatment with SFN did not reduce CYP3A4 induction. Treatment with SFN alone also did not affect CYP3A4 activity in the cohort as a whole, although in the subset with the highest basal CYP3A4 activity there was a statistically significant increase in midazolam AUC (i.e., decrease in CYP3A4 activity). A parallel study in humanized PXR mice yielded similar results. The parallel effects of SFN between humanized PXR mice and human subjects demonstrate the predictive value of humanized mouse models in situations where species differences in ligand-receptor interactions preclude the use of a native mouse model for studying human ligand-receptor pharmacology.
Keywords: Sulforaphane, cytochrome P450, CYP3A4, midazolam, pregnane X-receptor, NR1I2, phase I clinical trial, humanized PXR mice, isolated hepatocytes
Introduction
Sulforaphane (SFN), an isothiocyanate found in Brassica vegetables, especially broccoli and broccoli sprouts, has been of great interest as a putative anticarcinogenic agent. SFN is an effective activator of the transcription factor NRF2, leading to subsequent up-regulation of certain antioxidant genes such as nitroquinone oxidoreductase 1 (NQ01) (1–3). SFN also invokes a number of other putative chemoprotective responses including induction of apoptosis and inhibition of histone deacetylase (4–8). The observed chemoprotective effects have been demonstrated in vivo, as SFN has been shown to be protective against both tumor initiation and progression in a number of animal cancer models (2, 9–11).
We previously demonstrated in human hepatocytes and other human cell-based models that SFN is an effective in vitro antagonist of ligand activation of the human pregnane and xenobiotic receptor (PXR) (12). PXR (also known as SXR or NR1I2) is a ligand-activated transcription factor important in the regulation of many genes involved in xenobiotic metabolism and transport, such as CYP3A4 and MDR1 (13), and the anti-apoptotic gene BCL2 (14). CYP3A4 is the most abundant xenobiotic metabolizing cytochrome P450 in the human liver and small intestine and is involved in the biotransformation of over 50% of available Pharmaceuticals (15). CYP3A4 is highly inducible, and activation of PXR-mediated transcription is perhaps the most important contributor to CYP3A4 induction by xenobiotics (16).
Ligand-mediated binding to PXR and the subsequent increase in CYP3A4 enzyme activity is a common cause of drug-drug interactions (17). For example, administration of rifampicin (RIF) significantly induces CYP3A4 expression through activation of PXR, thereby contributing to adverse drug interactions frequently associated with RIF treatment for tuberculosis. Indeed, the use of RIF with many HIV protease inhibitors is contraindicated to avoid treatment failures (18). An effective, non-toxic PXR antagonist could potentially prevent such clinically important drug interactions that result from ligand activation of PXR and associated CYP3A4 induction, when co-administration of the CYP3A inducer with a sensitive substrate is a clinical imperative.
Various methods are used for pre-clinical testing of drug-drug interactions, with the most common being in vitro assays, such as sub-cellular systems (e.g., microsomes), immortalized cells, or human-derived primary cell cultures, such as human hepatocytes. However, pharmacokinetic behavior of xenobiotics may be substantially different in vitro, than what occurs in vivo. Indeed, the intracellular concentration of SFN (and metabolites) can be 100-fold higher than extracellular concentrations, likely due to accumulation of the GSH conjugate. However, in vivo, rapid conjugate formation and removal may prevent accumulation of unchanged compound and thus intracellular levels attained in vitro may be very different from that in vivo (19, 20).
In order to overcome the limitations of in vitro studies, in vivo animal studies are widely used to predict in vivo human response. Unfortunately these models are not always suitable due to species differences in enzyme and transporter structure and function. This is particularly true for activation of PXR because of well-described species differences in the ligand binding domain of the protein (13, 21). For example, mouse and rat PXR are not readily activated by the prototypical human PXR ligand RIF, and, conversely, the human PXR (hPXR) is not readily induced by the prototypical rodent inducer, pregnenolone 16α-carbonitrile (PCN) (22). Consequently, SFN does not inhibit ligand activation of mouse or rat PXR in vitro, even though it was highly effective at inhibiting RIF induction of CYP3A4 in human hepatocytes in primary culture (12). Thus rodents are not appropriate in vivo models to evaluate the ability of SFN to prevent ligand-mediated activation of human PXR in vivo 12). To overcome such limitations that result from structural differences in single genes / proteins, a growing number of transgenic mouse models have been generated which express humanized versions of specific genes, including genes for a number of transcription factors. Several transgenic mouse strains expressing the human PXR gene in a mouse PXR null background have recently become available (22–24) as well as mice that express humanized CAR (23), or humanized PXR and CAR (23). To determine the validity of such ‘humanized’ mouse models it is important to show that these mice can accurately predict human outcomes. Seldom are there opportunities to conduct phase I clinical trials in humans that can also be directly compared with ‘humanized’ transgenic mice to directly validate its predictive abilities for human response.
Prior to the availability of the humanized PXR mouse, we began a phase I clinical trial to evaluate the effectiveness of SFN as an antagonist of RIF-mediated activation of PXR. When the humanized PXR mice became available in the early stages of the clinical trial, it afforded us the opportunity to directly compare the response of these mice with humans. The purpose of this study was to test two hypotheses: 1) Can sulforaphane effectively antagonize PXR activation by the potent ligand, rifampicin, in vivo in healthy human subjects?, and 2) can humanized PXR mice provide a suitable in vivo animal model that accurately reflects human response to a PXR ligand (rifampicin) and putative PXR antagonist (sulforaphane)? Thus, this study reports the comparison of humanized PXR mice with healthy human adults given SFN with and without RIF. Similar to our previous findings with human hepatocytes (12), in this study we show that SFN is a highly effective antagonist to ligand activation of human PXR in vitro in humanized PXR mouse hepatocytes. However, when administered in vivo SFN failed to antagonize the RIF activation of PXR and subsequent induction of CYP3A4 or Cyp3all in both humans and transgenic mice containing human PXR, respectively. Interestingly, antagonism of the basal level of CYP3A4 activity was seen in human participants who fell into the highest three quartiles of basal CYP3A4 activity. In addition to showing that SFN, at the doses used, was not an effective antagonist to ligand activation of human PXR in vivo, this study demonstrated for the first time that transgenic mice expressing human genes accurately predict in vivo human response to those genes in a situation where species differences in the gene of interest precluded the use of ‘wild type’ mice (22)
Methods
Human Phase I clinical trial
Sulforaphane/Broccoli Sprout Preparation
Lyophilized broccoli sprout extract was obtained from Drs. Paul Talalay and Jed Fayhey at The Johns Hopkins University. The extract was prepared as described previously (25). Briefly, seeds were sprouted for 3 days, then harvested and plunged into boiling water for 30 minutes. The resulting extract of the water-soluble glucosinolates present in the broccoli sprouts contained ~5 µmoles/ml glucoraphanin, a carbohydrate conjugate of SFN. This glucosinolate extract was then treated with myrosinase, derived from daikon (radish) sprouts, for 4 hours to hydrolyze the sugar moiety from the isothiocyanates. The resulting extract contained approximately 3 µmoles SFN/ml extract. This extract was further concentrated by lyophilization, and the resulting powder was analyzed by HPLC. Individual 2 kg bags of lyophilized broccoli sprout extract were tested and found to contain between 200 and 240 µmoles/g powder. Repeated analysis of these lots demonstrated that the levels of SFN were stable for at least 2.5 years from the time of initial preparation. The powder was tested and found to be free of microbial contaminates using standard analytical methods. The aliquot of powder we received contained 229 µmoles SFN/g of powder and less than 0.13 µmoles glucosinolate/g of powder, demonstrating that myrosinase hydrolysis was more than 99.9% complete.
The powder was divided into 2 g aliquots that provided ~450 µmoles (~80mg) SFN. The powdered extract was either mixed into a bowl of cheese-based soup (1/2 cup), or dissolved in 25–50 ml water which was consumed in a bolus, and then followed by the cheese-based soup, based on participant preference.
Study Design
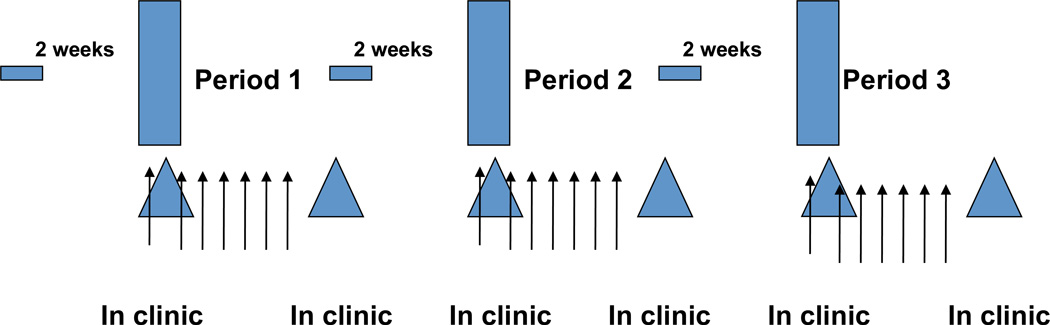
The three armed, randomized, crossover trial consisted of: 1) powdered extract containing ~450 µmoles of SFN + cheese soup, 2) SFN + cheese soup and 300 mg RIF once daily for 7 days, or 3) cheese soup and 300 mg RIF alone. The order of the three treatment periods was randomized prior to the start of the study. RIF was taken orally by capsule immediately following soup consumption. The dose of RIF is approximately 50% of the standard therapeutic dose used in the treatment of tuberculosis. Participants refrained from consuming grapefruit and any cruciferous vegetables other than the study materials starting at two weeks prior to the first visit and through the conclusion of their last visit (Figure 1). The study design was reviewed and approved by the University of Washington Institutional Review Board, and the General Clinical Research Center (Institute for Translational Health Sciences). All subjects were properly consented before initiation of any study activities.
Figure 1.
Participant Recruitment and Exclusion Criteria
Participants were recruited via advertisements in a student daily newspaper and flyers. Exclusion criteria for the participants were age <20 or >40 y, BMI over 30 kg/m2, active smoking or exposure to tobacco smoke, exposure to certain other chemical agents, inability to tolerate the soup or comply with study schedule, use of prescription medications, and clinical laboratory test values outside the normal range during screening, including tests for liver and kidney function, and hematology. Participants were asked to refrain from medications, supplements and cruciferous vegetables and grapefruit products for the duration of their participation.
Sample Collection
Participants were asked to refrain from food consumption for 4 hours before the start of the study day. Baseline blood samples were then drawn for routine serum chemistry (ALT, AST, BUN, alkaline phosphatase), lipid analysis (total lipids, LDL, HDL and total cholesterol), and WBC. Midazolam (1 mg) was administered orally and time set as 0; blood was drawn just prior to dosing and at 15, 30, 45, 60, 90, 120, 180, 240, 360 min. Blood was collected in heparinized Vacutainer tubes and placed on ice-water and processed within 2 hours. Plasma was obtained by 15-min centrifugation at 1,350×g at 4°C, aliquoted into cryovials and frozen. Urine was collected at baseline, from 0–6 hours, and from 6–24 hours; 1 ml aliquots of each collection were stored at −80°C.
Cyclocondensation Assay for total urinary isothiocyanates (ITC)
Urine was analyzed for total ITC content with the cyclocondensation method described by Zhang et al, with slight modifications (26). Briefly, 250 µl urine was added to a mix of 450 µl acetonitrile and 250 µl K3PO4. Then 50 µl of 80 mM benzene-dithiol in methanol was added for a total reaction volume of lml. Samples were vortexed and incubated at 70° for 1 h in a water bath, then spun at 14,000×g in a microcentrifuge for 30 min. Supernatant samples were analyzed by reverse-phase HPLC on a Hewlett-Packard P 1090 HPLC. Two hundred µl of each sample was injected onto a 26 cm × 5 mm C18 HPLC column and run isocratically at 80% methanol / 20% H20 for 15 min with a 10 min post run. Samples were monitored with a UV detector set at 365 nm.
Plasma concentration of MDZ
MDZ levels were measured in plasma using LC-MS. A 0.5 ml aliquot of plasma was processed through Varian Certify extraction tubes. Plasma was loaded onto columns, rinsed with distilled water, 1M acetic acid, methanol, then eluted with 78:20:2 methylene chloride:IPA:ammonium hydroxide. The eluate was dried at 40° and reconstituted in 75 µl 55:45 pH=4 10 mM ammonium acetate:acetonitrile. Samples were analyzed by an Agilent 1100 LC coupled to a G1956 MS. Five µl of each sample was injected onto a Zorbax C18 column and run isocratically with 75:55:45 pH 4 10 mM ammonium acetate:acetonitrile for 10 min. The MS monitored selected ion masses of 326.00 for midazolam, 330.00 for midazolam-d4, 342.10 for 1'-OH-midazolam, and 346.10 for 1'-OH-midazolam-d4.
Pharmacokinetic analysis
Non-compartmental analysis of plasma concentration vs. time was performed using the pharmacokinetic modeling software WinNonLin (Pharsight, Mountain View, CA). Significance in difference between day one and day eight of each trial period was determined using paired two-tailed t-tests. P values of < 0.05 were considered to be significant.
Mouse housing and genotyping
Humanized PXR breeder mice (developed on a C57BL/6 background) were obtained from Dr. Frank Gonzales at The National Institutes of Health (22). Briefly, humanized PXR mice were generated by insertion of a Bacterial Artificial Chromosome (BAC) onto a PXR null background. The BAC contained the entire human PXR gene including 5’ and 3’ flanking sequences. These mice express human PXR in the liver and intestine, and respond to the prototypical human PXR activating ligand, RIF, thus mimicking human expression patterns (22).
A breeding colony of these humanized PXR mice was established in the transgenic animal core at the University of Washington. All experiments were reviewed and approved by the University of Washington Institutional Animal Care and Use Committee. Mice were housed in standard conditions, with a 12 hours light/dark cycles, with rodent food and water available ad libitum. Mice were back-crossed for at least two generations prior to utilization for the following experiments, although significant genetic variation in other loci was evident, as evidenced by coat color difference in off-spring. Mouse tail tips were genotyped for the presence of the humanized PXR gene. No method was available to distinguish between hemi and heterozygotes.
Primers for hPXR were as follows:
hPXR 5′ UTR 2F : 5′ - GCA CCT GCT GCT AGG GAA TA-3′
hPXR 5′ UTR 2R : 5′ - CTC CAT TGC CCC TCC TAA GT-3′
Murine epoxide hydrolase was included as a positive control; primers used were:
MEH F0R:5′-AAG TGA GTT TGC ATG GCG CA-3′
MEH REV : 5′-CCC TTT AGC CCC TTC CCT CTG-3′
Final conditions for PCR utilized 20–50 ng template DNA, 0.2 mM DNTPs, 2 mM MgCl2 and 1 U Taq polymerase with lx Taq reaction buffer. PCR cycling was done as follows: 92 degrees for 15 s; 92 degrees for 10 s; 60 degrees for 30 s; 72 degrees for 60 s. Cycles were repeated 30 times, followed by 72 degrees for 10 min. PCR products were separated by 1% agarose gel electrophoresis to identify the presence of the humanized gene.
Mouse hepatocyte preparations
Mice were anesthetized with ketamine and xylazine and monitored until they were unresponsive to toe-pinch. The abdominal wall was cleaned with ethanol then opened to expose the liver. The liver was perfused through the portal vein with Ca-free Hanks buffer at 37° C for 4.5 min, followed by perfusion with a collagenase (10mg/ 100ml media) solution for a further 6 min until the connective tissue matrix holding the cells was digested. Hepatocytes were released from Gleason’s capsule and placed into 15 ml Williams E flow media (WEFM). The dissociated hepatocytes were then passed through a nylon mesh filter that was then rinsed with 2.5ml WEFM. The recovered cells were then spun at 600 rpm at 4 for 5 min and resuspended in Hepatozyme media (Invitrogen). The cells were then placed on collagen-coated plates and allowed to equilibrate for 4–5 hr. Media was changed to Williams E culture media and hepatocytes were treated as indicated.
In vivo activation of PXR with SFN
Mice were treated with vehicle, RIF (10 mg/kg-d), or RIF + SFN for 3 days, according to the schedule in Table 1; 3 days of RIF treatment at this dose has been shown to substantially increase hepatic Cyp3all mRNA (~12x) and protein (~2.5x) levels in these mice (22). All treatments were administered by gavage in a volume of 5 ml/kg (2 mg RIF/ml). RIF was obtained from Sigma and SFN was obtained from LKT laboratories. Eight hours after the last dose of SFN and/or RIF, animals were euthanized by CO2 narcosis followed by cervical dislocation and liver tissue was collected. Half of the tissue sample was stored in RNAlater® for mRNA analysis, and the other half was homogenized in phosphate buffered saline for Cyp3all (mouse homologto human CYP3A4) activity analysis.
Table 1.
Urinary excretion of total isothiocyanate (ITC) over the 18 hr period following soup consumption in the two treatment groups administered broccoli sprouts. Rifampicin alone arm is included for comparison. Cumulative excretion of ITC in umole was calculated using total urine volumes. % Recovery is based on the administered dose of 450 µmoles of ITC.
| Total ITC* | Broccoli Sprouts + RIF | Broccoli Sprouts alone | RIF alone |
|---|---|---|---|
| Concentration µM | 170 ± 143 | 181 ± 136 | 1 ± 2 |
| Amount Excreted µmoles excreted in 18hrs | 173 ± 131 | 188 ± 109 | 1 ± 2 |
| % recovery | 38 ± 33% | 42 ± 27% | N/A |
Mean ± SD
mRNA isolation and analysis
mRNA was isolated from mouse liver using Trizol®, according to the manufacturer’s protocol. Approximately 50 mg of mouse liver tissue was placed in 2 ml Trizol® and put on ice and then homogenized using a hand-held Teflon homogenizer. Chloroform was added and mixed, the sample was then spun and the supernatant removed to a clean tube. The mRNA was precipitated with isopropanol and then washed with 70% ethanol. ‘Real Time’ RT-PCR was performed using an Applied Biosystems 7900HT Fast Real-Time PCR system. PCR was monitored using a TaqMan®-based system. The PCR mixture consisted of forward and reverse primers, TaqMan® probe, and TaqMan® universal master mix. To normalize for difference in amount of total RNA added to each RT-reaction, β-actin was used as the normalizing control gene. The Ct value of the target gene was divided by Ct value for β-actin to determine absolute value.
The primers and probes came predesigned from Agilent Technologies. The context sequences for mouse Cyp3All was: CTCTGCCCAACAAGGCACCTCCCAC
β-actin was the housekeeping gene used for normalization; primers and probes were as follows.
| FP | CCCTAAGGCCAACCGTGAAA (mouse/rat) |
| RP | ACGACCAGAGGCATACAGGGA (mouse/rat) |
| Probe | ATGACCCAGATCATGTTTGAGACCTTCAACAC (human, rat, mouse) |
Loss of Righting Reflex (LORR) Assay
Mice were administered 250 mg/kg of 2,2,2-tribromoethanol, which is metabolically cleared only via Cyp3all (27), via IP injection (12.5 mg/ml; 20ml/kg). Once immobilized, the mice were placed on their backs under a heat lamp and monitored for the ability to right themselves twice in one minute. All mice were observed until their righting reflexes were definitively recovered. A baseline LORR time for each mouse was established for the administered dose of 2,2,2-tribromoethanol. Following a two week recovery period, each mouse was then administered RIF (40 mg/kg, in DMSO and H2O, by gavage) for three days and the righting reflex experiment was repeated. Mice were then allowed to recover from the second period for two weeks before the third treatment arm of the study. For the third arm, mice were dosed with RIF (40 mg/kg once per day, at a volume of 5 ml/kg) and SFN (50 mg/kg, 3 times per day via IP at a volume of 5 ml/kg) for a further three days.
Statistics
2-sample, 2-tailed Students t-tests were performed to examine differences between treated and control Cyp3all mRNA levels. Paired 2-tailed Students t-tests were performed to examine differences between groups in the LORR experiment. P values of less than 0.05 were considered to be significant.
RESULTS
Human clinical study
A total of 29 participants entered the study (15 women and 14 men). The powdered broccoli extract imparted an unpleasant, bitter taste to the cheese soup used as a vehicle. Therefore we had all potential participants try the soup before committing to participating. Three participants who initially did not object to the taste did drop out after entering the study and did not finish any of the study arms due to dislike or intolerance of the extract. Two of these participants became nauseated, one also had vomiting but it was determined that the subject was suffering from the flu and thus the response was deemed by our attending physician not to be solely treatment-related. Three additional participants did not complete all their study periods (one developed apparent lactose intolerance to the soup, one did not routinely comply with study activities, and one relocated out of state after the second study period).
We analyzed samples from a total of 23 healthy adults who completed the study, 12 males and 11 females, average age of 23.7 years (range 20–34), average BMI of 23.4 kg/m2 (range 19.2 to 29.3, SD 2.7). Seventeen (74%) of the participants were Caucasian, with others self-identifying as Asian American (5; 22%) or African American (1; 4%). All participants had normal liver and kidney function and hematology as measured at the beginning of the trial. There were no severe treatment-related adverse events noted after this dose of ~450 µmoles (~80 mg) of SFN in the form of powdered broccoli sprout extract, the highest level yet given during a clinical trial.
Urinary ITC as an assessment of protocol compliance and bioavailability of SFN
Analysis of the ITC concentrations in the urine collected at baseline on day 1 showed that subjects were compliant with the requests to abstain from other cruciferous vegetable consumption, with most having no detectable ITC in their baseline urine (detection limit = 0.5 µM). Urinary ITC levels showed extremely large increases following powdered broccoli sprout administration – pooled urine from each individual collected for 18 hours after dose had an average concentration of 170 ± 143 µM ITC after the powder and RIF arm, and 181 ± 136 µM ITC after the powder alone arm. During the RIF alone arm, urinary ITC concentrations were unchanged from baseline, with ITCs only detectable in a few samples at low concentrations (1 ± 2 µM), such that overall urinary ITC concentrations in this arm were not significantly above the detection limit. Total ITC recoveries were calculated using total collected urine volumes. The recovery after the two powder containing arms was 180 ± 120 µmoles, yielding a mean urinary recovery of 40% of the ~450 µmole dose administered (Table 1).
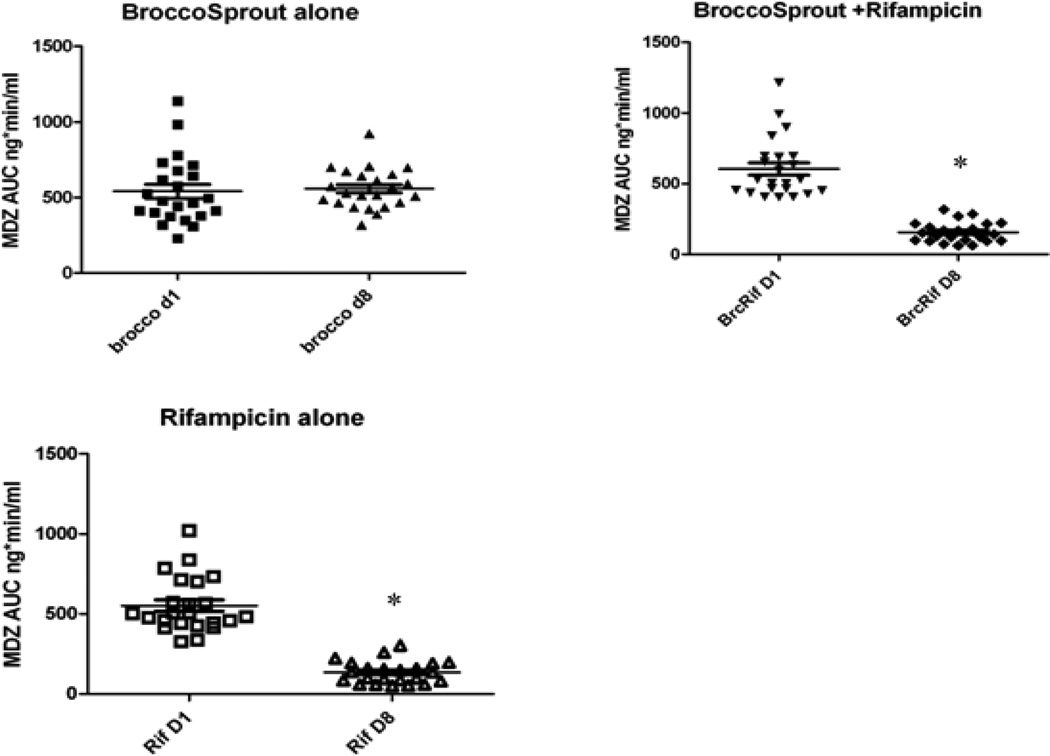
The metabolic clearance of MDZ was accelerated substantially following RIF treatment (Table 2, Fig. 2), consistent with what has been reported previously (28). Mean peak serum concentrations of both MDZ and l’-OH-MDZ decreased by 50%, following RIF administration, although no changes in the ratio of MDZ-to-l’-OH-MDZ AUC were observed. Midazolam AUC was reduced by 75% and oral clearance (i.e., systemic clearance offset by bioavailability, CL/F) was increased by 4.75-fold following treatment with RIF alone. Treatment with SFN (broccoli-sprout powder) alone had no effect on MDZ peak serum concentrations, AUC, clearance or terminal half-life. Most importantly, co-administration of SFN did not moderate the inductive effect of RIF in that none of the parameters of MDZ elimination changed significantly when compared with RIF alone. Thus, at the dose and rate of administration in this study, SFN in the broccoli-sprout powder was not able to effectively antagonize RIF-mediated activation of PXR, as reflected in the increase in intestinal and/or hepatic CYP3A activity (i.e., increased clearance of orally administered MDZ).
Table 2.
Mean ± standard deviation (SD) of MDZ pharmacokinetic parameters observed in the three treatment groups.
| Broccoli Sprouts + Rifampicin | Broccoli Sprouts Alone | Rifampicin Alone | ||||
|---|---|---|---|---|---|---|
| Day 1 | Day 8 | Day 1 | Day 8 | Day 1 | Day 8 | |
| Peak MDZ ng/ml | 4.0±1.8 | 1.9±1.6* | 4.1±1.5 | 4.7±1.2 | 4.4±1.6 | 2.2±1.8* |
| Peak 1’OH-MDZ ng/ml | 1.1±0.9 | 0.6±1.1* | 1.4±0.5 | 1.7±1.0 | 1.5±0.6 | 0.6±0.6* |
| Peak 1’OH-MDZ/MDZ | 0.29 | 0.31 | 0.33 | 0.36 | 0.33 | 0.30 |
| AUC (ng·min/ml) | 552±171 | 135±70** | 541±222 | 558±134 | 604±212 | 156±72** |
| Terminal Half-life (min) | 135±41 | 98±67** | 145±62 | 136±39 | 131±38 | 103±66* |
| Oral Clearance (ml/min) | 1697±526 | 8754±4400** | 1840±760 | 1649±437 | 1590±437 | 7410±3689 |
Statistically different from Day 1,
p<0.05;
P<0.01
Figure 2.
Individual (symbols) and mean (bar) MDZ AUC before and after treatment for each of three arms: broccoli sprouts alone; broccoli sprouts +RIF; RIF alone. Stars denote P<.05
Although, overall, there were no statistically significant effects of the broccoli sprout powder on basal CYP3A levels, as determined by comparison of mean midazolam pharmacokinetic parameters in the same subject before and after 8 days SFN treatment, there was a large range in CYP3A4 activity across the 23 subjects. It is plausible that endogenous ligands of PXR drive some, but not all, of the constitutive expression of CYP3A4. If this assumption is correct, individuals with relatively low CYP3A4 activity (high MDZ AUC), may have little basal PXR-mediated CYP3A4 activity, and thus inhibition of endogenous ligand activation by SFN would be less evident. To test this hypothesis, the MDZ AUC data were divided into quartiles and the lower three quartiles from subjects with the higher CYP3A4 activity were examined, i.e., the quartile with the lowest basal CYP3A4 expression was excluded. For this subset of the study population, 7 days of SFN treatment caused a 20% decrease in CYP3A4 activity, as reflected by both an increase in MDZ AUC or a decrease in MDZ oral clearance (P<0.001; Table 3). The mean peak plasma concentration increased from 3.6 ± 1.2 ng/ml on day 1 to 4.4 ± 1.0 ng/ml on day 8 (P=0.03). Thus, it appears that SFN present in the broccoli sprout extract decreased intestinal and hepatic CYP3A4 activity in those members of the population with higher ‘basal’ CYP3A4 activity, consistent with the hypothesis that SFN is able to at least partially antagonize endogenous ligand mediated activation of PXR, even though it was not able to effectively antagonize the potent exogenous ligand, RIF.
Table 3.
Mean MDZ parameters for the 3 quartiles with the highest CYP3A4 activity (lowest AUC)
| Broccoli Sprouts + Rifampicin | Broccoli Sprouts Alone | Rifampicin Alone | ||||
|---|---|---|---|---|---|---|
| Day 1 | Day 8 | Day 1 | Day 8 | Day 1 | Day 8 | |
| AUC ng-min/ml | 499±112 | 144+73** | 437±111 | 527±104* | 529±136 | 146±66** |
| Oral Clearance ml/min | 1848±490* | 8141±4510** | 2116±707 | 1741±404* | 739±352 | 7830±4070** |
Differences between Day 8 and Day 1:
P<0.001;
P<0.02
Results in humanized Mice
Soon after the human trial was initiated, humanized PXR mice became available, which provided an opportunity to determine if ‘humanized PXR’ transgenic mice would be a suitable model for predicting human response to PXR ligands and antagonists. In human primary hepatocytes, RIF causes a dramatic up-regulation in CYP3A4 levels that is completely blocked by the addition of 50 µM SFN (12). To ascertain the suitability of hPXR mice as a model, isolated primary mouse hepatocytes from humanized PXR mice were prepared and the in vitro response to RIF and SFN were determined in a manner similar to what was done previously in human hepatocytes (12): hPXR mouse primary hepatocytes (5 independent experiments; 2 males and 3 females) were treated with SFN and/or RIF, followed by Cyp3all mRNA analyses.
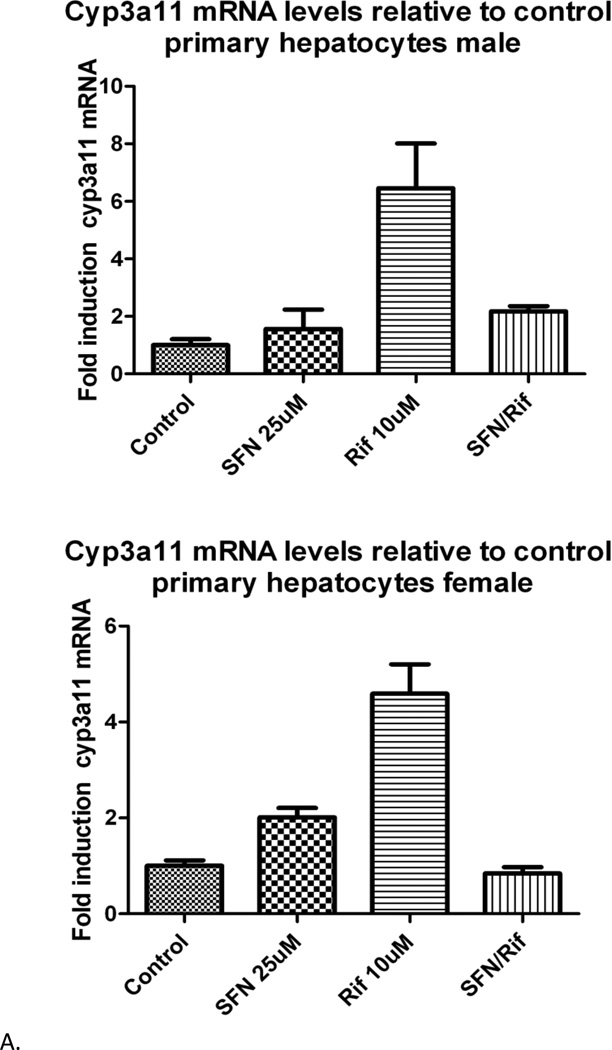
Treatment of humanized PXR mouse hepatocytes with RIF substantially, but variably, induced Cyp3all mRNA, and the induction was completely blocked by the addition of SFN in all tested hepatocytes (Figure 3), similar to what was seen in human hepatocytes (12). The inhibition of induction tended towards statistical significance (P = 0.1) but did not reach it due to high variance of RIF induction in the presence of two outlier mice (33- and 56-fold induction over control). Post-hoc removal of those mice from the data set led to a p value of less than 0.05.
Figure 3.
Hepatocyte cyp3all mRNA levels from representative mice. Top panel. A single male humanized PXR mouse. Bottom panel. A single female humanized PXR mouse. Both sets of hepatocytes show strong induction by RIF and antagonism of that induction by SFN. mRNA levels are reported as fold change relative to control. Error bars represent two separate mRNA measurements.
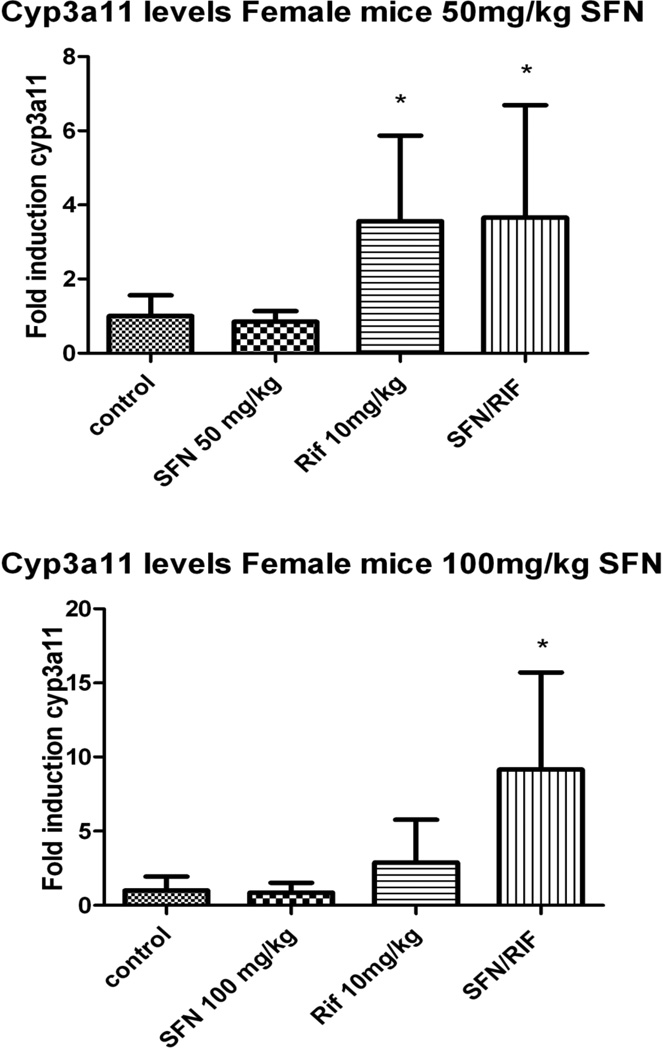
Satisfied that the PXR in the hepatocytes of the humanized mice was inhibited by SFN in vitro in a manner similar to what was seen previously in human hepatocytes, we then evaluated the ability of SFN to antagonize the ligand activation of PXR in vivo, using a study design modeled after the human clinical trial. Four dose groups of 4 animals each were prepared and treated with SFN alone, SFN plus RIF, RIF alone, or vehicle alone (Figure 4). Cyp3all levels were significantly elevated in the two RIF treatment groups, although the magnitude of induction was highly variable. When administered at 50 mg/kg-day for 3 days, SFN treatment had no effect on basal expression of Cyp3all, and co-administration with RIF had no significant effect on the magnitude of RIF induction.
Figure 4.
Female mice were split into four groups: (a) received vehicle, (b) 10mg/kg RIF, (c) 50 mg/kg SFN/day, and (d) 10mg/kg RIF and 50mg/kg/day SFN. Mice were administered SFN and/or RIF by gavage daily for 3 days and sacrificed 8 hours after the final dose. Tissue was preserved for RNA later, and three separate 50mg portions from each liver were analyzed for mRNA. Cyp3all content was measured relative to β-actin, numbers are reported as a percentage of control. B. Identical design to figure A, but with a SFN dose of 100 mg/kg. N=4 in all groups.
The SFN dose rate was then increased to 100 mg/kg/day for 3 days to see if higher doses might effectively antagonize RIF induction. Again, RIF administration led to a large but highly variable (and thus not significant) increase in Cyp3all mRNA that was not abrogated by co-administration of SFN. In fact, in these mice, the 100 mg dose of SFN led to an higher levels of cyp3all mRNA induction relative to RIF alone ; i.e., 3.53 ± 0.76 to 12.9 ± 7.3 fold.
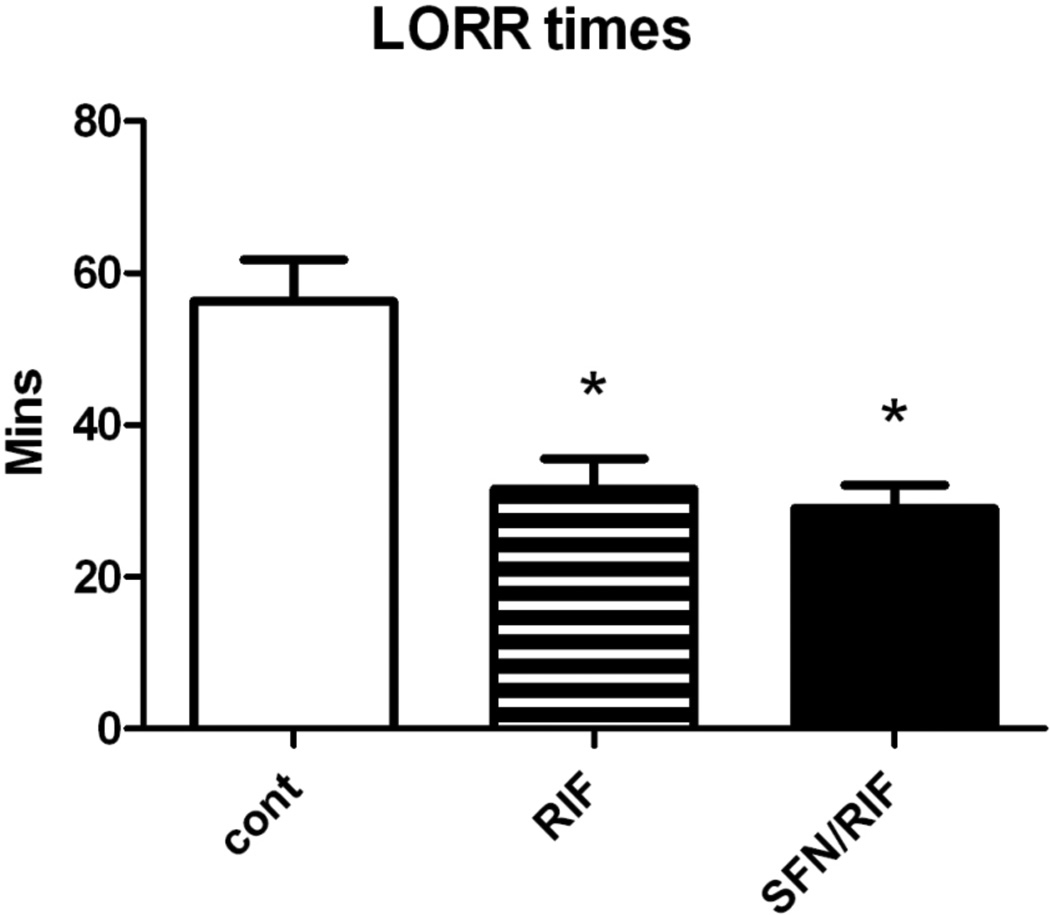
As an alternate approach to determine if SFN could prevent the PXR-mediated induction of Cyp3all in humanized PXR mice in vivo, we utilized the “Loss of Righting Reflex” (LORR) assay which measures the capacity of the liver to eliminate the anesthetic, 2,2,2 tribromoethanol, a Cyp3all-specific substrate. This assay allows an evaluation of the extent of induction of Cyp3all after RIF dosing without sacrificing the mouse.
Consistent with the induction of Cyp3all mRNA following RIF, mice administered 40mg/kg/d of RIF had a significantly reduced LORR time following a dose of 2,2,2-tribromoethanol (Figure 5). Co-administration of SFN at either 25 or 50 mg/kg dose had no effect on the ability of RIF to induce Cyp3all, as indicated by LORR times (Figure 5).
Figure 5.
The mean LORR time for each of the three different treatments: RIF 40 mg/kg, SFN 25 or 50 mg/kg 3X per day (SFN doses were combined as no effect was seen at either dose). Stars indicate significant differences from control as measured by paired t-test. N=8.
Discussion
The ligand-activated nuclear transcription factor PXR plays a key role in the regulation of a number of xenobiotic biotransformation enzymes. As numerous drugs are either activating ligands for PXR and/or substrates for enzymes that are regulated by PXR, ligand activation of PXR is a major mechanism responsible for a host of drug-drug interactions. Development of a PXR antagonist has potential therapeutic uses as a co-drug and may be useful in other cases of inappropriate PXR activation. For example, resistance to chemotherapy has in some instances been associated with high levels of PXR activity (29–31). We previously demonstrated that, in vitro, SFN is an effective antagonist of ligand activation of human PXR, inhibiting both basal expression of CYP3A4 as well as ligand (e.g., RIF) activation of CYP3A4 expression in human hepatocytes in primary culture [12]. However, for the potential therapeutic utility of SFN to be realized, it must be demonstrated to be efficacious in vivo. This study represents the first human trial to determine the efficacy of SFN as an in vivo antagonist of ligand activation of the human PXR in vivo.
Bioavailability of SFN from a broccoli sprout extract
Although SFN has not been approved as a pharmaceutical agent, several clinical studies have utilized broccoli sprouts containing relatively high levels of the sulforaphane precursor, glucoraphinin. In one of the first studies to evaluate bioavailability of SFN following administration of broccoli sprouts, Shapiro et al. (32) monitored urinary ITC levels following administration of a single dose of 111 µmoles of ITCs or glucosinolates. They found that 80% and 12%, of ITCs were recovered in the urine 72 hrs following administration hydrolyzed and unhydrolyzed sprouts, respectively, demonstrating the greatly improved bioavailability of SFN and other isothiocyanates following myrosinase pretreatment of broccoli sprout homogenates (32). In a subsequent study, Shapiro et al. (33) administered untreated or myrosinase-hydrolyzed broccoli sprout extracts containing the equivalent of 25 µmoles of total ITCs 3 times daily (75 mg total daily dose), for 7 days. Analysis of the glucosinolates in the untreated broccoli sprout extract showed that ~75% was in the form of glucoraphanin, the precursor to SFN, and ~24% in the form of glucoreucin plus small amounts of several other glucosinolates. Isothiocyanate excretion in the individuals receiving glucosinolates ranged from 8% to 31%, suggesting variable and incomplete hydrolysis and/or absorption of SFN (33). However, apparent bioavailability was substantially greater when the broccoli sprouts were hydrolyzed with myrosinase prior to administration; average urinary ITC excretion was ~70% of the administered dose, again demonstrating that prior hydrolysis greatly increased bioavailability of SFN and other ITCs. In a study that utilized a glucosinolate rich tea derived from broccoli sprouts, Kensler et al. reported that urinary ITC output over 12 hours exhibited large interindividual variability ranging from 4–45% of a given (400 µmoles) glucoraphanin dose (34). This observation was confirmed by a crossover study from Kensler et al. performed in Qidong China, comparing urinary SFN excretion after administration of a broccoli sprout tea prepared either with or without myosinase treatment, mean urinary excretion of SFN was 70% after consumption of the myrosinase treated tea, and 5% after consumption of the untreated tea (34). Fahey et al. confirmed these numbers with a study conducted in Baltimore Maryland, also seeing large interindividual variation in urinary excretion (35).
Given the highly variable and relatively poor bioavailability of SFN from direct administration of broccoli sprout preparations, in the present study we utilized a protocol developed by Shapiro et al. (33) in which the broccoli sprouts were placed in boiling water briefly to inactivate endogenous enzymes that favor the conversion to the biologically inactive sulforaphane nitrile, followed by addition of daikon radish sprouts which contain relatively high levels of myrosinase that selectively cleaves SFN from the glucosinolate moiety. Analysis of the lyophilized, myrosinase-treated broccoli sprout extract demonstrated that greater than 98% of the glucoraphanin had been converted to SFN. Although we did not directly assess the bioavailability of SFN from the lyophilized broccoli sprout extract, over 50% of the administered dose of SFN was recovered in the urine during the first 18 hrs after administration of the first dose of SFN.
Safety of SFN administered as a hydrolyzed broccoli sprout extract
The study of Shapiro et al (33) was a double-blind, placebo-controlled, randomized clinical study of the safety and tolerance of repeated doses of broccoli sprout extracts. Clinical evaluation included routine blood and urine analyses, tests for thyroid and liver function, and thorough symptom evaluation by trained observers. Six subjects were evaluated following doses of 75 or 300 µmoles of unhydrolyzed glucosinolates daily (25 or 100 µmoles every 8 hrs) for 7 days. Three subjects received 75 µmoles of hydrolyzed glucosinolates (~75% as SFN) daily for 7 days, for a total dose of ~525 µmoles. No treatment related adverse effects were identified. In the present study, we administered a daily dose of 450 µmoles of SFN (given orally as myrosinase-hydrolyzed broccoli sprout extract) for 7 consecutive days. Although a few subjects in the pre-screening evaluation withdrew from the study because they found the soup containing the hydrolyzed broccoli sprout extract unpalatable, of the 23 subjects completing all three arms of the trial, no treatment-related adverse effects were reported, and all clinical parameters evaluated remained in the range of normal. Thus, this study provides further data demonstrating that oral doses of SFN as high 450 µmoles per day can be administered safely for at least one week in healthy human volunteers.
Biological effects of SFN in humans following administration of broccoli sprout extracts
In the present study, daily doses of ~ 450 µmoles SFN/day for 7 days did not have the hypothesized effect of preventing rifampicin-mediated activation and subsequent induction of CYP3A4 that was seen in human hepatocytes in vitro. Because we were unable to replicate in vivo the robust inhibition of PXR activation by SFN seen in vitro in human hepatocytes, it is necessary to explore possible reasons for the lack of efficacy in vivo. The first possibility may simply be that an inadequate dose of SFN reaches the liver following the ingestion of the broccoli sprout extract. As noted above, there is ample evidence that SFN is well absorbed from the gastrointestinal tract, as previous studies using nearly identical preparations of broccoli sprouts have demonstrated recovery of total ITCs of 70–80%, and we were able to recover ~ 50% of the administered dose in urine in the first 18 hrs following administration. In another trial, a single dose of 200 µmoles SFN in the form of broccoli sprout extract given to 8 healthy volunteers led to readily detectable metabolite levels (~2 pmoles/mg tissue) in breast tissue approximately 1.5 hr after dosing (25). Therefore, it is unlikely that poor absorption could explain the lack of in vivo efficacy.
It is important to note that the above calculations represent total SFN, including metabolites. Thus, it is possible that gastrointestinal conjugation of SFN with GSH may have greatly reduced the bioavailability of ‘free’ (unconjugated) SFN to the liver. However, results from another study demonstrated that a single dose of 102 µmoles of SFN in broccoli sprout homogenate was able to cause up-regulation of phase II enzymes in the upper respiratory tract without causing deleterious side effects (36). Recently Yanaka et al (37) found that two months of daily intervention with broccoli sprouts containing ~420 µmoles unhydrolyzed glucoraphanin reduced the sequelea of infection in Helicobacter pylori infected patients (37). In another human trial, Myak et al reported that consumption of 68 g broccoli sprouts containing the equivalent of ~105 mg SFN (~560 µmoles) in the glucosinolate form inhibited HDAC activity 3 hours after broccoli sprout consumption (9). In aggregate, these studies indicate oral absorption of unchanged SFN in amounts sufficient to elicit NRF2 activation.
As noted above, studies in which glucosinolates rather than ITCs were administered relies upon plant and/or enteric myrosinase activity for the conversion of the precursor into the biologically active form, which likely contributes to incomplete conversion of glucosinolate to ITC and large differences in exposure between individuals. Thus, it is unlikely that participants in previous trials that utilized doses of more than 400 µmoles glucosinolate were exposed to levels of SFN as high as the 450 µmoles of SFN given in our trial, since we used a hydrolyzed broccoli sprout extract in which nearly all of the glucosinolate was present as ‘free’ SFN.
Gasper et al. (33) administered to healthy volunteers broccoli soup containing a cultivar of ‘super broccoli’ containing the equivalent of ~345 µmoles SFN. The florets were cooked briefly and then homogenized. Although the conversion of glucosinolates to free SFN was not reported specifically, the authors stated that preliminary studies demonstrated that “plant thioglucosidases remain active and glucosinolates are converted to isothiocyanates without nitrile formation.” Gaspar et al. (38) measured 24 hour urinary ITC excretion and reported a recovery of 55% of the administered dose, which is close to our measured average 18 hour urinary excretion of 40% of the 450 µmole dose (38). The study by Gasper et al. showed that average plasma ITC levels peaked at ~7 µM 1.5 hours after oral administration of 345 µmoles of ITC. This value represented total SFN plus glutathione conjugate-derived metabolites. Over the 24 hr period of the study, approximately 45% of the plasma AUC was unchanged (free) SFN, with the remaining distributed among various GSH-derived metabolites (SFN-GSH, SFH-Cys, SFN-Cys-Gly and SFN-NAC).
In this clinical trial with healthy volunteers we saw, as expected, robust induction of CYP3A4 activity by RIF as measured by MDZ AUC and mean peak MDZ plasma concentration. The drug was able to reduce the AUC for MDZ by over 70%. However, concurrent dosing with 450 µmoles of SFN in the broccoli sprout extract had no effect on the magnitude of induction of CYP3A4 activity after RIF treatment. In our previous In vitro study, the IC50 for the antagonistic effect of SFN on PXR-mediated activation of CYP3A4 expression in human hepatocytes was 16 µM, and there was significant inhibition at 10 µM. However, at 1 µM there was only slight inhibition of RIF-activation of PXR by SFN in a HepG2 cell system transfected with human PXR (12). Others have suggested a Kd for RIF binding to the ligand binding domain of PXR in the low micromolar range (39–41) SFN has been associated with a number of chemopreventative effects in numerous cell lines with a range of different IC50s, but many of the effects may be achieved at the low micromolar range, while our in vitro data suggests that hepatic concentrations in excess of 5 µM free SFN may be necessary to significantly inhibit RIF activation of SFN. If, over the 24 hr period following administration of SFN, an average of 45% of the administered dose of SFN was present as ‘free’ SFN (33), then peak plasma concentrations of free SFN in our study may have been on the order of 3–4 µM. In cell culture, intracellular levels of total (free plus GSH-conjugates) SFN can be upwards of 100 fold higher than those in the media due to intracellular conjugation with glutathione and the inability of the conjugate to readily pass through the cell membrane (19). In vivo, active transport of the SFN-GSH conjugate out of the liver may prevent intracellular accumulation of total SFN. It is impossible to know what the actual concentration of free SFN was in liver tissue in our subjects, but the lack of any effect of SFN on RIF activation of PXR suggests that it may have been in the low micromolar range, or less. It has been suggested that the SFN-GSH adduct is unstable and can result in spontaneous hydrolysis, thus serving as a reservoir for ‘free’ intracellular SFN in vitro 42). Thus, it is possible that such conjugate transport differences between the in vitro and in vivo conditions could result in differential intracellular availability even when cells in vivo and in vitro are exposed to similar extracellular (plasma or media) concentrations. It is also possible that SFN accumulated to significant concentrations within the hepatocytes, causing an initial inhibition of PXR-mediated CYP3A4 transcription in vivo, but that this was a transient inhibition lost at later time points as SFN has a short half-life and is rapidly eliminated. If there is an effect in vivo but it diminishes rapidly as SFN is excreted, inhibition may become apparent after repeated dosing, or perhaps dietary supplementation with SFN if either approach were to result in higher ‘steady state’ levels of parent SFN in liver.
The specific mechanism by which SFN inhibits PXR activation by RIF and other ligands has not yet been elucidated. Preliminary studies in our laboratory failed to identify SFN-lysine adducts in the PXR ligand binding domain via peptide mass spectrometry, and site-directed mutagenesis of putative isothiocyanate-modifiable amino acid residues did not identify potential sites for direct adduction (Poulton and Eaton, unpublished observations). It is thus unclear what other mechanisms, apart from differences in pharmacokinetics of SFN and rifampicin in vitro vs. in vivo, could account for the discrepancy between the effective antagonism of SFN seen in vitro, and lack of observed antagonism in vivo, following rimfampicin treatment. Whether SFN might antagonize other PXR ligands in vivo has not been determined. While not feasible in a human study, the humanized PXR mouse may be an appropriate model to test the effects of different rates of SFN administration on PXR activation by different activating ligands, such as hyperforin, which has a reported EC50 for PXR ligand binding slightly lower than RIF, or omeprazole, which has a reported EC50 substantially greater than RIF (41).
Although SFN failed to effectively antagonize the induction of CYP3A4 by the potent exogenous ligand, RIF, there did appear to be an effect of SFN on basal CYP3A4 expression, which is driven in part by activation of PXR with endogenous ligands. When all participants were included, basal levels of PXR were not affected by consumption of broccoli sprout extract. However, when the data were divided into quartiles and the 25% with the lowest CYP3A4 activity (presumably because of low endogenous PXR activation) were removed from the analysis, there is a statistically significant 20% decrease in CYP3A4 activity seen after 7 days of dosing with broccoli sprout extract. A similar but not significant trend was seen with the l’-OH metabolite of MDZ. Although these changes are not likely to be of clinical importance, the fact that they can be seen suggests that the mechanism of antagonism seen in vitro may still be functionally possible in vivo, and that it is dosing or pharmacokinetic limitations rather than a complete failure of the antagonistic mechanism that resulted in the lack of SFN inhibition of RIF mediated PXR activation in vivo. Importantly, these data demonstrate that inhibition of CYP3A4 expression is not likely to be an important ‘adverse effect’ following repeated dosing with SFN for chemoprevention purposes.
It is interesting to speculate on the potential significance of SFN inhibition of putative endogenous ligands of PXR. In isolated human hepatocytes, 50 µM SFN nearly completely blocked transcription of CYP3A4, apparently by inhibiting unknown endogenous ligands (12). It remains uncertain exactly what endogenous molecules serve as endogenous ligands for human PXR. Some bile acids, especially lithocholic acid (LCA), a hepatotoxic secondary bile acid, are effective PXR agonists of both mouse and human PXR (43). It was initially hypothesized that LCA-mediated activation of PXR and subsequent induction of Cyp3a provided a protective pathway to limit LCA-induced hepatotoxicity (43), although a more recent report with PXR null mice found that LCA induced enzymes that decreased its hepatotoxicity independently of PXR, and the induction occurred primarily in intestinal cells (44). LCA is an effective ligand of the Vitamin D receptor, as well as PXR (45). Dussault et al (46) demonstrated that an intermediate in the catabolism of cholesterol to bile acids, 5β-cholestane-3α,7 α,12 α-triol, was an effective and relatively potent ligand of mouse PXR, but was ineffective toward human PXR. Interestingly, the PXR-driven induction of Cyp3a by 5β-cholestane-3α,7 α,12 α-triol appears to ameliorate the neurotoxicity - of this cholesterol metabolite in mice (46). Thus, although the specific endogenous ligand(s) for human PXR remain unclear, it seems unlikely that modest inhibition of endogenous ligand activation of PXR by SFN is likely to have any adverse effects, since the magnitude of reduction of ‘basal’ CYP3A4 in SFN-treated subjects in this study was small and not likely to be pharmacologically significant.
SFN is not an antagonist to normal mouse or rat PXR, making those animals unsuitable for use as a model. However, the development of a transgenic hPXR mouse provides a suitable animal model to test the inhibitory properties of SFN in vivo. Indeed, primary mouse hepatocytes from hPXR mice mimic almost exactly the response previously seen in primary human hepatocytes. In addition, in whole animals SFN did not appear to affect Cyp3all mRNA or activity levels at any tested doses or time points just as was seen in the phase I trial. While it was outside the scope of this study, these animals appear to be a suitable model to study the phenomena of PXR inhibition. Future work using these mice could include testing other, weaker PXR agonists or lower concentrations of RIF.
The only effect noticed in the human trial that was not duplicated in the mouse model was the basal CYP3A4 inhibition. The end points tested in the mouse model, mRNA and recovery from anesthesia, were probably less sensitive than the MDZ parameters used in the human trial. Also, the effects observed in humans were modest; they would not have been detected if we were not able to use each subject as their own control. A crossover study in the mouse model was not feasible.
Many transgenic mouse models have been developed for use in research and many more are likely to be developed in the future. If these models are to be used to generate data for extrapolation into human settings it is important to validate that such ‘humanized’ mouse models are indeed more predictive of human response than ‘wild type’ mice. We show here that in this case for drug-drug interactions the humanized transgenic mouse model prove to be a good predictive model for human response, when validated against a controlled human trial with a similar design.
In summary, we demonstrated that, although SFN is an effective inhibitor of the hPXR in vitro, it does not appear to be a viable in vivo antagonist of exogenous (drug) activation of PXR, at least at the dose rate and conditions used in this study. However, the ability of SFN to repress basal level CYP3A4 activity in a subset of the study population suggests that the mechanism of inhibition seen in vitro is viable in vivo, but dosing, pharmacokinetic, or other limitations prevent it from acting as an effective antagonist of ligand activation of PXR. The development of a functional in vivo PXR antagonist remains a therapeutic need.
Highlights.
Comparison of Humanized PXR Mice with a Human Phase I Clinical Trial Evaluating Sulforaphane (SFN) as a Potential Antagonist of the Pregnane X-Receptor (PXR)
Rifampicin increased CYP3A4 activity in humans by 70%, based on reduction in midazolam AUC;
SFN had no significant effect on rifampicin-mediated induction of CYP3A4;
SFN was an effective antagonist of PXR activation in ‘humanized PXR’ mouse hepatocytes;
SFN co-administration had no effect on rifampicin induction of Cyp3a11 in humanized PXR mice;
antagonism of PXR activation by SFN seen in vitro is not readily achievable in vivo.
Acknowledgements
This research was supported NIGMS R01GM079280-01A1 (DLE), R01GM063666 (KT), and NIEHS Training Grant T32ESO7032 (EJP). Mass Spectrometry support was provided through NIGMS Program Project grant P01GM032165 (DDS). Genotyping and other functional genomics analyses were provided by NIEHS Center Grant P30ES007033 (DLE). Clinical support was provided through the Institute for Translational Health Sciences CTSA grant UL1 RR025014. The authors would like to thank Jed Fahey and Professor Paul Talalay at The Johns Hopkins University for their assistant in acquiring and characterizing the lyophilized, hydrolyzed broccoli sprout extract used in this study, and for their helpful suggestions in the study design. The authors also thank Dr. Frank Gonzalez at the National Cancer Institute for making the humanized PXR mice available to us.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McWalter GK, Higgins LG, McLellan LI, Henderson CJ, Song L, Thornalley PJ, Itoh, K., Yamamoto M, Hayes JD. Transcription factor Nrf2 is essential for induction of NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J Nutr. 2004;134:3499S–3506S. doi: 10.1093/jn/134.12.3499S. [DOI] [PubMed] [Google Scholar]
- 4.Chuang LT, Moqattash ST, Gretz HF, Nezhat F, Rahaman J, Chiao JW. Sulforaphane induces growth arrest and apoptosis in human ovarian cancer cells. Acta Obstet Gynecol Scand. 2007;86:1–6. doi: 10.1080/00016340701552459. [DOI] [PubMed] [Google Scholar]
- 5.Choi WY, Choi BT, Lee WH, Choi YH. Sulforaphane generates reactive oxygen species leading to mitochondrial perturbation for apoptosis in human leukemia U937 cells. Biomed Pharmacother. 2008;62:637–644. doi: 10.1016/j.biopha.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Terce F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Research. 2000;60:1426–1433. [PubMed] [Google Scholar]
- 7.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Research. 2004;64:5767–5774. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 9.Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med (Maywood) 2007;232:227–234. [PMC free article] [PubMed] [Google Scholar]
- 10.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis . 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 11.Gills JJ, Jeffery EH, Matusheski NV, Moon RC, Lantvit DD, Pezzuto JM. Sulforaphane prevents mouse skin tumorigenesis during the stage of promotion. Cancer Lett . 2006;236:72–79. doi: 10.1016/j.canlet.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Zhou C, Poulton EJ, Grun F, Bammler TK, Blumberg B, Thummel KE, Eaton DL. The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol Pharmacol. 2007;71:220–229. doi: 10.1124/mol.106.029264. [DOI] [PubMed] [Google Scholar]
- 13.Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, Tomkinson NC, LeCluyse EL, Lambert MH, Willson TM, et al. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Molecular Endocrinology . 2000;14:27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- 14.Bauer B, Yang X, Hartz AMS, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS. In Vivo Activation of Human Pregnane X Receptor Tightens the Blood-Brain Barrier to Methadone through P-Glycoprotein Up-Regulation. Mol Pharmacol. 2006;10:1212–1219. doi: 10.1124/mol.106.023796. [DOI] [PubMed] [Google Scholar]
- 15.Paine MF, Criss AB, Watkins PB. Two major grapefruit juice components differ in intestinal CYP3A4 inhibition kinetic and binding properties. Drug Metab Dispos. 2004;32:1146–1153. doi: 10.1124/dmd.104.000547. [DOI] [PubMed] [Google Scholar]
- 16.Burk O, Koch I, Raucy J, Hustert E, Eichelbaum M, Brockmoller J, Zanger UM, and Wojnowski L. The Induction of Cytochrome P450 3A5 (CYP3A5) in the Human Liver and Intestine Is Mediated by the Xenobiotic Sensors Pregnane X Receptor (PXR) and Constitutively Activated Receptor (CAR) Journal of Biological Chemistry. 2004;279:38379–38385. doi: 10.1074/jbc.M404949200. [DOI] [PubMed] [Google Scholar]
- 17.Willson TM, Kliewer SA. PXR, CAR and drug metabolism. Nat Rev Drug Discov . 2002;1:259–266. doi: 10.1038/nrd753. [DOI] [PubMed] [Google Scholar]
- 18.Brooks JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol Biomarkers Prev. 2001;10:949–954. [PubMed] [Google Scholar]
- 19.Zhang Y. Molecular mechanism of rapid cellular accumulation of anticarcinogenic isothiocyanates. Carcinogenesis. 2001;22:425–431. doi: 10.1093/carcin/22.3.425. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Callaway EC. High cellular accumulation of sulphoraphane, a dietary anticarcinogen, is followed by rapid transporter-mediated export as a glutathione conjugate. Biochem J. 2002;364:301–307. doi: 10.1042/bj3640301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tirona RG, Leake BF, Podust LM, Kim RB. Identification of Amino Acids in Rat Pregnane X Receptor that Determine Species-Specific Activation. Mol Pharmacol. 2004;65:36–44. doi: 10.1124/mol.65.1.36. [DOI] [PubMed] [Google Scholar]
- 22.Ma X, Shah Y, Cheung C, Guo GL, Feigenbaum L, Krausz KW, Idle JR, Gonzalez FJ. The PREgnane X receptor gene-humanized mouse: a model for investigating drug-drug interactions mediated by cytochromes P450 3A. Drug Metab Dispos. 2007;35:194–200. doi: 10.1124/dmd.106.012831. [DOI] [PubMed] [Google Scholar]
- 23.Scheer N, Ross J, Rode A, Zevnik B, Niehaves S, Faust N, Wolf CR. A novel panel of mouse models to evaluate the role of human pregnane X receptor and constitutive androstane receptor in drug response. J Clin Invest. 2008;118:3228–3239. doi: 10.1172/JCI35483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, Brunt EM, Guzelian PS, Evans RM. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–439. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- 25.Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, Chen MS, Stierer T, Garrett-Mayer E, Argani P, et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Wade KL, Prestera T, Talalay P. Quantitative determination of isothiocyanates, dithiocarbamates, carbon disulfide, and related thiocarbonyl compounds by cyclocondensation with 1,2-benzenedithiol. Anal Biochem. 1996;239:160–167. doi: 10.1006/abio.1996.0311. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Li H, Moore LB, Johnson MDL, Maglich JM, Goodwin B, Ittoop ORR, Wisely B, Creech K, Parks DJ, et al. The Phytoestrogen Coumestrol Is a Naturally Occurring Antagonist of the Human Pregnane X Receptor. Molecular Endocrinology. 2008;22:838–857. doi: 10.1210/me.2007-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Backman J, Olkkola K, Neuvonen P. The area under the plasma concentration-time curve for oral midazolam is 400-fold larger during treament with itraconazole than with rifampicin. European Journal of Clinincal Pharmacology. 1998;54:53–58. doi: 10.1007/s002280050420. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Tang Y, Wang MT, Zeng S, Nie D. Human pregnane X receptor and resistance to chemotherapy in prostate cancer. Cancer Research. 2007;67:10361–10367. doi: 10.1158/0008-5472.CAN-06-4758. [DOI] [PubMed] [Google Scholar]
- 30.Ling V. Multidrug resistance: molecular mechanisms and clinical relevance. Cancer Chemother Pharmacol. 1997;40(Suppl):S3–S8. doi: 10.1007/s002800051053. [DOI] [PubMed] [Google Scholar]
- 31.Mensah-Osman EJ, Thomas DG, Tabb MM, Larios JM, Hughes DP, Giordano TJ, Lizyness ML, Rae JM, Blumberg B, Hollenberg PF, et al. Expression levels and activation of a PXR variant are directly related to drug resistance in osteosarcoma cell lines. Cancer. 2007;109:957–965. doi: 10.1002/cncr.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prev. 2001;10:501–508. [PubMed] [Google Scholar]
- 33.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 34.Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, Ye L, Coady JL, Wang JB, Wu Y, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 35.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. PNAS. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway. Clin Immunol. 2009;130:244–251. doi: 10.1016/j.clim.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, Tauchi M, Suzuki H, Hyodo I, Yamamoto M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila) 2009;2:353–360. doi: 10.1158/1940-6207.CAPR-08-0192. [DOI] [PubMed] [Google Scholar]
- 38.Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Glutathione S-transferase Ml polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr . 2005;82:1283–1291. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 39.Huang H, Wang H, Sinz M, Zoeckler M, Staudinger J, Redinbo MR, Teotico DG, Locker J, Kalpana GV, Mani S. Inhibition of drug metabolism by blocking the activation of nuclear receptors by ketoconazole. Oncogene. 2007;26:258–268. doi: 10.1038/sj.onc.1209788. [DOI] [PubMed] [Google Scholar]
- 40.Navaratnarajah P, Steele BL, Redinbo MR, Thompson NL. Rifampicin- independent interactions between the pregnane X receptor ligand binding domain and peptide fragments of coactivator and corepressor proteins. Biochemistry. 2012;51:19–31. doi: 10.1021/bi2011674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao L, Nickbarg E, Wang W, Thomas A, Ziebell M, Prosise WW, Lesburg CA, Taremi SS, Gerlach, V.L, Le HV, et al. Evaluation of in vitro PXR-based assays and in silico modeling approaches for understanding the binding of a structurally diverse set of drugs to PXR. Biochem Pharmacol. 2011;81:669–679. doi: 10.1016/j.bcp.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Conaway C, Yang Y, Chung FI. Isothiocyanates as Cancer Chemopreventive Agents: Their Biological Activities and Metabolism in Rodents and Humans. Current Drug Metabolism . 2002;3:233–255. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- 43.Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, La Tour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Owen BM, Milona A, van Mil S, Clements P, Holder J, Boudjelal M, Cairns W, Parker M, White R, Williamson C. Intestinal detoxification limits the activation of hepatic pregnane X receptor by lithocholic acid. Drug Metab Dispos. 2010;38:143–149. doi: 10.1124/dmd.109.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reschly EJ, Krasowski MD. Evolution and function of the NR1I nuclear hormone receptor subfamily (VDR, PXR, and CAR) with respect to metabolism of xenobiotics and endogenous compounds. Curr Drug Metab. 2006;7:349–365. doi: 10.2174/138920006776873526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dussault I, Yoo HD, Lin M, Wang E, Fan M, Batta AK, Salen G, Erickson SK, Forman BM. Identification of an endogenous ligand that activates pregnane X receptor- mediated sterol clearance. Proc Natl Acad Sci USA. 2003;100:833–838. doi: 10.1073/pnas.0336235100. [DOI] [PMC free article] [PubMed] [Google Scholar]