Abstract
We found that absence of osteopontin (OPN) in immunocompromised Rag2−/− mice, which lack T and B cells, made the mice extremely susceptible to an opportunistic fungus Pneumocystis, although immunocompetent OPN-deficient mice could clear Pneumocystis as well as wild-type mice. OPN has been studied as an extracellular protein, and the role of an intracellular isoform of osteopontin (iOPN) is still largely unknown. Here, we elucidated the mechanism by which iOPN was involved in anti-fungal innate immunity. First, iOPN was essential for cluster formation of fungal receptors that detect Pneumocystis; including dectin-1, TLR2, and mannose receptor. Second, iOPN played a role as an adaptor molecule in TLR2 and dectin-1 signaling pathways, and mediated ERK activation and cytokine production by zymosan, which simultaneously activates TLR2 and dectin-1 pathways. Third, iOPN enhanced phagocytosis and clearance of Pneumocystis. Our study here suggests the critical involvement of iOPN in anti-fungal innate immunity.
Introduction
Pneumocystis is an opportunistic fungal pathogen that cause of serious morbidity and mortality in immunocompromised individuals. Host cells recognize Pneumocystis via pattern recognition receptors (PRRs), such as dectin-1, Toll-like receptor 2 (TLR2), and mannose receptor (MR), followed by phagocytosis, cytokine production, and killing the pathogen (1–3). These PRRs are recruited in proximity on the cell surface and cooperatively integrate their signals (4–7). However, the molecular mechanisms by which these PRRs collaborate are largely unknown.
Osteopontin (OPN) is a protein, which is expressed by a variety of tissues and cell types, including macrophages, dendritic cells (DCs), NK cells, neutrophils, and T and B lymphocytes. The role of OPN in innate immunity is reflected by its protective role in infectious diseases, but only limited numbers of reports are available on the involvement of OPN in host responses against fungi (8, 9). A recent microarray study, however, showed that expression of Opn mRNA had the highest upregulation 8 weeks after Pneumocystis infection in alveolar macrophages from immunosuppressed rats (10). OPN has two types of isoforms; secreted OPN (sOPN) and intracellular OPN (iOPN) that was recently characterized (11–17). Opn−/− mice showed attenuated resistance to a low dose Candida albicans infection (9), but depletion of OPN by antibody (Ab) in wild-type (WT) mice did not attenuate the resistance to C. albicans (9). The finding suggested that the host resistance to C. albicans is attributed to iOPN, but not sOPN, because OPN Ab only depletes sOPN.
OPN has been studied as extracellular protein, i.e., as sOPN, and the role of iOPN is much less characterized compared to that of sOPN. Thus, current understanding on iOPN is limited, but it is known that innate immune phagocytes, such as macrophages and DCs, constitutively express high levels of iOPN (and sOPN), but T cells prefer to express sOPN (12, 14, 18). Currently, iOPN is known to participate in TLR9 signaling, by interacting with MyD88, and enhances IFNα production by plasmacytoid DCs (14). These results suggested that iOPN plays a role as an adaptor molecule in innate immune signaling pathways.
Adaptive immunity is considered to be the ultimate mechanism to resolve opportunistic fungal infection. However, our study here demonstrated that iOPN in Rag2−/− mice, which lack adaptive immunity, make a significant difference in host resistance against opportunistic Pneumocystis fungal infection. In contrast, OPN-deficient immunocompetent mice can clear Pneumocystis, suggesting that OPN is not essential in the presence of adaptive immunity. However, OPN still matters in immunocompromised hosts -- patients who contract opportunistic fungal infections are immunocompromised. In this study, we also demonstrated that iOPN does not only play a critical role in formation of fungal PRR clusters on the cell surface, but iOPN is also involved in TLR2 and in dectin-1 signaling transduction pathways as an adaptor molecule.
Materials and Methods
Animals and Pneumocystis
C57BL/6 background mice were used in this study. Opn−/− mice were a gift from D. Denhardt and S. Rittling (Rutgers University), and backcrossed to C57BL/6 mice for 15 generations. Rag2−/− mice (Jackson Laboratory) were crossed to Opn−/− mice to generate Opn−/− Rag2−/− mice. All the mice were kept in a barrier facility. This study was approved by the Duke University Institutional Animal Care and Use Committee. Pneumocystis was obtained from ATCC (Pneumocystis murina, PRA-111), propagated in vivo by infecting Rag2−/− mice, and was harvested from lungs as previously described (19).
qPCR conditions and primer sequences
mRNA expression levels were determined by using the −ΔΔCt method of real-time PCR. qPCR primers/TaqMan probe for Pneumocystis rRNA (20) and PCMei2 (19) are previously described. Actb mRNA (encoding β-actin) was used as an internal control (14).
Confocal microscopy
To evaluate colocalization of Pneumocystis recognition receptors and iOPN, we stained cells with antibodies against OPN (2A1, Santa Cruz), MR (BioLegend), dectin-1 (BioLegend), and TLR2 (Molecular Probe) with fluorophore-conjugated secondary antibodies. The frequency (%) of cells that show clustering of iOPN and PRRs was calculated by enumerating cells that formed the cluster from at least 50 cells, which were either attached to Pneumocystis or had phagocytosed Pneumocystis.
Immunoprecipitation, immunoblotting, and ELISA
Immunoprecipitation was carried out with OPN antibody (2A1), followed by immunoblotting with IRAK1 antibody (Assay Designs) or Syk antibody (BioLengend) with Pam2CSK4 (TLR2 ligand) and curdlan (dectin-1 ligand), respectively. To evaluateERK activation by zymosan, peritoneal macrophages from WT and Opn−/− mice were used. To neutralize secreted OPN, we treated cells with a neutralizing antibody, AF808 (R&D Systems) at 10 μg/ml to the cells 10 min before Pneumocystis treatment. Opn−/− macrophages were infected using lentiviral vectors to express GFP and iOPN, as previously described (14). ERK and phospho-ERK were detected by immunoblotting. Levels of IL-1β and IL-10 in the culture supernatants of zymosan-stimulated macrophages were determined by ELISA (BD Pharmingen).
Analyzing phagocytosis, ROS production, and Pneumocystis clearance
Pneumocystis was labeled as previously reported (21), except for using Pneumocystis (1×107 cysts) with 2 μl of 10 mg/ml Alexa fluor 647 (Molecular Probe). Macrophages were stained with CD11b, and phagocytosis was evaluated by detecting CD11b and Alexa-647 double positive cells. To evaluate reactive oxygen species (ROS) generation by macrophage stimulation with Pneumocystis, dihydroethidium (DHE) staining was detected in CD11b-positive cells. Detection of Pneumocystis clearance was described previously (22).
Statistical analysis
Statistical analysis was evaluated using Student’s t tests. The criterion of significance was set as p<0.05. All results are expressed as mean ± SEM.
Results and Discussion
OPN-deficient Rag2−/− mice are susceptible to spontaneous Pneumocystis infection
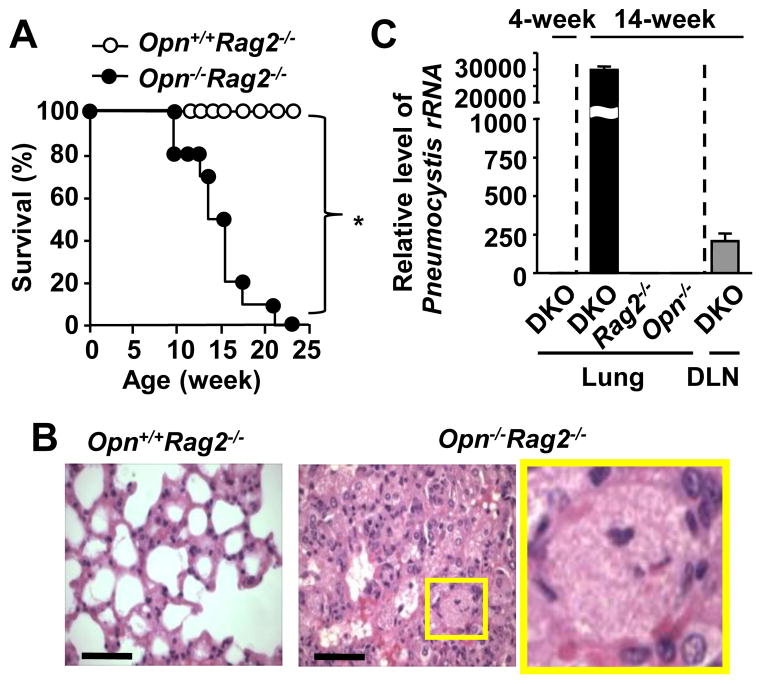
Immunocompromised Rag2−/− mice, which lack T and B cells, are susceptible to opportunistic fungal infection by Pneumocystis. Opn−/−Rag2−/− mice became sick after 12–13 weeks of age, under the environment that Opn+/+Rag2−/− mice were healthy and showing no signs of disease. Opn−/−Rag2−/− mice were sick and clearly smaller in size compared to the Opn+/+Rag2−/− mice at 13-week-old (Supplemental Fig. 1A), and started dying from 12–13 weeks of age (Fig. 1A). We performed pathology analyses on 14-week-old mice. Although the lungs from Opn+/+Rag2−/− mice appeared normal (Fig. 1B, left), those from the Opn−/−Rag2−/− mice indicated typical Pneumocystis histopathology, i.e., foamy exudates, stained in pink with H&E, which were filling alveolar spaces (Fig. 1B. center and right). Lung sections of Opn−/−Rag2−/− mice also showed extensive inflammatory cell infiltration, and lack of airway spaces (Fig. 1B). Opn−/−Rag2−/− mice could recruit cells into lungs (Fig. 1B), but the ability of cell recruitment did not appear to be a decisive factor to clear Pneumocystis, because of the high loads of Pneumocystis in Opn−/−Rag2−/− mice.
Figure 1. OPN-deficient Rag2−/− mice are extremely susceptible to spontaneous Pneumocystis.
(A) Survival ratio of Opn+/+Rag2−/− mice and Opn−/−Rag2−/− mice. n=10. (B) Representative H&E staining in the lungs of 13-week-old Opn+/+Rag2−/− mice and Opn−/−Rag2−/− mice. The image in a yellow rectangle in the middle panel is enlarged in the right panel. Scale bars = 50 μm. (C) PCMei2 mRNA was detected by qPCR, normalized with β-actin mRNA. First column denotes PCMei2 levels in lungs of 4-week-old Opn−/−Rag2−/− (DKO) mice, otherwise all the samples are from 14-week-old mice of DKO, and single KO of Rag2−/− or Opn−/−. The last column denotes values from lung draining LNs from 14-week-old DKO mice.
Pneumocystis cannot be cultured; therefore, it is not possible to assess the fungal loads by CFU (colony forming unit). Therefore, we confirmed Pneumocystis infection by detecting the expression of the mitochondrial large subunit rRNA of Pneumocystis (22) and Pneumocystis-specific gene PCMei2 (19). Four-week old Opn−/−Rag2−/− (double knockout; DKO) mice looked healthy, and the levels of the large subunit Pneumocystis rRNA and PCMei2 mRNA were around the detection limit in their lungs (Fig. 1C, supplemental Fig. 1B). However, 14-week-old Opn−/−Rag2−/− mice showed much higher expression levels of both transcripts than the 4-week-old Opn−/−Rag2−/− mice. This suggested that the mice developed intensive Pneumocystis infection. In addition to lungs, the presence of Pneumocystis was also detected in mediastinal (lung draining) lymph nodes (LNs) from the 14-week-old Opn−/−Rag2−/− mice. Lungs from single gene deficient mice, i.e., Opn+/+Rag2−/− and Opn−/−Rag2+/+ mice, at the age of 14 weeks did not have detectable levels of Pneumocystis rRNA and PCMei2 transcripts (Fig. 1C, supplemental Fig. 1B), suggesting that single knockouts remained highly resistant to opportunistic Pneumocystis infection. The data strongly suggested that OPN is not essential to protect hosts against opportunistic Pneumocystis when adaptive immunity is equipped. In summary, OPN plays a role to protect hosts against opportunistic Pneumocystis infection in innate immunity, but OPN is dispensable in the presence of adaptive immunity.
Co-localization of iOPN and fungal PRRs in macrophages stimulated by Pneumocystis
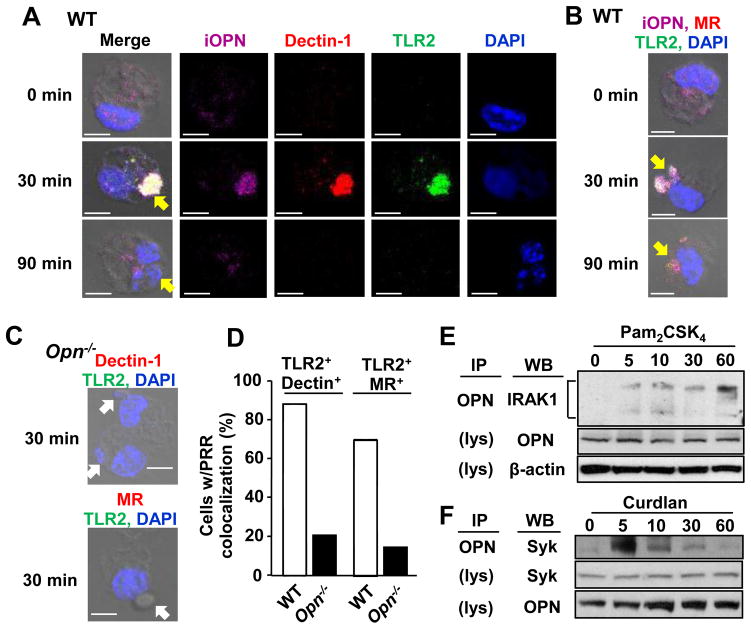
Macrophages contribute to the host immune responses to Pneumocystis by phagocytosis and subsequent killing of the fungus. TLR2, dectin-1, and MR are known to play a critical role in detection and clearance of Pneumocystis (1–3, 6, 23). Previous studies showed co-localization of the PRRs, and such physical proximity of the PRRs makes their signals integrated and synergized to enhance host responses (4–7). To evaluate involvement of iOPN in the association of fungal PRRs, we detected iOPN, TLR2, and dectin-1 or MR in wild-type (WT) macrophages stimulated with Pneumocystis. Thirty min after Pneumocystis treatment, cells showed co-localization of iOPN, TLR2, dectin-1, and MR upon contact with Pneumocystis (Fig. 2A, B; Supplemental Fig. 2A). On the other hand, Opn−/− macrophages failed to co-localize the PRR cluster (Fig. 2C, D; Supplemental Fig. 2B), suggesting that iOPN is essential for PRR clustering.
Figure 2. Interaction of iOPN and fungal PRRs by Pneumocystis treatment.
(A) Representative confocal microscopic images of OPN (magenta), dectin-1 (red), TLR2 (green) and DAPI (blue) staining in WT macrophages at indicated time points after co-culture with Pneumocystis. (B) Same as (A), except that MR is indicated with red. (C) No distinct cluster formation of PRRs in Opn−/− macrophages 30 min after Pneumocystis co-culture. (A–C) Yellow and white arrows represent phagocytosed Pneumocystis and Pneumocystis contacting to macrophages, respectively. Scale bars = 5 μm. See Supplemental Fig. 2A and 2B for single color staining images for (B) and (C), respectively. (D) Calculated frequency of macrophages with PRR colocalization (TLR2 and dectin-1 or MR) at 30 min after Pneumocystis co-culture. (E) Immunoprecipitation with OPN in RAW 264.7 cells stimulated with Pam2CSK4 (100 ng/ml) for 0, 5, 10, 30, and 60 min. Immunoblot were performed with anti-IRAK-1. OPN and β-actin were detected in non-immunoprecipitated cell lysates (indicated as “lys”). (F) Co-immunoprecipitation of iOPN and Syk in peritoneal macrophages stimulated with curdlan (100 μg/ml).
To elucidate a role of iOPN in downstream signaling pathways from TLR2, we first tested whether iOPN associates with IRAK1, which is one of downstream kinases of the TLR2 signaling pathway. IRAK1 was selected, because of possible interdependence between iOPN and IRAK1 from previous studies, demonstrating that iOPN and IRAK1 are required for TLR9-mediated IRF7 activation (14, 24). TLR2 stimulation with Pam2CSK4, a TLR2 ligand,induced OPN-IRAK1 association (Fig. 2E). This finding suggested the involvement of iOPN in TLR2 signaling. We also found that iOPN is involved in a dectin-1 signaling pathway by associating with Syk after stimulation with curdlan, a dectin-1 ligand (Fig. 2F). It should be noted that whole cell lysates include both iOPN and sOPN. Yet, OPN that associates with IRAK1 and Syk is iOPN, because iOPN, IRAK1 and Syk are cytosolic proteins but sOPN is segregated in secretory vesicles. In summary, we found that iOPN is essential for fungal PRR cluster formation and involved in signaling downstream of TLR2 and dectin-1 pathways by associating with IRAK1 and Syk, respectively.
iOPN facilitates phagocytosis and clearance of Pneumocystis
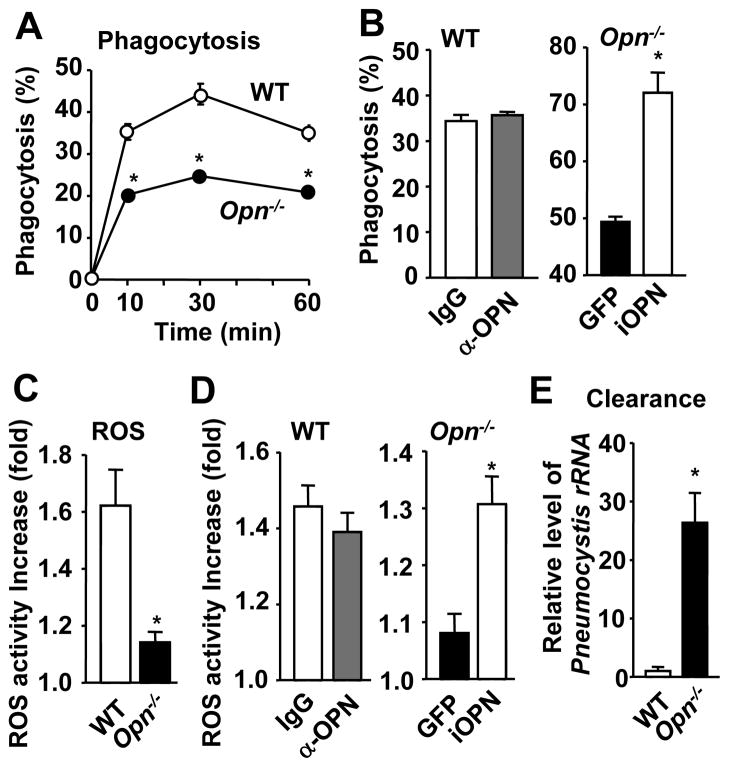
We next tested the requirement of iOPN in the phagocytosis of Pneumocystis. Pneumocystis labeled with Alexa-647 was cultured with WT or Opn−/− macrophages, and phagocytosis of Pneumocystis by macrophages was detected by flow cytometry. Phagocytosis was significantly reduced in Opn−/− macrophages, compared in WT macrophages (Fig. 3A; Supplemental Fig. 3A). We next evaluated which OPN, sOPN or iOPN, is involved in phagocytosis of Pneumocystis, by antibody-mediated sOPN depletion in WT macrophages and lentivirus-mediated iOPN expression in Opn−/−macrophages. sOPN depletion with OPN antibody (AF808; 10 μg/ml) in WT cell culture treatment did not reduce phagocytic activity (Fig. 3B, left panel), but expression of iOPN in Opn−/− macrophages rescued the OPN-deficient phenotype of decreased phagocytic ability (Fig. 3B, right panel). These findings strongly suggested that iOPN, but not sOPN, was critical for the phagocytosis of Pneumocystis by macrophages.
Figure 3. Involvement of iOPN in phagocytosis, ROS generation, and fungal clearance by macrophage.
(A) Phagocytosis of Pneumocystis by macrophages. Percentages of Alexa-647/CD11b double-positive cells over total CD11b positive cells were calculated (Supplemental Fig. 3A). (B) WT macrophages were cultured with Pneumocystis with OPN antibody (AF808, 10 μg/ml) or control IgG (left panel). iOPN was lentivirally expressed in Opn−/− macrophages (right panel). Phagocytic activity at 60 min. (C) ROS activity increase is calculated as MFI value increase (%) of DHE staining in the initial 1 h after Pneumocystis stimulation (Supplemental Fig. 3B). (D) Comparison of ROS generation by sOPN depletion in WT cells and by iOPN expression in Opn−/− cells, as described in (B). (E) Pneumocystis clearance was detected with Pneumocystis rRNA in macrophages.
Once pathogen is phagocytosed, ROS production is critical for its killing (25, 26). It was reported that ROS generation is impaired in dectin-1-deficient macrophages upon stimulation with Pneumocystis (2). We found that Opn−/− macrophages had significantly reduced ROS generation (Fig. 3C; Supplemental Fig. 3B). In addition, ROS activity was not affected by treatment with OPN antibody (Fig. 3D, left panel), but lentiviral reconstitution of iOPN expression in Opn−/− macrophages induced ROS generation (Fig. 3D, right panel). The result suggests that iOPN, not sOPN, is involved in ROS generation in macrophages that phagocytosed Pneumocystis. It is of note that deficiency of p91phox (Nox2), a component of the Nox2 NADPH oxidase that produces ROS, did not affect tissue damages in Pneumocystis-infected CD4 T cell-depleted immunocompetent mice (27). Since Sasaki et al. (28) demonstrated that Nox1 could compensate ROS generation in Nox2-deficient bone marrow macrophages/monocytes, the Nox1 NADPH oxidase may compensate resistance against Pneumocystis in Nox2-deficient mice. We also found that Pneumocystis was not effectively killed in Opn−/− macrophages as in WT macrophages. Higher levels of Pneumocystis rRNA and PCMei2 expression were detected in Opn−/− macrophages than wild-type macrophages, which phagocytosed Pneumocystis (Fig. 3E; Supplementary Fig. 3C). Collectively, our findings strongly suggested that iOPN in macrophages played a critical role to clear Pneumocystis by enhancing phagocytosis, ROS generation, and fungal killing.
iOPN enhances zymosan-induced cytokine production in macrophage
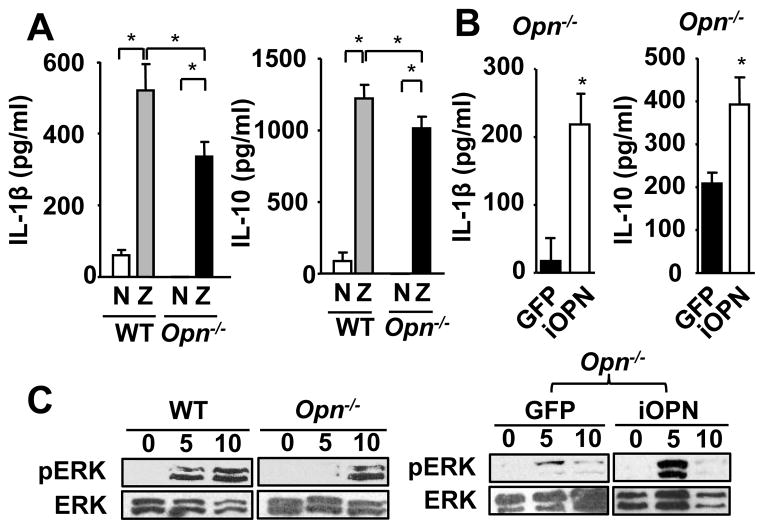
We next tested the requirement of iOPN in the cytokine production by macrophages stimulated with Pneumocystis. Although Opn−/− macrophages appeared to produce less IL-1β and IL-10 than WT macrophages when stimulated with Pneumocystis (Supplementary Fig. 3D), the levels of cytokines were not high enough by ELISA detection. To better assess the consequence of simultaneous stimulation of multiple receptors that detect Pneumocystis, we therefore used zymosan, a glucan from fungal cell wall. Zymosan, simultaneously ligates dectin-1 and TLR2, which are essential for the detection of Pneumocystis by host cells (5, 29–31). Opn−/− macrophages had significantly reduced production of both IL-1β and IL-10 (Fig. 4A), but not TNFα and IFNγ (Supplemental Fig. 3D), upon zymosan stimulation. Because iOPN is essential for clustering of dectin-1 and TLR2, and involved in PRR signaling as an adaptor molecule (Fig. 2), we asked whether iOPN is involved in the production of IL-1β and IL-10 by zymosan stimulation. iOPN expression successfully restored the production of IL-1β and IL-10 in Opn−/− macrophages (Fig. 4B), suggesting the involvement of iOPN in signal transduction pathways activated by zymosan. It is known that zymosan induces IL-10 production through ERK activation (30). Opn−/− macrophages had reduced ERK activation compared to WT macrophages, and iOPN expression in Opn−/− macrophages restored ERK activation (Fig. 4C). Collectively, our findings demonstrated that iOPN mediated signal transduction of dectin-1 and TLR2, by interacting with IRAK1 and Syk, leading to ERK-dependent cytokine production.
Figure 4. Involvement of iOPN in activating signal transduction triggered by zymosan.
(A) Expression of IL-1β and IL-10 by WTand Opn−/− macrophages stimulated with (indicated as “Z”) or without (indicated as “N”) 100 μg/ml zymosan for 24 hr. Macrophage culture supernatants were analyzed by ELISA. (B) Induction of IL-1β and IL-10 production by lentiviral iOPN expression by Opn−/− macrophages with zymosan stimulation. (C) Reduction of phospho-ERK (pERK) in zymosan-stimulated Opn−/−macrophages. (D) Induction of zymosan–induced ERK activation in Opn−/− macrophages by lentiviral iOPN expression.
In conclusion, we have shown that Opn−/−Rag−/− mice are extremely susceptible to spontaneous Pneumocystis infection. iOPN is essential for generating anti-fungal innate immune responses in PRR recognition, signal transduction, phagocytosis, and clearance of Pneumocystis, and cytokine production. It appears that iOPN not only clusters fungal PRRs together, but iOPN also functions as an adaptor molecule that transduces signals from PRRs (Supplemental Fig. 4). This study demonstrated a new mechanism by which a novel OPN isotype, iOPN, plays a critical role. Lack of iOPN in the innate immune system may explain the extremely susceptible phenotype of Opn−/−Rag−/− mice to opportunistic Pneumocystis infection.
Supplementary Material
Acknowledgments
We thank Drs. Minghua Zhu and Weiguo Zhang for technical comments, Drs. John Perfect and Joseph Heitman for discussion, Dr. Joshua Burgess for technical comments for handling Pneumocystis.
Grant support from Duke University School of Medicine Startup fund.
References
- 1.Ezekowitz RA, Williams DJ, Koziel H, Armstrong MY, Warner A, Richards FF, Rose RM. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature. 1991;351:155–158. doi: 10.1038/351155a0. [DOI] [PubMed] [Google Scholar]
- 2.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, Kinjo T, Nakamura K, Kawakami K, Iwakura Y. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 3.Wang SH, Zhang C, Lasbury ME, Liao CP, Durant PJ, Tschang D, Lee CH. Decreased inflammatory response in Toll-like receptor 2 knockout mice is associated with exacerbated Pneumocystis pneumonia. Microbes Infect. 2008;10:334–341. doi: 10.1016/j.micinf.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ip WK, Takahashi K, Moore KJ, Stuart LM, Ezekowitz RA. Mannose-binding lectin enhances Toll-like receptors 2 and 6 signaling from the phagosome. J Exp Med. 2008;205:169–181. doi: 10.1084/jem.20071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, Taylor PR, Kerrigan A, Tsoni SV, Gordon S, Meyer-Wentrup F, Adema GJ, Kullberg BJ, Schweighoffer E, Tybulewicz V, Mora-Montes HM, Gow NA, Williams DL, Netea MG, Brown GD. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tachado SD, Zhang J, Zhu J, Patel N, Cushion M, Koziel H. Pneumocystis-mediated IL-8 release by macrophages requires coexpression of mannose receptors and TLR2. J Leukoc Biol. 2007;81:205–211. doi: 10.1189/jlb.1005580. [DOI] [PubMed] [Google Scholar]
- 7.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 8.Nishikaku AS, Scavone R, Molina RF, Albe BP, da Cunha SC, Burger E. Osteopontin involvement in granuloma formation and in the severity of Paracoccidioides brasiliensis infection. Med Mycol. 2009;47:495–507. doi: 10.1080/13693780802342537. [DOI] [PubMed] [Google Scholar]
- 9.Sato I, Yamamoto N, Rittling SR, Denhardt DT, Hino M, Morimoro J, Sakai F, Fujie A, Uede T. Osteopontin is dispensable for protection against high load systemic fungal infection. Int Immunopharmacol. 2008;8:1441–1448. doi: 10.1016/j.intimp.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Cheng BH, Liu Y, Xuei X, Liao CP, Lu D, Lasbury ME, Durant PJ, Lee CH. Microarray studies on effects of Pneumocystis carinii infection on global gene expression in alveolar macrophages. BMC Microbiol. 2010;10:103. doi: 10.1186/1471-2180-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantor H, Shinohara ML. Regulation of T-helper-cell lineage development by osteopontin: the inside story. Nat Rev Immunol. 2009;9:137–141. doi: 10.1038/nri2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci U S A. 2008;105:7235–7239. doi: 10.1073/pnas.0802301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, Cantor H. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki K, Zhu B, Rittling SR, Denhardt DT, Goldberg HA, McCulloch CA, Sodek J. Colocalization of intracellular osteopontin with CD44 is associated with migration, cell fusion, and resorption in osteoclasts. J Bone Miner Res. 2002;17:1486–1497. doi: 10.1359/jbmr.2002.17.8.1486. [DOI] [PubMed] [Google Scholar]
- 16.Zhu B, Suzuki K, Goldberg HA, Rittling SR, Denhardt DT, McCulloch CA, Sodek J. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J Cell Physiol. 2004;198:155–167. doi: 10.1002/jcp.10394. [DOI] [PubMed] [Google Scholar]
- 17.Zohar R, Suzuki N, Suzuki K, Arora P, Glogauer M, McCulloch CA, Sodek J. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J Cell Physiol. 2000;184:118–130. doi: 10.1002/(SICI)1097-4652(200007)184:1<118::AID-JCP13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 18.Shinohara ML, Jansson M, Hwang ES, Werneck MB, Glimcher LH, Cantor H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc Natl Acad Sci U S A. 2005;102:17101–17106. doi: 10.1073/pnas.0508666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess JW, Kottom TJ, Limper AH. Pneumocystis carinii exhibits a conserved meiotic control pathway. Infect Immun. 2008;76:417–425. doi: 10.1128/IAI.00986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motz GT, Eppert BL, Sun G, Wesselkamper SC, Linke MJ, Deka R, Borchers MT. Persistence of lung CD8 T cell oligoclonal expansions upon smoking cessation in a mouse model of cigarette smoke-induced emphysema. J Immunol. 2008;181:8036–8043. doi: 10.4049/jimmunol.181.11.8036. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler RT, Kombe D, Agarwala SD, Fink GR. Dynamic, morphotype-specific Candida albicans beta-glucan exposure during infection and drug treatment. PLoS Pathog. 2008;4:e1000227. doi: 10.1371/journal.ppat.1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson MP, Metz AE, Li S, Lowell CA, Steele C. The absence of Hck, Fgr, and Lyn tyrosine kinases augments lung innate immune responses to Pneumocystis murina. Infect Immun. 2009;77:1790–1797. doi: 10.1128/IAI.01441-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Wang SH, Liao CP, Lasbury ME, Durant PJ, Tschang D, Lee CH. Toll-like receptor 2 knockout reduces lung inflammation during Pneumocystis pneumonia but has no effect on phagocytosis of Pneumocystis organisms by alveolar macrophages. J Eukaryot Microbiol. 2006;53(Suppl 1):S132–133. doi: 10.1111/j.1550-7408.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 24.Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, Matsuda M, Coban C, Ishii KJ, Kawai T, Takeuchi O, Akira S. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction. J Exp Med. 2005;201:915–923. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forman HJ, Torres M. Signaling by the respiratory burst in macrophages. IUBMB Life. 2001;51:365–371. doi: 10.1080/152165401753366122. [DOI] [PubMed] [Google Scholar]
- 26.Henricks PA, Nijkamp FP. Reactive oxygen species as mediators in asthma. Pulm Pharmacol Ther. 2001;14:409–420. doi: 10.1006/pupt.2001.0319. [DOI] [PubMed] [Google Scholar]
- 27.Swain SD, Wright TW, Degel PM, Gigliotti F, Harmsen AG. Neither neutrophils nor reactive oxygen species contribute to tissue damage during Pneumocystis pneumonia in mice. Infect Immun. 2004;72:5722–5732. doi: 10.1128/IAI.72.10.5722-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki H, Yamamoto H, Tominaga K, Masuda K, Kawai T, Teshima-Kondo S, Matsuno K, Yabe-Nishimura C, Rokutan K. Receptor activator of nuclear factor-kappaB ligand-induced mouse osteoclast differentiation is associated with switching between NADPH oxidase homologues. Free Radic Biol Med. 2009;47:189–199. doi: 10.1016/j.freeradbiomed.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Lamkanfi M, Malireddi RK, Kanneganti TD. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem. 2009;284:20574–20581. doi: 10.1074/jbc.M109.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodridge HS, Simmons RM, Underhill DM. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J Immunol. 2007;178:3107–3115. doi: 10.4049/jimmunol.178.5.3107. [DOI] [PubMed] [Google Scholar]
- 31.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.