Disturbances in cardiac sodium channel function are associated with inherited arrhythmia susceptibility. Mutations in SCN5A, which encodes the cardiac sodium channel (NaV1.5), cause congenital long QT syndrome type 3 (LQT3), Brugada syndrome (BrS) and a variety of cardiac conduction disorders (CCD) [1,2]. These disorders have can have complex genotype-phenotype relationships [3,4]. Here we report the clinical features of an LQTS family segregating a novel amino acid deletion mutation (N1472del) in SCN5A that produces a unique pattern of biophysical disturbances consistent with the clinical phenotype.
Nine members of a three-generation Italian LQTS family were evaluated clinically by the Cardiology Division of the Monaldi Hospital (Naples, Italy) or in other hospitals (Fig. 1A). Investigations included a complete medical history, physical examination, 12-lead ECG recording, 24-hour Holter recording and echocardiogram at different times during a follow-up of at least 3 years. Informed consent for genetic studies was obtained from all subjects.
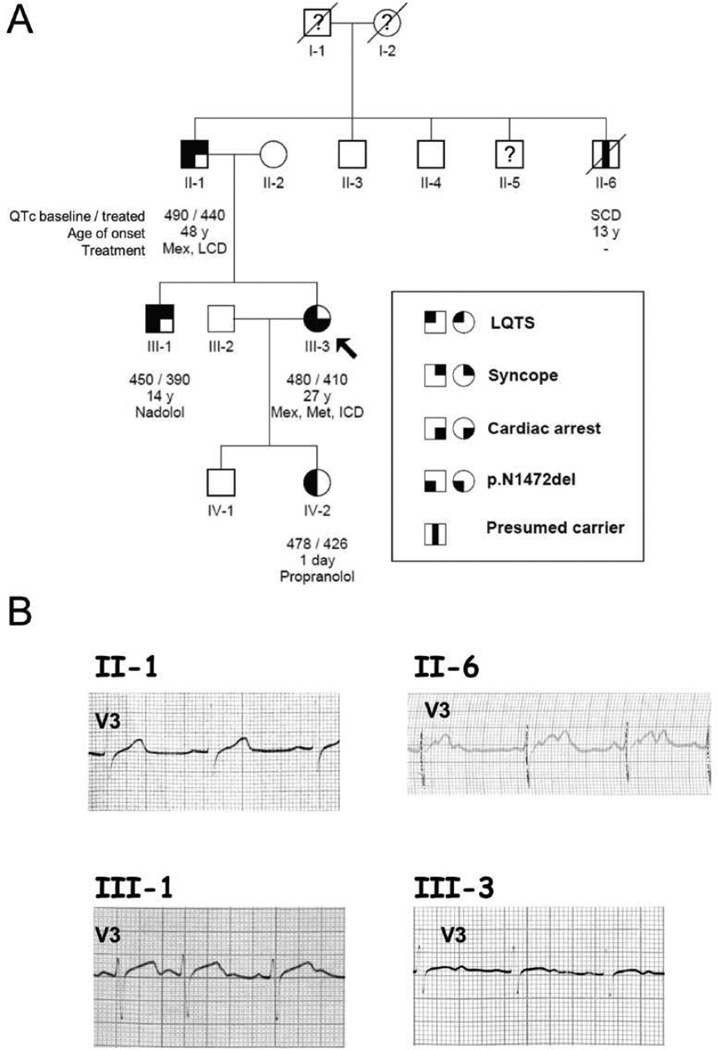
Figure 1. Pedigree of a LQTS family with representative ECG traces.
(A) Genotype and phenotype is defined according to the legend inset. Open symbols represent subjects with a negative genotype and phenotype. An arrow marks the proband (III-3). Symbols with a question mark represent subjects with unknown phenotype. The QTc interval, the age of onset and the treatment are reported below each subject symbol (abbreviations: Mex, mexiletine; Met, Metoprolol; LCD, left cardiac sympathetic denervation; ICD, implantable cardioverter defibrillator; SCD, sudden cardiac death). (B) Representative ECG traces from four genotype positive family members.
The proband (III-3, Fig. 1A) was a 27-year-old female who had postpartum cardiac arrest shortly after an uncomplicated Caesarian section while receiving erythromycin. An ECG performed during adolescence reportedly exhibited normal QT intervals, but her QTc was prolonged (480 ms) following successful resuscitation (Fig. 1B). She was treated with metoprolol, mexiletine and had an ICD implanted. During the following 8 years, she remained asymptomatic with a QTc ranging between 410 and 445 ms without conduction disturbances. There was a significant family history of unexplained syncope and sudden death. Particularly, the proband’s uncle (II-6, Fig. 1A) experienced syncope at age 12, then died suddenly overnight at age 13. In retrospect, an ECG obtained at age 12 demonstrated a prolonged QTc and periods of 2:1 AV block (Fig. 1B).
The proband was screened for mutations in SCN5A, KCNQ1, KCNH2, KCNE1 and KCNE2 genes. Genetic analysis revealed a novel SCN5A in-frame deletion mutation (c.4414-4416delAAC) predicting the deletion of asparagine-1472 (p.N1472del). The mutation deletes a conserved residue within the DIII-DIV cytoplasmic loop, which is an essential structural determinant of sodium channel fast inactivation [5,6]. The mutation was present in all clinically affected relatives who were genotyped (Fig. 1A) but was absent in 500 chromosomes from control subjects.
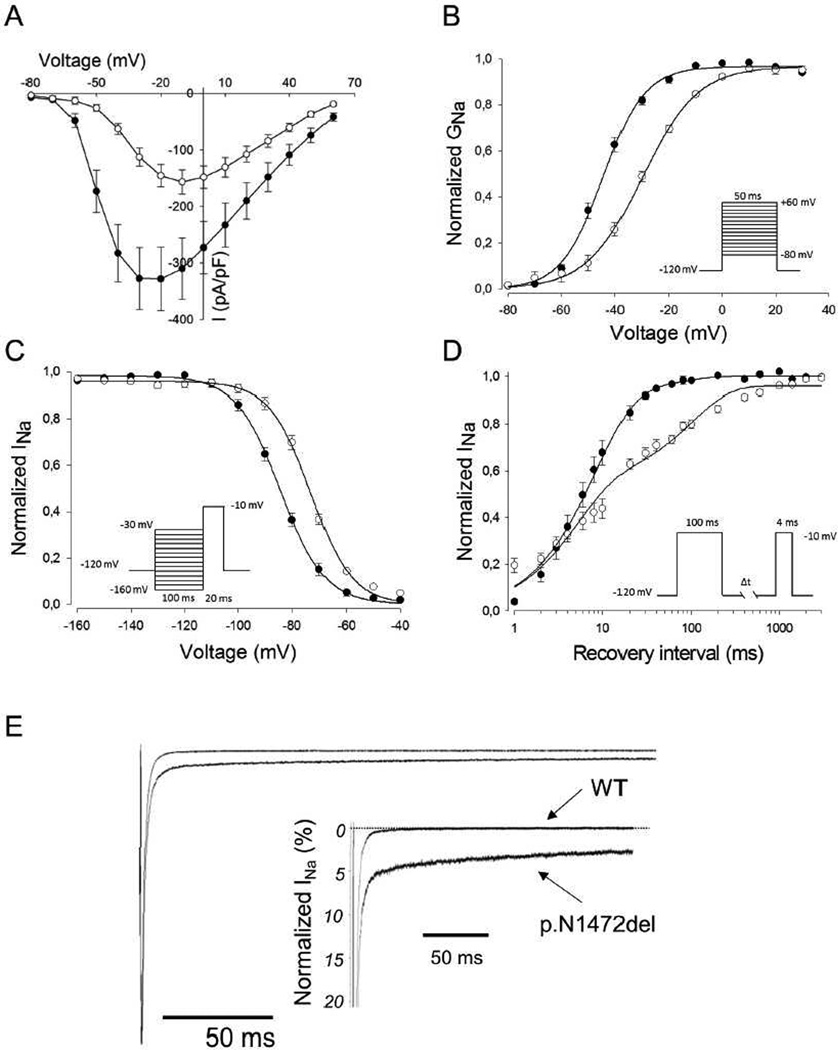
Mutation N1472del was generated by site-directed mutagenesis of recombinant human NaV1.5 (GenBank M77235) inserted into the expression vector pRc/CMV. To determine the functional consequence of the mutant channel, sodium current was recorded by whole cell patch-clamp in transiently transfected tsA201 cells as previously described [7]. Peak current density recorded from cells expressing N1472del was ~50% smaller than that of cells expressing WT-NaV1.5 (p.N1472del: 160.5 ± 21 pA/pF, n = 25; WT-NaV1.5: 344.8 ± 52 pA/pF, n = 21; p<0.05) and the peak current of N1472del was shifted to a more positive potential (Fig. 2A). Correspondingly, there was a +15 mV depolarizing shift in the voltage dependence of activation observed for mutant channels (N1472del, V½: −28.9±0.7 mV, n=23; WT-NaV1.5, V½: −44.4±1.1 mV, n=21; p<0.001) (Fig. 2B). Mutant channels also exhibited a +12 mV depolarizing shift in steady-state inactivation (N1472del, V½: −72.6±0.5 mV, n=21; WT-NaV1.5, V½: −85.0±2.2 mV, n=21; p<0.001) (Fig. 2C). In addition, the time course of recovery from fast inactivation observed for WT-NaV1.5 was monoexponential described by a single time constant (6.8 ± 0.01 ms, n = 10), whereas recovery for N1472del was biexponential characterized by fast (4.3 ± 0.4 ms, 60%; n=20) and slow time constants (200 ± 33 ms, 38%; n = 21). The additional slow component explains the significantly slower recovery from inactivation exhibited by the mutant channel (Fig. 2D). Finally, the N1472del channel showed a greater level of TTX-sensitive persistent sodium current compared with WT-NaV1.5 (N1472del: 2.6±0.2%, n=5; WT-NaV1.5: 0.1±0.02, n=5; p<0.001) (Fig. 2E), a common feature of SCN5A mutations associated with LQT3 [8].
Figure 2. Biophysical properties of WT-NaV1.5 and N1472del sodium channels.
(A) Current-voltage relationships for WT-NaV1.5 (filled circles) and N1472del (open circles). Currents were normalized for cell capacitance to give a measure of sodium current density. (B) Voltage dependence of activation for WT-NaV1.5 (filled circles) and N1472del (open circles) assessed using the voltage protocol illustrated in panel A. Conductance-voltage curves were fit with a Boltzmann distribution (solid lines). (C) The voltage dependence of steady-state inactivation for WT-NaV1.5 (filled circles) and N1472del (open circles). Currents were normalized to the peak current amplitude. Lines represent Boltzmann fits to the data. (D) Recovery from inactivation for WT-NaV1.5 (filled circles) and N1472del (open circles) measured with double-pulse protocol shown in the inset. The current-time curve was fit with a single (WT-NaV1.5) or double-exponential equation (N1472del). (E) Increased TTX-sensitive persistent sodium current for N1472. Persistent sodium current was recorded during a 200 ms depolarizing voltage pulse to −30 mV from a holding potential of −120 mV in the absence then presence of 30 µM TTX followed by offline digital subtraction of the currents. Zero current level is indicated by the dotted line. The inset shows an expanded y-axis scale to emphasize the relative proportion of WT-NaV1.5 and N1472del currents.
This family exhibited a heterogeneous phenotype with variability in severity and clinical presentation. Among the four documented and one presumed mutation carrier (Fig. 1A), the presentation ranged from late onset syncope (subject II-1) to sudden unexplained death during adolescence (subject II-6) or post-partum cardiac arrest (proband, III-3). The sudden death victim and presumed mutation carrier also exhibited ECG evidence of cardiac conduction system disease in the form of 2:1 AV block (Fig. 1B) that has been reported as a condition with higher life-threatening risk [9]. None of the mutation carriers exhibited bradycardia. Clinical variability is typical of LQTS and may be related to the variable exposure of individuals to circumstances promoting arrhythmia or co-inheritance of genetic factors other than the primary mutation that either potentiate or reduce the risk of arrhythmic events. The absence of bradycardia and the favorable response to monotherapy with β-blockers observed in some SCN5A mutation carriers (III-1, IV-2) are atypical of LQT3. Exposure of LQT3 subjects to β-blockers carries risk of evoking bradycardia that then potentiates arrhythmia risk [10].
There have been reports of other SCN5A deletion mutations within the DIII-DIV cytoplasmic loop but not all of these cause LQTS. In particular, delKPQ 1505–1507 and delQKP 1507–1509 are associated with LQTS [11,12], while K1479del and K1493del have been reported in subjects with BrS [13,14]. These two lysine mutations have not been functionally characterized. Furthermore, K1500del mutation has been reported in a family that exhibits features of LQTS, BrS and conduction disease in the same subjects [4]. These reports demonstrate the difficulty in predicting phenotype from genotype when considering only the structural domain involved.
The biophysical properties of the novel SCN5A N1472del mutation provided insight into the cause of LQTS in this family and might help explain other clinical features. This mutation elicits a defect in channel inactivation leading to increased persistent current, a hallmark of NaV1.5 dysfunction in LQT3 (Fig. 2E). Increased persistent sodium current causes a delay in repolarization that, in turn, elicits prolonged ventricular action potential duration. The pathogenic effects of increased persistent sodium current in LQTS have been strongly supported by computer simulations of cardiac action potentials [15,16] and direct electrophysiological investigations of native cardiac myocytes from genetically engineered mice carrying LQTS mutations [17,18]. Increased persistent current can be considered a ‘gain-of-function’ that accounts for the dominant nature of genetic arrhythmia risk in heterozygous mutation carriers. Potentially compounding this defect in N1472del channels is a depolarizing shift in voltage dependence of steady-state inactivation that increases channel availability (Fig. 2D), however this is offset by other functional abnormalities.
In addition to the ‘gain-of-function’ defect, our experiments revealed other biophysical properties that may impair channel function under physiological conditions (reduced peak current density, depolarized conductance-voltage relationship and slower recovery from inactivation) (Fig. 2A,B,D). The impact of these ‘loss-of-function’ effects on the clinical phenotype observed in mutation carriers cannot be known for certain, but we speculate that these features may have contributed to AV conduction block in subject II-6. The combination of ‘gain-of-function’ and ‘loss-of-function’ features has been reported with SCN5A mutations associated with mixed phenotypes in ‘overlap syndromes’ (e.g., LQT3 with BrS) [3,4]. Although the family we present did not have an overlap syndrome, provocative testing, to expose Brugada syndrome for example, was not performed and therefore we cannot exclude an additional latent phenotype.
ACKNOWLEDGMENTS
The work was support by grants from the NIH (HL083374 to A.L.G.), the Regione Campania (Convenzione CEINGE-Regione Campania, G.R. 27/12/2007), the Ministero dell'Istruzione, dell'Università e della Ricerca-Rome PS35-126/IND and from IRCCS – Fondazione SDN, and the Ministero della Salute, Rome, Italy. We thank Jean Ann Gilder (Scientific Communication srl) for text editing and Vittorio Lucignano, CEINGE – Biotecnologie Avanzate for technical assistance. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [19].
ABBREVIATIONS
- AV block
Atrio-ventricular block
- Bpm
Beats per minute
- BrS
Brugada syndrome
- CCD
Cardiac conduction defects
- ECG
Electrocardiogram
- HR
Heart rate
- ICD
Implantable cardioverter defibrillator
- K
Slope factor
- LQTS
Long QT syndrome
- QTc
QT interval corrected for heart rate
- TTX
Tetrodotoxin
- V½
Membrane potential at half-maximal activation or inactivation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.George AL., Jr Inherited disorders of voltage-gated sodium channels. J Clin Invest. 2005;115:1990–1999. doi: 10.1172/JCI25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George AL., Jr Zipes DP, Jalife J. Cardiac Electrophysiology: From Cell to Bedside. 5th. Philadelphia: Elsevier; 2009. Inheritable sodium channel diseases. [Google Scholar]
- 3.Veldkamp MW, Viswanathan PC, Bezzina C, Baartscheer A, Wilde AA, Balser JR. Two distinct congenital arrhythmias evoked by a multidysfunctional Na+ channel. Circ Res. 2000;86:E91–E97. doi: 10.1161/01.res.86.9.e91. [DOI] [PubMed] [Google Scholar]
- 4.Grant AO, Carboni MP, Neplioueva V, Starmer CF, Memmi M, Napolitano C, Priori S. Long QT syndrome, Brugada syndrome, and conduction system disease are linked to a single sodium channel mutation. J Clin Invest. 2002;110:1201–1209. doi: 10.1172/JCI15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patton DE, West JW, Catterall WA, Goldin AL. Amino acid residues required for fast Na+-channel inactivation: Charge neutralizatons and deletions in the III-IV linker. Proc Natl Acad Sci USA. 1992;89:10905–10909. doi: 10.1073/pnas.89.22.10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West JW, Patton DE, Scheuer T, Wang Y, Goldin AL, Catterall WA. A cluster of hydrophobic amino acid residues required for fast Na+-channel inactivation. Proc Natl Acad Sci USA. 1992;89:10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson DW, Wang DW, Dyment M, Knilans TK, Fish FA, Strieper MJ, Rhodes TH, George AL., Jr Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A) J Clin Invest. 2003;112:1019–1028. doi: 10.1172/JCI18062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett PB, Yazawa K, Makita N, George AL., Jr Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 9.Sarubbi B, Frisso G, Romeo E, Evangelista E, Cordella A, D'Alto M, Santarpia G, Russo MG, Salvatore F, Calabro R. Efficacy of pharmacological treatment and genetic characterization in early diagnosed patients affected by long QT syndrome with impaired AV conduction. Int J Cardiol. 2011;149:109–113. doi: 10.1016/j.ijcard.2010.12.099. [DOI] [PubMed] [Google Scholar]
- 10.Moss AJ, Zareba W, Hall WJ, Schwartz PJ, Crampton RS, Benhorin J, Vincent GM, Locati EH, Priori SG, Napolitano C, Medina A, Zhang L, Robinson JL, Timothy K, Towbin JA, Andrews ML. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 12.Keller DI, Acharfi S, Delacretaz E, Benammar N, Rotter M, Pfammatter JP, Fressart V, Guicheney P, Chahine M. A novel mutation in SCN5A, delQKP 1507–1509, causing long QT syndrome: Role of Q1507 residue in sodium channel inactivation. J Mol Cell Cardiol. 2003;35:1513–1521. doi: 10.1016/j.yjmcc.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Smits JP, Eckardt L, Probst V, Bezzina CR, Schott JJ, Remme CA, Haverkamp W, Breithardt G, Escande D, Schulze-Bahr E, LeMarec H, Wilde AA. Genotype-phenotype relationship in Brugada syndrome: electrocardiographic features differentiate SCN5A-related patients from non-SCN5A-related patients. J Am Coll Cardiol. 2002;40:350–356. doi: 10.1016/s0735-1097(02)01962-9. [DOI] [PubMed] [Google Scholar]
- 14.Kapplinger JD, Tester DJ, Salisbury BA, Carr JL, Harris-Kerr C, Pollevick GD, Wilde AA, Ackerman MJ. Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm. 2009;6:1297–1303. doi: 10.1016/j.hrthm.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clancy CE, Rudy Y. Linking a genetic defect to its cellular phenotype in a cardiac arrhythmia. Nature. 1999;400:566–569. doi: 10.1038/23034. [DOI] [PubMed] [Google Scholar]
- 16.Clancy CE, Rudy Y. Na+ channel mutation that causes both Brugada and long-QT syndrome phenotypes: a simulation study of mechanism. Circulation. 2002;105:1208–1213. doi: 10.1161/hc1002.105183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuyens D, Stengl M, Dugarmaa S, Rossenbacker T, Compernolle V, Rudy Y, Smits JF, Flameng W, Clancy CE, Moons L, Vos MA, Dewerchin M, Benndorf K, Collen D, Carmeliet E, Carmeliet P. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nature Med. 2001;7:1021–1027. doi: 10.1038/nm0901-1021. [DOI] [PubMed] [Google Scholar]
- 18.Blana A, Kaese S, Fortmuller L, Laakmann S, Damke D, van BK, Eckstein J, Piccini I, Kirchhefer U, Nattel S, Breithardt G, Carmeliet P, Carmeliet E, Schotten U, Verheule S, Kirchhof P, Fabritz L. A knock-in gain-of-function sodium channel mutation prolongs atrial action potentials and alters atrial vulnerability. Heart Rhythm. 2010;7:1862–1869. doi: 10.1016/j.hrthm.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Coats AJ, Shewan LG. Statement on authorship and publishing ethics in the international journal of cardiology. Int J Cardiol. 2011;153:239–240. doi: 10.1016/j.ijcard.2011.10.119. [DOI] [PubMed] [Google Scholar]