Abstract
OBJECTIVES:
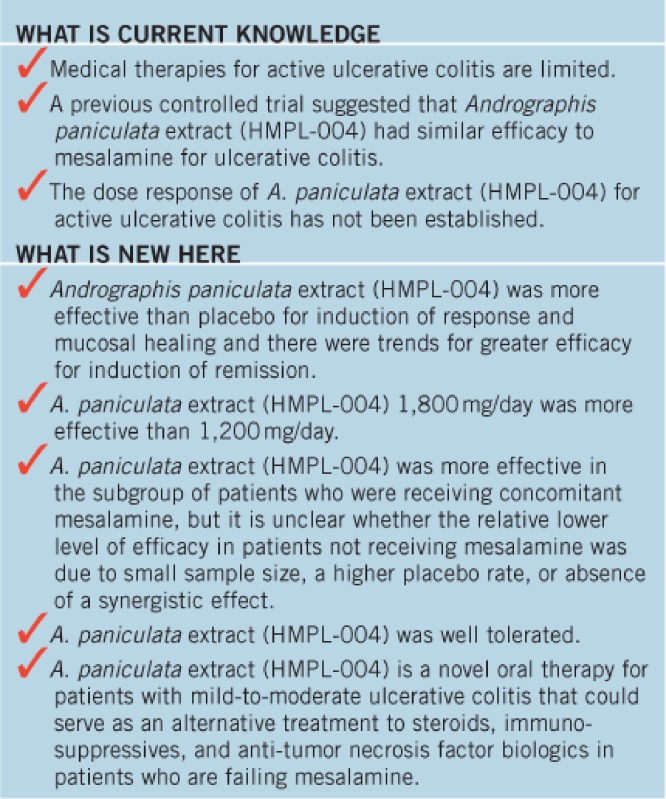
Andrographis paniculata has in vitro inhibitory activity against TNF-α, IL-1β and NF-κB. A pilot study of A. paniculata extract (HMPL-004) suggested similar efficacy to mesalamine for ulcerative colitis.
METHODS:
A randomized, double-blind, placebo-controlled trial evaluated the efficacy of A. paniculata extract (HMPL-004) in 224 adults with mild-to-moderate ulcerative colitis. Patients were randomized to A. paniculata extract (HMPL-004) 1,200 mg or 1,800 mg daily or placebo for 8 weeks.
RESULTS:
In total, 45 and 60% of patients receiving A. paniculata 1,200 mg and 1,800 mg daily, respectively, were in clinical response at week 8, compared with 40% of those who received placebo (P=0.5924 for 1,200 mg vs. placebo and P=0.0183 for 1,800 mg vs. placebo). In all, 34 and 38% of patients receiving A. paniculata 1,200 mg and 1,800 mg daily, respectively, were in clinical remission at week 8, compared with 25% of those who received placebo (P=0.2582 for 1,200 mg vs. placebo and P=0.1011 for 1,800 mg vs. placebo). Adverse events developed in 60 and 53% of patients in the A. paniculata 1,200 mg and 1,800 mg daily groups, respectively, and 60% in the placebo group.
CONCLUSIONS:
Patients with mildly to moderately active ulcerative colitis treated with A. paniculata extract (HMPL-004) at a dose of 1,800 mg daily were more likely to achieve clinical response than those receiving placebo.
INTRODUCTION
Ulcerative colitis is a chronic inflammatory disorder of the colon defined by relapsing and remitting episodes (1). Mild-to-moderate disease symptoms are treated with mesalamine (2,3). Patients who fail to respond to mesalamine are treated with systemic steroids, azathioprine, and infliximab (2,3), which are all associated with serious toxicities (4). Additional treatments that can be used as first-line or salvage therapy in patients failing mesalamine are needed.
Andrographis paniculata, a member of the plant family Acanthaceae, is an herbal remedy used in China, India, Thailand, and other Asian countries. A. paniculata has been used to treat upper respiratory tract infections (5,6,7). An ethanol extract of A. paniculata yields an herbal mixture containing mainly diterpene lactones including andrographolide. Andrographolide, which is used as a marker compound, comprises <10% of the mixture. A. paniculata extract has in vitro inhibitory activity against TNF-α, IL-1β, and NF-κB (8,9). A pilot study of A. paniculata extract (HMPL-004) 1,200 mg/day suggested similar efficacy to mesalamine for ulcerative colitis (10).
Our study was an 8-week, placebo-controlled trial comparing A. paniculata extract (HMPL-004) with placebo in patients with mildly to moderately active ulcerative colitis.
METHODS
The study was designed by two academic investigators William Sandborn and Stephan Targan, Vera Byers from Immunology Inc., and Tom Tang from Hutchison MediPharma Ltd. Data were collected by Clinical Research Management Inc. (Agawam, MA) and Omnicare Clinical Research (Frankfurt, Germany), and were analyzed by Everest Clinical Research Services Inc. (Ontario, Canada). William Sandborn wrote the first draft of the manuscript. The academic authors vouch for the veracity and completeness of the data and data analyses.
Patients
This multicenter, randomized, double-blind, placebo-controlled trial was conducted at 52 centers in 5 countries (United States, Canada, Germany, Romania, and Ukraine) between February 2008 and October 2009 (4 sites in Germany were activated but failed to recruit any patients). The institutional review board at each center approved the protocol. Patients gave written informed consent.
Eligible patients were at least 18 years of age and had a confirmed diagnosis of ulcerative colitis. Patients had a Mayo Score of 4–10 points (11) and mildly to moderately active disease on sigmoidoscopy (endoscopic subscore of at least 1) while receiving either oral mesalamine (or equivalent medications sulfasalazine, balsalazide, and olsalazine) for at least 4 weeks or no medical therapy.
Patients with Crohn's disease or indeterminate colitis, severe ulcerative colitis (Mayo Score of 11 or 12 points, toxic mega-colon, toxic colitis), previous colonic surgery or probable requirement for intestinal surgery within 12 weeks, enteric infection within 2 weeks, a history of tuberculosis, a positive chest X-ray or tuberculin protein-purified derivative skin test, active infection with hepatitis B or any infection with hepatitis C, infection with human immunodeficiency virus, cancer within 5 years, inadequate bone marrow, hepatic, or renal function, a history of alcohol or drug abuse that would interfere with the study, significant concurrent medical diseases, allergy to plants in the Acanthacea family, and women who were pregnant or breastfeeding were not eligible. Patients receiving oral or rectal steroids within 1 month, rectal mesalamine within 1 week, antibiotics within 2 weeks, or azathioprine, 6-mercaptopurine, anti-tumor necrosis factor agents, or immunosuppressive therapy within 6 weeks were also excluded.
Study design
Patients were randomly assigned to receive oral capsules containing A. paniculata ethanol extract (HMPL-004; Hutchison MediPharma Ltd., Shanghai, China) at doses of 1,200 mg or 1,800 mg or placebo, administered in three divided doses. Patients were treated for 8 weeks and followed through week 12. Randomization was performed centrally using a block randomization schedule stratified by concurrent mesalamine use (yes or no) and country/geographic region (North East USA, Mid-East USA, South East USA, Western USA, Canada, Ukraine, and Romania). Oral mesalamine was continued at a stable dose.
Follow-up, efficacy, and safety evaluations
A colonoscopy or flexible sigmoidoscopy was performed and the Mayo Score was determined at weeks 0 and 8. A partial Mayo Score (Mayo Score without endoscopy) was determined at all visits between weeks 0 and 8 inclusive. Clinical response was defined as a decrease from baseline in the total Mayo Score by at least 3 points and at least 30% with an accompanying decrease in rectal bleeding subscore of at least 1 point or a absolute rectal bleeding subscore of 0 or 1 point (12,13). Clinical remission was defined as a total Mayo Score of 2 points or lower, with no individual subscore exceeding 1 point (12,13). Mucosal healing was defined as a decrease from baseline in the endoscopy subscore by at least 1 point and an absolute endoscopy subscore of 0 or 1 point (12,13).
Adverse events and concomitant medications were followed through week 12. Blood samples were collected at weeks 0 and 8 for C-reactive protein (CRP) concentrations.
Statistical analysis
The primary efficacy end point was clinical response at week 8. Secondary efficacy end points included clinical remission at week 8; mucosal healing at week 8; time to partial Mayo Score response (defined as the time point at weeks 2, 4, 6, or 8 at which there was a decrease from baseline in the partial Mayo Score by at least 2 points); change from baseline in the partial Mayo Score at weeks 2, 4, 6, or 8; and the mean change from baseline in the total Mayo Score at week 8. Safety assessments on adverse events were conducted through week 12.
To control for a type I error of 0.05 or less, the primary end point analyses were conducted in a prespecified, sequential manner. The global null hypothesis was that the proportions of patients with clinical response at week 8 would not be different between the three treatment groups at a 0.05 (two-sided) significance level. If the global null hypothesis was rejected, then the combined A. paniculata dose groups, the A. paniculata 1,200 mg dose group, and the A. paniculata 1,800 mg dose group were compared with placebo at a 0.05 (two-sided) significance level. If the global null hypothesis was not rejected, then the combined dose group and individual dose group comparisons with placebo were considered as not statistically significant. Given the large number of prespecified secondary efficacy variables evaluated at multiple time points during the study, the P values for all secondary efficacy variables should be considered as nominal, as no adjustments were made for multiple comparisons.
Demographic and baseline characteristics were compared with the use of the χ2 or Fisher's exact test for categorical variables and with analysis of variance on van der Waerden normal scores for continuous variables. The statistical analysis plan stated that clinical response, clinical remission, and mucosal healing would be analyzed using logistic regression, and that last observation carried forward methodology would be used to handle missing data. At the request of regulatory authorities, the data were instead analyzed using the Cochran–Mantel–Haenszel test and missing data were handled using worst case methodology as described below. A two-sided Cochran–Mantel–Haenszel test with concurrent mesalamine use/non-use as strata was used to compare clinical response, clinical remission, and mucosal healing. Missing data were handled using a “worst case” intention-to-treat analysis in which patients with any missing component of the Mayo Score were considered not to be in clinical response, clinical remission, or to have mucosal healing. Given a significant overall treatment difference for clinical response, the specific comparisons of placebo vs. A. paniculata 1,200 mg, placebo vs. A. paniculata 1,800 mg, and placebo vs. the combined A. paniculata dose groups were analyzed. Time to partial Mayo Score response was compared with Cox regression and the results expressed as a hazard ratio (HR), interpreted as an odds ratio with 95% confidence interval (CI). A cumulative incidence Kaplan–Meier plot was also created. The change from baseline in partial Mayo Score at weeks 2, 4, 6, and 8 by treatment group and the mean treatment difference (placebo minus A. paniculata with respect to change from baseline) were plotted by post-baseline visit for each treatment group along with 95% CI at these assessment times. The earliest time at which the lower limit on the CI for the treatment difference above zero was defined to be the time to first significant partial Mayo Score difference. The mean treatment differences of Mayo Score change from baseline between placebo and A. paniculata treatment groups were tested using ANCOVA, with treatment as a fixed effect, country/region as a random effect, and age, gender, race, baseline value, and concomitant mesalamine use as covariates. Safety comparisons used the Fisher's exact test. All patients receiving at least one dose of study medication were analyzed for safety according to the treatment actually received.
To evaluate the consistency of treatment effect on clinical response between placebo, A. paniculata 1,200 mg, and A. paniculata 1,800 mg, 2 prespecified subgroup analyses (concurrent mesalamine and country/region) and 7 post hoc subgroup analyses (gender, race, age, weight, disease duration, elevated CRP, baseline Mayo Score) were performed. P values were calculated based on the Pearson χ2 test and 95% CI's were based on the normal approximation to the binomial.
For the primary end point of clinical response at week 8, it was estimated that 202 patients would allow 80% power to detect a difference in response rates of 21% between the combined A. paniculata dose groups and the placebo group using the logistic regression analysis, assuming a 51% rate of response to A. paniculata and a 30% rate of response to placebo.
RESULTS
Characteristics of the patients
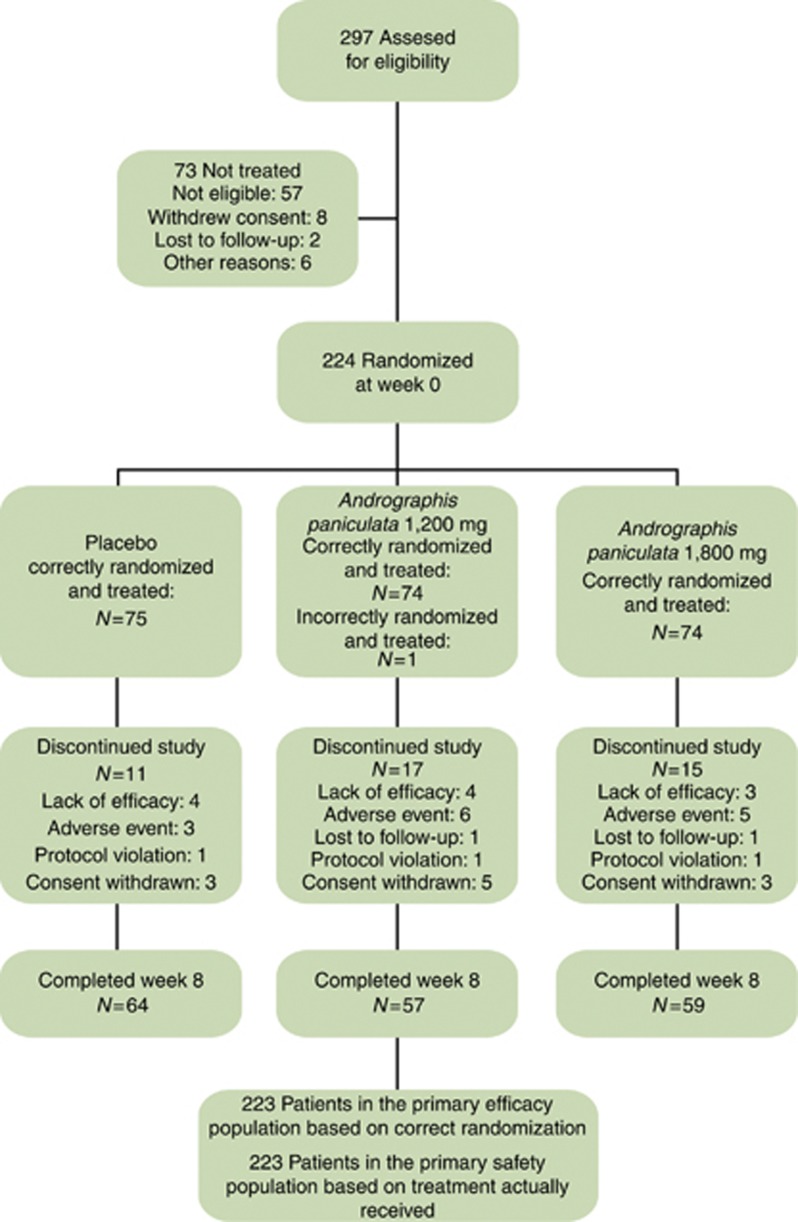
In total, 224 patients were randomized to treatment: 75 to placebo, 75 to the A. paniculata 1,200 mg daily, and 74 to A. paniculata 1,800 mg daily. One patient was incorrectly randomized and was excluded from the efficacy analyses. The baseline disease characteristics were similar in the three groups ( Table 1). In all, 180 of 224 patients (80.7%) completed the 8-week trial (Figure 1).
Table 1. Demographic and baseline disease characteristics.
| Placebo (N=75) | Andrographis paniculata 1,200 mg (N=74) | Andrographis paniculata 1,800 mg (N=74) | Total (N=223) | P valuea | |
|---|---|---|---|---|---|
| Male, no. (%) | 41 (54.7) | 40 (54.1) | 41 (55.4) | 122 (54.7) | 0.9864 |
| Caucasian, no. (%) | 67 (89.3) | 64 (86.5) | 64 (86.5) | 195 (87.4) | 0.7448 |
| Age (years) | |||||
| Mean±s.d. | 44.7±15.2 | 44.3±14.5 | 45.6±13.6 | 44.9±14.4 | 0.8454 |
| Median | 45.0 | 43.5 | 46.5 | 45.0 | |
| Body weight (kg) | |||||
| Mean±s.d. | 76.8±14.8 | 77.0±19.9 | 78.6±20.1 | 77.5±18.4 | 0.8030 |
| Median | 75.8 | 74.2 | 74.4 | 74.8 | |
| Disease duration (months) | 0.5615 | ||||
| Mean±s.d. | 63.4±70.3 | 68.6±83.4 | 72.6±74.7 | 68.2±76.0 | |
| Median | 42.9 | 32.4 | 43.4 | 40.7 | |
| C-reactive protein (mg/dl) | |||||
| Mean±s.d. | 0.58±0.82 | 0.64±0.70 | 1.14±2.70 | 0.79±1.69 | 0.5282 |
| Median | 0.30 | 0.31 | 0.30 | 0.30 | |
| Elevated CRP,b n (%) | 14 (18.7) | 21 (28.4) | 20 (27.0) | 55 (24.7) | 0.3290 |
| Mayo Score | |||||
| Mean±s.d. | 6.1±1.8 | 6.3±1.8 | 6.1±1.7 | 6.2±1.8 | 0.6748 |
| Median | 6.0 | 6.0 | 6.0 | 6.0 | |
| Concomitant mesalamine, n (%) | 52 (69.3) | 50 (67.6) | 51 (68.9) | 153 (68.6) | 0.9710 |
CRP, C-reactive protein.
P values for all categorical variables are based on a χ2 test. P values for continuous variables are based on analysis of variance if normal distribution assumption is met; otherwise P values are based on the Kruskal–Wallis test.
The normal range for CRP concentration is <0.8 mg/dl.
Figure 1.
Enrollment and treatment through week 8.
Efficacy
Primary end point
The overall treatment comparison of A. paniculata with placebo yielded a P value of 0.0465. In all, 52% of patients receiving A. paniculata (78 of 148) were in clinical response at week 8 as compared with 40% receiving placebo (30 of 75) (P=0.092; Table 2). A dose response for A. paniculata was demonstrated. In all, 45% of patients receiving A. paniculata 1,200 mg daily (33 of 74) were in clinical response at week 8 as compared with 40% receiving placebo (30 of 75) (P=0.592). In all, 60% of patients receiving A. paniculata 1,800 mg daily (44 of 74) were in clinical response at week 8 as compared with 40% receiving placebo (30 of 75) (P=0.018). The results of logistic regression analysis using last observation carried forward methodology were similar (Supplementary Table 1 online). The efficacy of A. paniculata was generally consistent among demographic and baseline disease characteristics (see subgroup logistic regression analyses in Supplementary Figure 1A (A. paniculata vs. placebo), Supplementary Figure 1B (A. paniculata 1,200 mg vs. placebo), and Supplementary Figure 1C (A. paniculata 1,800 mg vs. placebo)). The following subgroups showed trends toward a greater A. paniculata response compared with placebo: Mayo Score <6 points (primarily in the 1,200-mg group); endoscopy subscore <2 points; CRP >0.8 mg/dl; weight >85 kg; patients from Europe (primarily in the 1,800-mg group); concomitant mesalamine use (primarily in the 1,800-mg group); disease duration >5 years; white patients; patients aged 18–45 years; and male patients. All subgroup comparisons in the pooled A. paniculata vs. placebo analysis showed distinct divergence with the exception of the baseline CRP subgroup. In the 1,200-mg vs. placebo subgroup analysis only the endoscopy subscore <2 points (P=0.0241) and race (P=0.0780) subgroups were observed to be divergent. However, the 1,800-mg vs. placebo subgroup analysis showed distinct divergence in all subgroups with the exception of the baseline CRP subgroup. Disease duration was not confounded within age group as there was little difference in disease duration across the age group categories.
Table 2. Efficacy resultsa.
| End point | Dose | Andrographis paniculata | Placebo | P valueb | P valuec |
|---|---|---|---|---|---|
| Clinical response at week 8 | Combined 1,800+1,200 mg | 78/148 (52.0%) | 30/75 (40.0%) | 0.0922 | 0.0465 |
| 1,800 mg | 44/74 (59.5%) | 0.0183 | |||
| 1,200 mg | 33/74 (44.6%) | 0.5924 | |||
| Clinical remission at week 8 | Combined 1,800+1,200 mg | 53/148 (35.8%) | 19/75 (25.3%) | 0.1173 | 0.2516 |
| 1,800 mg | 28/74 (37.8%) | 0.1011 | |||
| 1,200 mg | 25/74 (33.8%) | 0.2718 | |||
| Mucosal healing at week 8 | Combined 1,800+1,200 mg | 65/148 (43.9%) | 25/75 (33.3%) | 0.1309 | 0.1025 |
| 1,800 mg | 37/74 (50.0%) | 0.0404 | |||
| 1,200 mg | 28/74 (37.8%) | 0.5821 |
Efficacy determined using worst case handling of missing data in which missing data were set to failure.
P values for the pair wise comparisons are based on the Cochran–Mantel–Haenszel with concomitant mesalamine use as strata.
P value is for the overall treatment comparison based on the Cochran–Mantel–Haenszel.
Secondary end points
In total, 36% of patients receiving A. paniculata (53 of 148) were in clinical remission at week 8 as compared with 25% receiving placebo (19 of 75) (P=0.1173; Table 2). The rates of clinical remission for the 1,800- and 1,200-mg doses were not significantly greater than placebo, although there was a trend toward significance for the 1,800-mg dose, P=0.1011. The results of logistic regression analysis using last observation carried forward methodology were generally similar to the worst case methodology, but using this analysis the difference between A. paniculata 1,800 mg and placebo was significant (Supplementary Table 1).
In all, 44% of patients receiving A. paniculata (65 of 148) achieved mucosal healing at week 8 as compared with 33% receiving placebo (25 of 75) (P=0.1309; Table 2). A dose response for A. paniculata was demonstrated; the rate of mucosal healing for the 1,800-mg dose was significantly greater than placebo whereas the 1,200-mg dose was not. The results of logistic regression analysis using last observation carried forward methodology were similar (Supplementary Table 1).
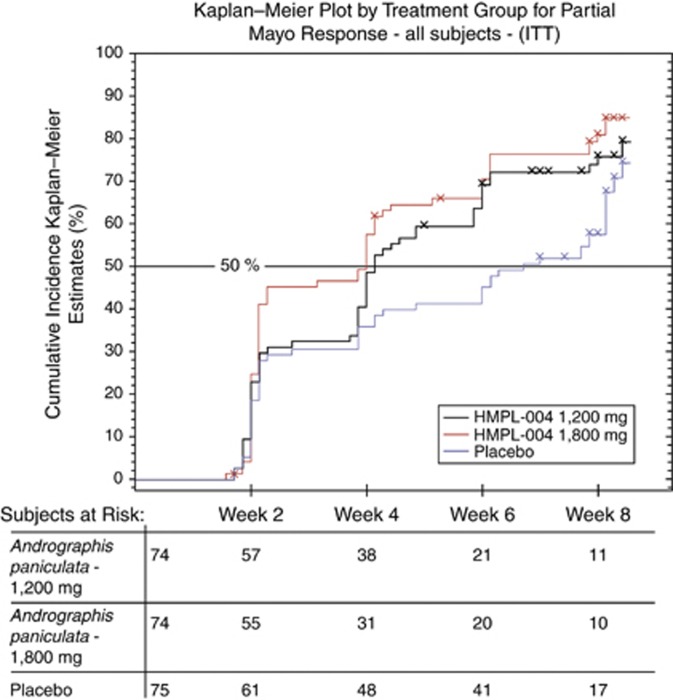
The times to first significant difference in partial Mayo Score response and first significant partial Mayo Score response were both 4 weeks in the combined A. paniculata groups, 4 weeks in the A. paniculata 1,800 mg group, and 4 weeks in the A. paniculata 1,200 mg group. The HR for the time to first significant difference in partial Mayo Score response was 1.57 (P=0.0096) for the combined A. paniculata groups over placebo; the HRs for the 1,800-mg and 1,200-mg groups were 1.72 (P=0.0060) and 1.44 (P=0.0679), respectively. The cumulative incidence Kaplan–Meier plot is shown in Figure 2. The mean improvement (95% CIs) from baseline in the total Mayo score at week 8 between placebo and A. paniculata from the ANCOVA model was 0.51 (−0.33, 1.35) points for the combined A. paniculata groups (P=0.2329), 0.58 (−0.24, 1.41) points, and 0.43 (−0.42, 1.29) points for the 1,800-mg and 1,200-mg groups (P=0.1652 and P=0.3208, respectively).
Figure 2.
Kaplan–Meier estimate of the proportion of patients free of clinical response as defined by the partial Mayo Score through 8 weeks for the combined Andrographis paniculata group, the A. paniculata 1,200 mg group, the A. paniculata 1,800 mg group, and the placebo group. ITT, intention-to-treat.
Safety
Through week 8, the incidence of adverse events was generally similar among groups ( Table 3). A rash occurred in 8% of patients receiving A. paniculata and 1% of patients receiving placebo. The rashes were mostly mild (with the rest moderate), reversible, and did not cause treatment discontinuation.
Table 3. Summary of safety findings through week 12a.
|
P valueb |
P valueb |
||||
|---|---|---|---|---|---|
| Placebo (N=75) | Andrographis paniculata 1,200 mg (N=75) | Andrographis paniculata 1,200 mg vs. placebo | Andrographis paniculata 1,800 mg (N=74) | Andrographis paniculata 1,800 mg vs. placebo | |
| Mean duration of treatment, days | 52 | 49 | 48 | ||
| Any adverse event, no. (%) | 45 (60) | 45 (60) | 1.0000 | 39 (53) | 0.3692 |
| Adverse events occurring in 4% of any treatment group, n (%) | |||||
| Abdominal pain | 6 (8) | 4 (5) | 4 (5) | ||
| Diarrhea | 2 (3) | 3 (4) | 4 (5) | ||
| Dyspepsia | 1 (1) | 3 (4) | 1 (1) | ||
| Flatulence | 1 (1) | 1 (1) | 4 (5) | ||
| Nausea | 2 (3) | 4 (5) | 3 (4) | ||
| Ageusia | 0 (0) | 3 (4) | 2 (3) | ||
| Dysgeusia | 0 (0) | 0 (0) | 3 (4) | ||
| Headache | 5 (7) | 8 (11) | 4 (5) | ||
| Influenza | 4 (5) | 2 (3) | 2 (3) | ||
| Nasopharyngitis | 3 (4) | 2 (3) | 2 (3) | ||
| Alanine aminotransferase increased | 0 (0) | 3 (4) | 0 (0) | ||
| Blood alkaline phosphatase increased | 1 (1) | 3 (4) | 0 (0) | ||
| Blood glucose increased | 0 (0) | 0 (0) | 3 (4) | ||
| Gamma-glutamyl transferase increased | 2 (3) | 3 (4) | 1 (1) | ||
| Rash | 1 (1) | 3 (4) | 3 (4) | ||
| Fatigue | 3 (4) | 2 (3) | 0 (0) | ||
| Anemia | 3 (4) | 0 (0) | 1 (1) | ||
| Basophilia | 3 (4) | 0 (0) | 0 (0) | ||
| Back pain | 3 (4) | 0 (0) | 3 (4) | ||
| Adverse events leading to study drug discontinuation, n (%) | 3 (4) | 7 (9) | 0.3268 | 6 (8) | 0.3268 |
| Serious adverse events, n (%) | 2 (3) | 2 (3) | 1.000 | 2 (3) | 1.000 |
A patient's treatment group is based on treatment received.
P values are based on Fisher's exact test if the frequency of at least 1 cell is <5; otherwise P values are based on a χ2 test.
DISCUSSION
Treatment with A. paniculata extract (HMPL-004) was more effective than placebo for induction of clinical response and mucosal healing at week 8 among patients with mildly to moderately active ulcerative colitis, most of whom were failing first-line therapy with mesalamine. The best efficacy was observed with the 1,800-mg dose. However, there were no significant differences between the A. paniculata and placebo groups in the worst case scenario analyses of clinical remission rates or mean improvement in total Mayo score at week 8. The end point of clinical remission was significant with the last observation carried forward analysis. Subgroup analysis indicated trend toward greater clinical response rates in subgroups of patients treated with A. paniculata who had baseline Mayo score <6 points and those with an endoscopy subscores <2 points; this was most apparent in those patients treated with the 1,200-mg dose. Age, gender, and geographic region were also assessed as covariates of clinical response. There was significant subgroup-by-treatment interaction observed in the 1,200-mg group for the baseline endoscopy subscore and race. There was also a trend toward a better clinical response to patients treated with A. paniculata in subgroups of patients with younger age, male sex, and European geographic location. A statistically significant improvement in clinical response was not observed in patients with North American geographic location. These subgroup analysis results should be interpreted with caution due to limited power based on small number of study subjects. The HRs for the time to first significant difference in partial Mayo Score response were greater in the A. paniculata-treated patients, but in the Kaplan–Meier analysis, the response rates equalized at week 8.
A previous pilot study demonstrated that treatment with A. paniculata 1,200 mg daily for 8 weeks resulted in a similar reduction from baseline in disease activity to that observed with oral mesalamine in patients with mildly to moderately active ulcerative colitis (10). We also found preliminary evidence of efficacy for A. paniculata 1,200 mg daily for 8 weeks as compared with placebo in patients with mildly to moderately active Crohn's disease (14). In the current study, we demonstrated dose response with A. paniculata 1,200 mg and 1,800 mg daily and no dose-dependent toxicity. Additional clinical trials to evaluate the safety and efficacy of even higher doses of A. paniculata extract (HMPL-004) should be undertaken in both ulcerative colitis and Crohn's disease.
Subgroup analysis showed a larger therapeutic effect for A. paniculata extract (HMPL-004) relative to placebo in patients who were currently failing oral mesalamine, and a smaller and non-significant effect relative to placebo among patients not receiving mesalamine. This latter result appears largely due to a greater rate of placebo response among patients not receiving mesalamine. This subgroup of patients was relatively small, and the study lacked sufficient statistical power to draw any meaningful conclusions regarding the efficacy of A. paniculata in this patient population. Additional adequately powered studies to assess the efficacy of A. paniculata extract (HMPL-004) as monotherapy in patients with ulcerative colitis are warranted. It should also be noted that the efficacy of A. paniculata extract (HMPL-004) for induction therapy in patients with ulcerative colitis who are failing various combinations of corticosteroids, azathioprine, and anti-tumor necrosis factor therapy with infliximab is unknown, as its efficacy as a maintenance agent. Additional studies in these patient populations are also needed.
The 40% placebo response rate in our study was slightly higher than expected. This study was patterned after two randomized double-blind placebo-controlled studies of infliximab in ulcerative colitis (12). In the first of these studies, the week 8 response rate was about 40% and in the second study the analogous response rate was 30%. The placebo response in our study was in this same general range. In the infliximab trials, the entry criteria specified baseline Mayo Scores of 6–12 points. In our study, the baseline Mayo Scores were 4–10 points, which may also have influenced the placebo response rate. Experience with the use of real-time central reading of endoscopy in clinical trials to reduce placebo rates is evolving, and in future trials central reading could potentially reduce the relatively high placebo rates that we observed in this trial.
In this short-term study, the overall incidence of adverse events observed in patients treated with A. paniculata extract (HMPL-004) was similar to patients receiving placebo, with the exception of rash which was higher in the A. paniculata extract (HMPL-004) groups. Longer term studies are needed to further assess the safety and tolerability of A. paniculata extract (HMPL-004) in patients with ulcerative colitis. If such studies continue to demonstrate a favorable safety profile, then it may be possible to combine A. paniculata extract (HMPL-004) with other drugs of known efficacy such as corticosteroids, azathioprine, and anti-tumor necrosis factor agents, with the goal of achieving synergistic efficacy without incurring synergistic toxicity.
The mechanism of action of A. paniculata extract should be further explored. In vitro inhibitory activity against TNF-α, IL-1β, and NF-κB has been reported (8,9). Additional preclinical studies in animal models of colitis should be undertaken to further explore the efficacy and biologic effects of A. paniculata extract and its major active components the diterpene lactones such as andrographolide across a range of doses
In conclusion, patients with mildly to moderately active ulcerative colitis treated with A. paniculata extract (HMPL-004) were more likely to achieve clinical response than those receiving placebo.
STUDY HIGHLIGHTS
APPENDIX
The study Principal Investigators are listed below in alphabetical order:
Orest Ostapovich Abrahamovych, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine;
Mohammed Azzouz, Gastroenterology and Liver Diseases, Warwick, RI, USA;
Daniel Baumgart, Charité - Campus Virchow, Berlin, Germany;
William J. Cohn, Gastrointestinal Research Associates, Setauket, NY, USA;
Hanns-Gerd Dammann, Klinische Forschung Hannover Mitte, Hannover, Germany;
Oleksiy Borisovich Datsenko, Municipal Clinical Hospital #2, Proctology department, Kharkov, Ukraine;
Frank Drouven, Bethlehem Krankenhaus, Stolberg, Germany;
J. Daniel Davis, Hope Research Institute, Phoenix, AZ, USA;
Steven Desautels, Physicians Research Option, Sandy, UT, USA;
Ion Dina, SC Endocenter Medicina Integrativa, Bucharest, Romania;
Dan Dumitrascu, Universitatea Iuliu Hatieganu, Cluj Napoca, Romainia;
Galyna Dmytrivna Fadieienko, Institute of Therapy by name L.T.Maloy of Ukrainian AMS, Kharkov, Ukraine;
Blanche Fung-Liu, Long Island Clinical Research Associates, Great Neck, NY, USA;
Syam P. Gaddam, Digestive and Liver Disease Specialists, Garden Grove, CA, USA;
B. Matthew Garner, St Bernards Research Center, Jonesboro, AR, USA;
Glenn L. Gordon, Center for Digestive and Liver Diseases, Inc., Mexico, MO, USA;
Robert Hardi, Metropolitan Gastroenterology Group, Chevy Chase Clinical Research, Chevy Chase, MD, USA
Robert Holmes, Piedmont Medical Research, Winston-Salem, NC, USA;
Steven Hong, Spokane Digestive Disease Center, Spokane, WA, USA;
Oleksandra Mykhaylivna Iemets, Medychnyy Centr Mriya, Kyiv, Ukraine;
Robert Kaplan, LeBauer Research Associates, Greensboro, NC, USA;
James H. Kopp, Hartwell Research Group, Anderson, SC, USA;
Bernard Leman, Iowa Digestive Disease Center, Clive, IA, USA;
Olena Mykhaylivna Levchenko, Odes′kyy oblasnyy centr gastroenterologiyi, Odessa, Ukraine;
John Lowe, Advanced Research Institute, Ogden, UT, USA;
Mitchell Mah'Moud, Boice-Willis Clinic, Clinical Research Department, Rocky Mount, NC, USA;
Nasrullah Manji, Pioneer Research Solutions, Houston, TX, USA;
Stefano Marcuard, Carolina Research, Greenville, NC, USA;
Hipolito G. Mariano, Research Center of Fresno, Fresno, CA, USA;
Ravi K. Moparty, Spring Clinical Research, Houston, TX, USA;
Michael J. Ostro, Toronto Digestive Disease Associates, Toronto, Ontario, Canada;
Daniel Pambianco, Charlottesville Medical Research, Charlottesville, VA, USA;
Edward Pensa, Pharma Resource, East Providence, RI, USA;
Corina Popa, SC Pelican Impex SRL, Oradea Jud Bihor, Romania;
Natarajan Ravendhran, Digestive Disease Associates, Baltimore, MD, USA;
Mark Reichelderfer, University of Wisconsin Hospital & Clinics, Madison, WI, USA;
Donato R. Ricci, Accord Clinical Research, Port Orange, FL, USA;
Dennis Riff, Advanced Clinical Research Institute, Anaheim, CA, USA;
Barry Sanders, North Texas Gastroenterology Consultants, Lewisville, TX, USA;
Omer Secil, Sp. Clinic de Urgenta Sf. Ioan, Bucharest, Romania;
Ursula Seidler, MHH, Hannover, Germany;
Ira Shafran, Shafran Gastroenterology Center, Winter Park, FL, USA;
Andreas Stallmach, Universitätsklinikum Jena, Jena Lobeda-Ost, Germany;
Carol Stanciu, Institutul de Gastroenterologie si Hepatologie, Lasi, Romania;
Ira Stein, Sarah Cannon Research Institute, Nashville, TN, USA;
Jawahar Taunk, Advanced Gastroenterology Associates, Palm Harbor, FL, USA;
James Underwood, Gastrointestinal Associates, Jackson, MS, USA;
Lawrence Wruble, Memphis Gastroenterology Group, Germantown, TN, USA;
June Yong, Hutchinson Clinic, Hutchinson, KS, USA;
Oren Zaidel, South Bay GI, Torrance, CA, USA;
Iurii Mykhailovich Zakharash, Central Clinical Hospital, National Medical University, Kyiv, Ukraine;
Salam Zakko, Connecticut Gastroenterology Institute, Bristol Hospital, Bristol, CT, USA.
Guarantors of the article: William J. Sandborn, MD and Stephan R. Targan, MD.
Specific author contributions: William J. Sandborn and Stephan R. Targan served on the executive committee of study investigators, and worked with Vera Byers of Immunology Inc., Dean Rutty of Everest Clinical Research Services, Inc., and Tom Tang of Hutchison Medipharma Ltd. to design the study. All authors have been involved in analysis and interpretation of the data. William J. Sandborn drafted the manuscript. All authors have critically reviewed the manuscript draft for intellectual content, and approved the final version of the manuscript for publication
Financial support: This work was supported by a research grant from Hutchison Medipharma Ltd., Shanghai, China.
Potential competing interests: Dr Sandborn reports having received consulting fees from Abbott Laboratories, ActoGeniX NV, AGI Therapeutics, Inc., Alba Therapeutics Corporation, Albireo, Alfa Wasserman, Amgen, AM-Pharma BV, Anaphore, Astellas Pharma, Athersys, Inc., Atlantic Healthcare Limited, Axcan Pharma (now Aptalis), BioBalance Corporation, Boehringer-Ingelheim Inc, Bristol Meyers Squibb, Celegene, Celek Pharmaceuticals, Cellerix SL, Cerimon Pharmaceuticals, ChemoCentryx, CoMentis, Cosmo Technologies, Coronado Biosciences, Cytokine Pharmasciences, Eagle Pharmaceuticals, Eisai Medical Research Inc., Elan Pharmaceuticals, EnGene, Inc., Eli Lilly, Enteromedics, Exagen Diagnostics, Inc., Ferring Pharmaceuticals, Flexion Therapeutics, Inc., Funxional Therapeutics Limited, Genzyme Corporation, Genentech (now Roche), Gilead Sciences, Given Imaging, Glaxo Smith Kline, Human Genome Sciences, Ironwood Pharmaceuticals (previously Microbia Inc.), Janssen (previously Centocor), KaloBios Pharmaceuticals, Inc., Lexicon Pharmaceuticals, Lycera Corporation, Meda Pharmaceuticals (previously Alaven Pharmaceuticals), Merck Research Laboratories, MerckSerono, Millennium Pharmaceuticals (subsequently merged with Takeda), Nisshin Kyorin Pharmaceuticals Co., Ltd., Novo Nordisk A/S, NPS Pharmaceuticals, Optimer Pharmaceuticals, Orexigen Therapeutics, Inc., PDL Biopharma, Pfizer, Procter and Gamble, Prometheus Laboratories, ProtAb Limited, Purgenesis Technologies, Inc., Receptos, Relypsa, Inc., Salient Pharmaceuticals, Salix Pharmaceuticals, Inc., Santarus, Schering Plough Corporation (acquired by Merck), Shire Pharmaceuticals, Sigmoid Pharma Limited, Sirtris Pharmaceuticals, Inc. (a GSK company), S.L.A. Pharma (UK) Limited, Targacept, Teva Pharmaceuticals, Therakos, Tillotts Pharma AG (acquired by Zeria Pharmaceutical Co., Ltd.), TxCell SA, UCB Pharma, Viamet Pharmaceuticals, Vascular Biogenics Limited (VBL), Warner Chilcott UK Limited, Wyeth (now Pfizer). He has received lecture fees from Abbott Laboratories, Bristol Meyers Squibb, and Janssen (previously Centocor). He has received research support from Abbott Laboratories, Bristol Meyers Squibb, Genentech, Glaxo Smith Kline, Janssen (previously Centocor), Millennium Pharmaceuticals (now Takeda), Novartis, Pfizer, Procter and Gamble Pharmaceuticals, Shire Pharmaceuticals, and UCB Pharma. Dr Targan reports having received consulting fees from Amgen, Biogen, Esai, GSK, Hutchison Medipharma, Lycera, Merck, Prometheus, Symbiotix, Takeda Pharmaceutical and Zyngenia. In addition, he reports having received research and meeting/travel support from Hutchison Medipharma. He also reports having received a grant for researching the mechanism of action for the drug published in this paper from Hutchison Medipharma. Dr Byers reports having received the following from Hutchison Medipharma: support for travel to meetings for the study and other purposes, receiving fees for review activity related to data monitoring and analysis, and for writing and reviewing the manuscript. Mr. Rutty reports having received the following from Hutchison Medipharma: support for travel to meetings for the study and other purposes, receiving fees for review activity related to data monitoring and analysis, and for writing and reviewing the manuscript. In addition, he reports Board membership for Steba Biotech SA and reports consulting for the following companies: Schering Corporation, Roche, Methylgene, Steba Biotech SA, Aderans Research Institute Inc., Stem Cell Theraputics, Genentech, Pearly Therapeutics, Sundise Chinese Medicine Technology Development Corp., Endocyte, Inc, and Generon Corporation Ltd. Mr Rutty is an employee of Everest Clinical Research Services Inc. Dr Tang reports being an employee of Hutchison Medipharma at the time of the clinical trial described herein and during the drafting of this manuscript. He is currently an employee of Generon (Shanghai). Dr Mu reports being an employee of Hutchison Medipharma. Dr Zhang reports being an employee of Hutchison Medipharma.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Supplementary Material
References
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- Kornbluth A, Sachar DB.Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee Am J Gastroenterol 2010105501–523.quiz 24. [DOI] [PubMed] [Google Scholar]
- Travis SPL, Stange EF, Lémann M, et al. European evidence-based consensus on the management of ulcerative colitis: current management. J Crohn′s Colitis. 2008;2:24–62. doi: 10.1016/j.crohns.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Lichtenstein GR, Abreu MT, Cohen R, et al. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:940–987. doi: 10.1053/j.gastro.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Poolsup N, Suthisisang C, Prathanturarug S, et al. Andrographis paniculata in the symptomatic treatment of uncomplicated upper respiratory tract infection: systematic review of randomized controlled trials. J Clin Pharm Ther. 2004;29:37–45. doi: 10.1046/j.1365-2710.2003.00534.x. [DOI] [PubMed] [Google Scholar]
- Coon JT, Ernst E. Andrographis paniculata in the treatment of upper respiratory tract infections: a systematic review of safety and efficacy. Planta Med. 2004;70:293–298. doi: 10.1055/s-2004-818938. [DOI] [PubMed] [Google Scholar]
- Saxena RC, Singh R, Kumar P, et al. A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection. Phytomedicine. 2010;17:178–185. doi: 10.1016/j.phymed.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Chao WW, Kuo YH, Lin BF. Anti-inflammatory activity of new compounds from Andrographis paniculata by NF-kappaB transactivation inhibition. J Agric Food Chem. 2010;58:2505–2512. doi: 10.1021/jf903629j. [DOI] [PubMed] [Google Scholar]
- Parichatikanond W, Suthisisang C, Dhepakson P, et al. Study of anti-inflammatory activities of the pure compounds from Andrographis paniculata (burm.f.) Nees and their effects on gene expression. Int Immunopharmacol. 2010;10:1361–1373. doi: 10.1016/j.intimp.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Tang T, Targan S, Li ZS, et al. Randomised clinical trial: herbal extract HMPL-004 in active ulcerative colitis: a double blind comparison with sustained release mesalazine. Aliment Pharmacol Ther. 2011;33:194–202. doi: 10.1111/j.1365-2036.2010.04515.x. [DOI] [PubMed] [Google Scholar]
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. a randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- D'Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- Sandborn W, Targan S, Byers V, et al. Randomized, double-blind, placebo-controlled trial of Andrographis paniculata extract (HMPL-004) in patients with moderately active Crohn's Disease Am J Gastroenterol 2010105(Suppl 1S429Abstract 1180. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.