Abstract
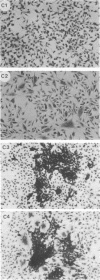
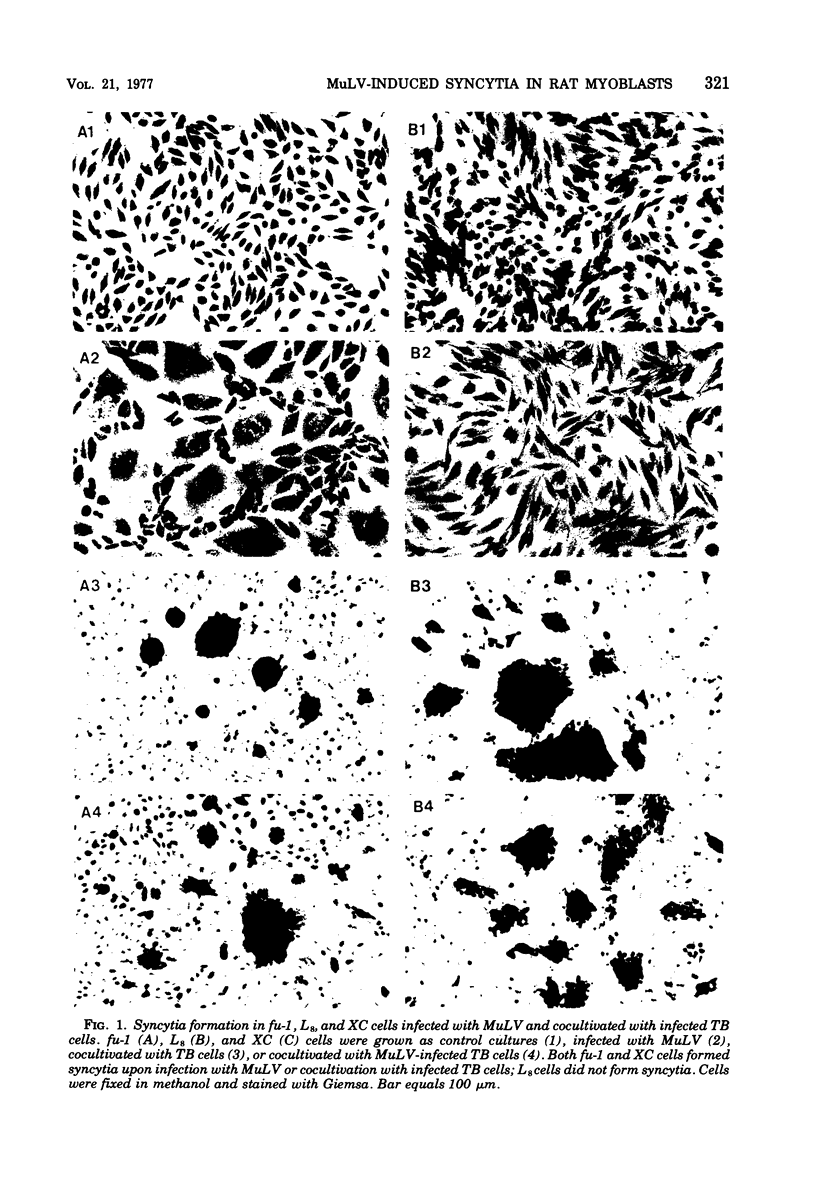
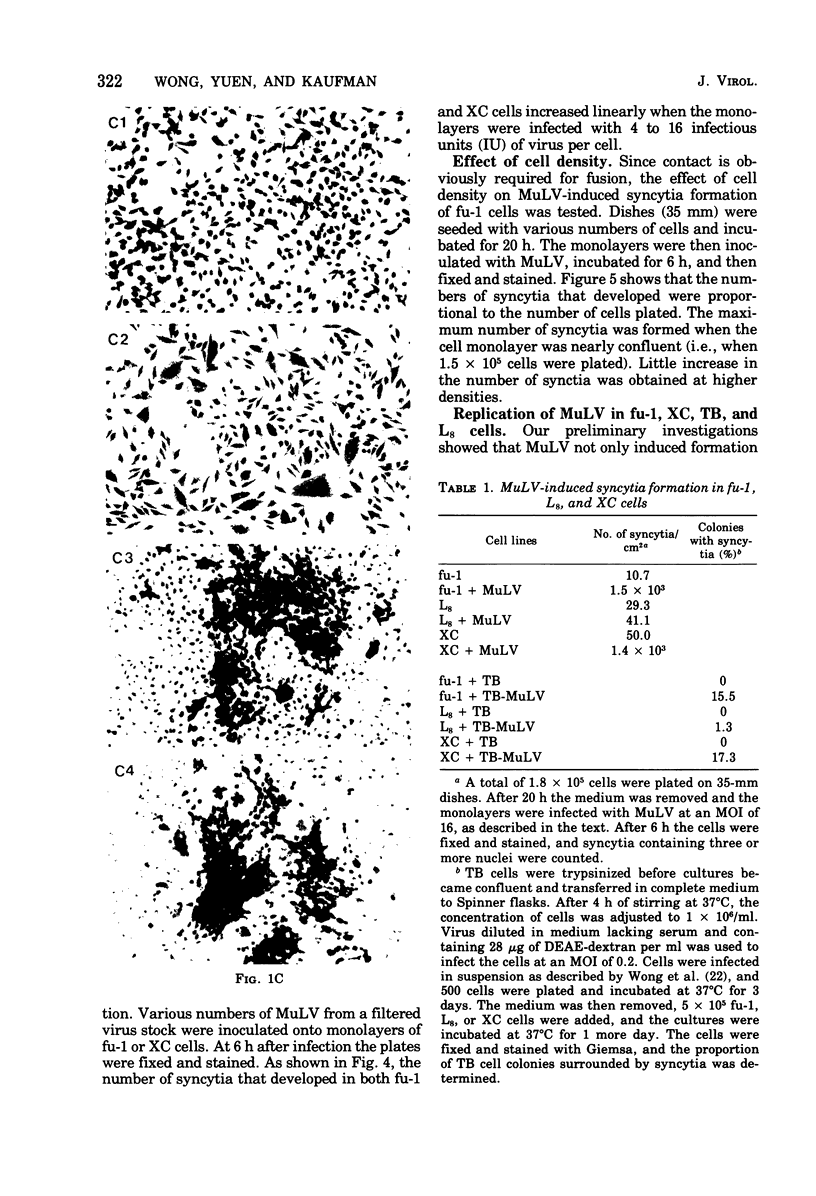
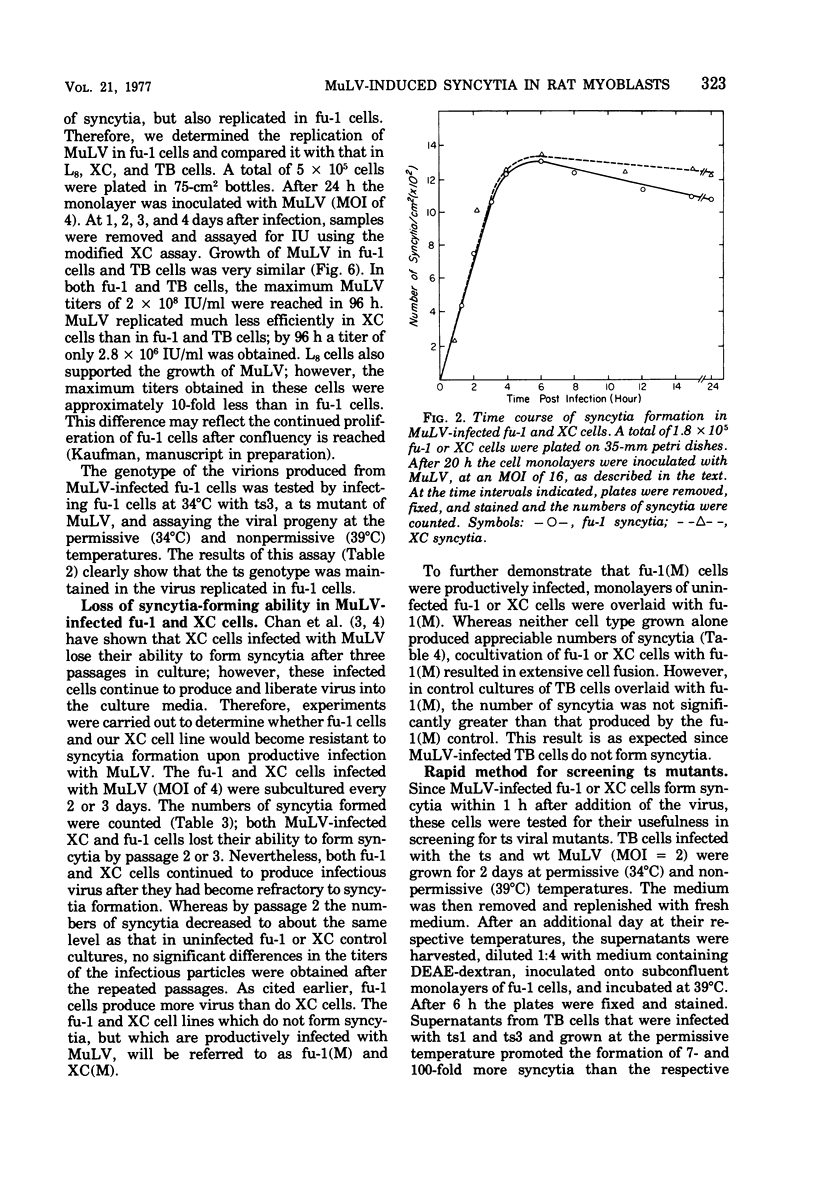
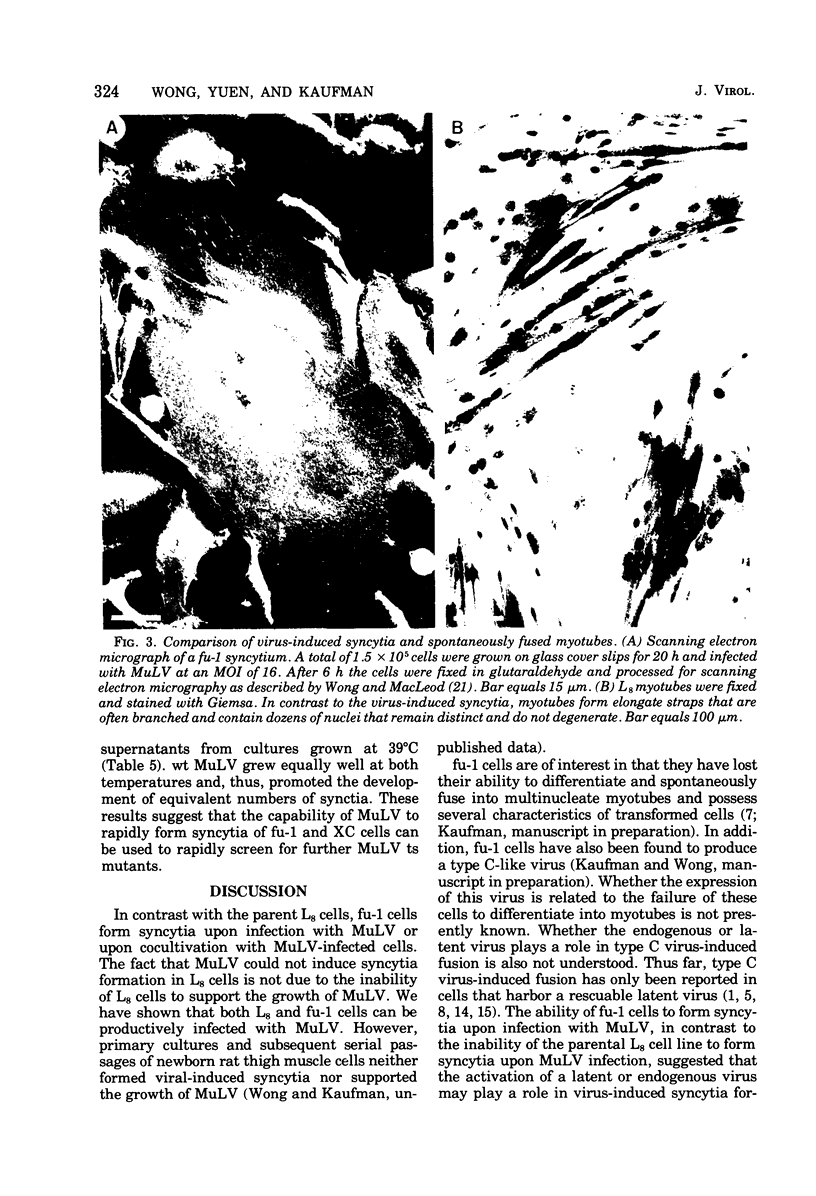
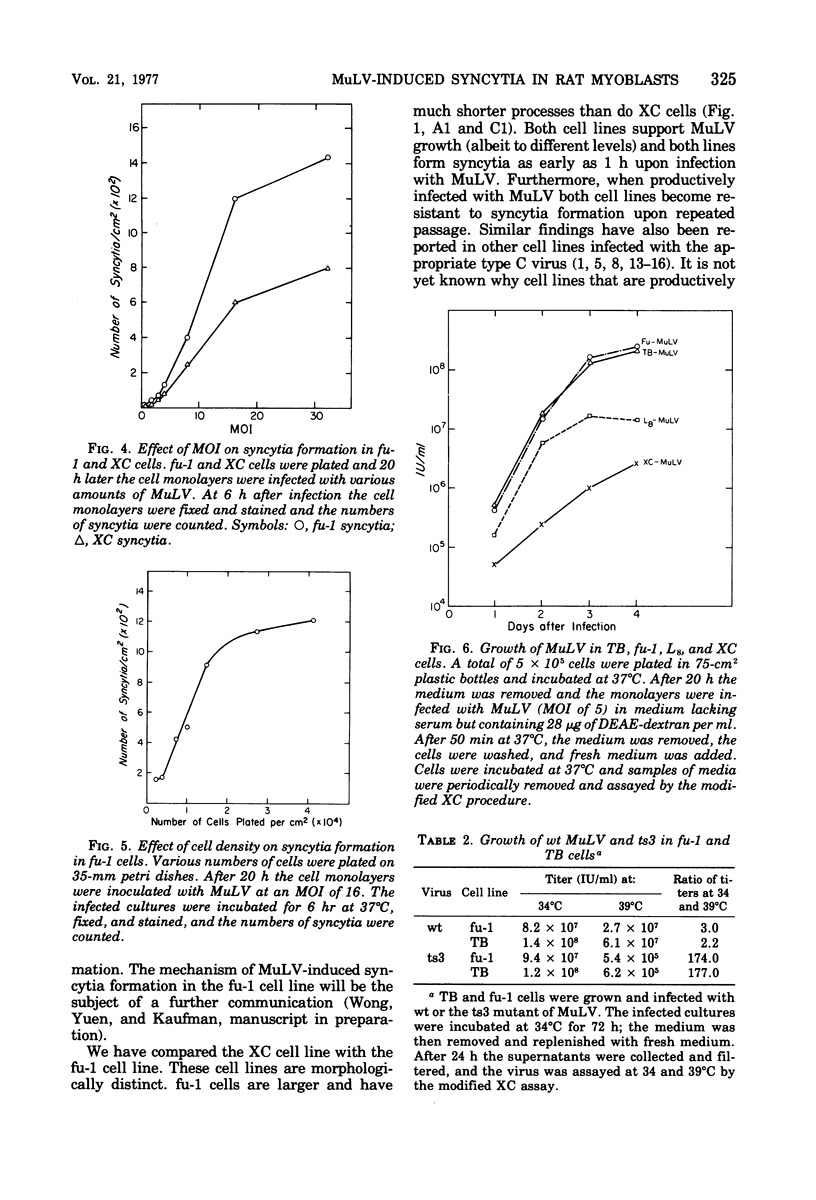
fu-1 cells, a nonfusing variant of the L8 line of rat myoblasts, form syncytia upon infection with murine leukemia virus (MuLV) or upon cocultivation with MuLV-infected cells; L8 cells do not form these syncytia, but do fuse into multinucleate myotubes. Syncytia of fu-1 cells form within 1 h after infection. The number of syncytia formed is proportional to the multiplicity of virus within a range of 4 to 16 and is maximum when the cell density is subconfluent. When either XC or fu-1 cells are productively infected with MuLV, they become resistant to syncytia formation by passage 3. The rapid formation of syncytia in fu-1 cells was found amenable for selection of temperature-sensitive mutants of MuLV and for screening additional variants of the L8 line.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M., Korol W., Larson D. L., Harewood K. R., Mayyasi S. A. Interactions between endogenous baboon type-C virus and oncogenic viruses. I. Syncytium induction and development of infectivity assay. Int J Cancer. 1975 Nov 15;16(5):747–755. doi: 10.1002/ijc.2910160507. [DOI] [PubMed] [Google Scholar]
- BALL J. K., HUH T. Y., MCCARTER J. A. ON THE STATISTICAL DISTRIBUTION OF EPIDERMAL PAPILLOMATA IN MICE. Br J Cancer. 1964 Mar;18:120–123. doi: 10.1038/bjc.1964.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cham J. C., Vera N., Allen P. T., East J. L., Knesek J. E. Alteration of the syncytium-forming property of XC cells by productive Moloney leukemia virus infection. Cancer Res. 1975 Jul;35(7):1854–1857. [PubMed] [Google Scholar]
- Chan J. C., Vera N., East J. L., Hiraki S., Dmochowski L. Lack of syncytium formation by a type C virus-producing XC cell line in the mixed culture cytopathogenicity test. Cancer Res. 1974 Mar;34(3):468–473. [PubMed] [Google Scholar]
- Hampar B., Rand K. H., Lerner R. A., Del Villano B. C., Jr, McAllister R. M., Martos L. M., Derge J. G., Long C. W., Gilden R. V. Formation of syncytia in human lymphoblastoid cells infected with type C viruses. Virology. 1973 Oct;55(2):453–463. doi: 10.1016/0042-6822(73)90187-6. [DOI] [PubMed] [Google Scholar]
- Johnson G. S., Friedman R. M., Pastan I. Analysis of the fusion of XC cells induced by homogenates of murine leukemia virus-infected cells and by purified murine leukemia virus. J Virol. 1971 Jun;7(6):753–758. doi: 10.1128/jvi.7.6.753-758.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement V., Rowe W. P., Hartley J. W., Pugh W. E. Mixed culture cytopathogenicity: a new test for growth of murine leukemia viruses in tissue culture. Proc Natl Acad Sci U S A. 1969 Jul;63(3):753–758. doi: 10.1073/pnas.63.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA Y. Analysis of giant polynuclear cell formation caused by HVJ virus from Ehrlich's ascites tumor cells. I. Microscopic observation of giant polynuclear cell formation. Exp Cell Res. 1962 Feb;26:98–107. doi: 10.1016/0014-4827(62)90205-7. [DOI] [PubMed] [Google Scholar]
- Poste G., Allison A. C. Membrane fusion reaction: a theory. J Theor Biol. 1971 Jul;32(1):165–184. doi: 10.1016/0022-5193(71)90144-5. [DOI] [PubMed] [Google Scholar]
- Poste G., Allison A. C. Membrane fusion. Biochim Biophys Acta. 1973 Dec 28;300(4):421–465. doi: 10.1016/0304-4157(73)90015-4. [DOI] [PubMed] [Google Scholar]
- Poste G. Mechanisms of virus-induced cell fusion. Int Rev Cytol. 1972;33:157–252. doi: 10.1016/s0074-7696(08)61451-5. [DOI] [PubMed] [Google Scholar]
- Rand K. H., Long C. W. Fusion of a Rous sarcoma virus transformed human cell line, KC, by RD-114 virus. J Gen Virol. 1973 Dec;21(3):523–532. doi: 10.1099/0022-1317-21-3-523. [DOI] [PubMed] [Google Scholar]
- Rand K. H., Long C. Syncytial assay for the putative human C-type virus, RD-114, utilizing human cells transformed by Rous sarcoma virus. Nat New Biol. 1972 Dec 6;240(101):187–190. doi: 10.1038/newbio240187a0. [DOI] [PubMed] [Google Scholar]
- Rand K., Davis J., Gilden R. V., Oroszlan S., Long C. Fusion inhibition: bioassay of type C viral protein. Virology. 1975 Mar;64(1):63–74. doi: 10.1016/0042-6822(75)90079-3. [DOI] [PubMed] [Google Scholar]
- Rangan S. R., Ueberhorst P. J., Wong M. C. Syncytial giant cell focus assay for viruses derived from feline leukemia and a simian sarcoma. Proc Soc Exp Biol Med. 1973 Apr;142(4):1077–1082. doi: 10.3181/00379727-142-37180. [DOI] [PubMed] [Google Scholar]
- SVOBODA J., CHYLE P., SIMKOVIC D., HILGERT I. Demonstration of the absence of infectious Rous virus in rat tumour XC, whose structurally intact cells produce Rous sarcoma when transferred to chicks. Folia Biol (Praha) 1963 Apr;9:77–81. [PubMed] [Google Scholar]
- Trowbridge S. T., Benyesh-Melnick M., Biswal N. Replication of the Moloney murine sarcoma-leukemia virus in XC cells. J Virol. 1973 Jan;11(1):146–149. doi: 10.1128/jvi.11.1.146-149.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., MacLeod R. Studies on the budding process of a temperature-sensitive mutant of murine leukemia virus with a scanning electron microscope. J Virol. 1975 Aug;16(2):434–442. doi: 10.1128/jvi.16.2.434-442.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., McCarter J. A. Studies of two temperature-sensitive mutants of Moloney murine leukemia virus. Virology. 1974 Apr;58(2):396–408. doi: 10.1016/0042-6822(74)90075-0. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Russ L. J., McCarter J. A. Rapid, selective procedure for isolation of spontaneous temperature-sensitive mutants of Moloney leukemia virus. Virology. 1973 Feb;51(2):424–431. doi: 10.1016/0042-6822(73)90441-8. [DOI] [PubMed] [Google Scholar]