Abstract
In healthy adults, activation of γ-aminobutyric acid (GABA)A and glycine receptors inhibits neurons as a result of low intracellular chloride concentration ([Cl–]i), which is maintained by the potassium-chloride cotransporter KCC2. A reduction of KCC2 expression or function is implicated in the pathogenesis of several neurological disorders, including spasticity and chronic pain following spinal cord injury (SCI). Given the critical role of KCC2 in regulating the strength and robustness of inhibition, identifying tools that may increase KCC2 function and, hence, restore endogenous inhibition in pathological conditions is of particular importance. We show that activation of 5-hydroxytryptamine (5-HT) type 2A receptors to serotonin hyperpolarizes the reversal potential of inhibitory postsynaptic potentials (IPSPs), EIPSP, in spinal motoneurons, increases the cell membrane expression of KCC2 and both restores endogenous inhibition and reduces spasticity after SCI in rats. Up-regulation of KCC2 function by targeting 5-HT2A receptors, therefore, has therapeutic potential in the treatment of neurological disorders involving altered chloride homeostasis. However, these receptors have been implicated in several psychiatric disorders, and their effects on pain processing are controversial, highlighting the need to further investigate the potential systemic effects of specific 5-HT2AR agonists, such as (4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine hydrobromide (TCB-2).
The neuron-specific K+-Cl– cotransporter KCC2 (encoded by the solute carrier family 12 member 5, Slc12a5) extrudes Cl– and is responsible for the low [Cl–]i in mature neurons (1–3), a prerequisite for hyperpolarizing inhibition mediated by GABAA receptors (GABAARs) and glycine receptors (GlyRs). The expression or the function of KCC2 is reduced in several neurological disorders (2, 4), and the resulting slight increase in [Cl–]i (depolarizing shift of the chloride equilibrium potential, ECl) dramatically compromises the inhibitory control of firing rate and excitatory inputs (5–7). Given the role of KCC2 in regulating the strength of inhibitory synaptic transmission, identifying tools that may increase KCC2 function and, hence, restore endogenous inhibition in pathological conditions is of particular importance.
Spasticity is a disabling complication affecting individuals with spinal cord injury (SCI) and is characterized by a velocity-dependent increase in muscle tone resulting from hyperexcitable stretch reflexes, spasms, and hypersensitivity to normally innocuous sensory stimulations (8, 9). Down-regulation of KCC2 after SCI in rats is implicated in the development of spasticity (10) and chronic pain (11, 12). Notably, the expression of KCC2 in the motoneuron membrane is reduced, and, concomitantly, the density of cytoplasmic clusters is higher, suggesting that the surface stability of the transporter is reduced in these pathological conditions (10).
Mounting evidence indicates that phosphorylation of KCC2 in the C-terminal intracellular domain dynamically regulates its activity and surface expression (1). In particular, phosphorylation by protein kinase (PK)C, enhances KCC2 activity and reduces endocytosis (13). Interestingly, activation of 5-hydroxytryptamine type 2 receptors (5-HT2Rs) to serotonin stimulates PKC and strengthens the left–right alternation of motor bursts observed during locomotion (14–16), which rely on reciprocal inhibition (17, 18). We hypothesized that 5-HT2R activity modulates KCC2 function and/or expression. Our results indicate that the activation of the 5-HT2AR subtype hyperpolarizes EIPSP via a PKC-dependent mechanism, increases KCC2 expression in the plasma membrane of motoneurons, and reduces SCI-induced spasticity.
Results
Negative Shift of EIPSP and Up-Regulation of KCC2.
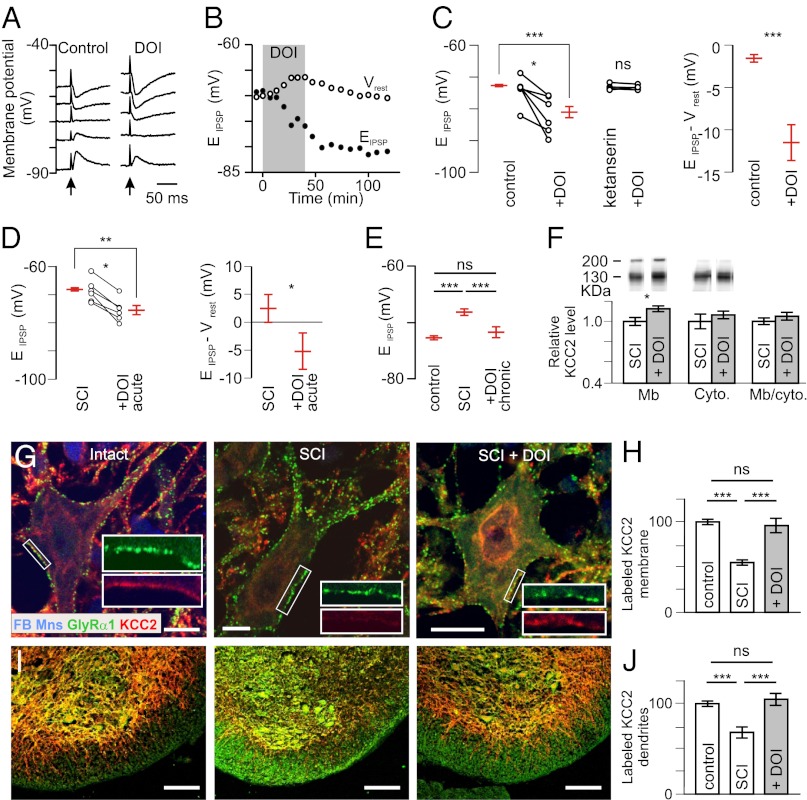
We first examined the effect of the 5-HT2A/2B/2CR agonist (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI) (10 µM; Table S1) on EIPSP in control neonatal rats [postnatal day (P)5–P7]. DOI-hyperpolarized EIPSP within 10–20 min (Fig. 1 A and B). This effect was long-lasting (at least 2 h; Fig. 1B). EIPSP was significantly more hyperpolarized when motoneurons were recorded in the presence of DOI compared with control (8 mV; Fig. 1C, Left). There was a concomitant trend toward a depolarization of the resting membrane potential (Vrest) by DOI (+2 mV; P > 0.05). As a result, the amplitude of hyperpolarizing IPSPs recorded at Vrest increased significantly (Fig. 1C, Right).
Fig. 1.
Activation of 5-HT2Rs hyperpolarizes EIPSP and increases membrane expression of KCC2. (A) IPSPs evoked by stimulation of the ventral funiculus of the spinal cord (arrow) at different holding potentials in a motoneuron from a P6 intact rat before and after adding DOI (10 µM). (B) Time course of the change in EIPSP and Vrest. (C) EIPSP and driving force (EIPSP-Vrest) measured in control conditions (n = 21) and after adding DOI (n = 8). ***P < 0.001 (Mann–Whitney test). Six motoneurons were tested before and after DOI. *P < 0.05 (Wilcoxon paired test). The effects of DOI were prevented in the presence of ketanserin (10 µM; n = 4) (Center). (D) Effects of DOI (1–1.5 µM) on EIPSP and driving force in motoneurons recorded 4–6 d after neonatal SCI (17 and 6 cells recorded in the absence and the presence of DOI, respectively). **P < 0.01, *P < 0.05 (Mann–Whitney test); *P < 0.05 (Wilcoxon paired test). (E) EIPSP was significantly more hyperpolarized after chronic treatment of DOI (n = 9) compared with SCI animals (n = 22) but not different compared with control animals (n = 21). ns, not significant (P > 0.05); ***P < 0.001 (one-way ANOVA, Tukey’s post tests). (F, Upper) Western blots of membrane and cytoplasmic fractions of lumbar spinal cords labeled with a KCC2-specific antibody. The 130- to 140-kDa and the >200-kDa bands correspond to the monomeric and oligomeric proteins, respectively (53). (F, Lower) Quantification of KCC2 expression in DOI-treated rats after neonatal SCI (percentage of untreated rats). *P < 0.05 (Mann–Whitney test; n = 6 in each group). (G) Dual labeling of GlyRα1 and KCC2 on FB-labeled ankle extensor motoneurons, in three conditions (P7): intact, neonatal SCI, and chronic DOI treatment. (Scale bars: 10 µm.) (H) Quantification of the density of membrane KCC2 labeling (ratios of labeled pixel surface per somatic perimeter) in intact rats (n = 34 motoneurons) and untreated (n = 41) or DOI-treated (n = 44) rats with neonatal SCI (three rats in each group). ***P < 0.001 (Kruskal–Wallis test, Dunn’s post tests). (I) Ventral part of the lumbar spinal cord exhibiting KCC2-immunopositive dendrites stretching out through the white matter. (Scale bars: 100 µm.) (J) Quantification of the KCC2 labeling in the white matter in intact rats (n = 34) and untreated (n = 41) or DOI-treated (n = 44) rats with neonatal SCI. ***P < 0.001 (Kruskal–Wallis test, Dunn’s post tests).
The next series of experiments was performed on animals that underwent a neonatal SCI. EIPSP was significantly more depolarized in those animals tested at P5–P7, compared with controls of the same age, as shown previously (19) (compare Fig. 1 C and D). Because of the increased sensitivity of neurons to 5-HT in those animals (15), DOI was tested at a lower concentration (1–1.5 µM) than in controls. DOI induced an ∼8-mV hyperpolarization of EIPSP (Fig. 1D, Left). As a result, EIPSP shifted from above to below Vrest (Fig. 1D, Right). Another set of animals was treated chronically with DOI from P4 to P6–P7 [0.15 mg/kg, i.p. (15, 20) twice a day]. EIPSP was more hyperpolarized in those DOI-treated animals than in untreated transected animals (Fig. 1E). Values were similar to those measured in control animals.
We performed subcellular fractionation of proteins from the lumbosacral spinal cord, followed by immunoblotting with a specific antibody against KCC2. The amount of KCC2 in the membrane fraction (KCC2Mb) was significantly increased after chronic DOI treatment, compared with NaCl-treated pups (Fig. 1F). There was a trend toward an increase in the amount of KCC2 in the cytosolic fraction. As a result, the ratio KCC2Mb/KCC2 cytoplasm was nonsignificantly increased. We then analyzed the expression of KCC2 by immunohistochemistry. All of the analyses were performed on a homogeneous population of retrogradely labeled lumbar motoneurons [triceps surae (TS) muscles (ankle extensors); Fig. 1G]. GlyRs are colocalized with the anchoring protein gephyrin and can, therefore, be used to label the plasma membrane; in addition, they are not affected by a neonatal spinal cord transection (20). We, therefore, immunolabeled the α1 subunit of this receptor to visualize the plasma membrane of motoneurons in control (intact), untreated (SCI), and DOI-treated cord-transected animals (SCI + DOI; Fig. 1G, Insets). After SCI, the intensity of KCC2 staining in the somatic membrane was reduced (Fig. 1H). DOI treatment increased KCC2 staining, so that no difference was observed compared with controls of the same age. We also quantified the KCC2 staining at the level of motoneuron dendrites that stretch out of the gray matter into the lateral and ventral funiculi (Fig. 1I). Similar to what was observed for the somatic membrane, DOI treatment restored the intensity of staining to values seen in controls (Fig. 1J). Altogether, these results indicate that the activation of 5-HT2R is able to restore chloride homeostasis (hyperpolarizing shift of EIPSP) after neonatal SCI. An increased expression of KCC2 in the plasma membrane, at least in part, provides the molecular basis of this restoration.
Contribution of 5-HT2ARs.
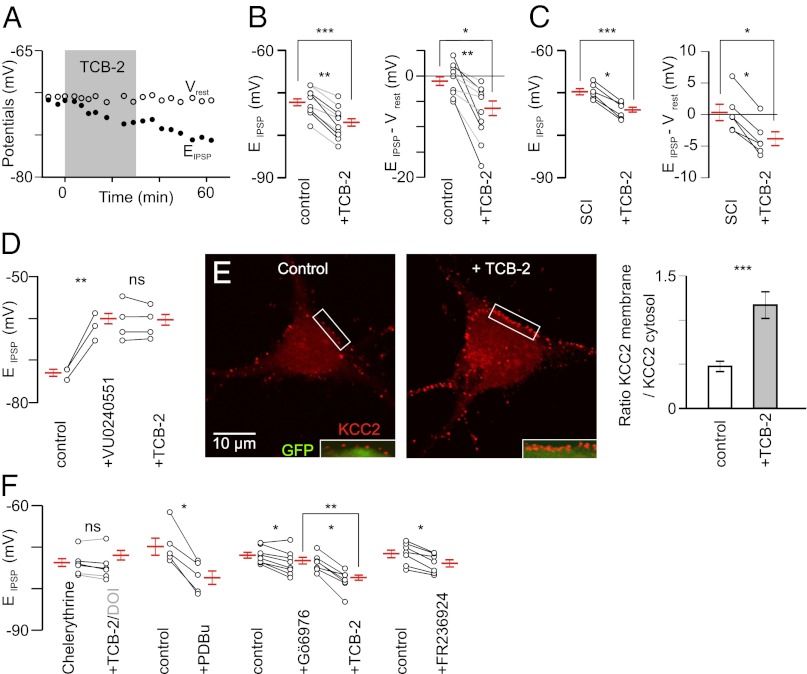
The effects of DOI on EIPSP were prevented in the presence of the 5-HT2R antagonist ketanserin (10 µM; n = 4; Fig. 1C, Center). Because DOI and ketanserin also bind to 5-HT2BRs and 5-HT2CRs (Table S1), to further identify the subtype of 5-HT2R that are involved, we used (4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)methylamine hydrobromide (TCB-2), a high-affinity 5-HT2AR agonist (21, 22) (Table S1). TCB-2 at low concentration (0.1 µM; n = 6) hyperpolarized EIPSP by ∼4 mV in control rats (Fig. 2 A and B, gray). Note that even values that were highly negative (−78 mV) were further hyperpolarized in the presence of TCB-2. The shift was larger (6.1 mV) at a higher concentration (10 µM; n = 4; Fig. 2B, black). TCB-2 had no effect on Vrest and the other electrical properties investigated (Fig. S1). As a result, the driving force (EIPSP-Vrest) was significantly increased (Fig. 2B; −5.3 mV). We next tested this compound on motoneurons recorded from animals with neonatal SCI. The hyperpolarizing shift (−4.2 mV) induced by TCB-2 (0.1µM) was similar to that seen in control animals (Fig. 2C). On average, EIPSP switched from above to below Vrest (Fig. 2C; P < 0.05; Wilcoxon paired test).
Fig. 2.
Involvement of 5-HT2ARs via a PKC-dependent signaling pathway. (A) TCB-2 (0.1 µM; 30 min) induces a hyperpolarizing shift of EIPSP without concomitant effect on Vrest (P6). (B, Left) Effect of TCB-2 (0.1 µM, gray; 10 µM, black) on 10 motoneurons recorded from control rats (P5–P6). ***P < 0.001 (Mann–Whitney test; n = 12 and n = 14 before and under TCB-2, respectively); **P < 0.01 (Wilcoxon paired test). The difference was also significant when considering only the effect of the lowest concentration [0.1 µM; n = 6; *P < 0.05 (Wilcoxon paired test)]. (B, Right) Effect of TCB-2 (0.1–10 µM) on the driving force (control, n = 12; TCB-2, n = 14). *P < 0.05 (Mann–Whitney test); **P < 0.01 (Wilcoxon paired test). (C) Effect of TCB-2 (0.1 µM) on EIPSP and the driving force of motoneurons (six and nine in the absence and in the presence of TCB-2, respectively) in animals with SCI at birth. ***P < 0.001, *P < 0.05 (Mann–Whitney test); *P < 0.05 (Wilcoxon paired test) (n = 6). (D) EIPSP was significantly depolarized by the application of the KCC2 blocker VU0240551 (25 µM), which prevented the hyperpolarizing effect of TCB-2 (0.1 µM; n = 11). Averages are taken from 3 motoneurons in control situation, 9 motoneurons under VU0240551, and 11 motoneurons in the presence of both VU0240551 and TCB-2. ns, P > 0.05; **P < 0.01 (Mann–Whitney test). (E) TCB-2 (0.1 µM; 30 min) significantly increased the expression of KCC2 at the plasma membrane of Hb9::eGFP transgenic motoneurons in culture (14 DIV). (E, Left and Center) Merge images (Insets) show the KCC2 immunolabeling at the periphery of GFP-expressing cytosol. (E, Right) Ratio of KCC2 expression between plasma membrane and cytosol. ***P < 0.001 (t test; n = 34 cells from two experiments analyzed in each condition). (F) Preincubation of the PKC inhibitor chelerythrine (20 µM; 30 min; n = 6) prevented the effect of DOI (10 µM; n = 3; gray) or TCB-2 (0.1 µM; n = 3). PDBu significantly hyperpolarized EIPSP. ns, P > 0.05; *P < 0.05 (Wilcoxon paired test). The Ca2+-dependent PKC inhibitor Gö6976 (2 µM; n = 8) induced a slight but significant hyperpolarization of EIPSP and a further ∼4-mV negative shift was observed after adding TCB-2 (0.1 µM; n = 16). **P < 0.001 (Mann–Whitney test); *P < 0.05 (Wilcoxon paired test). The activator of the Ca2+-independent PKCɛ (FR236924; 2–8 µM; n = 7) hyperpolarized EIPSP. *P < 0.05 (Wilcoxon paired test).
The use of 5-HT2A antagonists (ketanserin at 1 µM and MDL11,939 at 2 µM) and 5-HT2B/2C agonists and antagonists confirmed the involvement of 5-HT2AR in the negative shift of EIPSP and suggested that 5-HT2B/2C may have opposite effects (Fig. S2). To identify the mechanisms underlying these effects, we used VU0240551 (25 µM), a highly specific KCC2 blocker (23). This compound depolarized EIPSP by >10 mV (Fig. 2D). KCC2 blockade occluded the effect of TCB-2 (Fig. 2D, Right), which is consistent with the requirement of KCC2 for the EIPSP shift induced by the activation of 5-HT2AR. TCB-2 had no effect on the kinetics of IPSPs (Fig. S3), indicating that it modulates the strength of inhibition essentially through a modulation of KCC2 function.
We used immunolabeling to quantify the effect of TCB-2 on the expression of KCC2 at the plasma membrane of cultured motoneurons isolated from Hb9::eGFP transgenic mice (Fig. 2E, Left). Following activation of 5-HT2AR by TCB-2 (0.1 µM; 30 min), the ratio of KCC2 expression between plasma membrane and cytosol, visualized by eGFP fluorescence (Fig. 2E, Insets), was strongly increased (Fig. 2E, Right).
PKC-Dependent Pathway Is Involved.
We next investigated the second messenger pathway activated by TCB-2/DOI. 5-HT2ARs stimulate phospholipase C, leading to activation of PKC. Consistent with an involvement of PKC, the effects of both TCB-2 (Fig. 2F, black) and DOI (Fig. 2F, gray) were prevented by preincubation of PKC inhibitor chelerythrine (20 µM). In addition, activation of PKC with phorbol 12,13-dibutyrate (PDBu) (1 µM) induced an ∼9-mV negative shift of EIPSP (Fig. 2F), which switched on average from above (5.5 ± 3.7 mV) to below (−3.3 ± 2.1 mV) Vrest. PKCs are divided into subfamilies, based on their second messenger requirements. The indolocarbazole Gö6976 enables discrimination between Ca2+-dependent and -independent isoforms of PKC. Nanomolar concentrations of this compound inhibit the Ca2+-dependent isozymes, whereas even micromolar concentrations have no effect on the Ca2+-independent PKC subtypes (24). Gö6976 (2 µM) induced a small (∼1.5 mV) but significant hyperpolarization of EIPSP (Fig. 2F). Importantly, a further ∼4-mV negative shift was observed after adding TCB-2 (Fig. 2F). The potent and selective activator of the Ca2+-independent PKCɛ, FR236924, induced an ∼2-mV negative shift of EIPSP (Fig. 2F). Altogether, these results demonstrate that the activation of 5-HT2AR increases KCC2 function, at least in part, through a Ca2+-independent PKC.
Activation of 5-HT2AR Increases the Strength of Inhibition.
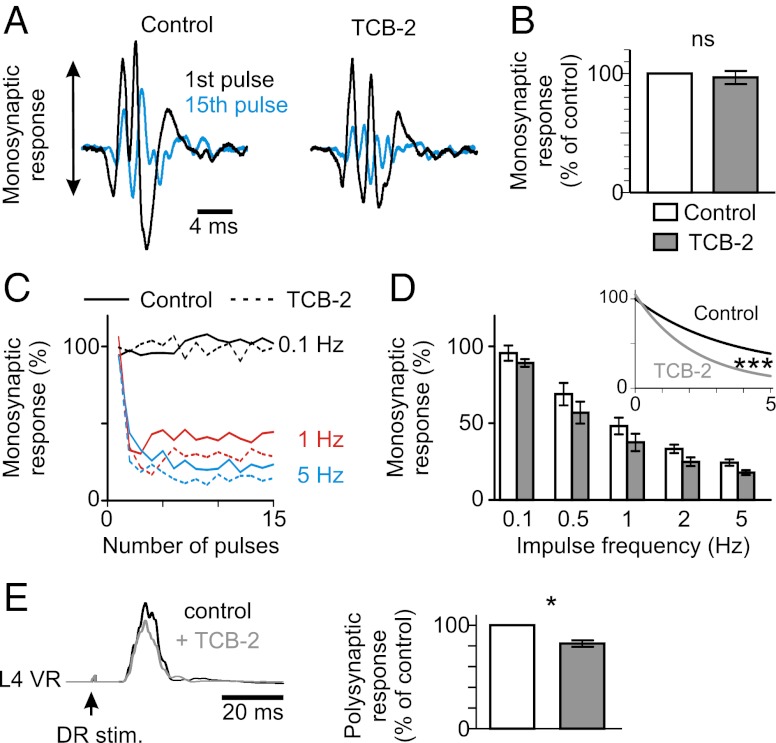
Supramaximal stimulation of a lumbar dorsal root elicits a response in the homonymous ipsilateral ventral root in vitro (Fig. 3A). The earliest component representing the monosynaptic excitation of motoneurons (25, 26) was not affected by TCB-2 (0.1–0.15 µM; Fig. 3 A and B). The amplitude of the monosynaptic response decreases when the dorsal root is stimulated repeatedly (Fig. 3A), an effect that gets stronger as the frequency of stimulation increases [rate-dependent depression (RDD); Fig. 3C]. Because KCC2 plays a key role in the depression of the response (Fig. S4), we hypothesized that hyperpolarizing EIPSP by TCB-2 would affect the RDD. As expected, TCB-2 reduced the amplitude of the monosynaptic response elicited by repetitive stimulation (Fig. 3 A, blue trace, and Fig. 3C, dotted lines). As a result, the RDD was significantly increased (Fig. 3D). This effect was prevented by VU0240551 (Fig. S5).
Fig. 3.
Effects of TCB-2 on ventral root responses to dorsal root stimulation in the intact spinal cord in vitro. (A) Responses evoked in the L5 ventral root by supramaximal stimulation of the ipsilateral homonymous dorsal root, in the in vitro spinal cord isolated from rats at P6. The earliest component represents the monosynaptic response of motoneurons. The amplitude of this response decreased when the dorsal root was stimulated repeatedly (response to the 15th pulse at 1 Hz is shown in blue, superimposed with the control response to the first pulse). The slight increase in latency is attributable to a delayed firing of motoneurons as the pulse number increases. The amplitude of the response to the 15th pulse was much smaller after adding TCB-2 (0.1–0.15 µM). (B) Relative amplitudes of the monosynaptic responses delivered every 30 s before and 20–30 min after adding TCB-2 [percentage of control before TCB-2; P > 0.05 (Wilcoxon paired test); n = 12]. (C) Relative amplitudes of the monosynaptic responses to 15 consecutive stimulations at different frequencies (0.1 Hz, black; 1 Hz, red; 5 Hz, blue) before (continuous line) and after adding TCB-2 (dotted line). This is the same experiment as in A. (D) Mean (± SEM) relative amplitudes of the monosynaptic reflex at different stimulation frequencies in seven animals at P5–P6 before [controls in artificial cerebrospinal fluid (aCSF)] and after TCB-2 application (0.1–0.15 μM). Twelve values (the first three discarded) were averaged for each animal. The RDD was significantly increased by TCB-2. ***P < 0.001 (one-phase exponential decay regression). Note that for each frequency of stimulation, except at 0.1 Hz, the difference between before and after TCB-2 was significant [P < 0.05 (Wilcoxon paired test)]. (E) Mean envelopes of the ventral root (VR) burst evoked by stimulation of the ipsilateral homonymous dorsal root (DR) before and after adding TCB-2 (0.15 µM). *P < 0.05 (Wilcoxon paired test; n = 7).
We next considered the polysynaptic response, which results from a mixture of excitation and inhibition in motoneurons (27, 28). The polysynaptic response is potentiated by VU0240551 within 15–20 min, as a result of decreased strength of inhibition (Fig. S6). This effect was reversible. TCB-2 reduced the polysynaptic response within 15–20 min, and this effect lasted at least 1 h (Fig. 3E), an effect that was occluded by prior application of picrotoxin and strychnine (to block GABAARs and GlyRs, respectively) or VU0240551 (Fig. S6). Therefore, TCB-2 increases the strength of postsynaptic inhibition in the intact spinal cord in vitro likely through a modulation of chloride homeostasis. In addition, such strengthening of inhibition by TCB-2 enabled to restore the alternating locomotor pattern that is disorganized after neonatal SCI (15) (Fig. S7).
Reduction of Spasticity After SCI.
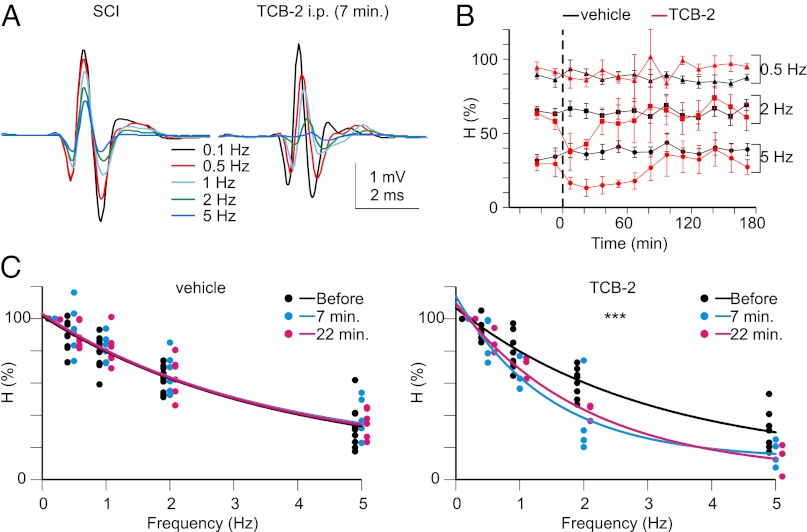
The H (or Hoffmann) reflex is commonly used to assess the excitability of the monosynaptic reflex loop in individuals suffering from spasticity. The H wave (Fig. 4A) resulting from the monosynaptic activation of motoneurons by type Ia afferents undergoes an RDD when using frequencies of stimulation higher than 0.1 Hz (29) (Fig. 4 A–C). Reduction of the RDD is a reliable correlate of spasticity after SCI (10, 29, 30). The down-regulation of KCC2 accounts, at least in part, for this reduction (10). We investigated the effects of TCB-2 (0.3 mg/kg, i.p.) on the RDD in paraplegic spastic adult rats (14–21 d post-SCI). The RDD remained quite stable after i.p. injection of the vehicle (NaCl; Fig. 4 B and C). Injection of TCB-2 did not affect the thresholds, maximal amplitudes, or stimulus intensities at which the maximal amplitude is obtained for both M and H waves at 0.1 Hz (Table S2). However, an effect was clearly present when frequencies of stimulation higher than 0.5 Hz were used (Fig. 4 B and C). Indeed, the amplitude of the H wave was smaller than it was before injection, with a maximal and highly significant effect 7–25 min after TCB-2 injection (Fig. 4 B and C).
Fig. 4.
Activation of 5-HT2ARs increases the RDD of the H reflex. (A) Hmax responses evoked in adult rats after SCI before (Left) and 7 min after i.p. injection of TCB-2 (0.3 mg/kg) (Right). Each trace is the mean response to 17 consecutive stimulations (the first three discarded) at 0.1, 0.5, 1, 2, or 5 Hz. (B) Time course of the change in H-reflex depression after i.p. injection of vehicle (NaCl; n = 6 rats) or TCB-2 (n = 4). (C) Mean relative amplitudes of the H reflex before (black) and after (blue, 7 min; red, 22 min) the injection of vehicle (Left) or TCB-2 (Right). The data of all experiments are represented (the dots that are slightly offset at each frequency of stimulation are for clarity) together with the one-phase exponential decay fit of these data. ***P < 0.001, comparison of the fits before and after TCB-2 (either 7 or 22 min). The fits before and after vehicle are not significantly different.
Discussion
This study demonstrates that the activation of a receptor to a neurotransmitter restores endogenous inhibition in pathological conditions by up-regulating KCC2 function. Activation of 5-HT2AR by TCB-2 in vitro leads to a hyperpolarizing shift of EIPSP and an increased cell surface expression of KCC2 within minutes. Blocking KCC2 occluded this shift, indicating that the effect of TCB-2 is likely mediated by posttranslational modification of KCC2, as opposed to changes in gene transcription or protein synthesis. Chronic activation of 5-HT2AR also induced a hyperpolarizing shift of EIPSP and a concomitant increased expression of KCC2. Changes in gene transcription or protein synthesis may occur in this situation, as suggested by the trend toward an increase in cytoplasmic KCC2.
Modulation of chloride homeostasis by 5-HT has been previously reported in the spinal cord [in zebrafish (31)] and several other systems (e.g., refs. 32 and 33). Here, we showed that the effect of 5-HT2AR activation on chloride homeostasis was critically dependent on PKC. In agreement with these findings, the activity of a number of protein kinases, including PKC and phosphatases, has been reported to influence KCC2 function. It was further demonstrated that KCC2 is directly phosphorylated by PKC activity and that the major site of phosphorylation within the C-terminal intracellular domain of this protein is serine 940 (Ser940). PKC-dependent phosphorylation of Ser940 increases KCC2 cell surface stability and activity by decreasing endocytosis from the plasma membrane (13). In HEK-293 cells, the entire cell surface population of KCC2 is internalized over a time course of 10 min, a process that is dramatically slowed by activation of PKC (13). Similarly, in cultured hippocampal neurons, PKC activation increases the cell surface expression of KCC2.
Activation of 5-HT2AR still induced a hyperpolarizing shift of EIPSP after blocking Ca2+-dependent PKC by Gö6976. In addition, this compound induced a small hyperpolarizing shift of EIPSP. These results suggest opposite effects of Ca2+-dependent and Ca2+-independentxPKC on KCC2 function. In support of this hypothesis, repetitive postsynaptic spiking induces a positive shift in ECl through a Ca2+-dependent down-regulation of KCC2 (34-36). Inhibition of Ca2+-dependent PKC abolishes the spiking-induced shift in ECl (34). In agreement with a negative effect of intracellular Ca2+ on KCC2 function, NMDA receptor activation leads to Ca2+ influx that ultimately causes protein phosphatase 1–mediated dephosphorylation of KCC2 residue Ser940 and depolarizing shift of ECl (37).
We showed that blocking 5-HT2BRs and 5-HT2CRs hyperpolarized EIPSP. Conversely, activating these receptors induced a depolarizing shift of EIPSP. Although we did not determine whether these effects are mediated by one or both of these receptors, these results suggest that 5-HT2ARs and 5-HT2B/2CRs have opposite effects on KCC2 function. Although the pharmacological characteristics of 5-HT2ARs and 5-HT2CRs are quite similar, owing, in part, to the high level of amino acid sequence homology between them (38), significant differences have been reported between the signaling cascades regulated by these receptors (39, 40). Consistent with these observations, 5-HT2ARs and 5-HT2CRs exert opposing effects on both locomotor activity in mice (41) and the long-lasting amplification of spinal reflexes in rats (42). Interestingly, 5-HT2BRs and 5-HT2CRs become constitutively active (spontaneously active without 5-HT) after SCI (43, 44). This constitutive activity may be partly responsible for the depolarizing shift of ECl after SCI (10). The modulatory effects of 5-HT2B/2CRs on chloride homeostasis require furtherinvestigation.
The activation of 5-HT2AR reduced spasticity after SCI, as revealed by the increased RDD of the H reflex. This effect was very likely due to the restoration of endogenous inhibition, because slight changes in ECl were shown to have an important impact on the strength of inhibition (5–7, 10). 5-HT2ARs are strongly expressed in the ventral horn (45) and are mainly localized in the plasma membrane of neurons, covering a large surface of cell bodies and dendrites (46). These receptors are dramatically up-regulated in the motoneuron somata and dendrites following SCI (47), an effect that starts as early as 1 d after transection (48). The 5-HT2AR displays ∼10-fold lower constitutive activity than the 5-HT2CR (38).
In the intact spinal cord, the neuromodulators released by descending pathways allow the motoneurons to exhibit sustained depolarizations (plateau potentials), which are attributable to slowly activating voltage-dependent persistent inward currents (PICs). Shortly after the injury, the excitability of the spinal cord decreases because this excitatory influence of the brainstem on motoneurons is lost. However, motoneurons slowly recover the capacity to generate PICs, which are, in large part, responsible for long-lasting reflexes and spasms (49, 50). Both 5-HT2BRs and 5-HT2CRs, but not 5-HT2ARs, are involved in this up-regulation of PICs (44). To conclude, by restoring endogenous inhibition through an up-regulation of KCC2 function, without depolarizing motoneurons and activating PICs, activation of 5-HT2ARs may represent an innovative therapeutic strategy to reduce spasticity after SCI. However, these receptors have been implicated in several psychiatric disorders (e.g., 51), and their effects on pain processing are controversial (52), highlighting the need to further investigate the potential systemic effects of specific 5-HT2AR agonists, such as TCB-2.
Materials and Methods
In Vitro Electrophysiological Recordings.
After hypothermic anesthesia, we dissected out the spinal cords (sacral segments up to T8–T9) and L3–L5 dorsal and ventral roots of newborn rats. Dissections and electrophysiological procedures were performed under continuous perfusion with an oxygenated artificial CSF.
In Vivo Electrophysiological Recordings.
We measured the H reflex in adult rats under ketamine anesthesia (100 mg/kg, i.p.) using a pair of stainless steel needle electrodes transcutaneously inserted into the vicinity of the tibial nerve for stimulation. We placed the recording electrode into the flexor digitorum muscle beneath the ankle and the reference electrode s.c. into the foot.
Western Blots.
The amount of KCC2 in the lumbar enlargement of the spinal cord was determined by Western blots as described previously (10). The procedures used for separating membrane and cytoplasmic fractions are detailed in SI Materials and Methods.
Immunohistochemistry.
Fast blue (FB) [3 µL; 0.5% in 0.9% NaCl; F-5756 (Sigma)] was injected bilaterally in the TS muscles (ankle extensors) anesthetized animals at P5 for retrograde identification. Animals were killed 2 d later. After fixation, spinal cords were cut transversally (20-µm-thick sections). Immunohistochemistry was performed by using a mixture of affinity-purified rabbit KCC2-specific polyclonal antibody and GlyR monoclonal antibody. We then revealed the labeling with a mixture of donkey Cy3-conjugated rabbit-specific antibody and donkey AlexaFluor 488–conjugated mouse-specific antibody.
Motoneuron Cultures and Immunocytochemistry.
Motoneurons were isolated and cultured. At 13–14 d in vitro (DIV), TCB-2 was freshly prepared, diluted in complete culture medium (0.1 µM), and added for 30 min.
Further experimental details are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Mouloud Bouhadfane for helpful discussions throughout the experiments and Florian Gackière and Frédéric Brocard for their comments on the manuscript. This work was supported by the Christopher and Dana Reeve Foundation Grants VB1-0502-2 and VB2-0801-2, the International Spinal Research Trust Grant STR110, the French Agence Nationale pour la Recherche Grant ANR-2010-BLAN-1407-01, and the French Institut pour la Recherche sur la Moelle épinière et l’Encéphale (to L.V.). P.B., G.H., and H.B. are supported by the Institut National de la Santé et de la Recherche Médicale, and K.S. is supported by Association Française contre les Myopathies Grant 13912.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213680110/-/DCSupplemental.
References
- 1.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61(6):820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Payne JA, Stevenson TJ, Donaldson LF. Cation-chloride co-transporters in neuronal communication, development and trauma. J Biol Chem. 1996;271(27):16245–16252. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- 3.Payne JA, Rivera C, Voipio J, Kaila K. Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. Trends Neurosci. 2003;26(4):199–206. [Google Scholar]
- 4.Kahle KT, et al. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4(9):490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- 5.Jean-Xavier C, Mentis GZ, O’Donovan MJ, Cattaert D, Vinay L. Dual personality of GABA/glycine-mediated depolarizations in immature spinal cord. Proc Natl Acad Sci USA. 2007;104(27):11477–11482. doi: 10.1073/pnas.0704832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyon N, et al. Efficacy of synaptic inhibition depends on multiple, dynamically interacting mechanisms implicated in chloride homeostasis. PLOS Comput Biol. 2011;7(9):e1002149. doi: 10.1371/journal.pcbi.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prescott SA, Sejnowski TJ, De Koninck Y. Reduction of anion reversal potential subverts the inhibitory control of firing rate in spinal lamina I neurons: Towards a biophysical basis for neuropathic pain. Mol Pain. 2006;2:32. doi: 10.1186/1744-8069-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biering-Sørensen F, Nielsen JB, Klinge K. Spasticity-assessment: A review. Spinal Cord. 2006;44(12):708–722. doi: 10.1038/sj.sc.3101928. [DOI] [PubMed] [Google Scholar]
- 9.Boulenguez P, Vinay L. Strategies to restore motor functions after spinal cord injury. Curr Opin Neurobiol. 2009;19(6):587–600. doi: 10.1016/j.conb.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Boulenguez P, et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med. 2010;16(3):302–307. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- 11.Cramer SW, et al. The role of cation-dependent chloride transporters in neuropathic pain following spinal cord injury. Mol Pain. 2008;4:36. doi: 10.1186/1744-8069-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y, Zheng J, Xiong L, Zimmermann M, Yang J. Spinal cord injury-induced attenuation of GABAergic inhibition in spinal dorsal horn circuits is associated with down-regulation of the chloride transporter KCC2 in rat. J Physiol. 2008;586(Pt 23):5701–5715. doi: 10.1113/jphysiol.2008.152348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HH, et al. Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J Biol Chem. 2007;282(41):29777–29784. doi: 10.1074/jbc.M705053200. [DOI] [PubMed] [Google Scholar]
- 14.Pearlstein E, Ben Mabrouk F, Pflieger JF, Vinay L. Serotonin refines the locomotor-related alternations in the in vitro neonatal rat spinal cord. Eur J Neurosci. 2005;21(5):1338–1346. doi: 10.1111/j.1460-9568.2005.03971.x. [DOI] [PubMed] [Google Scholar]
- 15.Norreel JC, et al. Reversible disorganization of the locomotor pattern after neonatal spinal cord transection in the rat. J Neurosci. 2003;23(5):1924–1932. doi: 10.1523/JNEUROSCI.23-05-01924.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan LM, Sławińska U. Chapter 12—modulation of rhythmic movement: Control of coordination. Prog Brain Res. 2011;188:181–195. doi: 10.1016/B978-0-444-53825-3.00017-6. [DOI] [PubMed] [Google Scholar]
- 17.Grillner S. The motor infrastructure: From ion channels to neuronal networks. Nat Rev Neurosci. 2003;4(7):573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 18.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 19.Jean-Xavier C, Pflieger JF, Liabeuf S, Vinay L. Inhibitory postsynaptic potentials in lumbar motoneurons remain depolarizing after neonatal spinal cord transection in the rat. J Neurophysiol. 2006;96(5):2274–2281. doi: 10.1152/jn.00328.2006. [DOI] [PubMed] [Google Scholar]
- 20.Sadlaoud K, et al. Differential plasticity of the GABAergic and glycinergic synaptic transmission to rat lumbar motoneurons after spinal cord injury. J Neurosci. 2010;30(9):3358–3369. doi: 10.1523/JNEUROSCI.6310-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLean TH, et al. 1-Aminomethylbenzocycloalkanes: Conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists. J Med Chem. 2006;49(19):5794–5803. doi: 10.1021/jm060656o. [DOI] [PubMed] [Google Scholar]
- 22.Fox MA, French HT, LaPorte JL, Blackler AR, Murphy DL. The serotonin 5-HT(2A) receptor agonist TCB-2: A behavioral and neurophysiological analysis. Psychopharmacology (Berl) 2010;212(1):13–23. doi: 10.1007/s00213-009-1694-1. [DOI] [PubMed] [Google Scholar]
- 23.Delpire E, et al. Small-molecule screen identifies inhibitors of the neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci USA. 2009;106(13):5383–5388. doi: 10.1073/pnas.0812756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martiny-Baron G, et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem. 1993;268(13):9194–9197. [PubMed] [Google Scholar]
- 25.Fulton BP, Walton K. Electrophysiological properties of neonatal rat motoneurones studied in vitro. J Physiol. 1986;370:651–678. doi: 10.1113/jphysiol.1986.sp015956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lev-Tov A, Pinco M. In vitro studies of prolonged synaptic depression in the neonatal rat spinal cord. J Physiol. 1992;447:149–169. doi: 10.1113/jphysiol.1992.sp018996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallis DI, Wu J. FAST and SLOW ipsilateral and contralateral spinal reflexes in the neonate rat are modulated by 5-HT. Gen Pharmacol. 1992;23(6):1035–1044. doi: 10.1016/0306-3623(92)90283-p. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res. 1999;816(2):493–499. doi: 10.1016/s0006-8993(98)01199-8. [DOI] [PubMed] [Google Scholar]
- 29.Thompson FJ, Reier PJ, Lucas CC, Parmer R. Altered patterns of reflex excitability subsequent to contusion injury of the rat spinal cord. J Neurophysiol. 1992;68(5):1473–1486. doi: 10.1152/jn.1992.68.5.1473. [DOI] [PubMed] [Google Scholar]
- 30.Grey MJ, et al. Post-activation depression of soleus stretch reflexes in healthy and spastic humans. Exp Brain Res. 2008;185(2):189–197. doi: 10.1007/s00221-007-1142-6. [DOI] [PubMed] [Google Scholar]
- 31.Brustein E, Drapeau P. Serotoninergic modulation of chloride homeostasis during maturation of the locomotor network in zebrafish. J Neurosci. 2005;25(46):10607–10616. doi: 10.1523/JNEUROSCI.2017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer SE, Sanders-Bush E. 5-Hydroxytryptamine type 2A and 2C receptors linked to Na+/K+/Cl- cotransport. Mol Pharmacol. 1994;45(5):991–996. [PubMed] [Google Scholar]
- 33.Broadbelt KG, Paterson DS, Rivera KD, Trachtenberg FL, Kinney HC. Neuroanatomic relationships between the GABAergic and serotonergic systems in the developing human medulla. Auton Neurosci. 2010;154(1-2):30–41. doi: 10.1016/j.autneu.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiumelli H, Cancedda L, Poo MM. Modulation of GABAergic transmission by activity via postsynaptic Ca2+-dependent regulation of KCC2 function. Neuron. 2005;48(5):773–786. doi: 10.1016/j.neuron.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 35.Woodin MA, Ganguly K, Poo MM. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl- transporter activity. Neuron. 2003;39(5):807–820. doi: 10.1016/s0896-6273(03)00507-5. [DOI] [PubMed] [Google Scholar]
- 36.Fiumelli H, Woodin MA. Role of activity-dependent regulation of neuronal chloride homeostasis in development. Curr Opin Neurobiol. 2007;17(1):81–86. doi: 10.1016/j.conb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Lee HH, Deeb TZ, Walker JA, Davies PA, Moss SJ. NMDA receptor activity downregulates KCC2 resulting in depolarizing GABAA receptor-mediated currents. Nat Neurosci. 2011;14(6):736–743. doi: 10.1038/nn.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aloyo VJ, Berg KA, Spampinato U, Clarke WP, Harvey JA. Current status of inverse agonism at serotonin2A (5-HT2A) and 5-HT2C receptors. Pharmacol Ther. 2009;121(2):160–173. doi: 10.1016/j.pharmthera.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Berg KA, Clarke WP, Sailstad C, Saltzman A, Maayani S. Signal transduction differences between 5-hydroxytryptamine type 2A and type 2C receptor systems. Mol Pharmacol. 1994;46(3):477–484. [PubMed] [Google Scholar]
- 40.Berg KA, Stout BD, Maayani S, Clarke WP. Differences in rapid desensitization of 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptor-mediated phospholipase C activation. J Pharmacol Exp Ther. 2001;299(2):593–602. [PubMed] [Google Scholar]
- 41.Halberstadt AL, et al. 5-HT(2A) and 5-HT(2C) receptors exert opposing effects on locomotor activity in mice. Neuropsychopharmacology. 2009;34(8):1958–1967. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Machacek DW, Garraway SM, Shay BL, Hochman S. Serotonin 5-HT(2) receptor activation induces a long-lasting amplification of spinal reflex actions in the rat. J Physiol. 2001;537(Pt 1):201–207. doi: 10.1111/j.1469-7793.2001.0201k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murray KC, et al. Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat Med. 2010;16(6):694–700. doi: 10.1038/nm.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray KC, Stephens MJ, Ballou EW, Heckman CJ, Bennett DJ. Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J Neurophysiol. 2011;105(2):731–748. doi: 10.1152/jn.00774.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearlstein E, Bras H, Deneris ES, Vinay L. Contribution of 5-HT to locomotion - the paradox of Pet-1(-/-) mice. Eur J Neurosci. 2011;33(10):1812–1822. doi: 10.1111/j.1460-9568.2011.07679.x. [DOI] [PubMed] [Google Scholar]
- 46.Doly S, et al. The 5-HT2A receptor is widely distributed in the rat spinal cord and mainly localized at the plasma membrane of postsynaptic neurons. J Comp Neurol. 2004;472(4):496–511. doi: 10.1002/cne.20082. [DOI] [PubMed] [Google Scholar]
- 47.Kong XY, Wienecke J, Hultborn H, Zhang M. Robust upregulation of serotonin 2A receptors after chronic spinal transection of rats: An immunohistochemical study. Brain Res. 2010;1320:60–68. doi: 10.1016/j.brainres.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 48.Kong XY, Wienecke J, Chen M, Hultborn H, Zhang M. The time course of serotonin 2A receptor expression after spinal transection of rats: An immunohistochemical study. Neuroscience. 2011;177:114–126. doi: 10.1016/j.neuroscience.2010.12.062. [DOI] [PubMed] [Google Scholar]
- 49.Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol. 2004;91(5):2247–2258. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- 50.Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127(Pt 10):2247–2258. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- 51.González-Maeso J, Sealfon SC. Psychedelics and schizophrenia. Trends Neurosci. 2009;32(4):225–232. doi: 10.1016/j.tins.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 53.Blaesse P, et al. Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. J Neurosci. 2006;26(41):10407–10419. doi: 10.1523/JNEUROSCI.3257-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.