Abstract
Parasitic helminths are a major cause of chronic human disease, affecting more than 3 billion people worldwide. Host protection against most parasitic helminths relies upon Type 2 cytokine production, but the mechanisms that regulate interleukin (IL) 4 and 13 production from CD4+ T helper 2 cells (TH2) and innate lymphoid type 2 cells (ILC2s) remain incompletely understood. The epithelial cell-derived cytokines IL-25 and IL-33 promote Type 2 responses, but the extent of functional redundancy between these cytokines is unclear and whether Type 2 memory relies upon either IL-25 or IL-33 is unknown. Herein, we demonstrate a pivotal role for IL-33 in driving primary and anamnestic immunity against the rodent hookworm Nippostrongylus brasiliensis. IL-33–deficient mice have a selective defect in ILC2–derived IL-13 during both primary and secondary challenge infections but generate stronger canonical CD4+ T helper 2 cells responses (IL-4, IgE, mast cells, and basophils) than WT controls. Lack of IL-13 production in IL-33–deficient mice impairs resistin-like molecule beta (RELMβ) expression and eosinophil recruitment, which are two mechanisms that eliminate N. brasiliensis parasites from infected hosts. Thus, IL-33 is requisite for IL-13 but not IL-4–driven Type 2 responses during hookworm infection.
Keywords: mucosal immunity, gastrointestinal nematode, inflammation
Type 2 immunity underlies host protection against diverse helminth species and most allergic disorders (1, 2). Type 2 responses are characterized by interleukins (ILs) 4, 5, 9 13, 25, and 33; expansion of CD4+ T helper 2 (TH2) cells; IgE production; eosinophilia; mastocytosis; basophilia; alternatively activated macrophages; smooth muscle hypercontractility; and goblet cell metaplasia (1, 3). Although CD4+TH2 cells were previously considered central drivers of Type 2 immunity, recent discoveries of Type 2 cytokine-producing innate lymphoid cells (ILC2s) have brought new insight(s) to our global understanding of inflammatory responses (4–6). Prevailing hypotheses suggest that IL-25 and IL-33 release from damaged epithelia promotes the rapid expansion of ILC2s following allergen challenge or helminth infection (4, 6–8). Whether similar mechanisms regulate cytokine release from both TH2 cells and ILC2s remains unclear.
IL-33 is an IL-1 family cytokine that regulates a wide array of pathological states associated with cardiovascular disease, rheumatoid arthritis, anaphylaxis, ulcerative colitis, and pathogen infestation (9, 10). Like other IL-1 family members, IL-33 can be cleaved by caspase-1, although it appears that this cleavage leads to functional inactivation (11), leading to speculation that IL-33 primarily functions as a chromatin-associated nuclear factor (12). However, bioactive IL-33 can be generated following pro–IL-33 cleavage by other proteases such as neutrophil elastase and cathepsins, suggesting that bioactive IL-33 may be released into the extracellular environment (13). Indeed, mucosal damage caused by allergen or helminth infection elicits IL-33 production from epithelial cells, macrophages, and inflammatory dendritic cells (DCs) (14). Extracellular IL-33 exerts its biological activities, at least in part, through the suppressor of tumorigenicity (TI/ST2) receptor that signals through MyD88-mediated activation of MAP kinases and NF-κb (15). This may partially explain how rIL-33 administration to rodents promotes clearance of the gastrointestinal helminth Trichuris muris (16). Although TI/ST2 was considered expressed only on TH2 cells and mast cells, IL-33 signaling via TI/ST2 regulates the function(s) of fibroblasts, DCs, macrophages, eosinophils, and ILC2s/nuocytes (6) (15, 17). Despite clear evidence that IL-33 can promote Type 2 responses, whether IL-33 is necessary for primary and/or secondary Type 2 responses remains controversial (18–21).
Mouse infection with the hookworm parasite Nippostrongylus brasiliensis is widely used for understanding Type 2 immunity (22). In this system, infectious larvae (L3) migrate from the skin into the pulmonary tract and cause hemorrhagic lung injury within 1–3 d postinfection. TH2 cell expansion and systemic Type 2 cytokines start to increase from 3 to 5 d postinfection, as worms migrate from the lung into the small intestine (23). Between 6–12 d postinfection, IL-4– and IL-13–dependent effects cause intestinal epithelial cells (IECs) to expel adult worms from the intestinal lumen through mechanisms that require resistin-like molecule beta (RELMβ), a goblet cell-specific protein that interferes with worm nutrition (23, 24). If previously infected animals are rechallenged, STAT-6–dependent processes drive rapid expulsion of worms, potentially via IL-4 and CD4+T cells, but there is lack of consensus on the exact mechanism (25–28). Given that human hookworm infections are characterized by poor memory responses and high rates of reinfection (29), it is possible that better understanding of host protection in rodents will lead to novel approaches for reducing the burden of human disease.
These data show that IL-33 is necessary for immunity against primary and secondary N. brasiliensis infection. IL-33–deficient mice (IL-33KO) mice failed to clear worms despite a strong induction of IL-4, IgE, basophil, and mast cell responses. Instead, IL-33KO mice generated insufficient IL-13 production for IEC-derived RELMβ and eosinophil recruitment. IL-33 was essential for the early expansion of IL-13+ ILC2s and was partially responsible for the increase of IL-13–producing CD4+ T cells within the lung tissue. Taken together, this suggests that IL-33 promotes in vivo IL-13 production that drives worm expulsion independently of canonical TH2 responses.
Results
IL-4 and IL-13 Are Differentially Induced and Regulated During N. brasiliensis Infection.
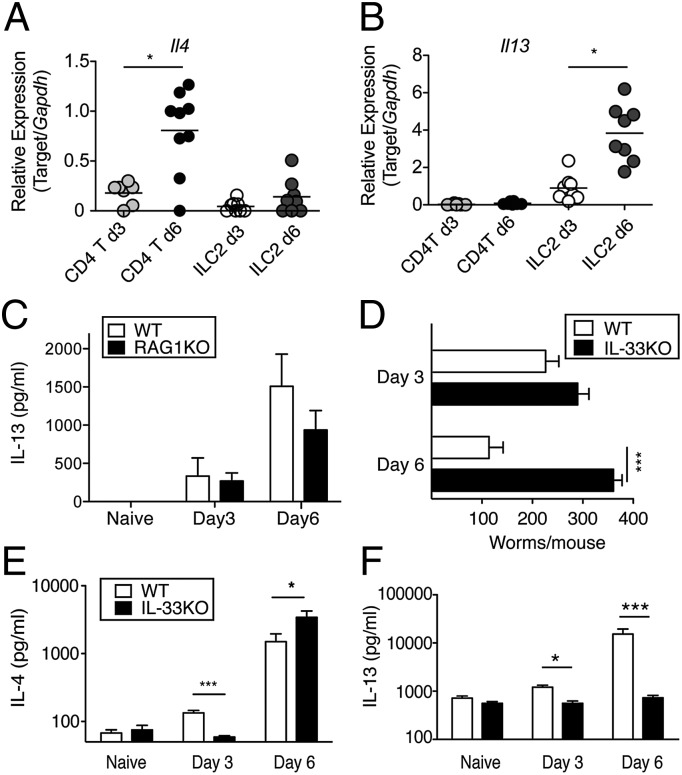
IL-4Rα–mediated STAT-6 activation is necessary for immunity against N. brasiliensis infection. However, IL-4KO mice have a moderate defect in host resistance, whereas IL-13 deficiency severely impairs immunity (30, 31). To determine whether innate vs. adaptive lymphocytes differentially produced IL-4 and IL-13 during primary N. brasiliensis infection, we sorted lineage-negative lymphocytes and CD4+T cells from the lung and mesenteric lymph nodes of wild-type C57BL/6 (WT) mice at d 3 and d 6 postinfection. ILC2s were defined as B220−, F4/80−, CD5−, CD4−, CD3−, CD27−, NK1.1−, TCRβ−, CD11b−, CD11c−, CD45+, c-kit+, CD90.2+, and CD127+, and CD4+T cells were identified by TCRβ+ and CD4+ (32). cDNA was generated from 1 × 104 cells from each population and qRT-PCR was used to determine IL-4 and IL-13 relative expression levels. ILC2s did not show an infection-induced increase of IL-4 expression, but CD4+T cells increased IL-4 expression levels from d 3 to d 6 (Fig. 1A). Conversely, ILC2s expressed slightly higher IL-13 levels than CD4+T cells at d 3 but nearly fourfold higher IL-13 levels than CD4+T cells by d 6 (Fig. 1B). To determine whether ILC2-derived IL-13 required the presence of T and B cells, systemic IL-13 levels at d 6 postinfection were compared between WT and RAG-1–deficient mice using the in vivo cytokine capture assay (IVCCA) (33). Both strains generated similar increases in IL-13 (Fig. 1C), which demonstrates that T/B cells were not required for most of the IL-13 produced up to d 6 following primary N. brasiliensis infection.
Fig. 1.
IL-33 is necessary for IL-13 but not IL-4 induction during primary N. brasiliensis infection. 1 × 104 CD4+ T cells or ILC2s were sorted from lung and mesenteric lymph nodes of WT C57BL/6 mice that were infected with 750 N. brasiliensis L3, and mRNA transcript levels for (A) IL-4 and (B) IL-13 were determined at the times indicated. Mean ± SE of five mice/group analyzed from two independent experiments. (C) Serum IL-13 levels in WT and RAG1KO mice as determined by IVCCA. (D) Numbers of N. brasiliensis worms recovered from the intestinal lumen of WT and IL-33KO mice. Serum levels of (E) IL-4 and (F) IL-13 in WT and IL-33KO mice determined by IVCCA. Data shown represent the mean ± SE of 6–12 mice/group infected with 750 L3 of three independent experiments. *P < 0.05 and ***P < 0.001.
IL-25 and IL-33 can have redundant roles for Type 2 immunity (6); therefore, IL-33KO mice were generated to determine whether IL-33 was essential for any aspect of a Type 2 response. This mouse strain had no differences in body weight, blood chemistry, or organ development. Compared with WT controls, IL-33KO mice did not show defects in thymic T-cell ratios, T-cell activation status (as determined by CD25 expression), or myeloid subsets within the lung tissue (Fig. S1).
To investigate whether IL-33 was necessary for immunity against hookworms, WT and IL-33KO mice were inoculated s.c. with 750 N. brasiliensis infectious stage larvae (L3). Although worm numbers at d 3 postinfection were equivalent between strains, by d 6 IL-33KO had threefold greater intestinal worm numbers than WT mice (Fig. 1D). Moreover, IL-33KO mice remained chronically infected for >21 d following a primary inoculation, whereas WT mice cleared their parasites by d 10 postinfection (Fig. S2). Regarding cytokine production, IL-33KO mice produced less IL-4 than WT at d 3, but more IL-4 than WT by d 6 (Fig. 1E). However, IL-33KO mice failed to increase their IL-13 levels over baseline at d 3 or d 6 postinfection (Fig. 1F). Therefore, N. brasiliensis up-regulated IL-4 and IL-13 production from distinct lymphocyte populations, with IL-33 serving an essential role for IL-13, but only a transient role for IL-4 production.
IL-33 Selectively Drives ILC2-Derived IL-13 for RELMβ and Eosinophil Responses.
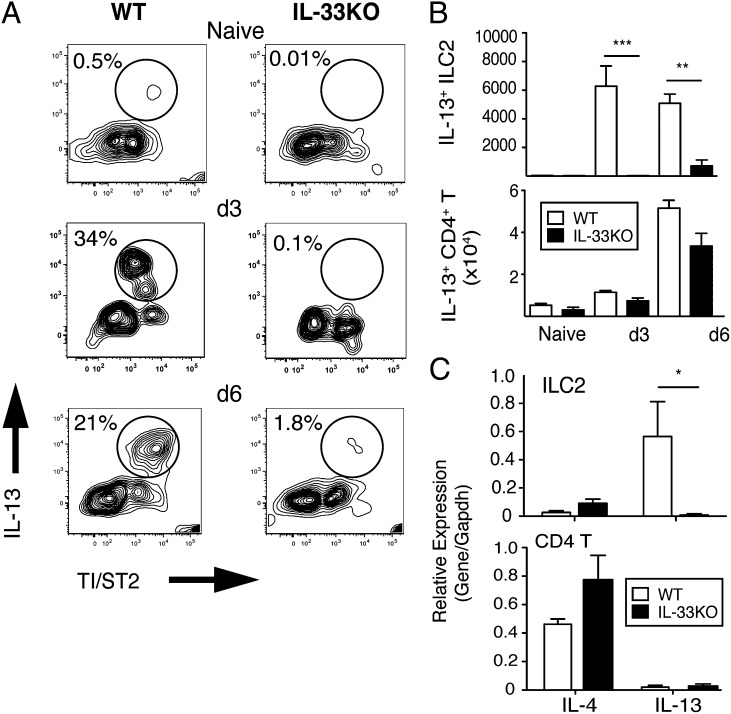
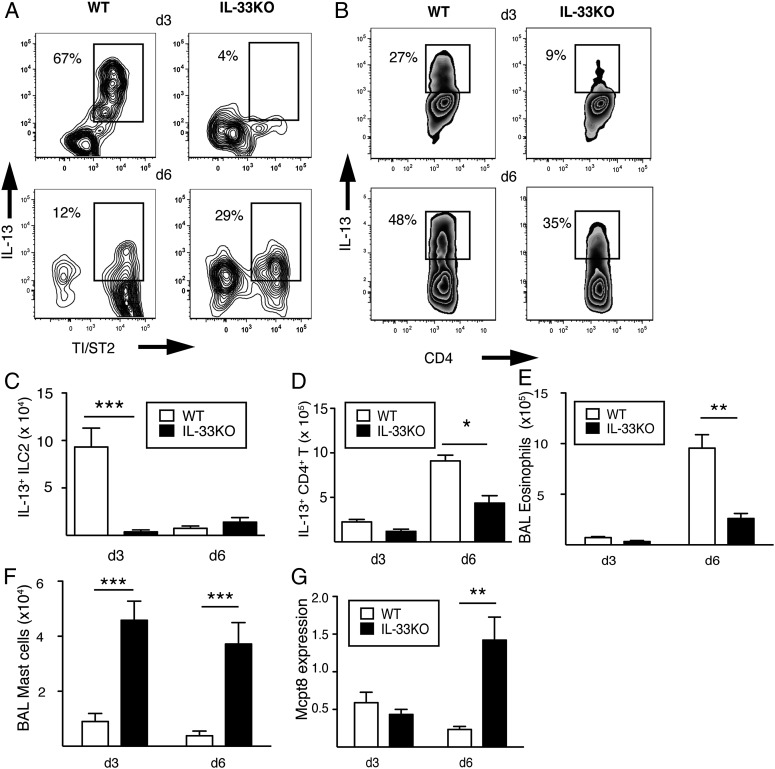
To investigate whether IL-33 was requisite for different lineages of IL-13–producing lymphocytes, we compared intracellular IL-13 levels between ILC2s and CD4+T cells of WT and IL-33KO mice. In WT, the percentage of IL-13+ST2+ ILC2s within lung tissue changed from 1% (naïve) to 34% (d 3) and 21% (d 6), but in IL-33KO, the IL-13+ST2+ ILC2 population changed from 0.01% (naïve) to only 1.8% by d 6 postinfection (Fig. 2A). Total numbers of IL-13+ST2+ ILC2s in WT lung tissue ranged between 5,000–6,000 cells/lung between d3–d6, whereas in IL-33KO tissues, the numbers were <500 cells/lung up to d 6 (Fig. 2B). On the other hand, the numbers of IL-13+CD4+T cells that expanded in response to N. brasiliensis infection were equivalent between strains at d 3 and d 6 postinfection (Fig. 2B).
Fig. 2.
IL-33 drives hookworm infection-induced IL-13 production from ILC2s and CD4+ T cells during a primary response. (A) TI/ST2 expression and percentage of intracellular IL-13 within lung ILC2s of WT and IL-33KO mice at the indicated time points following infection with 750 N. brasiliensis L3. Representative plots are shown. (B) Total numbers of lung IL-13+ ILC2s (Upper) and lung IL-13+CD4+ TCRβ+ cells (Lower) from WT and IL-33KO mice at the indicated time points following infection with 750 N. brasiliensis L3. Data shown represent the mean ± SE of 4–6 mice/group analyzed from three independent experiments. (C) IL-4 and IL-13 mRNA levels in sorted ILC2s (Upper) and CD4 T (Lower) from WT and IL-33KO mice 6 d following 750 L3 infection. *P < 0.05, **P < 0.01, and ***P < 0.001.
Next, relative expression levels for IL-4 and IL-13 were determined from ILC2s that were sorted from the lung and CD4+ sorted from the mesenteric lymph nodes at d 6 postinfection. The gating strategy used to identify N. brasiliensis-induced ILC2 from the lung and and CD4+T cells from the mesenteric lymph nodes is shown in Fig. S3. Interestingly, both ILC2 and CD4+T-cell populations from IL-33KO mice expressed slightly higher IL-4 levels than from WT, whereas IL-33KO ILC2s completely lacked IL-13 expression (Fig. 2C). Moreover, by d 14 postinfection, IL-33KO produced systemic levels of IL-4, IgE, and mast cells (mast cell protease 1, MCPT-1) that were greater than or equal to WT levels (Fig. S4). Hence, IL-33 was necessary for ILC2-derived IL-13 but was not required for CD4+ T-cell–derived IL-4, IgE, or mast cells during the primary response.
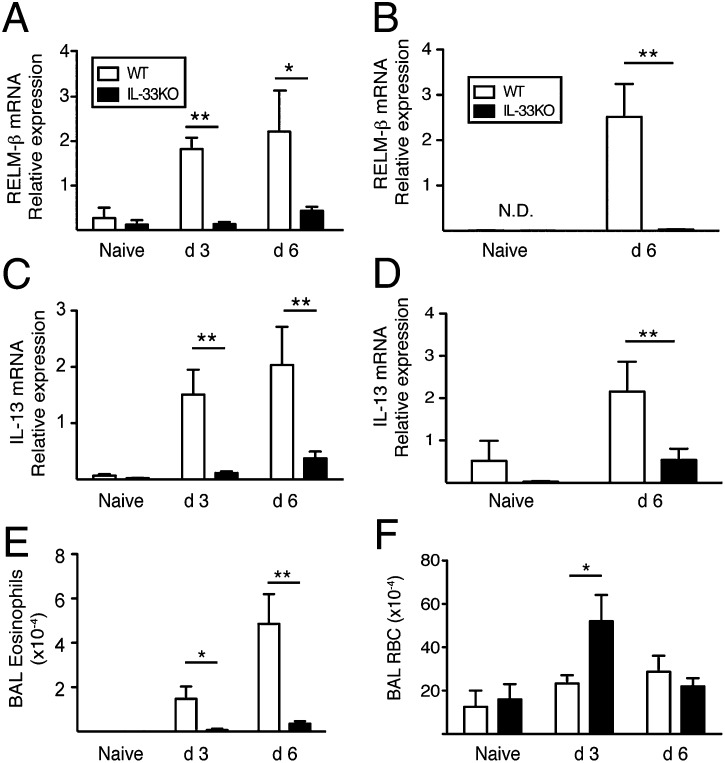
IL-4/IL-13–dependent effects on epithelia drive expulsion of N. brasiliensis adult worms from the intestine through RELMβ, a cytokine that interferes with worm nutrition (23). In comparison with WT mice, RELMβ mRNA transcripts in IL-33KO lung and intestines were significantly reduced at both d 3 and d 6 postinfection (Fig. 3 A and B). Reduced RELMβ levels were associated with fewer IL-13 mRNA transcripts in the lung and intestines of IL-33KO compared with WT mice (Fig. 3 C and D). Also, IL-33KO mice generated significantly fewer airway eosinophils than WT at d 3 and d 6 postinfection (Fig. 3E).
Fig. 3.
IL-33 is required for IL-13 production, RELMβ expression and pulmonary pathogenesis during primary infection with N. brasiliensis . RELMβ mRNA transcript levels in the (A) lung and (B) jejunum at the indicated time points following infection with 750 N. brasiliensis L3. IL-13 mRNA levels in the (C) lung and (D) gut at indicated time points postinfection. Data shown represent the mean ± SE of 6–12 mice/group analyzed from two independent experiments. (E) Total numbers of eosinophils and (F) red blood cells within the BAL fluid in WT and IL-33KO mice at d 3 and d 6 following 750 L3 infection. Data shown represent the mean ± SE of 4–6 mice/group analyzed from three independent experiments. *P < 0.05 and **P < 0.01.
Erythrocyte (RBC) numbers within bronchoalveolar lavage (BAL) fluid were quantified to determine whether IL-33 restrained lung injury caused by N. brasiliensis infection. RBCs in IL-33KO BAL fluid were threefold greater than WT BAL at d 3 postinfection, but no differences were observed between strains by d6 (Fig. 3F). Therefore, IL-33 deficiency impaired IL-13–dependent RELMβ expression, reduced eosinophil recruitment to the lung, and increased the apex of N. brasiliensis-induced lung injury following primary infection.
IL-33 Mediates Anamnestic Immunity Against Secondary Infection.
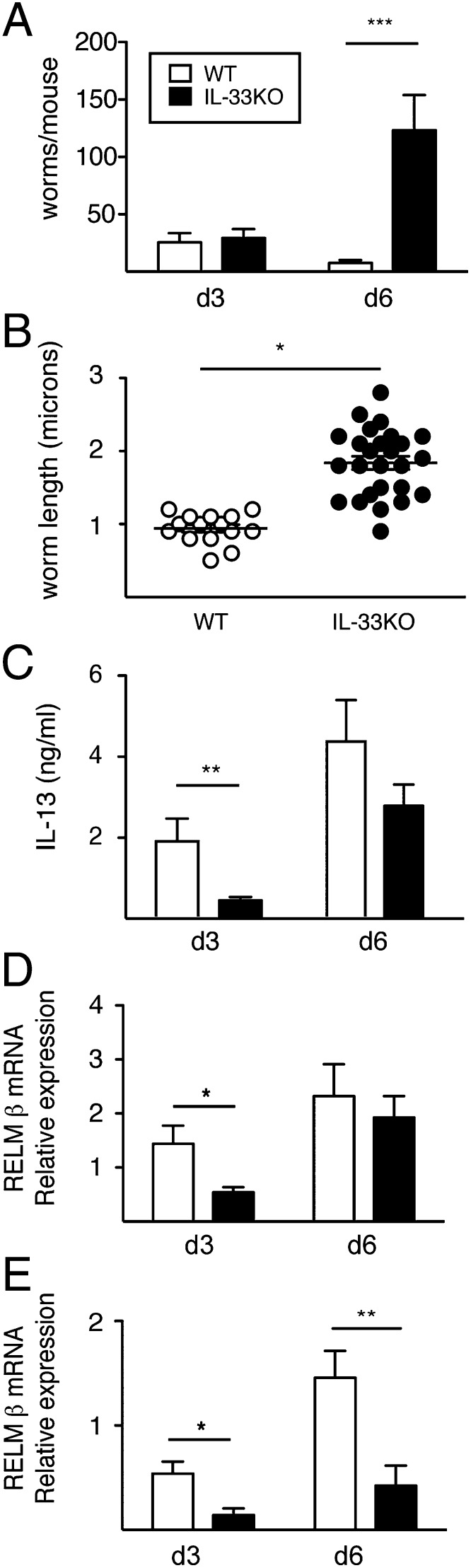
Given the profound importance for IL-33 in primary host resistance, we postulated an additional role for IL-33 for secondary immunity against reinfection. To investigate, WT and IL-33KO mice were infected with 750 L3, treated with the antihelminthic drug pyrantel pamoate on d 14, rechallenged with 500 L3 on d 28, and monitored for intestinal worms and immune responses at d 3 and d 6 following reinfection. Despite equivalent worm numbers between strains at d 3, there were 10-fold higher worms in IL-33KO mice vs. WT by d 6 postinfection (Fig. 4A). Comparison of worm lengths between strains revealed significantly longer parasites in IL-33KO mice compared with WT mice (Fig. 4B). Thus, IL-33 deficiency impaired worm clearance during secondary challenge, most likely due to a failure in limiting parasite development.
Fig. 4.
IL-33 is required for worm expulsion and up-regulation of the IL-13/RELMβ axis during rechallenge. (A) Numbers of intestinal worms recovered from WT and IL-33KO mice at d 3 and d 6 following secondary challenge. (B) Worm lengths at d 6 postinfection in experiment described in A. Each individual dot represents an individual parasite. (C) Systemic IL-13 production, (D) lung RELMβ mRNA transcript levels, and (E) intestinal RELMβ mRNA levels in WT and IL-33KO mice at d 3 and d 6 following secondary challenge. Data shown represent the mean ± SE of 6–8 mice/group analyzed from three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001.
RELMβ inhibits the ability of hookworms to feed upon their hosts (23, 24); therefore, we investigated whether IL-33 was necessary for inducing an IL-13/RELMβ axis upon rechallenge. IL-33KO mice produced significantly less IL-13 than WT mice at d 3 but not at d 6 postinfection (Fig. 4C). Both lung and intestinal RELMβ mRNA transcripts were significantly reduced in IL-33KO compared with WT at d 3 postinfection, but only intestinal RELMβ expression levels remained suppressed in IL-33KO tissues at d 6 (Fig. 4 D and E).
To identify the cellular sources of IL-13 during secondary responses to N. brasiliensis, we determined both the percentage and number of lung IL-13+ST2+ ILC2s and IL-13+CD4+T cells at d 3 and d 6 following reinfection. At d 3, the percentage of lung 13+ ILC2s in WT mice was 14-fold higher than in IL-33KO (Fig. 5A), and the percentage of IL-13+CD4+T cells in WT was twofold higher than in IL-33KO (Fig. 5B). In total cell number, the WT IL-13+ST2+ ILC2 population was 10-fold greater than in IL-33KO at d 3 (Fig. 5C), but these numbers decreased to levels that were no different from IL-33KO by d 6. In addition, IL-33KO lung tissues also had fewer IL-13+ CD4+T cells than WT lung tissues at d 6 postinfection (Fig. 5D).
Fig. 5.
IL-33 is necessary for the early expansion of IL-13+ ILC2, eosinophil recruitment, and late accumulation of IL-13+ CD4+ T cells but not mast cell or basophil responses following rechallenge. (A) Flow cytometry contour plots show TI/ST2 expression and percentage of intracellular IL-13 within the lung ILC2/nuocyte populations of WT and IL-33KO mice at d 3 and d 6 following secondary challenge. (B) Zebra plots show percentage of IL-13 within lung CD4+ TCRβ+ cells at d 3 and d 6 following secondary challenge. (C) Total numbers of lung IL-13+ ILC2s within gate shown in A. (D) Total numbers of lung IL-13+CD4+ TCRβ+ cells within gate shown in B. (E) Total numbers of eosinophils and (F) mast cells within the BAL fluid and (G) intestinal MCPT8 mRNA transcript levels in WT and IL-33KO mice at d 3 and d 6 following secondary challenge. Data shown represent the mean ± SE of 4–6 mice/group analyzed from two independent experiments. *P < 0.05, **P < 0.01, and***P < 0.001.
Lastly, BAL fluid and intestinal tissues were examined to determine whether IL-33 regulated granulocyte recruitment during the secondary response. Lung eosinophil numbers were fourfold lower in IL-33KO compared with WT mice at d 6 (Fig. 5E). Conversely, lung mast cell numbers were 4–5-fold greater in IL-33KO BAL fluid compared with WT (Fig. 5F). Also, IL-33KO expressed higher intestinal mRNA levels for MCPT-8 (basophil-specific granule protein) compared with WT (Fig. 5G) (25, 34). Therefore, secondary immune responses were IL-33–dependent and strongly associated with coordinated IL-13 production from ILC2s and CD4+T cells. This coordinated IL-13 production most likely contributed to intestinal RELMβ production and eosinophil recruitment but was not essential for mast cell or basophil responses.
Discussion
TH2-associated cytokines predominate the immune responses generated against helminths and allergens, but mechanisms that govern Type 2 response initiation, maintenance, and resolution are incompletely understood (3, 29). Herein, the rodent-specific hookworm, N. brasiliensis, was used to investigate how IL-33 regulates host resistance during a primary and secondary infection. Surprisingly, IL-33 was necessary for both phases of immunity. Mice lacking IL-33 were unable to generate (i) primary and secondary expansion of IL-13+ ILC2s, (ii) secondary expansion of IL-13+ CD4+ T cells, (iii) primary and secondary RELMβ expression, and (iv) primary and secondary eosinophil-recruitment. On the other hand, canonical Type 2 responses were enhanced in IL-33KO mice, such as (i) systemic IL-4 production, (ii) IgE secretion, (iii) mast cell, and (iv) basophil responses. Taken together, these data imply that IL-33 preferentially instructs IL-13–driven inflammation instead of IL-4–mediated immunity (35, 36), which provides greater insight into the in vivo regulation of Type 2 responses.
IL-33 was initially described as an epithelial/endothelial cell-specific cytokine. More recently, we and others have shown that IL-33 is also produced from myeloid lineage cells within hours of pathogen exposure (8, 14), which is consistent with a hypothesis that IL-33 functions as an “alarmin” that instructs adaptive immune responses (12). Our demonstration that IL-33KO mice were highly susceptible to both primary and secondary N. brasiliensis infection is consistent with reciprocal experiments that demonstrated IL-33 administration promoted the rapid expulsion of the gastrointestinal helminth T. muris (16). Our results are similar to the phenotype of N. brasiliensis-infected mice lacking IL-25, a cytokine functionally related to IL-33 and one that is also produced by epithelia and professional antigen presenting cells (APCs) (37). Whether IL-25KO mice also have defects in secondary responses against N. brasiliensis is presently unclear, but our work suggests that IL-25 and IL-33 may not function in an entirely redundant manner for Type 2 immunity (5, 7).
We focused on how IL-33 regulated IL-13–producing lymphocytes (ILC2 and CD4+T) within the lung, which is a major target organ of hookworm infection in both mice and humans (38). Migratory parasites cause hemorrhagic tissue injury within this organ as they molt from L3 to L4, break out of the alveoli, and migrate into the gastrointestinal tract by 3 d postinfection (23). Within 1 d of N. brasiliensis infection, IL-33 levels increased dramatically within the lung (14). Within 3 d, the IL-13+ ILC2 population increased >100-fold in number through an IL-33–dependent mechanism. Importantly, ILC2s were present within IL-33KO lung tissues but fail to produce IL-13, implying that IL-33 provided a “license” for IL-13 expression. Whether IL-33 drives lymphocyte-specific IL-13 transcription and/or indirectly promotes IL-13 production through effects on professional APC remains unclear. IL-33–dependent IL-13 induction was important for mucosal tissue repair because lung hemorrhage was exacerbated in IL-33KO compared with WT. This suggestion of IL-13–dependent tissue repair is consistent with evidence that IL-13+ ILC2 expansion during influenza infection promotes regeneration of lung epithelia via amphiregulin, an EGF family cytokine (32).
The lung is also a critical site for TH2 cell priming during N. brasiliensis infection (39). At d 3 postinfection, IL-13–producing CD4+ T cells expand within the mediastinal lymph nodes (14), but this population does not accumulate within lung tissues until d 6 postinfection. IL-33 deficiency also significantly decreased both the percentage and number of lung IL-13+ CD4+ T cells during secondary immune responses, but IL-33 was not essential for expanding IL-4–producing CD4+ T cells. In fact, IL-4 expression in CD4+T cells and systemic IL-4 levels in the sera of infected IL-33KO mice was generally higher than in WT animals. Based on our cell-sorting experiments and experiments performed with WT vs. RAG1KO mice, our interpretation is that primary immune responses against N. brasiliensis are shaped by the combined actions of IL-4–producing CD4+T cells and IL-13–producing ILC2s. However, the IL-13–dependent effector functions have a dominant role in host protection, because IL-33KO mice remain chronically infected for >21 d despite elevated IL-4 responses. During the completion of this manuscript, Yasuda et al. demonstrated that IL-33 from alveolar type 2 cells promoted IL-13 production and host immunity against the gastrointestinal (GI) nematode Strongyloides venenzuelensis, but whether IL-33 was essential for Type 2 memory responses was not addressed (40).
Demonstration that IL-33 was essential for secondary immunity against reinfection was particularly intriguing because anamnestic responses are considered mechanistically distinct from primary immunity against N. brasiliensis (27, 41, 42). Considerable debate has centered upon the site of worm killing and the types of granulocytes required for immunity against rechallenge (26, 27, 39). Our data showed no differences in worm burdens between WT and IL-33KO mice at d 3 postsecondary challenge. However, by d 6 postinfection, WT mice eliminated >95% of their worms, and those still present were pale and poorly developed. Conversely, worms recovered during secondary challenge of IL-33KO mice at d 6 were larger and were feeding upon the host. Although a direct role for RELMβ in secondary immunity has not been demonstrated, impaired RELMβ expression in the lung and intestines of IL-33KO mice during both primary and secondary responses is entirely consistent with a central role for RELMβ in IL-13–mediated immunity (23).
IL-33 deficiency reduced eosinophil recruitment, which was another potential explanation for the impaired anamnestic response. Immunity against N. brasiliensis rechallenge was impaired in mice made eosinophil deficient by targeted deletion of the erythroid transcription factor (GATA-1) or IL-5 (27, 28, 43). Mechanistically, IL-33 could promote eosinophilic lung inflammation through inducing IL-5 and IL-13 production from CD4+ T cells and ILC2s (19), or by directly activating eosinophils through TI/ST2. On the other hand, IL-33KO mice generated higher levels of mast cells and expressed higher intestinal MCPT-8 mRNA transcripts than WT mice. Intact mast cell and basophil responses in IL-33KO mice were associated with enhanced IL-4 and IgE levels. This dichotomy between IL-33–driven IL-13 versus IL-4-driven inflammatory responses resolves the controversy over whether signaling via T1/ST2 is necessary for Type 2 immunity (18, 21, 44). The source of IL-4 within IL-33KO mice was CD4+T cells but also could include mast cells and basophils. Indeed, basophils are a major source of IL-4 during secondary immune responses, and were shown to promote secondary immunity against N. brasiliensis (26, 45–47). Thus, while multiple redundant mechanisms are functioning during secondary challenge infection, our data support a hypothesis that eosinophils serve a predominant role in worm destruction during anamnestic immunity against GI nematodes (48, 49).
Overall, our data indicate that within hours of a primary N. brasiliensis infection, IL-33 release drives initial expansion of IL-13+ILC2/nuocytes followed by IL-13+ CD4+ T cells within three days. This accumulation of IL-13 production instructs IEC to produce RELMβ and recruits eosinophils, which together result in parasite destruction. Upon rechallenge, this process occurs more rapidly, perhaps due to the progressive accumulation of alternatively activated macrophages that can provide a larger pool of IL-33 for IL-13–driven immunity (50).
Materials and Methods
Mice and Parasites.
A targeting construct that replaced exons 1–6 was injected into 129 ES cells, and chimeras were backcrossed onto the C57BL/6 background >10 generations and confirmed using a 377 SNP panel for microsatellite analysis. All experiments were conducted with age- and sex-matched WT or IL-33–deficient mice on a C57BL/6 background obtained from Taconic and have been described elsewhere (51). N. brasiliensis was maintained in the laboratory using established protocols (23). The Institutional Animal Care and Use Committee at the Cincinnati Children’s Hospital Medical Center and University of California at San Francisco approved all procedures.
qRT-PCR.
RNA was DNase I–treated and cDNA prepared using SuperScript II Reverse Transcriptase (Invitrogen). Real-time PCR was carried out on a Biorad iCycler (Hercules) or CFX Connect (Bio-Rad) with the Syber Green detection reagent. Cycle threshold (CT) values for genes evaluated were determined and expressed using the 1/∆∆ct method, as described previously (52).
Flow Cytometry.
Whole lung tissues were perfused with 1× PBS and minced with scissors followed by digestion in serum-free RPMI containing Liberase TL (0.25 mg/mL, Roche) and DNase I (0.5 mg/mL, Sigma) RPMI for 45 min at 37 °C. Samples were passed through a 70 μm cell strainer to obtain a single cell suspension. Single cell suspensions of lung tissue were stained with one or more of the following fluorescently labeled mAb: TCRβ (clone H57-597), CD4 (clone GK1.5), F4/80 (clone BM8), CD5 (clone 53–7.3), CD27 (clone LG.7F9), NK1.1 (clone PK136), CD45R/B220 (clone RA3-6B2), CD11c (clone N418), CD11b (clone M1/70), CD3 (clone 17A2), CD127 (clone A7R34), CD117/cKit (clone 2B8), Ly-6G/Gr-1 (clone RB-8C5), ST2L/IL-1R4 (clone 245707) and anti–IL-13 (clone eBio13A), and isotype control (MOPC-173) (eBioscience).
Statistical Analysis.
Statistical significance was assessed by either two-tailed Student t test (two groups) or ANOVA for multiple groups with a post hoc tukey test to determine significance; all were performed using Prism Graph Pad 4.0 software (* P < 0.05, ** P < 0.01, ***P < 0.001).
Supplementary Material
Acknowledgments
The authors thank Judy Appleton for critical reading of this manuscript and Danielle Kellar and Charles Perkins, who provided technical assistance. This work was supported by National Institutes of Health Grants R01 GM083204 and AI095289 (to D.R.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206587110/-/DCSupplemental.
References
- 1.Finkelman FD, Urban JF., Jr The other side of the coin: The protective role of the TH2 cytokines. J Allergy Clin Immunol. 2001;107(5):772–780. doi: 10.1067/mai.2001.114989. [DOI] [PubMed] [Google Scholar]
- 2.Locksley RM. Asthma and allergic inflammation. Cell. 2010;140(6):777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 4.Barlow JL, McKenzie AN. Nuocytes: expanding the innate cell repertoire in type-2 immunity. J Leukoc Biol. 2011;90(5):867–874. doi: 10.1189/jlb.0311160. [DOI] [PubMed] [Google Scholar]
- 5.Price AE, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107(25):11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barlow JL, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129(1):191, e191–198. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Chang YJ, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12(7):631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7(6):321–329. doi: 10.1038/nrrheum.2011.53. [DOI] [PubMed] [Google Scholar]
- 10.Milovanovic M, et al. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012;52(1-2):89–99. doi: 10.1007/s12026-012-8283-9. [DOI] [PubMed] [Google Scholar]
- 11.Zhao W, Hu Z. The enigmatic processing and secretion of interleukin-33. Cell Mol Immunol. 2010;7(4):260–262. doi: 10.1038/cmi.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Küchler AM. Interleukin-33 cytokine of dual function or novel alarmin? Trends Immunol. 2009;30(5):227–233. doi: 10.1016/j.it.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Lefrançais E, et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA. 2012;109(5):1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wills-Karp M, et al. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med. 2012;209(3):607–622. doi: 10.1084/jem.20110079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chackerian AA, et al. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179(4):2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 16.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180(4):2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 17.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121(6):1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoshino K, et al. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J Exp Med. 1999;190(10):1541–1548. doi: 10.1084/jem.190.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurowska-Stolarska M, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181(7):4780–4790. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- 20.Rank MA, et al. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123(5):1047–1054. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191(6):1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finkelman FD, et al. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: Lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 23.Herbert DR, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206(13):2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artis D, et al. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA. 2004;101(37):13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnmacht C, et al. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33(3):364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 2010;184(1):344–350. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 27.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203(6):1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knott ML, et al. Impaired resistance in early secondary Nippostrongylus brasiliensis infections in mice with defective eosinophilopoeisis. Int J Parasitol. 2007;37(12):1367–1378. doi: 10.1016/j.ijpara.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Bethony J, et al. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet. 2006;367(9521):1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 30.Finkelman FD, Wynn TA, Donaldson DD, Urban JF. The role of IL-13 in helminth-induced inflammation and protective immunity against nematode infections. Curr Opin Immunol. 1999;11(4):420–426. doi: 10.1016/S0952-7915(99)80070-3. [DOI] [PubMed] [Google Scholar]
- 31.Finkelman FD, et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 32.Monticelli LA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12(11):1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finkelman F, Morris S, Orekhova T, Sehy D. The in vivo cytokine capture assay for measurement of cytokine production in the mouse. Curr Protoc Immunol. 2003;Chapter 6(Unit 6):28. doi: 10.1002/0471142735.im0628s54. [DOI] [PubMed] [Google Scholar]
- 34.Wada T, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest. 2010;120(8):2867–2875. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang HE, et al. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13(1):58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grünig G, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282(5397):2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fallon PG, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203(4):1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197–288. doi: 10.1016/S0065-308X(04)58004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harvie M, et al. The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect Immun. 2010;78(9):3753–3762. doi: 10.1128/IAI.00502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yasuda K, et al. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci USA. 2012;109(9):3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sullivan BM, Locksley RM. Basophils: A nonredundant contributor to host immunity. Immunity. 2009;30(1):12–20. doi: 10.1016/j.immuni.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan BM, et al. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12(6):527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knott ML, Matthaei KI, Foster PS, Dent LA. The roles of eotaxin and the STAT6 signalling pathway in eosinophil recruitment and host resistance to the nematodes Nippostrongylus brasiliensis and Heligmosomoides bakeri. Mol Immunol. 2009;46(13):2714–2722. doi: 10.1016/j.molimm.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 44.Mangan NE, Dasvarma A, McKenzie AN, Fallon PG. T1/ST2 expression on Th2 cells negatively regulates allergic pulmonary inflammation. Eur J Immunol. 2007;37(5):1302–1312. doi: 10.1002/eji.200636520. [DOI] [PubMed] [Google Scholar]
- 45.Min B, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200(4):507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seder RA, et al. Production of interleukin-4 and other cytokines following stimulation of mast cell lines and in vivo mast cells/basophils. Int Arch Allergy Appl Immunol. 1991;94(1-4):137–140. doi: 10.1159/000235345. [DOI] [PubMed] [Google Scholar]
- 47.Khodoun MV, Orekhova T, Potter C, Morris S, Finkelman FD. Basophils initiate IL-4 production during a memory T-dependent response. J Exp Med. 2004;200(7):857–870. doi: 10.1084/jem.20040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Connell AE, et al. Major basic protein from eosinophils and myeloperoxidase from neutrophils are required for protective immunity to Strongyloides stercoralis in mice. Infect Immun. 2011;79(7):2770–2778. doi: 10.1128/IAI.00931-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herbert DR, et al. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. J Immunol. 2000;165(8):4544–4551. doi: 10.4049/jimmunol.165.8.4544. [DOI] [PubMed] [Google Scholar]
- 50.Reece JJ, et al. Hookworm-induced persistent changes to the immunological environment of the lung. Infect Immun. 2008;76(8):3511–3524. doi: 10.1128/IAI.00192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beamer CA, et al. IL-33 mediates multi-walled carbon nanotube (MWCNT)-induced airway hyper-reactivity via the mobilization of innate helper cells in the lung. Nanotoxicology. 2012 doi: 10.3109/17435390.2012.702230. 10.3109/17435390.2012.702230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herbert DR, et al. Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis. J Immunol. 2010;184(11):6438–6446. doi: 10.4049/jimmunol.0902009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.