Abstract
The basal ganglia–thalamocortical circuitry plays a central role in selecting actions that achieve reward-seeking outcomes and avoid aversive ones. Inputs of the nucleus accumbens (NAc) in this circuitry are transmitted through two parallel pathways: the striatonigral direct pathway and the striatopallidal indirect pathway. In the NAc, dopaminergic (DA) modulation of the direct and the indirect pathways is critical in reward-based and aversive learning and cocaine addiction. To explore how DA modulation regulates the associative learning behavior, we developed an asymmetric reversible neurotransmission-blocking technique in which transmission of each pathway was unilaterally blocked by transmission-blocking tetanus toxin and the transmission on the intact side was pharmacologically manipulated by local infusion of a receptor-specific agonist or antagonist. This approach revealed that the activation of D1 receptors and the inactivation of D2 receptors postsynaptically control reward learning/cocaine addiction and aversive learning in a direct pathway-specific and indirect pathway–specific manner, respectively. Furthermore, this study demonstrated that aversive learning is elicited by elaborate actions of NMDA receptors, adenosine A2a receptors, and endocannabinoid CB1 receptors, which serve as key neurotransmitter receptors in inducing long-term potentiation in the indirect pathway. Thus, reward and aversive learning is regulated by pathway-specific neural plasticity via selective transmitter receptors in the NAc circuit.
Keywords: avoidance, decision-making, drug addiction, reward-based learning, synaptic plasticity
The basal ganglia circuitry plays a central role in integrating neural information from the cerebral cortex and thalamus to facilitate selection of actions that achieve reward-seeking outcomes and avoid aversive outcomes (1, 2). Dysfunction of the basal ganglia leads to devastating cognitive and psychiatric disorders in Parkinson disease, schizophrenia, and drug addiction (3–5). The projection neurons of the striatum and the nucleus accumbens (NAc), which is a ventral part of the striatum, are divided into two subpopulations (i.e., the striatonigral neurons in the direct pathway and the striatopallidal neurons in the indirect pathway). Inputs of these two parallel pathways converge at the substantia nigra pars reticulata (SNr) and control the dynamic balance of the basal ganglia–thalamocortical circuitry (6–8). In this circuit, dopamine (DA) from the ventral tegmental area (VTA) is essential for associative learning by dichotomously controlling glutamatergic synaptic plasticity in the direct and indirect pathways of the NAc via D1 and D2 receptors, respectively (9, 10). Furthermore, several key neurotransmitters including NMDA receptors, adenosine A2a receptors, and endocannabinoid CB1 receptors have been shown to be involved in DA-modulated synaptic plasticity in either or both pathways of the corticostriatal circuit (10–13). However, the regulatory mechanisms of these two parallel pathways in associative learning behaviors and, in particular, the synaptic mechanisms involved in aversive learning have still largely remained to be elucidated.
In a previous study, we developed a gene-manipulating technique termed reversible neurotransmission blocking (RNB) in which transmission of the direct or the indirect pathway is separately and reversibly blocked by pathway-specific expression of the transmission-blocking tetanus toxin in a doxycycline-regulated manner (14). Blockade of the direct pathway markedly attenuates both appetitive reward learning and cocaine sensitization. In contrast, when the indirect pathway is blocked, the ability to induce reward-based learning and cocaine sensitization is retained but aversive learning is impaired.
The function of the basal ganglia circuitry becomes defective only when both sides of the NAc circuit are simultaneously impaired in the brain hemispheres (15, 16). We thus extended the RNB technique to an asymmetric RNB (aRNB) technique. In this technique, one side of the NAc was blocked by the RNB technique and the other intact side was treated with an agonist or antagonist specific for a neurotransmitter receptor (17). This aRNB technique allowed us to examine what types of neurotransmitter receptors were responsible for learning in a pathway-specific and learning stage–dependent manner. Here, we report that the pathway-specific D1 receptor activation and D2 receptor inactivation distinctly control reward-based learning and aversive learning, respectively, and that a set of the neurotransmitter receptors that induce long-term potentiation (LTP) in the indirect pathway are indispensable for inducing aversive learning.
Results
Analytical Strategy for Assessing Pathway-Specific, DA Receptor–Dependent Behavior.
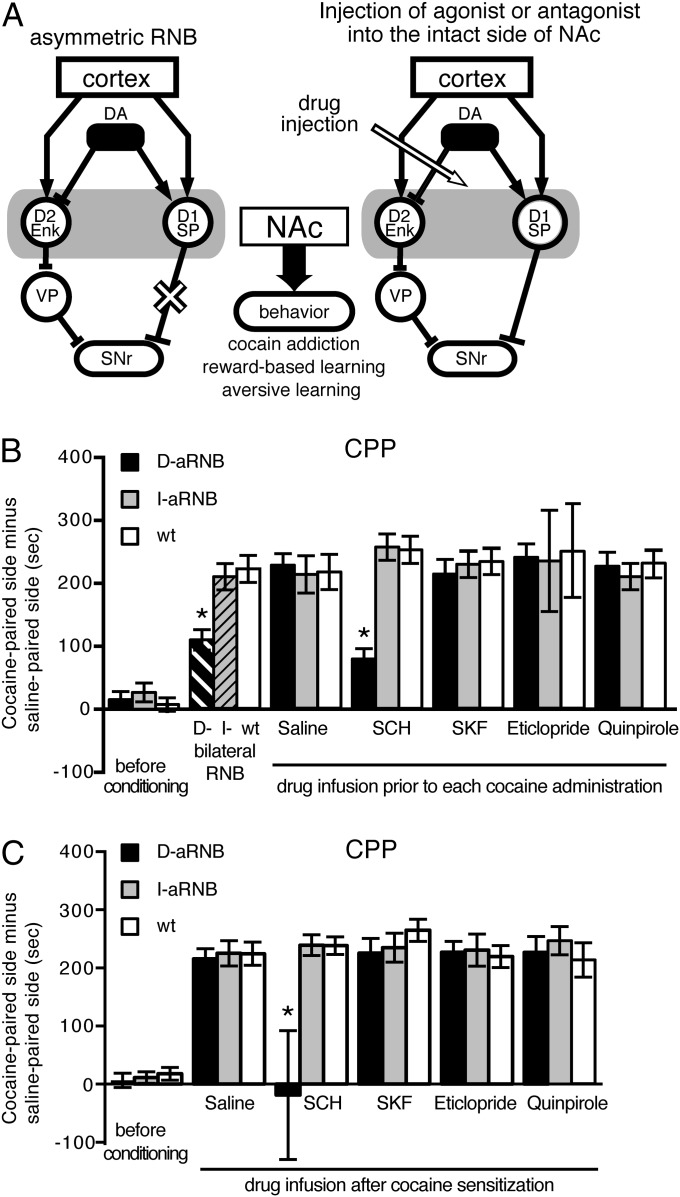
In the RNB technique, bilateral transmission blockade of either the direct pathway (D-RNB mice) or the indirect pathway (I-RNB mice) was achieved by the pathway-specific expression of transmission-blocking tetanus toxin, which was driven by interaction of the tetracycline-repressive transcription factor (tTA) and the tetracycline-responsive element (TRE) (14). The specific expression of tTA was made by using the adeno-associated virus (AAV)-mediated expression system, in which the tTA expression in the direct and indirect pathways was driven by the substance P and the enkephalin promoters, respectively (14). In the aRNB technique (Fig. 1A), transmission in either the direct pathway (D-aRNB mice) or the indirect pathway (I-aRNB mice) of the NAc was unilaterally blocked by the RNB technique. Then, 2–3 wk after the viral injection, a neurotransmitter agonist or antagonist was injected into the intact side of the NAc through an implanted cannula. Animal behavior was analyzed 20 min after drug infusion (Fig. 1A). After the behavioral analysis had been completed, the location of the implanted cannula was confirmed. A D1 agonist, SKF81297 (SKF), and a D1 antagonist, SCH23390 (SCH), were used to examine involvement of D1 receptors; whereas a full D2 agonist, quinpirole, a partial D2 agonist, aripiprazole, and a D2 antagonist, eticlopride, were used to examine engagement of D2 receptors.
Fig. 1.
Acquisition and expression of cocaine-induced CPP by D1 receptor activation in the direct pathway. (A) Schema of the asymmetric RNB technique combined with pharmacological analysis. One side of transmission of the direct or indirect pathway in the NAc was blocked by the RNB technique (as indicated by the ×), and the other intact side of the NAc was injected with saline or an agonist or antagonist specific for the targeted receptor. Arrowed and blocked lines note excitatory and inhibitory transmissions, respectively. D1, D1 receptors; D2, D2 receptors; Enk, enkephalin; SNr, substantia nigra pars reticulata; SP, substance P; VP, ventral pallidum. (B) Saline or the indicated DA agent was injected into the NAc 20 min before place conditioning of mice with administration of 10 mg/kg cocaine at one fixed chamber and saline at the other chamber. This conditioning was daily conducted for 3 d, and CPP was tested on day 4 without injection of the DA agent and cocaine administration (n = 5–6). (C) Mice were conditioned by administration of 10 mg/kg cocaine for 3 d. On day 4, saline or the indicated DA agent was injected; and 20 min later, CPP was measured without cocaine administration (n = 5–6). Columns and bars represent the mean ± SEM, respectively. D-RNB vs. I-RNB/WT or D-aRNB vs. I-aRNB/WT, *P < 0.05.
Selective Role of D1 Receptor–Dependent Direct Pathway in Cocaine Addiction.
Conditioned place preference (CPP) is associative learning between repeated cocaine administration and a cocaine-paired chamber. Two weeks after the viral injection into the NAc, the CPP test was conducted by administering cocaine at one fixed chamber and saline at the other chamber for 3 d. On day 4, place preference to visit the cocaine-paired chamber was determined without administration of cocaine (Fig. 1B). As reported previously (14), bilateral blockade of the direct pathway, but not that of the indirect pathway, significantly reduced the cocaine-induced CPP. In the control, infusion of saline into the intact side of the NAc of the D-aRNB or I-aRNB mice showed unchanged cocaine-induced CPP, comparable to that of cocaine-administered, saline-injected WT mice (Fig. 1B). This finding verified that blockade of one side of transmission had no effect on cocaine sensitization. Then, the regulation of acquisition of cocaine-induced adaptation by DA receptors was examined by drug infusion 20 min before each cocaine administration (Fig. 1B). When a D1 antagonist was infused into the D-aRNB mice, they showed a significant reduction in CPP, similar to that of the bilaterally blocked D-RNB mice. In contrast, infusion of a D1 agonist, a D2 agonist, or a D2 antagonist in the D-aRNB mice showed no such reduction in the cocaine-induced CPP. Furthermore, none of the DA agents prevented the cocaine-induced CPP in either the virus-transfected WT mice or the I-aRNB ones (Fig. 1B). As controls, no alteration of locomotion activity was observed in both D-aRNB and I-aRNB mice after treatments with DA agonists and antagonists (17). These results indicated that activation of D1 receptors in the direct pathway is critical for inducing cocaine sensitization.
Next, we addressed whether DA receptors could be involved in the expression of cocaine sensitization once such sensitization had been developed by repeated cocaine administration (Fig. 1C). In this test, animals were conditioned by daily administration of cocaine for 3 d. On day 4, saline or a DA agent was injected into the intact side of the NAc; 20 min later, the CPP test was conducted without cocaine administration. The D1 antagonist markedly prevented expression of CPP in the D-aRNB mice, and this prevention was not only D1 receptor antagonist-selective but also direct pathway–specific (Fig. 1C). These results demonstrated that the activation of D1 receptors in the direct pathway is necessary for both acquisition and expression of cocaine-induced addictive behavior.
D1 Receptor Regulation of the Direct Pathway in Acquisition of Appetitive Reward Learning.
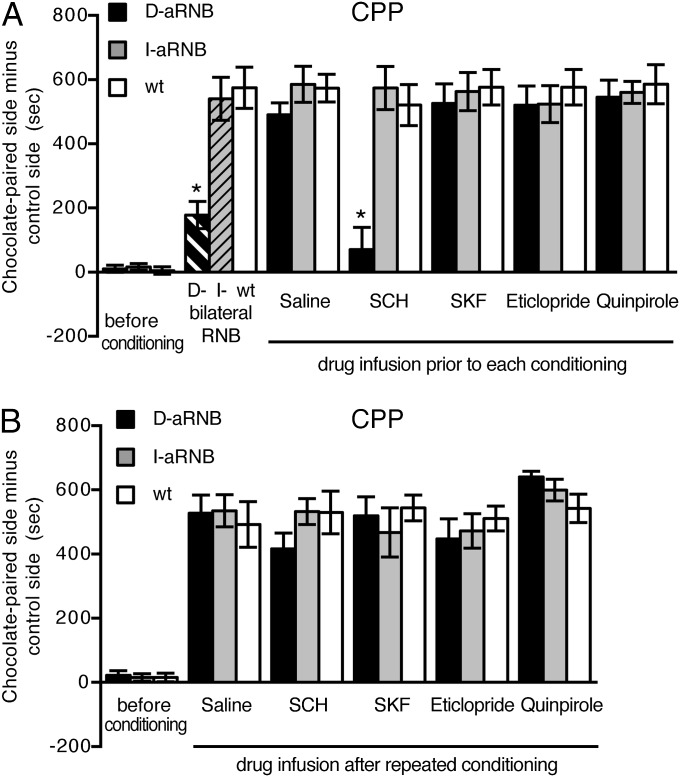
Regulation of DA receptors in acquisition and expression of appetitive reward learning was examined by measuring CPP of palatable chocolate over the standard food with the same procedures used for CPP of cocaine administration (Fig. 2). Bilateral blockade of the direct pathway, but not the indirect pathway, markedly impaired appetitive reward learning (14) (Fig. 2A). In contrast, unilaterally blocked, saline-infused D-aRNB or I-aRNB mice showed normal ability to learn a chocolate-paired chamber in the CPP test. Then, when a specific DA agonist or antagonist was injected into the intact side of the NAc 20 min before each conditioning with chocolate, the D1 antagonist-treated D-aRNB mice were severely impaired in acquisition of appetitive reward learning (Fig. 2A). This impairment was selective for D1 receptors in the direct pathway; none of the other groups of drug-infused animals were defective in acquisition of appetitive reward learning (Fig. 2A). Notably, once the D-aRNB mice had learned a reward by repeated appetitive conditioning for 3 d, infusion of the D1 antagonist failed to abrogate appetitive reward learning (Fig. 2B). Also, none of the other groups of drug-infused animals showed impaired expression of appetitive reward learning (Fig. 2B). These results indicated that the activation of D1 receptors in the direct pathway is essential for acquisition of appetitive reward learning but is not required for expression of this learning behavior.
Fig. 2.
Acquisition but not expression of appetitive reward learning by D1 receptor activation in the direct pathway. (A) Saline or the indicated DA agent was injected into the NAc 20 min before conditioning of mice with chocolate at one fixed chamber and a standard food at the other chamber. This conditioning was repeated for 3 d, and CPP was tested on day 4 without injection of saline or the DA agent (n = 5–6). (B) Mice were place-conditioned with chocolate for 3 d. On day 4, saline or the indicated DA agent was injected; 20 min later, CPP was tested (n = 5–6). Columns and bars represent the mean ± SEM, respectively. D-RNB vs. I-RNB/WT or D-aRNB vs. I-aRNB/WT, *P < 0.05.
D2 Receptor Dependency of the Indirect Pathway in Aversive Behavior.
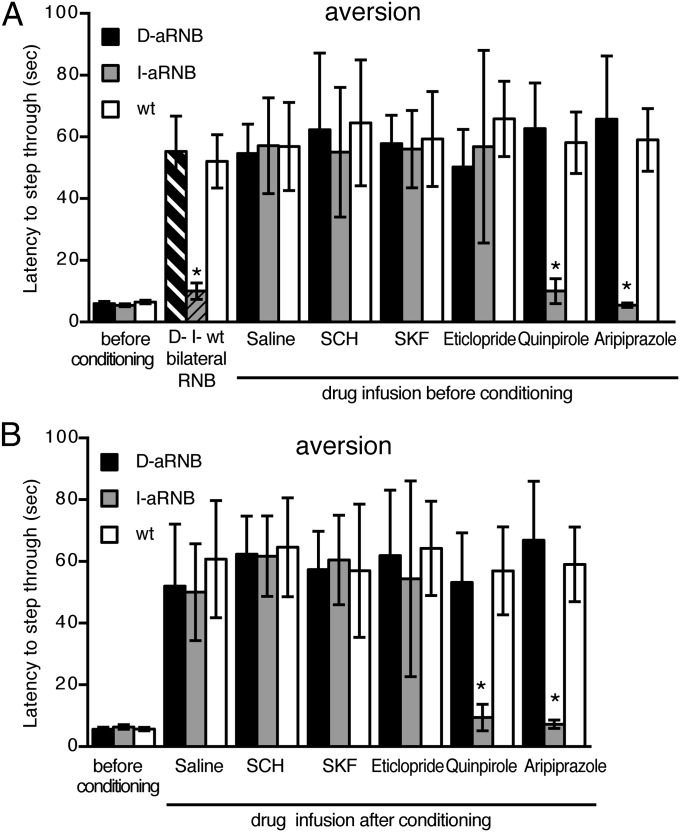
Aversive learning was then tested by performing the one-trial inhibitory avoidance task (Fig. 3). In this test, mice received electric shocks when they entered a preferred dark chamber from a lighted chamber. Aversive behavior was then tested 24 h later by measuring latencies to enter the dark chamber, where the mice were electrically shocked (14). In the absence of aversive conditioning, all three groups of animals (WT, D-aRNB, and I-aRNB) rapidly entered the preferred dark chamber with no statistical difference regardless of infusion of DA receptor agonists or antagonists. Then, bilateral blockade, but not unilateral blockade, of the indirect pathway impaired aversive learning to avoid entering the electrically shocked chamber (Fig. 3A). When quinpirole or aripiprazole was infused into the NAc 20 min before aversive conditioning, either of these D2 agonists effectively impaired avoidance learning of the I-aRNB mice but not that of the D-aRNB or WT mice (Fig. 3A). No such impairment was elicited by infusion of the D1 agonist, D1 antagonist, or D2 antagonist into the I-aRNB mice or by infusion of any of the DA agents into the WT or D-aRNB mice (Fig. 3A).
Fig. 3.
Acquisition and expression of aversive learning by D2 receptor inactivation in the indirect pathway. (A) Aversive learning was tested by the one-trial inhibitory avoidance task. Saline or the indicated DA agent was injected into the NAc; 20 min later, the mice were subjected to electric shocks when they entered the dark chamber from the lighted chamber. Memory retention was tested 24 h later by measuring latencies for the mice to enter the dark chamber (n = 5–6). (B) Mice were conditioned with electric shocks at the dark chamber; 24 h later, saline or the indicated DA agent was injected. Twenty minutes later, latencies to enter the dark chamber were measured (n = 4–6). Columns and bars represent the mean ± SEM, respectively. I-RNB vs. D-RNB/WT or I-aRNB vs. D-aRNB/WT, *P < 0.05.
The effects of DA agents on the expression of avoidance learning were then tested (Fig. 3B). Animals were conditioned with electric shocks in the dark chamber and subsequently kept in their home cage for 24 h. DA agents were then infused into the NAc; 20 min later, the animals were tested for their ability to avoid the electrically shocked chamber. This analysis showed selective impairments of aversive behavior by the activation of the D2 receptor with its agonists, quinpirole and aripiprazole, only in the I-aRNB mice (Fig. 3B). The results thus indicated that the inactivation of D2 receptors in the indirect pathway is indispensable for both acquisition and expression of aversive behavior.
Critical Function of Postsynaptic D2L Receptors in Aversive Behavior.
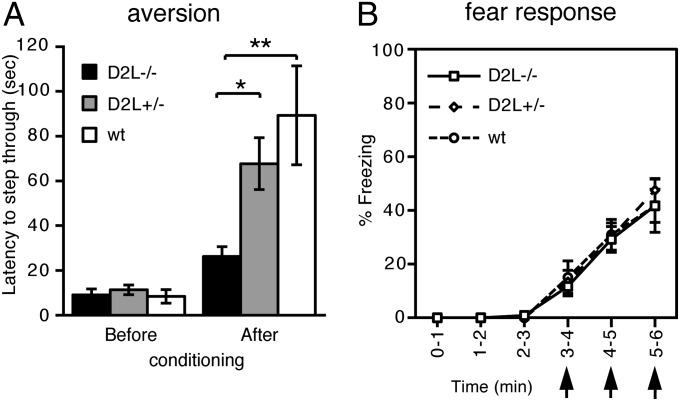
In the expression of D2 receptors, a short form (D2S) and a long form (D2L) of D2 receptors are generated by the respective exclusion and inclusion of a 29-amino acid sequence in the third cytoplasmic domain of the D2 receptors via alternative splicing (18–20). The D2L receptors have been shown to be responsible for DA transmission at the postsynaptic site of striatal neurons (21, 22). We thus addressed whether the observed aversive learning was mediated by the postsynaptic D2 receptors in DA transmission of the striatal neurons by examining aversive behavior of D2L knockout mice (23). Upon one-trial inhibitory avoidance analysis, the D2L knockout mice showed significant impairment in aversive learning in a gene dosage–dependent manner (Fig. 4A). This deficit was not due to impairments of the fear system, because the three groups of animals (WT, D2L+/−, and D2L−/− mice) showed comparable postshock freezing responses by repeated presentation of footshocks (Fig. 4B). These data thus indicated that the postsynaptic D2 receptors are critical for regulating DA transmission in aversive behavior.
Fig. 4.
Impaired aversive learning in the D2L knockout mice. (A) Latencies for mice to enter the dark chamber from the lighted chamber were measured before and 24 h after conditioning with electric shocks at the dark chamber. n = 6 for D2L−/− mice, 8 for D2L +/− mice, and 5 for WT mice. Columns and bars represent the mean ± SEM, respectively. *P < 0.05, **P < 0.01. (B) Percentages of freezing were determined for a 1-min period by giving electric shocks at 3, 4, and 5 min (arrows) (n = 4 each). Symbols and bars represent the mean ± SEM, respectively. ANOVA with repeated measures among the three groups of mice; for genotype, P = 0.90; for time, P < 0.001; interaction genotype × time, P = 0.94.
Involvement of Additional Key Neurotransmitter Receptors in Aversive Learning.
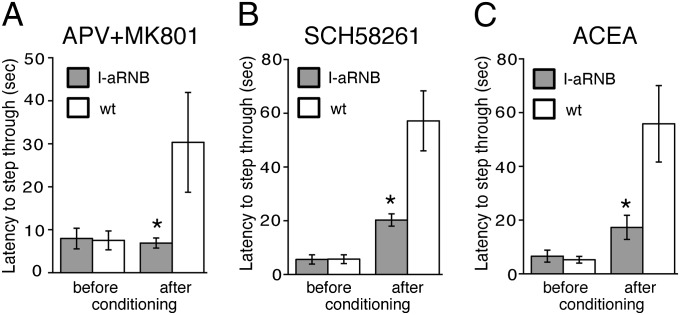
The inactivation of the inhibitory D2 receptors would most likely enhance the efficacy of input transmission of aversive stimuli in the indirect pathway and would thus induce aversive learning. The induction of LTP in glutamatergic transmission in the indirect-pathway neurons has been reported based on extensive electrophysiological studies using striatal slice preparations (10, 11, 24). In the indirect-pathway neurons, D2 receptors and adenosine A2a receptors are colocalized postsynaptically and counteract each other (10, 24, 25). The activation of postsynaptic A2a receptors efficiently impedes the synthesis of endocannabinoids and results in suppression of the presynaptic CB1 receptors in glutamatergic neurons through reduced retrograde transmission of endocannabinoids (13, 25). Electrophysiological studies have indicated that when the D2 receptors in the indirect-pathway neurons are inhibited or the striatal DA levels are reduced, the NMDA receptors and A2a receptors are coactivated and that elaborate synaptic modulation via the NMDA, A2a, and CB1 receptors induces LTP in glutamatergic transmission in the indirect-pathway neurons (10–12). Therefore, we examined whether these key neurotransmitter receptors could be involved in aversive learning by infusing the inhibitors or activator of these respective receptors into the intact side of the NAc of the I-aRNB mice. When a mixture of NMDA receptor inhibitors, D-(-)-2-amino-5-phosphonovalerate (APV) and MK801, was unilaterally injected into the NAc, an abnormal ipsilateral rotation was evoked in both the I-aRNB mice and WT mice, but this abnormality gradually disappeared thereafter. No such abnormal turning was detected when the A2a receptor antagonist SCH58261 or the CB1 receptor agonist arachidonyl-2-chloroethylamide (ACEA) was injected into the NAc of the I-aRNB and WT mice. Notably, the drug-treated, unconditioned I-aRNB and WT mice still retained preference to step through from the lighted chamber to the preferred dark chamber with no statistical difference between these two groups (Fig. 5). Then, 20 min after drug injection, the animals were conditioned with electric shocks in the dark chamber, and retention of aversive learning was tested 24 h after electric shocks by performing the one-trial inhibitory avoidance task. Treatments with the NMDA receptor inhibitors abolished aversive learning of the I-aRNB mice, but not that of the WT mice (Fig. 5A). Similarly, infusion of the A2a receptor antagonist SCH58261 significantly impaired the aversive learning of the I-aRNB mice compared with that of the WT mice (Fig. 5B). Furthermore, treatments with the CB1 receptor agonist ACEA hampered the aversive learning of the I-aRNB mice only (Fig. 5C). These results thus indicated that aversive learning behavior is regulated by the neural plasticity involving the elaborate actions of the postsynaptic NMDA receptors and A2a receptors and the presynaptic CB1 receptors at the glutamatergic synapses of the indirect-pathway neurons.
Fig. 5.
Aversive learning by activation of NMDA and A2a receptors and inhibition of CB1 receptors in the indirect pathway. Twenty minutes after injection of NMDA receptor antagonists APV and MK801 (A), A2a receptor antagonist SCH58261 (B), or a CB1 receptor agonist ACEA (C), latencies to step through from the lighted chamber to the preferred dark chamber were measured in mice before conditioning with electric shocks. They were then subjected to electric shocks at the dark chamber; latencies for the mice to enter the dark chamber were measured 24 h after shocking (n = 5 each). Columns and bars represent the mean ± SEM, respectively. I-aRNB vs. WT, *P < 0.05.
Discussion
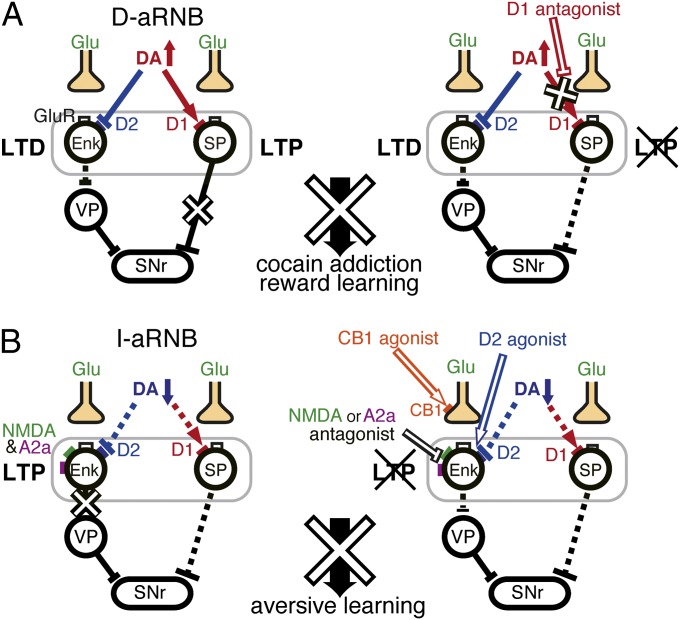
This study aimed at exploring the regulatory mechanisms of the NAc circuit in reward and aversive learning by combining the pathway-specific blockade of the NAc transmission and pharmacological analysis. Learning deficits in the D1 antagonist–treated D-aRNB mice and the D2 agonist–treated I-aRNB mice both faithfully reflected those in the bilaterally blocked D- and I-RNB mice, respectively. The activation of D1 receptors in the direct pathway and the inactivation of D2 receptors in the indirect pathway are thus essential for reward-based learning and aversive learning, respectively. The present study further revealed that the postsynaptic D2 receptors serve as a prerequisite determinant that modulates a set of the LTP-evoking synaptic receptors (NMDA, A2a, and CB1 receptors) for induction of aversive learning. The NAc comprises a minor cell population of cholinergic and GABAergic interneurons that express D1-like or D2-like DA receptors and NMDA receptors (11). Pharmacological treatments in the aRNB mice may thus act on some of these interneurons and could influence the activity of the direct-pathway or indirect-pathway neurons (26, 27). The mechanisms underlying the distinct role of each pathway thus need to be carefully interpreted. The present study has demonstrated that not only the pathway-specific postsynaptic D1 and D2 receptors but also several key receptors characteristic of the corticostriatal synapses play a pivotal role in the associative reward-based and aversive learning (10–12). We thus propose a mechanistic model for reward and aversive learning by focusing on the transmission modulation of the corticostriatal synapses in the NAc (Fig. 6).
Fig. 6.
A mechanistic model for reward-based and aversive learning by neural plasticity in the direct and indirect pathways of the NAc. For explanation of a mechanistic model of pathway-specific regulation of reward learning/cocaine addiction (A) and aversive learning (B) via selective transmitter receptors, see Discussion. The solid and dashed lines indicate the active and inactive states of input transmission, respectively. Arrowed and blocked lines note excitatory and inhibitory transmissions, respectively. A2a, A2a receptors; CB1, CB1 receptors; D1, D1 receptors; D2, D2 receptors; GluR, glutamate receptors; NMDA, NMDA receptors.
Rewarding stimuli elicit a burst of phasic firings in DA neurons (28), and these stimuli as well as cocaine administration vastly increase synaptic concentrations of DA in the NAc (29, 30). This increase effectively activates the low-affinity D1 receptors and results in induction of LTP in striatonigral neurons of the direct pathway (10, 11, 31). The simultaneous activation of the high-affinity, inhibitory D2 receptors, however, keeps the indirect pathway inactive. The D1 receptor antagonist thus disrupted cocaine-induced and reward-based behavior in a direct pathway–specific manner (Fig. 6A).
An electric footshock has been shown to inhibit about 90% of DA neurons in the VTA (32) and would reduce DA levels in the NAc. This reduction in DA relieves the inhibitory actions of the high-affinity D2 receptors but has no effects on the low-affinity D1 receptors. The resultant selective transmission enhancement in the indirect pathway would induce aversive learning. The exogenously applied D2 receptor agonist thus counteracted the inactivation of D2 receptors and selectively disrupted aversive learning (Fig. 6B). D2 receptors are located both presynaptically and postsynaptically, but the D2L receptors are located postsynaptically (23, 33). The D2L deficiency has been shown to abolish postsynaptic responses (21, 22) but keep presynaptic functions intact, including transmission by GABA, glutamate, and DA in the NAc (23, 33–36). In the present investigation, the D2L knockout mice exhibited impaired aversive learning. Thus, taking into account impairments of the D2 agonist-treated I-aRNB mice, the results indicated that the postsynaptic D2 receptors in the NAc play a pivotal role for induction of aversive learning. Furthermore, consistent with our proposed model, previous studies have indicated that D2L knockout mice retain the normal ability for CPP after repeated cocaine administration (37, 38). Importantly, previous electrophysiological studies have indicated that several key neurotransmitter receptors play a critical role in inducing LTP in glutamatergic transmission in the indirect pathway (10, 11). Our behavioral study explicitly demonstrated that these LTP-evoking receptors, when pharmacologically manipulated, all prevented aversive learning in the I-aRNB mice. Our model thus holds that aversive learning is induced by inactivation of the postsynaptic D2 receptors and then the sequential mechanistic events mediated by the key neurotransmitter receptors at the NAc circuit of the indirect pathway.
Interestingly, pharmacological manipulation by DA receptor agonist or antagonist before and after learning conditioning impaired cocaine sensitization and aversive learning, but once appetitive reward learning had been developed, such manipulation was no more effective in blocking this learning. Thus, dopaminergic synaptic modulation is required for both acquisition and expression of cocaine sensitization and aversive learning, but this modulation is specifically involved in acquisition but not expression of appetitive reward learning. The corticostriatal circuitry is functionally segregated in parallel loops conveying limbic, associative, and sensory motor information (6). Because transmission blockade was restricted to the NAc in this investigation, appetitive rewarding signals may shift from the NAc loop to other striatal loops and additional neural circuits. The difference in encoding information about abusive drugs versus natural rewards would be important for a better understanding of abnormal responses to abusive drugs. In conclusion, reward and aversive learning is controlled by the pathway-specific, segregated synaptic modulation in the NAc via selective transmitter receptors; this finding should open additional perspectives on the development of specific and efficient treatments for drug addiction and psychiatric disorders.
Materials and Methods
Animals.
All animal handling procedures were approved by the animal research committees of Osaka Bioscience Institute, Kyoto University Graduate School of Medicine, and Kitasato University. The RNB and aRNB mice were generated by the respective bilateral and unilateral injection of the recombinant AAV viruses into four sites of each NAc by stereotaxic techniques (14, 17). Virus-injected WT littermates were used as controls. The heterozygous D2L+/− mice (23) were backcrossed to a C57BL/6 genetic background for more than six generations; homozygous D2L−/−, heterozygous D2L+/−, and WT mice were generated by mating the heterozygous D2L+/− mice.
Drug Infusion in aRNB Mice.
After the recombinant virus had been unilaterally injected into the NAc of anesthetized mice, the other side of the NAc was implanted with a cannula aimed toward the NAc for drug infusion as described previously (17). The concentrations of infused drugs were 100 µM SCH23390, 300 µM SKF81297, 1 mM quinpirole, 400 µM eticlopride (all from SIGMA), 100 µM aripiprazole (Toronto Research Chemicals), 25 mM APV, 20 mM MK801 (both from Wako), 100 µM SCH58261 (Tocris), and 300 µM ACEA (abcam). After behavioral analysis, injection sites of drugs were confirmed by direct visualization of a series of slice sections of the NAc region. When the injection site was found to be outside the NAc, these data were discarded (about 2% of animals analyzed).
Behavior Tests.
Behavioral analysis was started 2–3 wk after viral injection together with surgery for drug infusion. The CPP test and the one-trial inhibitory avoidance test were performed as described previously (14). Cocaine was obtained from Shionogi. Fear responses were analyzed by scoring percentages of positive freezing responses at 2-s intervals in a 1-min period following electric shocks (14). All tests of animal behaviors were conducted in a blind fashion.
Statistical Analysis.
Statistical analysis was conducted by using StatView 5.0. Data were analyzed by two-way ANOVA or repeated-measured ANOVA.
Acknowledgments
We thank A. Uto for preparing figures. This work was supported by Research Grants-in-Aid 2222005 (to S.N.), 23120011 (to S.N., T.H., and S.Y.), and 23680034 (to T.H.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; and by grants from Japan Science and Technology Agency, Precursory Research for Embryonic Science and Technology (to T.H.); TK Project of Medical Innovation Center of Kyoto University (to T.H.); and the Ministry of Health, Labor and Welfare of Japan (to T.H.); the Takeda Science Foundation (to S.N.); the Naito Foundation (to T.H.); the Uehara Memorial Foundation (to T.H.); and the Senri Life Science Foundation (to T.H.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank MJ. Computational models of motivated action selection in corticostriatal circuits. Curr Opin Neurobiol. 2011;21(3):381–386. doi: 10.1016/j.conb.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 4.Israel Z, Bergman H. Pathophysiology of the basal ganglia and movement disorders: From animal models to human clinical applications. Neurosci Biobehav Rev. 2008;32(3):367–377. doi: 10.1016/j.neubiorev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65(5):585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 7.Graybiel AM. The basal ganglia. Curr Biol. 2000;10(14):R509–R511. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- 8.Deniau JM, Mailly P, Maurice N, Charpier S. The pars reticulata of the substantia nigra: A window to basal ganglia output. Prog Brain Res. 2007;160:151–172. doi: 10.1016/S0079-6123(06)60009-5. [DOI] [PubMed] [Google Scholar]
- 9.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321(5890):848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010;13(8):958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445(7128):643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- 14.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66(6):896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8(6):805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- 16.Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex. 2007;17(7):1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- 17.Yawata S, Yamaguchi T, Danjo T, Hikida T, Nakanishi S. Pathway-specific control of reward learning and its flexibility via selective dopamine receptors in the nucleus accumbens. Proc Natl Acad Sci USA. 2012;109(31):12764–12769. doi: 10.1073/pnas.1210797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dal Toso R, et al. The dopamine D2 receptor: Two molecular forms generated by alternative splicing. EMBO J. 1989;8(13):4025–4034. doi: 10.1002/j.1460-2075.1989.tb08585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giros B, et al. Alternative splicing directs the expression of two D2 dopamine receptor isoforms. Nature. 1989;342(6252):923–926. doi: 10.1038/342923a0. [DOI] [PubMed] [Google Scholar]
- 20.Monsma FJ, Jr, McVittie LD, Gerfen CR, Mahan LC, Sibley DR. Multiple D2 dopamine receptors produced by alternative RNA splicing. Nature. 1989;342(6252):926–929. doi: 10.1038/342926a0. [DOI] [PubMed] [Google Scholar]
- 21.Lindgren N, et al. Distinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sites. Proc Natl Acad Sci USA. 2003;100(7):4305–4309. doi: 10.1073/pnas.0730708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaulieu JM, et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27(4):881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, et al. Dopamine D2 long receptor-deficient mice display alterations in striatum-dependent functions. J Neurosci. 2000;20(22):8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flajolet M, et al. FGF acts as a co-transmitter through adenosine A(2A) receptor to regulate synaptic plasticity. Nat Neurosci. 2008;11(12):1402–1409. doi: 10.1038/nn.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuxe K, Ferré S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92(1-2):210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 26.Chuhma N, Tanaka KF, Hen R, Rayport S. Functional connectome of the striatal medium spiny neuron. J Neurosci. 2011;31(4):1183–1192. doi: 10.1523/JNEUROSCI.3833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Threlfell S, et al. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75(1):58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 28.Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. J Neurophysiol. 1994;72(2):1024–1027. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- 29.Tsai HC, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324(5930):1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47(2):255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Tan KR, et al. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73(6):1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Usiello A, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408(6809):199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 34.Centonze D, et al. Differential contribution of dopamine D2S and D2L receptors in the modulation of glutamate and GABA transmission in the striatum. Neuroscience. 2004;129(1):157–166. doi: 10.1016/j.neuroscience.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 35.Centonze D, et al. Dopamine D2 receptor-mediated inhibition of dopaminergic neurons in mice lacking D2L receptors. Neuropsychopharmacology. 2002;27(5):723–726. doi: 10.1016/S0893-133X(02)00367-6. [DOI] [PubMed] [Google Scholar]
- 36.Rougé-Pont F, et al. Changes in extracellular dopamine induced by morphine and cocaine: Crucial control by D2 receptors. J Neurosci. 2002;22(8):3293–3301. doi: 10.1523/JNEUROSCI.22-08-03293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith JW, Fetsko LA, Xu R, Wang Y. Dopamine D2L receptor knockout mice display deficits in positive and negative reinforcing properties of morphine and in avoidance learning. Neuroscience. 2002;113(4):755–765. doi: 10.1016/s0306-4522(02)00257-9. [DOI] [PubMed] [Google Scholar]
- 38.Welter M, et al. Absence of dopamine D2 receptors unmasks an inhibitory control over the brain circuitries activated by cocaine. Proc Natl Acad Sci USA. 2007;104(16):6840–6845. doi: 10.1073/pnas.0610790104. [DOI] [PMC free article] [PubMed] [Google Scholar]