Abstract
Urate is the end product of purine metabolism in humans, owing to the evolutionary disruption of the gene encoding urate oxidase (UOx). Elevated urate can cause gout and urolithiasis and is associated with cardiovascular and other diseases. However, urate also possesses antioxidant and neuroprotective properties. Recent convergence of epidemiological and clinical data has identified urate as a predictor of both reduced risk and favorable progression of Parkinson’s disease (PD). In rodents, functional UOx catalyzes urate oxidation to allantoin. We found that UOx KO mice with a constitutive mutation of the gene have increased concentrations of brain urate. By contrast, UOx transgenic (Tg) mice overexpressing the enzyme have reduced brain urate concentrations. Effects of the complementary UOx manipulations were assessed in a mouse intrastriatal 6-hydroxydopamine (6-OHDA) model of hemiparkinsonism. UOx KO mice exhibit attenuated toxic effects of 6-OHDA on nigral dopaminergic cell counts, striatal dopamine content, and rotational behavior. Conversely, Tg overexpression of UOx exacerbates these morphological, neurochemical, and functional lesions of the dopaminergic nigrostriatal pathway. Together our data support a neuroprotective role of endogenous urate in dopaminergic neurons and strengthen the rationale for developing urate-elevating strategies as potential disease-modifying therapy for PD.
Urate, the anionic component of uric acid, predominates at physiological pH. As an apparent consequence of mutations in the urate oxidase (UOx) gene during primate evolution, urate constitutes the enzymatic end product of purine metabolism in humans (1). There remains controversy over how the loss of UOx activity and the resultant high urate concentrations in hominoids may have been beneficial and whether it still is. On one hand, urate is considered a pathogenic factor in gout, urolithiasis, and nephropathy, and hyperuricemia is associated with other medical conditions, such as hypertension, cardiovascular disease, and metabolic syndrome (2). On the other hand, the loss of UOx activity through multiple independent mutations in hominoids presumably conferred evolutionary advantages. Urate possesses potent antioxidant properties. High urate levels may have provided an antioxidant defense against aging and cancer, thereby contributing to a prolonged hominoid life span (3). In addition, increased urate may mediate blood pressure homeostasis in low-salt environments. Furthermore, higher urate has been suggested to enhance human intelligence or motivational behaviors or promote neuronal integrity and function (4).
Recently a series of population and clinical epidemiology studies have lent support to a potential neuroprotective effect of urate (5, 6). These studies demonstrated a robust inverse link between urate levels and both the risk and clinical progression of Parkinson’s disease (PD), one of the most common neurodegenerative diseases. Given the putative role of oxidative stress in the pathogenesis of PD (7), these studies have identified urate as not only a unique biomarker for PD risk and progression but also a potential new target for treatment of PD (5, 6). A clinical trial of a urate precursor in PD has been launched as part of an effort to explore urate elevation as a possible disease-modifying strategy for PD (8).
To better understand the biological basis for a role of urate in PD and better gauge the therapeutic potential of urate in the treatment of neurodegenerative disease, we investigated the effects of urate manipulation in a well-established mouse 6-hydroxydopamine (6-OHDA) model of PD. Urate concentrations were altered by modifying the UOx gene, which in rodents encodes a functional enzyme catalyzing the degradation of urate to allantoin. Comprehensive pathological, neurochemical, and behavioral outcome measures were evaluated to determine nigrostriatal dopaminergic pathway deficits after unilateral intrastriatal 6-OHDA infusion in UOx KO and transgenic (Tg) mice, in which respective elevations and reductions were achieved in brain concentrations of urate.
Results
Altered Urate but Not Its Precursors in UOx KO and Tg Mouse Brain.
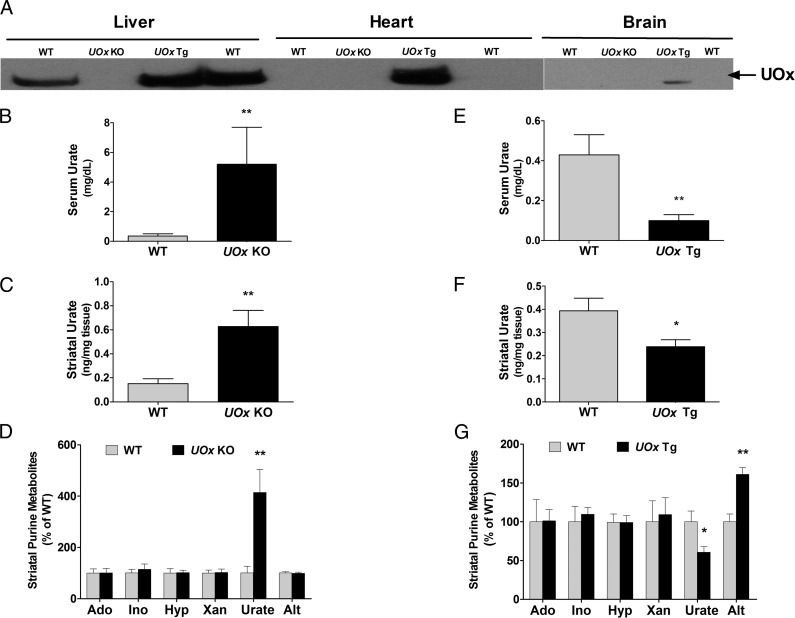
Western blotting was performed to confirm deletion and overexpression of UOx in peripheral tissues and brain of adult mice from UOx KO and Tg lines. As expected, there was no detectable UOx in liver, heart, or brain in UOx KO mice. In contrast, UOx was expressed in all organs examined in UOx Tg mice. Littermate WT animals did not have detectable UOx in brain and heart, consistent with a previous report that UOx is a liver-specific enzyme (Fig. 1A) (9).
Fig. 1.
Altered urate in serum and brain in UOx KO and Tg mice. (A) Western blot of UOx showing the absence of UOx in liver, heart, and brain in a UOx KO mouse (10 mo old). UOx is expressed in liver, heart, and brain in a UOx Tg mouse (12 mo old), and it is not detectable in heart and brain in WT mice. HPLC analysis indicates elevated urate levels in blood (B) and brain (C) in UOx KO mice. (D) Changes in urate levels are not accompanied by changes in concentrations of urate precursors adenosine (Ado), inosine (Ino), hypoxanthine (Hyp), xanthine (Xan), or urate metabolite allantoin (Alt) in brain in UOx KO mice. Conversely, UOx Tg mice have lower urate levels in blood (E) and brain (F). Overexpression of UOx also leads to an increase in striatal Alt in the Tg mice (G). There are no significant differences in striatal Ado, Ino, Hyp, or Xan between UOx Tg mice and the non-Tg WT littermate controls (G). Data are expressed as mean ± SEM. n = 6, WT and UOx KO (8–10 mo old); n = 9, WT and UOx Tg (4–5 mo old). *P < 0.05 vs. WT; **P < 0.01 vs. WT.
Urate and its purine precursors—adenosine, inosine, hypoxanthine, and xanthine—in serum and brain tissue (striatum) were quantified by HPLC coupled with UV and electrochemical detection. In UOx KO mice, serum urate reached 5.2 mg/dL, more than 10-fold greater than in their WT littermates (P < 0.01, t test) (Fig. 1B). Despite the absence of UOx in the brain of naïve mice and presumably minimal local central nervous system (CNS) effects of UOx disruption, the increase of urate in the periphery was accompanied by a significant increase in urate in brain. Striatal urate in UOx KO animals was four times higher than in WT littermate controls (P < 0.01, t test) (Fig. 1C). Disruption of UOx did not result in changes in purine precursors of urate in brain. Similarly, striatal levels of the urate metabolite allantoin in UOx KO mice, which was quantified by LC-MS, was not different from WT mice, in agreement with undetectable UOx activity in WT brain (9) and therefore minimal local enzymatic contribution to CNS levels of allantoin (Fig. 1D).
In UOx Tg animals, HPLC analysis revealed a more than fivefold reduction in serum urate when compared with WT non-Tg littermates (P < 0.01, t test) (Fig. 1E). Striatal urate was also significantly lower in the Tg mice (P < 0.05, t test) but to a lesser extent than in serum despite broad expression of UOx transgene driven by a β-actin promoter (10) (Fig. 1F). The similar gradient and yet tight correlation in urate concentrations between blood and brain in both UOx KO and Tg mice may reflect a role of blood–brain barrier in regulating brain concentrations of urate (6). No significant differences in adenosine, inosine, hypoxanthine, or xanthine were observed between UOx Tg mice and their WT non-Tg littermates. However, striatal allantoin was elevated in UOx Tg mice, consistent with locally increased UOx activity in these mice (Fig. 1G).
Impaired Renal Function in UOx KO Mice but Not UOx Tg Mice.
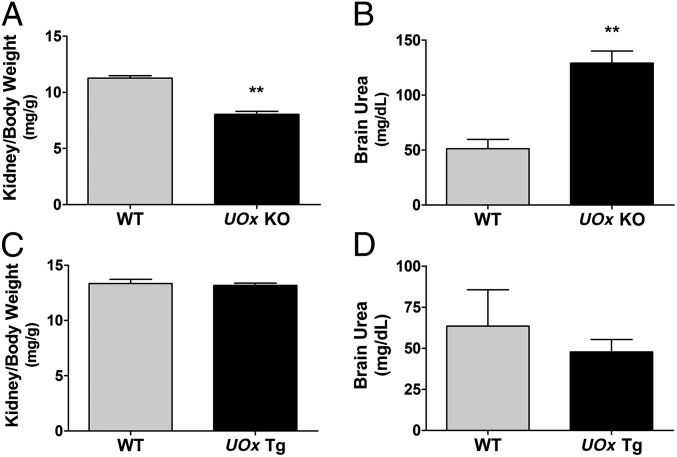
Given that altered urate levels are often associated with renal dysfunction and that urate nephropathy has been reported in UOx KO mice (11), we monitored kidney and body weights and urea levels, an indicator of kidney function, in both the KO and Tg mice. As shown in Fig. 2A, adult (4 mo old) UOx KO had 30% lower kidney to body weight ratio, compared with WT littermates (P < 0.01, t test). Body weight in UOx KO mice was slightly lower but not statistically different from that in WT littermates during the entire experimental course. Brain urea levels in the KO animals were more than twice as high as in WT littermates (P < 0.01, t test) (Fig. 2B). UOx Tg mice had normal gross renal morphology, as well as kidney to body weight ratio, compared with WT littermates (Fig. 2C). Brain urea was not changed in these mice (Fig. 2D).
Fig. 2.
Kidney to body weight ratios and urea levels in UOx KO and Tg mice. (A) UOx KO have significantly lower kidney to body weight ratio than WT mice (both kidneys from each animal; 4 mo old; n = 11 and 8 WT and UOx KO, respectively). (B) HPLC demonstrates a marked increase in brain urea level in UOx KO mice (3–4 mo old; n = 5, both WT and UOx KO). (C) Kidney to body weight ratio in UOx Tg mice (both kidneys from each animal. 6 mo old; n = 11 and 14 for WT and UOx Tg, respectively). (D) Brain urea is not changed in UOx Tg mice (4–5 mo old; n = 5, WT and UOx Tg). Data are expressed as mean ± SEM. **P < 0.01 vs. WT.
UOx Disruption or Overexpression Changes Levels of Protein Carbonyls.
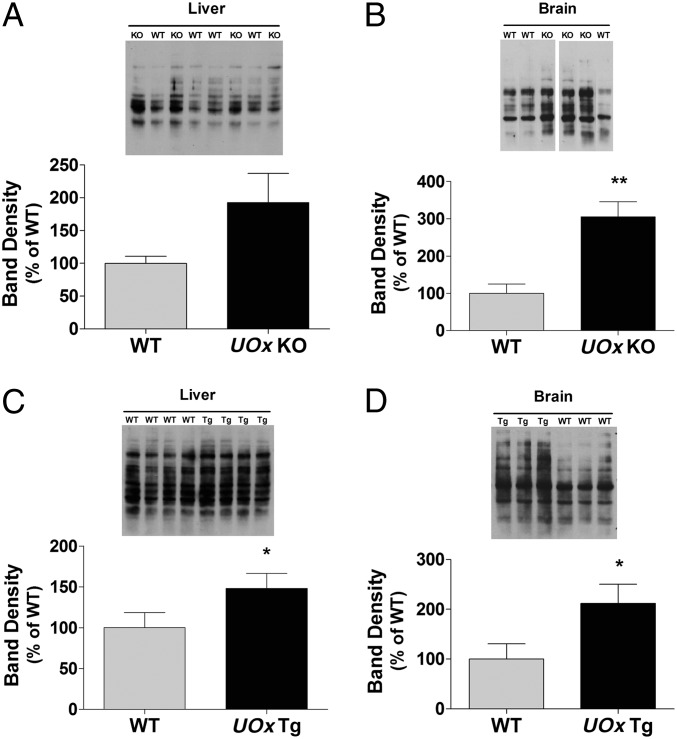
Urate is known to have antioxidant properties; altered urate levels resulting from UOx gene manipulation may therefore change susceptibility to oxidative stress. To evaluate oxidative stress status, levels of protein carbonyls, a general marker of oxidative damage, were assessed by Western blotting of immunoreactivity to derivitized protein carbonyl groups (Oxyblot) with tissues from adult UOx KO and Tg mice. Band densities were normalized with Ponceau staining of the blots. The results did not demonstrate decreased levels of protein carbonyls, as one might expect, but instead a trend toward increased levels of protein carbonyls in liver of the KO animals (Fig. 3A) and significantly higher levels in brain (P < 0.01, t test) (Fig. 3B) compared with those in WT littermates. Overexpression of UOx also increased protein carbonyl content in both liver and brain, as shown in Fig. 3 C and D; relative band densities in liver and brain tissues from UOx Tg mice were higher than in WT non-Tg littermates (P < 0.05, both liver and brain, t test).
Fig. 3.
UOx disruption or overexpression changes levels of protein carbonyls in mice. Protein carbonyls were assessed by Oxyblot. Band density was normalized with Ponceau staining of the proteins. (A) A trend toward increased levels of protein carbonyls in liver in UOx KO animals. (B) Protein carbonyls are higher in brain in UOx KO mice. Overexpression of UOx leads to increased protein carbonyl content in both liver (C) and brain (D) in Tg mice. Data are expressed as mean ± SEM n = 6, WT and UOx KO (3–4 mo old); n = 5, WT and UOx Tg (4–5 mo old). *P < 0.05; **P < 0.01 vs. WT.
UOx KO Mice Are Resistant to 6-OHDA Neurotoxicity.
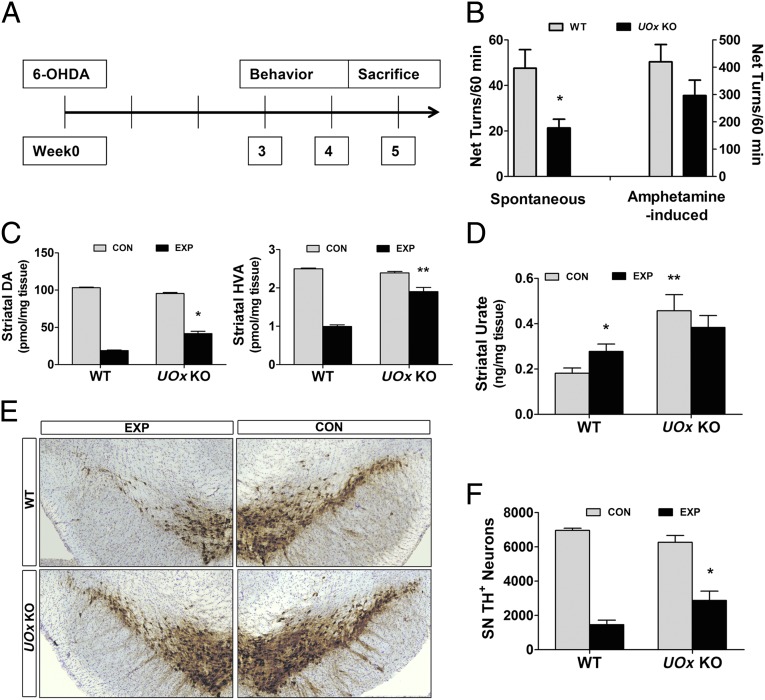
To evaluate the effects of UOx disruption and urate elevation in a standard mouse model of PD, young adult UOx KO mice (average age, 3 mo) and their WT littermates received unilateral intrastriatal infusion of 6-OHDA, a dopaminergic toxin. Spontaneous and amphetamine-induced rotational behaviors were recorded 3 and 4 wk after lesioning, as behavioral indices of ipsilateral dopaminergic deficit. Animals were killed at 5 wk after lesion (Fig. 4A). UOx KO mice showed markedly reduced spontaneous net ipsilateral rotations (P < 0.05, t test) and a trend toward reduced amphetamine-induced rotations ipsilateral to the lesion (Fig. 4B). Neurochemical analysis demonstrated significantly higher levels of residual dopamine (DA) (P < 0.05, Tukey’s post hoc test) and its metabolite homovanillic acid (HVA) on the lesion side of UOx KO animals compared with their WT littermate controls (P < 0.01, Tukey’s post hoc test) (Fig. 4C). Increased striatal levels of urate were confirmed in the KO animals compared with WT controls (P < 0.01, Tukey’s post hoc test), in which the intrastriatal 6-OHDA infusion produced a long-lasting local increase in urate content compared with that of the unlesioned striatum (Fig. 4D). This 6-OHDA–induced increase in urate may reflect persistent changes in oxidative stress status or energy metabolism after 6-OHDA. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), another commonly used parkinsonian toxin, has also been reported to increase striatal urate in mice (12). A representative set of sections stained for tyrosine hydroxylase (TH), a marker for dopaminergic neurons, showed few remaining TH-positive neurons in the substantia nigra (SN) on the lesion side in a WT mouse and preservation of TH positive neurons in a UOx KO mouse (Fig. 4E). Finally, stereological quantification of TH-positive nigral neurons indicated 46% survival of TH-positive neurons on the lesioned vs. unlesioned side in KO mice, a twofold increase over the percentage of surviving neurons in WT littermates (Fig. 4F) (P < 0.05, Tukey’s post hoc test).
Fig. 4.
UOx KO mice are more resistant to 6-OHDA neurotoxicity. (A) 6-OHDA (15 μg) was infused into the left striatum of UOx KO mice (average age, 3 mo). Spontaneous and 5 mg/kg amphetamine-induced rotational behavior were recorded at 3 and 4 wk after the lesion. Animals were killed at 5 wk after 6-OHDA lesion. (B) Spontaneous net ipsilateral rotations in UOx KO mice are attenuated (*P < 0.05 vs. WT), with a similar trend for attenuated amphetamine-induced net ipsilateral turns (WT n = 11; UOx KO n = 9). (C) UOx KO animals have significantly higher levels of DA and HVA on the experimental (lesion) side compared with their WT littermates (WT n = 11; UOx KO n = 8). *P < 0.05; **P < 0.01 vs. WT experimental side. (D) HPLC-electrochemical detection (ECD) confirms an increased level of urate in the UOx KO group. Injection of 6-OHDA induces an increase in urate in WT mice (n = 11 and 9 WT and UOx KO, respectively). *P < 0.05; **P < 0.01 vs. WT control side. (E) Preservation of SN dopaminergic neurons (TH positive) on the lesion side in a UOx KO mouse and disruption of SN TH neurons in a WT mouse. (F) Stereological quantification of TH neurons in the SN demonstrates more surviving dopaminergic neurons in the KO mice (n = 8 both WT and UOx KO groups). *P < 0.05 vs. WT experimental side. Data are expressed as mean ± SEM. CON, control nonlesion side; EXP, experimental lesion side.
UOx Tg Mice Are Susceptible to 6-OHDA Neurotoxicity.
The asymmetric turning behavior reflecting the extent of ipsilateral dopaminergic deficits was significantly exacerbated in UOx Tg mice at 3 and 4 wk after 6-OHDA infusion for both spontaneous (P < 0.01, t test) and amphetamine-induced (P < 0.05, t test) rotations in UOx Tg mice (average age, 5 mo) compared with WT non-Tg littermates (Fig. 5A). Consistent with the neurochemical phenotype of UOx Tg mice shown in Fig. 1F, their unlesioned striata had a significantly lower urate content than in WT non-Tg littermates. 6-OHDA lesioning induced an increase in local urate in WT mice, and even in the presence of excess UOx, in their Tg counterparts (Fig. 5B). DA and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) in the striatum decreased by ∼70% after 6-OHDA in WT non-Tg mice. In UOx Tg mice, DA content on the lesion side was further reduced to 13% of that of the nonlesion control side, a significant difference from WT littermates. DOPAC in the Tg mice was reduced to 20% of control nonlesion side, significantly lower than in WT mice (P < 0.05, DA and DOPAC, Tukey’s post hoc test) (Fig. 5C). Quantitative stereological analysis demonstrated a significant decrease in the number of residual TH-positive nigral neurons on the lesion side in UOx Tg mice, compared with WT non-Tg littermates (P < 0.01, Tukey’s post hoc test) (Fig. 5D). The difference was still significant statistically when expressed as percentage of control (CON) to normalize for the small difference on the control (unlesioned) side between the two groups of animals (P < 0.01, Tukey’s post hoc test). The subtle reduction in TH-positive neurons but not in DA content in UOx Tg remains to be further characterized. Representative sections of the ventral mesencephalon stained for TH depicted the extensive disruption of dopaminergic neurons in the SN in a UOx Tg mouse at 5 wk after intrastriatal 6-OHDA (Fig. 5E).
Fig. 5.
UOx Tg mice are more susceptible to 6-OHDA neurotoxicity. UOx Tg mice (average age, 5 mo) received intrastriatal 6-OHDA infusion. Behavioral tests were performed and animals killed at time points indicated in Fig. 4A. (A) Marked increases in both spontaneous and amphetamine-induced net ipsilateral rotations in UOx Tg mice after 6-OHDA infusion compared with WT non-Tg mice (n = 11 WT; n = 14 UOx Tg). *P < 0.05; **P < 0.01 vs. WT. (B) UOx Tg mice had lower urate levels in the striatum, and 6-OHDA induces local increases in urate in both WT and Tg mice (n = 11 and 14 WT and UOx Tg, respectively). **P < 0.01 vs. WT nonlesion control side; #P < 0.05 vs. WT experimental side, and vs. UOx Tg control side. (C) Significant further reductions in DA and DOPAC on experimental side in UOx Tg animals after 6-OHDA lesion (n = 11 and 14 WT and UOx Tg, respectively). *P < 0.05 vs. WT experimental side. (D) A significant decrease in the number of nigral TH-positive neurons on the experimental side in UOx Tg mice, compared with WT littermates. The difference is still significant statistically when expressing as percentage of CON to normalize for the difference on control side between the two genotypes (n = 6 and 7 WT and UOx Tg, respectively). *P < 0.05 vs. WT nonlesion control side; ##P < 0.01 vs. WT experimental side. (E) Few remaining TH-positive neurons in the SN in a UOx Tg mouse after intrastriatal 6-OHDA. Data are expressed as mean ± SEM. CON, control nonlesion side; EXP, experimental lesion side.
Discussion
In contrast to the established and hypothesized deleterious effects of urate on human health, its putative protective effects against disease have taken on particular relevance for CNS function and disorders. Among neurodegenerative diseases, PD has been most closely linked to low urate by convergent epidemiological and clinical findings (5, 6). In pursuing their translation toward therapeutics it is important to understand whether and how urate may have disease-modifying effects in preclinical models of PD. By using complementary genetic approaches disrupting and overexpressing UOx, we have demonstrated that disruption of the UOx gene with a resultant rise in urate protects the nigrostriatal dopaminergic system, and conversely that transgenic overexpressson of UOx with a resultant fall in urate exacerbates dopaminergic neurodegeneration and resultant neurochemical and behavioral deficits in a 6-OHDA mouse model of PD.
Neuroprotective effects of urate have been reported in various in vitro and in vivo experimental models of neurological disorders, including ischemic brain injury (13, 14), multiple sclerosis (15), and spinal cord injury (16, 17). However, evidence regarding urate in PD models is sparse and largely restricted to cellular models of the disease. Urate blocked DA-induced apoptotic cell death, and it protected against 6-OHDA toxicity in PC12 cells (18, 19). In dopaminergic neurons, it reduced mitochondrial dysfunction and cell death occurring spontaneously in culture or induced by pesticides rotenone or iron ions (20, 21). We report here that UOx KO mice are more resistant to 6-OHDA neurotoxicity. UOx disruption with elevated urate levels can prevent DA loss, promote long-term survival of dopaminergic neurons, and preserve functional performances after 6-OHDA lesion.
Conversely, Tg mice overexpressing UOx demonstrate enhanced susceptibility to 6-OHDA neurotoxicity. Lower urate levels have been associated with higher risk of PD, as well as more rapid clinical progression of PD (5, 6) and possibly other neurodegenerative diseases (22, 23). Similarly, lower urate levels have been consistently reported in PD patients compared with control subjects (24, 25). However, no experimental evidence has directly linked hypouricemia to neurodegeneration in vivo. Overexpression of UOx in mice leads to significant reduction in urate both in blood and in brain. The exacerbated neurotoxicity of 6-OHDA on the nigrostriatal dopaminergic pathway in UOx Tg mice entails greater DA depletion, more extensive neuron loss, and exacerbated asymmetry of movement. These findings in vivo are consistent with our recent report that this UOx transgene exacerbates dopaminergic neuron degeneration in a cellular model of PD (26).
Our findings thus provide a demonstration that genetic modulation of UOx modifies brain concentrations of urate and neurodegeneration in an established model of PD, supporting a contention that the known neuroprotective effects of urate itself may account for the complementary phenotypes of these opposing genetic manipulations in the 6-OHDA model of PD. However, altering UOx may have had other effects, particularly on purine metabolism, that could provide alternative explanations for the observed phenotypes. Blocking or accelerating purine catabolism at the level of UOx might also be expected to alter steady-state levels of its product allantoin as well as the multiple precursors of urate, including adenosine and inosine, which are themselves capable of modifying neuronal viability (27, 28). However, we have found that brain concentrations of major purine precursors upstream of urate from adenosine to xanthine are unaltered in UOx KO and Tg mice, arguing strongly against a proximal metabolic alteration as the basis of their phenotypes. Similarly, in UOx KO mice levels of brain allantoin were unaltered, confirming the absence of functional endogenous UOx in WT brain and supporting the hypothesis that the increased brain concentration of urate in UOx KO mice is the basis of their neuroprotective phenotype. By contrast, in UOx Tg mice allantoin was significantly increased in brain, raising the possibility of an alternative explanation for their exacerbated neurotoxicity other than the commensurate reduction in brain urate. However, the possibility that elevated allantoin mediates the UOx Tg phenotype presumes that allantoin can act as a neurotoxicant. However, the only available data of relevance indicate that allantoin actually displays neuroprotective properties in a 6-OHDA model of PD (29). Collectively, these data suggest that alterations in urate, rather the those in allantoin or another purine metabolite, are the basis for the observed UOx KO and Tg phenotypes.
Urate is a potent antioxidant, and antioxidant properties of urate have been proposed to mediate its neuroprotective effects in most aforementioned studies (13–21). We investigated oxidative stress status in UOx KO and Tg mice and found higher protein carbonyls, one of the most commonly used markers of oxidative stress, in both. Although an increase in basal levels of oxidative protein modification in UOx Tg animals is consistent with their lower levels of antioxidant urate, the converse hypothesis of lower levels of protein carbonyls in UOx KO mice is not supported. It is uncertain why protein carbonyls changed in the same direction despite urate level modulation in opposite directions. However, we are not the first to observe dissociation between urate and oxidative stress indices, protein carbonyl levels in particular. Clinical studies have revealed higher or unchanged protein carbonyls in patients with high urate, including refractory gout patients (30–32). Furthermore, urate has the capacity to act as a prooxidant under some circumstances (33–35).
In addition to this possible dual role of urate, it is particularly noteworthy that UOx KO mice develop nephropathy early in their lives despite pre- and perinatal allopurinol treatment. The severe kidney damage we and the others have documented (11, 36) in UOx KO mice may have confounded the testing of our hypothesis that elevated urate could confer antioxidant protection under basal conditions. Excessive oxidative stress has been linked to various renal pathologies (37), and urea, specifically, has been shown both in vitro and in vivo to induce reactive oxidative species (38). It is possible that an offsetting systemic effect of chronic nephropathy may predominate in determining the basal levels of oxidative stress in UOx KO mice. Therefore, despite the known oxidative mechanisms of 6-OHDA neurotoxicity (39) and antioxidant properties of urate, the basis for attenuated and exacerbated neurodegeneration in UOx KO and Tg mice, respectively, remains to be established.
Our complementary genetic approaches targeting UOx effectively manipulated urate in mice both peripherally and, perhaps more importantly in this study, in their brains. The findings that UOx KO (with higher urate) are more resistant to local 6-OHDA lesioning and that UOx Tg (with lower urate) are more susceptible to this neurotoxin support the possibility of a neuroprotective role for urate and PD. Together with previous epidemiological and clinical evidence (5, 6), these findings strengthen the rationale for investigating urate-elevating agents as potential therapeutic approaches to PD and possibly other neurodegenerative diseases. As proof-of-concept, our genetic study together with newly published pharmacological data (40) provides critical experimental evidence in vivo that urate may have beneficial CNS actions in the context of PD, and it provides a basis and justification for further mechanistic investigation. Nevertheless, further efforts to investigate the therapeutic potential of urate elevation—even within what is considered a “normal range”—must be tempered by known and potential risks of excessive urate.
Methods
Experimental Animals.
UOx KO mice, originally constructed by Wu et al. (11) by homologous recombination disrupting exon 3 of the UOx gene, were obtained from the Jackson Laboratory. Our initial characterization indicated that whereas homozygous mice demonstrated significantly elevated urate in both serum and brain (Fig. 1), heterozygous UOx KO animals did not have a urate elevation phenotype (11). We therefore used only homozygous mice (generated by heterozygote × heterozygote crosses) for this study. Allopurinol (150 mg/L) was provided in the drinking water of breeders and pups until weaning for rescue from perinatal lethality of hyperuricemia (11). UOx Tg mice were obtained from Kenneth L. Rock, Department of Immunology, University of Massachusetts, Worcester, MA. UOx transgene expression is driven by a strong constitutive (β-actin) promoter (10). Hemizygous UOx Tg mice were used. Both UOx KO and Tg mice had been back-crossed to C57BL/6 (Jackson Laboratory) for at least eight generations. UOx KO, UOx Tg mice and their littermate controls were maintained in home cages at constant temperature with a 12-h light/dark cycle and free access to food and water. All animal protocols were approved by the Massachusetts General Hospital Animal Care and Use Committee.
Measurement of Urate and Urate Precursors.
Animals were killed at indicated times via cervical dislocation. Whole blood was collected, and striatal tissues were dissected. Samples were prepared, and adenosine, inosine, hypoxanthine, xanthine, and urate concentrations were determined simultaneously using an HPLC method that we developed and recently reported (41).
Measurement of Allantoin.
The urate metabolite allantoin was determined by Bioanalytical Systems. Animals were killed via cervical dislocation. Fresh frozen striatal tissues were weighed and homogenized in water. A volume of 100 μL was taken for extraction. Calibrator, quality control standard, and sample homogenates were extracted with acetonitrile in a 96-well plate after adding isotope-labeled allantoin as internal standard. LC-MS was used for detection.
Western Blot.
For Western blot analysis of UOx, liver, heart, or brain tissues were obtained. Proteins were extracted and electrophoresed. After transferring, the membrane was treated with rabbit anti-UOx antibody (Santa Cruz Biotechnology, catalog no. SC33830) at 1:200, followed by secondary antibody (Thermo Scientific, catalog no. 32460). Densitometric analysis of band intensity was performed by using the Image J system (National Institutes of Health).
Protein Carbonylation.
Protein carbonlys in liver and brain were detected using the Oxyblot Protein Oxidation Detection Kit (Millipore, catalog no. S7150) according to the manufacturer’s instructions. Densitometric analysis of band intensity was performed by using the Image J system.
6-OHDA Lesion.
Mice received a unilateral intrastriatal injection of 6-OHDA (42). Animals were pretreated with desipramine (Sigma-Aldrich). A total dose of 15 μg 6-OHDA was infused into the left striatum at the following coordinates: anterior–posterior (AP), +0.09 cm; medial–lateral (ML), +0.22 cm; dorsal-ventral (DV), −0.25 cm relative to bregma.
Rotational Behavior Assessment.
Spontaneous and 5 mg/kg amphetamine-induced rotational behavior in mice was tested at 3–4 wk after the 6-OHDA lesion using an automated rotometry system (San Diego Instruments) as previously described (42). Results were expressed as ipsilateral net turns (net difference between ipsilateral and contralateral turns) per 60 min.
Measurement of DA and Metabolite.
Five weeks after 6-OHDA lesion, mice were killed by rapid cervical dislocation, and their striata were dissected. DA and metabolites DOPAC and HVA were determined by standard HPLC with electrochemical detection, as previously described (42).
TH Immunohistochemistry and Stereological Cell Counting.
Immunostaining for TH was performed as described previously (43). Five weeks after 6-OHDA lesioning mice were killed by rapid cervical dislocation. The hinder brain block containing midbrain was immediately fixed and cryoprotected. Every fourth section from a complete set of coronal midbrain sections was processed. The primary antibody was mouse monoclonal anti-TH (Sigma-Aldrich, catalog no. T1299) at 1:800. Stereologic analysis was performed with the investigator blinded to genotypes using the Bioquant Image Analysis System (R&M Biometrics) (43). For each animal, the SN on both sides of the brain was analyzed. For each section, the entire SN was identified and outlined as the region of interest. The number of TH-positive neurons in each counting frame (50 μm × 50 μm) was then determined under 40× objective by focusing down through the section using the optical dissector method. Our criterion for counting an individual TH-positive neuron was the presence of its nucleus either within the counting frame or touching the right or top frame lines (green), but not touching the left or bottom lines (red). The total number of TH-positive neurons for each side of the SN was calculated: total number = raw counts × 4 (every fourth section) × 6.25 (area of grid 125 μm × 125 μm/area of counting frame 50 μm × 50 μm).
Statistic Analysis.
All values are expressed as mean ± SEM. The difference between two groups was analyzed by Student t test. Multiple comparisons among groups were performed by one-way ANOVA and Tukey’s post hoc test. All statistical analyses were performed using SigmaStat software (SPSS). P < 0.05 is considered statistically significant.
Acknowledgments
The authors thank Kenneth L. Rock and Hajime Kono for generously providing UOx Tg mice and for advice on their use; and Alberto Serrano-Pozo for his assistance in stereological cell counting. This work is supported by the RJG Foundation, Michael J. Fox Foundation, American Federation for Aging Research Beeson Collaborative Program, and by National Institutes of Health Grants R21NS058324, K24NS060991, and DoD W81XWH-11-1-0150.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Wu XW, Lee CC, Muzny DM, Caskey CT. Urate oxidase: primary structure and evolutionary implications. Proc Natl Acad Sci USA. 1989;86(23):9412–9416. doi: 10.1073/pnas.86.23.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson RJ, Lanaspa MA, Gaucher EA. Uric acid: A danger signal from the RNA world that may have a role in the epidemic of obesity, metabolic syndrome, and cardiorenal disease: Evolutionary considerations. Semin Nephrol. 2011;31(5):394–399. doi: 10.1016/j.semnephrol.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc Natl Acad Sci USA. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Álvarez-Lario B, Macarrón-Vicente J. Uric acid and evolution. Rheumatology (Oxford) 2010;49(11):2010–2015. doi: 10.1093/rheumatology/keq204. [DOI] [PubMed] [Google Scholar]
- 5.Constantinescu R, Zetterberg H. Urate as a marker of development and progression in Parkinson’s disease. Drugs Today (Barc) 2011;47(5):369–380. doi: 10.1358/dot.2011.47.5.1591834. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Wu G, Schwarzschild MA. Urate in Parkinson’s disease: More than a biomarker? Curr Neurol Neurosci Rep. 2012;12(4):367–375. doi: 10.1007/s11910-012-0282-7. [DOI] [PubMed] [Google Scholar]
- 7.Schapira AH, Tolosa E. Molecular and clinical prodrome of Parkinson disease: Implications for treatment. Nat Rev Neurol. 2010;6(6):309–317. doi: 10.1038/nrneurol.2010.52. [DOI] [PubMed] [Google Scholar]
- 8. ClinicalTrials.gov. Safety of Urate Elevation in Parkinson’s Disease (SURE-PD). Available at http://clinicaltrials.gov/ct2/show/NCT00833690. Accessed December 2, 2012.
- 9.Usuda N, Reddy MK, Hashimoto T, Rao MS, Reddy JK. Tissue specificity and species differences in the distribution of urate oxidase in peroxisomes. Lab Invest. 1988;58(1):100–111. [PubMed] [Google Scholar]
- 10.Kono H, Chen CJ, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest. 2010;120(6):1939–1949. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, et al. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc Natl Acad Sci USA. 1994;91(2):742–746. doi: 10.1073/pnas.91.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serra PA, et al. The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induces apoptosis in mouse nigrostriatal glia. Relevance to nigral neuronal death and striatal neurochemical changes. J Biol Chem. 2002;277(37):34451–34461. doi: 10.1074/jbc.M202099200. [DOI] [PubMed] [Google Scholar]
- 13.Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. 1998;53(5):613–625. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Romanos E, Planas AM, Amaro S, Chamorro A. Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab. 2007;27(1):14–20. doi: 10.1038/sj.jcbfm.9600312. [DOI] [PubMed] [Google Scholar]
- 15.Koprowski H, Spitsin SV, Hooper DC. Prospects for the treatment of multiple sclerosis by raising serum levels of uric acid, a scavenger of peroxynitrite. Ann Neurol. 2001;49(1):139. doi: 10.1002/1531-8249(200101)49:1<139::aid-ana28>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 16.Du Y, Chen CP, Tseng CY, Eisenberg Y, Firestein BL. Astroglia-mediated effects of uric acid to protect spinal cord neurons from glutamate toxicity. Glia. 2007;55(5):463–472. doi: 10.1002/glia.20472. [DOI] [PubMed] [Google Scholar]
- 17.Scott GS, Cuzzocrea S, Genovese T, Koprowski H, Hooper DC. Uric acid protects against secondary damage after spinal cord injury. Proc Natl Acad Sci USA. 2005;102(9):3483–3488. doi: 10.1073/pnas.0500307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu TG, et al. Protective effects of urate against 6-OHDA-induced cell injury in PC12 cells through antioxidant action. Neurosci Lett. 2012;506(2):175–179. doi: 10.1016/j.neulet.2011.10.075. [DOI] [PubMed] [Google Scholar]
- 19.Jones DC, Gunasekar PG, Borowitz JL, Isom GE. Dopamine induced apoptosis is mediated by oxidative stess and is enhanced by cyanide in differentiated PC12 cells. J Neurochem. 2009;74:2296–2304. doi: 10.1046/j.1471-4159.2000.0742296.x. [DOI] [PubMed] [Google Scholar]
- 20.Guerreiro S, et al. Protection of midbrain dopaminergic neurons by the end-product of purine metabolism uric acid: Potentiation by low-level depolarization. J Neurochem. 2009;109(4):1118–1128. doi: 10.1111/j.1471-4159.2009.06040.x. [DOI] [PubMed] [Google Scholar]
- 21.Duan W, et al. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J Neurochem. 2002;80(1):101–110. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 22.Irizarry MC, et al. Plasma urate and progression of mild cognitive impairment. Neurodegener Dis. 2009;6(1-2):23–28. doi: 10.1159/000170883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Auinger P, Kieburtz K, McDermott MP. The relationship between uric acid levels and Huntington’s disease progression. Mov Disord. 2010;25(2):224–228. doi: 10.1002/mds.22907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Church WH, Ward VL. Uric acid is reduced in the substantia nigra in Parkinson’s disease: effect on dopamine oxidation. Brain Res Bull. 1994;33(4):419–425. doi: 10.1016/0361-9230(94)90285-2. [DOI] [PubMed] [Google Scholar]
- 25.Bogdanov M, et al. Metabolomic profiling to develop blood biomarkers for Parkinson’s disease. Brain. 2008;131(Pt 2):389–396. doi: 10.1093/brain/awm304. [DOI] [PubMed] [Google Scholar]
- 26.Cipriani S, et al. Urate and its transgenic depletion modulate neuronal vulnerability in a cellular model of Parkinson’s disease. PLoS ONE. 2012;7(5):e37331. doi: 10.1371/journal.pone.0037331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29(11):647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7(7):575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 29. Terpstra B. Purine nucleoside mediated neuroprotection in the 6-hydroxydopamine rodent model of Parkinson’s disease. Available at http://etd.ohiolink.edu/view.cgi?acc_num=ucin1298395215. Accessed December 2, 2012.
- 30.Hershfield MS, et al. Treating gout with pegloticase, a PEGylated urate oxidase, provides insight into the importance of uric acid as an antioxidant in vivo. Proc Natl Acad Sci USA. 2010;107(32):14351–14356. doi: 10.1073/pnas.1001072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinha S, Singh SN, Ray US. Total antioxidant status at high altitude in lowlanders and native highlanders: Role of uric acid. High Alt Med Biol. 2009;10(3):269–274. doi: 10.1089/ham.2008.1082. [DOI] [PubMed] [Google Scholar]
- 32.Tsukimori K, Yoshitomi T, Morokuma S, Fukushima K, Wake N. Serum uric acid levels correlate with plasma hydrogen peroxide and protein carbonyl levels in preeclampsia. Am J Hypertens. 2008;21(12):1343–1346. doi: 10.1038/ajh.2008.289. [DOI] [PubMed] [Google Scholar]
- 33.Bagnati M, et al. When and why a water-soluble antioxidant becomes pro-oxidant during copper-induced low-density lipoprotein oxidation: A study using uric acid. Biochem J. 1999;340(Pt 1):143–152. [PMC free article] [PubMed] [Google Scholar]
- 34.Patterson RA, Horsley ET, Leake DS. Prooxidant and antioxidant properties of human serum ultrafiltrates toward LDL: Important role of uric acid. J Lipid Res. 2003;44(3):512–521. doi: 10.1194/jlr.M200407-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Santos CX, Anjos EI, Augusto O. Uric acid oxidation by peroxynitrite: Multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophys. 1999;372(2):285–294. doi: 10.1006/abbi.1999.1491. [DOI] [PubMed] [Google Scholar]
- 36.Kelly SJ, et al. Diabetes insipidus in uricase-deficient mice: A model for evaluating therapy with poly(ethylene glycol)-modified uricase. J Am Soc Nephrol. 2001;12(5):1001–1009. doi: 10.1681/ASN.V1251001. [DOI] [PubMed] [Google Scholar]
- 37.Kao MP, Ang DS, Pall A, Struthers AD. Oxidative stress in renal dysfunction: Mechanisms, clinical sequelae and therapeutic options. J Hum Hypertens. 2010;24(1):1–8. doi: 10.1038/jhh.2009.70. [DOI] [PubMed] [Google Scholar]
- 38.D’Apolito M, et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J Clin Invest. 2010;120(1):203–213. doi: 10.1172/JCI37672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson-Lewis V, Blesa J, Przedborski S. Animal models of Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S183–S185. doi: 10.1016/S1353-8020(11)70057-8. [DOI] [PubMed] [Google Scholar]
- 40.Gong L, et al. Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson’s disease: Linking to Akt/GSK3β signaling pathway. J Neurochem. 2012;123(5):876–885. doi: 10.1111/jnc.12038. [DOI] [PubMed] [Google Scholar]
- 41.Burdett TC, et al. Efficient determination of purine metabolites in brain tissue and serum by high-performance liquid chromatography with electrochemical and UV detection. Biomed Chromatogr. 2012 doi: 10.1002/bmc.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao D, et al. Forebrain adenosine A2A receptors contribute to L-3,4-dihydroxyphenylalanine-induced dyskinesia in hemiparkinsonian mice. J Neurosci. 2006;26(52):13548–13555. doi: 10.1523/JNEUROSCI.3554-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kachroo A, et al. Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and parkinsonian mice. J Neurosci. 2005;25(45):10414–10419. doi: 10.1523/JNEUROSCI.3660-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]