Abstract
The pH (low) insertion peptide (pHLIP) family enables targeting of cells in tissues with low extracellular pH. Here, we show that ischemic myocardium is targeted, potentially opening a new route to diagnosis and therapy. The experiments were performed using two murine ischemia models: regional ischemia induced by coronary artery occlusion and global low-flow ischemia in isolated hearts. In both models, pH-sensitive pHLIPs [wild type (WT) and Var7] or WT-pHLIP–coated liposomes bind ischemic but not normal regions of myocardium, whereas pH-insensitive, kVar7, and liposomes coated with PEG showed no preference. pHLIP did not influence either the mechanical or the electrical activity of ischemic myocardium. In contrast to other known targeting strategies, the pHLIP-based binding does not require severe myocardial damage. Thus, pHLIP could be used for delivery of pharmaceutical agents or imaging probes to the myocardial regions undergoing brief restrictions of blood supply that do not induce irreversible changes in myocytes.
Keywords: acidity, cardiac, fluorescence imaging, targeted delivery
The pH (low) insertion peptide (pHLIP) enables targeting of cells in tissues with low extracellular pH (1). In this publication, we show that ischemic myocardial tissues are targeted, potentially opening new avenues for diagnosis and therapy. The pHLIP family consists of water-soluble polypeptides originally derived from the bacteriorhodopsin C helix, which is triggered by low pH to insert and fold across a membrane to form a stable transmembrane α helix as a consequence of titrating two carboxyl groups in the transmembrane (TM) helix to render them uncharged (2). In solution, at pH values above 7.0–7.2, a typical pHLIP partitions as a mainly unstructured peptide to the surface of a lipid bilayer, inserting when the pH drops from neutral or high to slightly acidic (7.0–6.5 and less). The insertion process is unidirectional and rapid (<2 min in lipid vesicles) (3–5). Variations of the pHLIP sequence can be used to change the time required for insertion across and exit from the membrane, ranging from milliseconds to minutes (6). The apparent pK of insertion across a liposomal bilayer membrane is 6.0 for the original WT-pHLIP sequence (2). pHLIP has been shown to locate imaging agents in tumors and arthritic inflammation (7–11), and to accomplish pH-selective cytoplasmic delivery of peptide nucleic acids, fluorescent dyes, and anticancer agents (3, 12, 13). Low pH is associated with a number of important pathological states including myocardial ischemia. According to the American Heart Association (14), ∼9 million Americans are estimated to experience angina pectoris, and a similar incidence of angina has been estimated for most European countries (15). It has been demonstrated that extracellular pH is lowered in ischemic heart models (16, 17) and clinically in patients during pacing-induced ischemia in coronary artery disease (18). To follow targeting by pHLIP, fluorescent dyes and fluorescently labeled liposomes coated with pHLIP were used as imaging agents. Experiments were carried out using two murine models of myocardial ischemia: regional ischemia induced by coronary artery occlusion in situ and low-flow ischemia in isolated perfused hearts. In this paper, we demonstrate that either pHLIP peptides or pHLIP-coated liposomes selectively accumulate in ischemic myocardium.
Results
Local Myocardial Ischemia in Situ.
We used three forms of pHLIP to study whether pHLIP peptides accumulate selectively in ischemic myocardium in the area at risk: WT, a slow inserting form; Var7, a fast inserting form with E substitutions for the two D positions in the TM helix; and kVar7, a noninserting form with K substitutions for the TM D positions. pHLIP variants were injected 3 min before arterial occlusion, and no acute changes of electrocardiogram (ECG) parameters were seen from the injection (SI Data, Table S1). Subsequent occlusion of the left coronary artery induced tissue discoloration and an elevation of the S-T segment of the ECG that is typical of myocardial ischemia within several seconds (Fig. 1A). The size of the area at risk expressed as a percentage of left ventricular volume did not differ among the experimental groups: 46 ± 4%, 45 ± 7%, 40 ± 6%, and 43 ± 4% (number of samples; n = 5, 5, 5, and 4 for saline, kVar7, WT, and Var7, respectively).
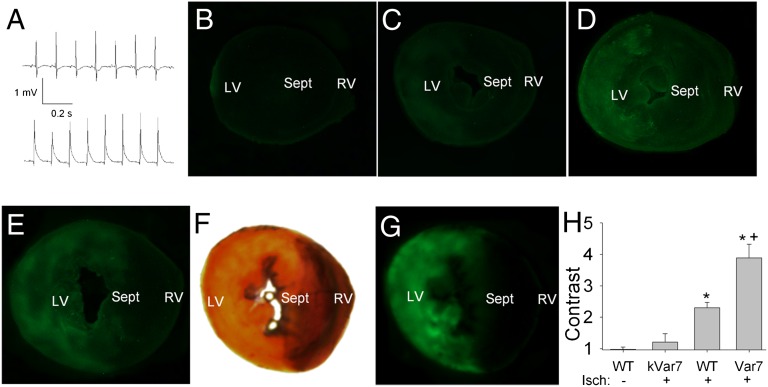
Fig. 1.
Experiments with local myocardial ischemia in situ. (A) Example of lead II ECG recordings at baseline (Upper) and at 30 s of occlusion (Lower) showing typical elevation of ST segment induced by left coronary artery occlusion. (B–E) Typical fluorescence images of sections from the hearts of four mice subjected to different experimental interventions: (B) sham procedure (instead of ischemia induction) with injection of pH-sensitive WT-pHLIP; (C) ischemia, pH-insensitive kVar7; (D) ischemia, pH-sensitive WT; and (E) ischemia, pH-sensitive Var7. LV, left ventricle; RV, right ventricle; Sept, septum. (F and G) Area at risk delineated with Evans Blue (light yellow in white-light image F) coinciding with area of bright fluorescence (fluorescence image G) in a section of the heart from a mouse injected with Var7. (H) Contrast of fluorescence in the area at risk vs. intact myocardium. Mean ± SEM values are shown; n = 5, 5, 5, 4 for no ischemia, kVar7, WT, and Var7, respectively. *P < 0.05 vs. no ischemia or kVar7; +P < 0.05 vs. WT.
The main purpose of the experiments with local ischemia was to test whether pHLIP accumulates selectively in ischemic myocardium in the area at risk. This was done by comparing the mean fluorescence intensity of Alexa 488-pHLIPs in the area at risk with the normal myocardium. The contrast of fluorescence was higher in the hearts from the mice injected with pH-sensitive WT and Var7, compared with the pH-insensitive kVar7, and was maximal in the group injected with Var7 (Fig. 1 B–D and H). We also compared the mean intensity of fluorescence of Alexa 488-Var7 in the areas at risk in the mice subjected to ischemia and in the sham-operated mice not subjected to ischemia. The mean intensity of fluorescence was significantly higher in the hearts subjected to local ischemia than in the sham group with no ischemia [54 ± 7 vs. 13 ± 4 arbitrary units (A.U.); n = 4 and 5; P < 0.05]. Anatomically, the region of relatively bright fluorescence in the presence of pH-sensitive pHLIPs coincides with the area at risk (Fig. 1 F and G). These findings suggest that pH-sensitive WT and Var7 selectively target ischemic myocardium, but not the pH-insensitive kVar7 pHLIP.
Low-Flow Myocardial Ischemia in Isolated Hearts.
In another approach, we evaluated the ability of the pH peptides to selectively target the ischemic myocardium using a model of low-flow global ischemia in isolated hearts, where better control of coronary flow can be achieved. As shown by the increased mean intensity of fluorescence, the Alexa 488-Var7 was present in significantly higher amounts in the ventricles of the hearts subjected to low-flow global ischemia than in the normally perfused heart controls (Fig. 2 A, C, and D). These results are consistent with the observation that Var7 is selectively bound to ischemic myocardium in situ.
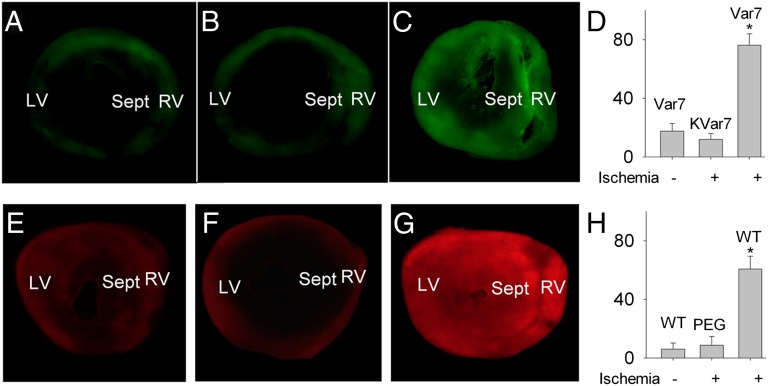
Fig. 2.
Experiments with low-flow ischemia in isolated mouse hearts. (A–C) Alexa 488 fluorescence images of sections from three isolated mouse hearts: (A) not subjected to low-flow ischemia and perfused with pH-sensitive Var7; (B) subjected to low-flow ischemia and perfused with pH-insensitive kVar7; and (C) subjected to low-flow ischemia and perfused with pH-sensitive Var7. (D) Summary data for mean intensity of fluorescence (in arbitrary units). Mean ± SEM values are shown; n = 5 for all groups. *P < 0.05 vs. Var7 minus ischemia and kVar7 plus ischemia. (E–G) Rhodamine fluorescence images of sections from three isolated mouse hearts: (A) not subjected to low-flow ischemia and perfused with liposomes coated with pH-sensitive WT; (B) subjected to low-flow ischemia and perfused with liposomes coated with PEG; and (C) subjected to low-flow ischemia and perfused with liposomes coated with pH-sensitive WT. (G) Summary data for mean intensity of fluorescence. Mean ± SEM values are shown; n = 5, 4, and 5 for WT minus ischemia, PEG plus ischemia, and WT plus ischemia, respectively. *P < 0.05 vs. WT minus ischemia or PEG plus ischemia.
To test whether pH sensitivity is important for pHLIP binding to ischemic myocardium, we used the pH-insensitive kVar7 and found that the mean intensity of fluorescence for pH-insensitive Alexa 488-kVar7 was significantly less than for the pH-sensitive Alexa 488-Var7. In the normally perfused hearts, however, binding of labeled kVar7 does not differ from Alexa 488-Var7 (Fig. 2 B and D), supporting the idea that pH sensitivity is the key for selective binding of pHLIP to ischemic myocardium.
Using the model of low-flow myocardial ischemia in isolated hearts, we also tested the ability of WT-pHLIP–coated liposomes to target ischemic myocardium. Significantly higher values of the mean intensity of fluorescence reveal more WT-pHLIP liposomes in the ventricular myocardium of the hearts subjected to ischemia than in the normally perfused hearts (Fig. 2 E, G, and H). Control liposomes coated with carboxyl-terminated PEG (designed to have the same density of negative charges on the liposome surface as in the case of the pHLIP coating) did not accumulate in ischemic myocardium (Fig. 2 F and H), showing that the presence of pHLIP is the agent for targeting.
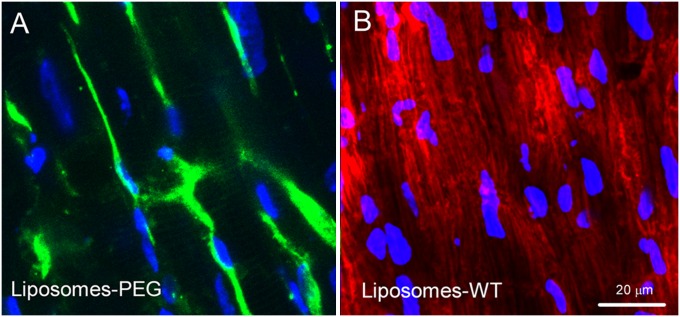
Histological examination revealed that the pattern of staining with Alexa 488-WT-pHLIP and WT-pHLIP–coated liposomes is different (Fig. 3). Alexa 488-WT-pHLIP stained plasma membranes (Fig. 3A), as had been shown previously in tumor models in vivo (9), whereas rhodamine-labeled lipids from the targeted liposomes appear to have reached intracellular organelles like mitochondria (Fig. 3B).
Fig. 3.
Confocal images showing details of staining of two isolated hearts subjected to low-flow ischemia and perfused either with Alexa 488-WT-pHLIP (green; A) or with liposomes containing Rho-lipids and coated with WT-pHLIP (red; B). DAPI-stained nuclei are blue.
Isolated Left Atrial Appendages.
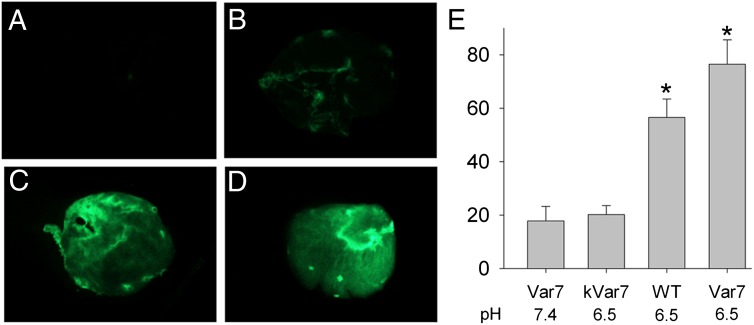
We began experiments with isolated atrial appendages by examining the effect of pH on pHLIP binding to the myocardial tissues. With the pH-sensitive Alexa 488-Var7, accumulation of pHLIP (assessed by the mean intensity of fluorescence) was significantly higher when the pH was 6.5 compared with 7.4 (Fig. 4 A, D, and E). An equally bright fluorescence at low pH 6.5 was found with the pH-sensitive Alexa 488-WT (Fig. 4 C and E) but not with the pH-insensitive Alexa 488-kVar7 (Fig. 4 B and E). Thus, pH-sensitive pHLIP variants accumulate in the atrial myocardium to a greater extent at lower pH than at higher pH.
Fig. 4.
(A–D) Fluorescence images of four mouse left atria exposed to (A) Var7 at the normal pH, (B) kVar7 at pH 6.5, (C) WT at pH 6.5, and (D) Var7 at pH 6.5. (E) Summary data for mean intensity of fluorescence (in arbitrary units). Mean ± SEM values are shown; n = 5 for all groups. *P < 0.05 vs. Var7 at pH 7.4 or kVar7 at pH 6.5.
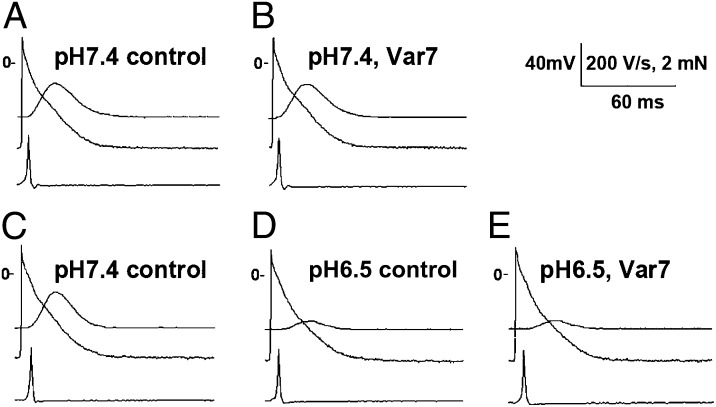
The main goal of this series of experiments was to assess the effects of the pH-sensitive Alexa 488-Var7 pHLIP on the electrical and mechanical activity of paced left atrial appendages in the normal and low pH environment. At pH 7.4, Var7 had no effect either on the force developed or on parameters of transmembrane potential (Fig. 5 A and B; Table S2). The decrease in pH by itself caused a significant reduction of the force developed without any significant change in the parameters of transmembrane potential (Fig. 5 C and D; Table S2). Administration of Var7 at low pH had no additional effect on the force developed or on the transmembrane potential (Fig. 5 D and E; SI Data, Table S2). Thus, exposure to the pH-sensitive Var7-pHLIP did not significantly influence the mechanical or electrical activity in preparations of left atrial appendages of mice.
Fig. 5.
Typical recordings showing the absence of Var7 effects on transmembrane potential and contractile activity in the left atrium at the normal (A and B) and low (C–E) pH. (A and B) Recordings of developed force (top trace), transmembrane potential (middle trace), and maximum upstroke velocity (peak of the first derivative of transmembrane potential shown in the bottom trace) in predrug control (A) and after 30 min in the presence of 10 μM Var7 (B) obtained from a preparation of the left atrium at pH 7.4. (C–E) Recordings at the normal pH (C), after 20 min at pH 6.5 (D), and after 30 min in the presence of 10 μM Var7 at pH 6.5 (B) obtained from another preparation of the left atrium. Notice a marked reduction in the developed force induced by introduction of low pH.
Discussion
During the past few years, several techniques for passive and active imaging and drug delivery in diseased cardiac tissue have been developed (19, 20), including various types of nanoparticles for drug delivery such as liposomes, drug–polymer conjugates, polymeric micelles, dendrimers, nanoshells, and nucleic acid-based carriers (21). Passive drug delivery is based on the fact that the endothelial lining of the blood vessel wall becomes more permeable in infarcted areas so that large molecules and even relatively large nanoparticles (micelles and liposomes) can leave the vascular bed and accumulate inside the infarcted region (22–24). For active targeting, monoclonal antibodies against characteristic components of ischemic myocardium are the most frequently used vector molecules. Cellular components that are normally localized intracellularly but exposed extracellularly due to membrane disruptions in necrotic myocytes (e.g., intracellular cardiac myosin, phosphatidylserine, cytoskeleton) are used as biomarkers (19, 24–27). P-selectin expression, which reaches a peak of up-regulation at 4 h after myocardial infarction, has also been used as a biomarker (28). Analysis of the existing methods for selective drug delivery in diseased cardiac tissue shows that these techniques have a general distinctive feature: they need severe myocardial ischemia or infarction (leaky vasculature for passive delivery or cell apoptosis for active targeting) and are therefore useful only in tissue repair or regenerative strategies.
In contrast, we find in this study that pH-sensitive WT and Var7-pHLIPs and WT-pHLIP–coated liposomes selectively target ischemic myocardium in tissues that are less damaged than is required for the targeting strategies previously used. The results were obtained in two murine ischemia models: regional ischemia induced by coronary artery occlusion and global low-flow ischemia in isolated hearts. Also, we find that the controls, pH-insensitive kVar7 and liposomes coated with PEG, do not target the ischemic myocardium in our models. The selective binding of pH-sensitive pHLIPs in ischemic myocardium is consistent with the original concept of targeting of acidic tumors (5, 8, 9, 29), while it is not a trivial consequence thereof. Importantly, accumulation of pHLIP in ischemic myocardium did not influence its mechanical or electrical activity. This result is in accordance with our previous data showing that the interaction of pHLIP with cellular membranes at both neutral and low pH does not lead to membrane leakage (4, 30) or cellular toxicity (7) over a range of peptide concentrations. A distinctive feature of pHLIP-based targeting of ischemic myocardium is that pHLIP binding does not require severe myocardial damage and should work in the myocardial region undergoing brief restrictions of blood supply that do not induce irreversible changes in cardiomyocytes. Such a brief restriction of blood supply is typical for angina pectoris. The major problem with medical treatment for angina is that all antianginal drugs have cardiac and noncardiac side effects that significantly restrict their utilization (15). Most of the adverse effects result from the fact that the antianginal drugs affect tissues and organs throughout the body. The results of the present study suggest that pHLIP might be used as a carrier for delivering pharmaceutical agents specifically into ischemic regions during anginal attacks. Although there are no direct measurements of intracellular or extracellular pH in ischemic areas during angina attacks in humans, there is evidence that pH drops to values low enough to allow pHLIP binding to ischemic myocardium: a range of pH values can be estimated indirectly using the fact that angina episodes are sensed as a chest pain, which results from the activation of cardiac sensory neurons by acidosis (31). It has been shown that chest pain caused by myocardial ischemia is mediated by cardiac sympathetic (C-fiber) afferents (32). Specifically, the acid-sensing ion channel 3 (ASIC3), which is expressed at extremely high levels in sensory neurons innervating the heart, functions as a pH sensor (33, 34). The activation threshold for the channel is about 7.0 and the half-activation value is between 6.7 and 6.6 (33–36). Thus, sensing chest pain during angina episode implies pH values within the pHLIP insertion range, which allows us to hypothesize that pHLIP can effectively bind and accumulate in the region of restricted blood flow. Because a decrease in pH occurs very rapidly (within a few minutes) after the onset of ischemia, we suggest that pHLIP might quickly deliver antianginal agents to ischemic regions, decrease the oxygen supply/demand ratio and therefore cell damage, and manage the anginal attack avoiding the multiple side effects of antianginal drugs. Because pHLIP can deliver polar cell-impermeable molecules intracellularly, a number of new therapeutic compounds might be formulated. Moreover, pHLIP-coated liposomes might be used to deliver a mixture of pharmaceuticals to the risk zone. Thus, we assume that pHLIP technology might have a significant impact on the treatment of chronic angina.
pHLIP could deliver therapeutic agents, imaging agents, or both at the same time, acting as a theranostic. The most straightforward cardiac application of pHLIP could be for imaging ischemic regions in myocardium, and might be especially important for the diagnosis of myocardial ischemia in pediatric patients. The problem with current tests is that the ST-segment elevation in ECG used as a sign of myocardial ischemia in adults is often misleading in the young (37), whereas radiography may reveal significant anatomical abnormalities conducive to myocardial ischemia but not myocardial ischemia per se. Obviously, it would be advantageous to have an agent visualizing ischemic myocardium at the stage of basic X-ray examination of children. To diagnose myocardial ischemia in children, exercise or pharmacologic cardiac stress is induced. We expect that pHLIP will target the acidic myocardium during the acute ischemia associated with cardiac stress and deliver contrast agents to any ischemic region, opening new opportunities in use of existing imaging modalities for the diagnosis of myocardial ischemia in children.
In conclusion, the results of our study demonstrate that pHLIPs or pHLIP-coated liposomes target ischemic but not normal regions of myocardium. pHLIP accumulation does not influence either the mechanical or the electrical activity of ischemic myocardium. Importantly, pHLIP recognizes ischemic tissue due to a drop in extracellular pH. Therefore, pHLIP can deliver pharmaceutical agents or imaging probes to the myocardial region undergoing brief restrictions of blood supply, which do not induce irreversible changes in myocytes. This is an important advantage that distinguishes pHLIP-based technologies from other existing methods of ischemic myocardium targeting.
Materials and Methods
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (38) and the rules of the Columbia University Institutional Animal Care and Use Committee. The experimental techniques were generally similar to those described by us previously (39, 40).
Constructs Used in Study.
The goal of our study was to evaluate ability of fluorescently labeled pHLIPs and pHLIP-coated liposomes to target ischemic myocardium. Several pHLIP variants were used in the study as follows: WT, ACEQNPIY WARYADWLFTTPLLLLDLALLV DADEGT; Var7, ACEEQNP WARYLEWLFPTETLLLEL; and kVar7, ACEEQNP WARYLKWLFPTKTLLLKL (underlining indicates transmembrane domain in peptides).
WT is a well-characterized pHLIP peptide that targets acidic tissue by virtue of the titration and neutralization of the carboxyl groups in the TM region. Var7 is a recently introduced truncated version of WT, which has a higher pK of insertion (substituting Glu for Asp in the TM) and much faster blood clearance. It was designed for PET/single-photon emission computed tomography imaging. kVar7 is a pH-insensitive peptide that does not target acidic tissue, because the protonatable Glu residues are replaced by positively charged Lys residues. Each peptide was conjugated with the Alexa 488 fluorescent dye. Additionally, we tested WT-pHLIP–coated and PEG-coated liposomes containing fluorescent rhodamine-PE lipids. The following liposome formulations (mol %) were investigated for targeting of ischemic myocardium: WT-pHLIP–coated liposomes: 90% DOPC, 5% Rho-PE, 5% pHLIP-PE; and PEG-coated liposomes: 90% DOPC, 5% Rho-PE, 5% DSPE-PEG2000-COOH.
The experiments with pHLIP peptides and liposomes were carried out using two murine ischemia models: regional ischemia induced by coronary artery occlusion and global low-flow ischemia in isolated hearts.
Preparation of Constructs.
pHLIP peptides were prepared by large-scale solid-phase peptide synthesis using 9-fluorenylmethyloxycarbonyl (Fmoc) chemistry at the W. M. Keck Foundation Biotechnology Resource Laboratory at Yale by James Elliott (Yale University, New Haven, CT). The detailed description of peptide conjugations with fluorescent dyes and lipids can be found in SI Data. Liposomes of two different compositions coated with pHLIP (5 mol % pHLIP-PE, 5% Rho PE, 90% DOPC) and control liposomes coated with PEG (5% DSPE-PEG2000-COOH, 5% Rho-PE, 90% DOPC) were prepared by extrusion; the details are presented in SI Data.
Local Myocardial Ischemia in Situ.
Male C57B mice weighing 23–32 g were anesthetized with sodium pentobarbital (50–70 mg/kg, i.p.; added per need in the course of experiment) and artificially ventilated at a rate of 120 pulse/min with a tidal volume of 0.5 mL (Harvard Apparatus Respirator; model 707). Mice received heparin (1,000 mg/kg, i.p.) and a snare around the left coronary artery was periodically tightened (for 10 min) and reperfused (for 5 s) to imitate an obstructed coronary flow. Tested pHLIP variants (80 μM in 100 μL of PBS) were injected into the femoral vein 3 min before the first occlusion. At the end of the protocol, the heart was excised with the left coronary artery still occluded, the aorta was cannulated, and 1% Evans Blue was injected into the aorta to delineate the area at risk. Frozen ventricles were sectioned and fluorescent or white light images were obtained with Nikon SMZ1000 UV dissection microscope. The contrast of fluorescence was measured by comparison of the mean intensities of fluorescence in the area at risk and in the intact myocardium.
Low-Flow Myocardial Ischemia in Isolated Hearts.
Mouse hearts were excised and placed into a cold (4 °C) physiologic solution of the following composition (in mmol/L): 150 NaCl, 4 KCl, 1.8 CaCl2, 1.0 MgCl2, 5.5 dextrose, and 10 Hepes equilibrated with 100% oxygen (pH 7.4) and promptly mounted for retrograde Langendorff perfusion with the same solution at 37 °C and constant flow rate of 4 mL/min while being continuously paced at a cycle length of 200 ms. Global low-flow ischemia was induced by reducing perfusion rate to 0.2 mL/min during 15 min. pHLIP or liposomes coated with pHLIP in concentration of 1 μM were administered during perfusion at a slow rate. SI Data contain more details of the experimental procedures.
Isolated Preparations of Left Atrial Appendages.
Preparations of left atrial appendages were mounted epicardial surface up in a chamber perfused with the same physiologic solution as used for experiments with isolated Langendorff perfused heats. Preparations were paced at a cycle length of 250 ms; tension transducer and conventional microelectrode techniques were used to register isometric developed force and transmembrane potential. Effects of 30-min exposure to 10 μM pHLIP were studied at normal and low pH (7.4 and 6.5, respectively). Further details of experimental techniques can be found in SI Data.
Statistical Analysis.
The data are presented as mean ± SEM. One- or two-way analysis of the variance for repeated measures were used for statistical analysis, and Bonferroni test was used for post hoc comparisons when appropriate.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants CA133890 and GM073857 (to O.A.A., D.M.E., and Y.K.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220038110/-/DCSupplemental.
References
- 1.Andreev OA, Engelman DM, Reshetnyak YK. pH-sensitive membrane peptides (pHLIPs) as a novel class of delivery agents. Mol Membr Biol. 2010;27(7):341–352. doi: 10.3109/09687688.2010.509285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt JF, Rath P, Rothschild KJ, Engelman DM. Spontaneous, pH-dependent membrane insertion of a transbilayer alpha-helix. Biochemistry. 1997;36(49):15177–15192. doi: 10.1021/bi970147b. [DOI] [PubMed] [Google Scholar]
- 3.Reshetnyak YK, Andreev OA, Lehnert U, Engelman DM. Translocation of molecules into cells by pH-dependent insertion of a transmembrane helix. Proc Natl Acad Sci USA. 2006;103(17):6460–6465. doi: 10.1073/pnas.0601463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reshetnyak YK, Segala M, Andreev OA, Engelman DM. A monomeric membrane peptide that lives in three worlds: In solution, attached to, and inserted across lipid bilayers. Biophys J. 2007;93(7):2363–2372. doi: 10.1529/biophysj.107.109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreev OA, Reshetnyak YK. Mechanism of formation of actomyosin interface. J Mol Biol. 2007;365(3):551–554. doi: 10.1016/j.jmb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Karabadzhak AG, et al. Modulation of the pHLIP transmembrane helix insertion pathway. Biophys J. 2012;102(8):1846–1855. doi: 10.1016/j.bpj.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreev OA, et al. Mechanism and uses of a membrane peptide that targets tumors and other acidic tissues in vivo. Proc Natl Acad Sci USA. 2007;104(19):7893–7898. doi: 10.1073/pnas.0702439104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vāvere AL, et al. A novel technology for the imaging of acidic prostate tumors by positron emission tomography. Cancer Res. 2009;69(10):4510–4516. doi: 10.1158/0008-5472.CAN-08-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reshetnyak YK, et al. Measuring tumor aggressiveness and targeting metastatic lesions with fluorescent pHLIP. Mol Imaging Biol. 2011;13(6):1146–1156. doi: 10.1007/s11307-010-0457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daumar P, et al. Efficient (18)F-labeling of large 37-amino-acid pHLIP peptide analogues and their biological evaluation. Bioconjug Chem. 2012;23(8):1557–1566. doi: 10.1021/bc3000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macholl S, et al. In vivo pH imaging with (99m)Tc-pHLIP. Mol Imaging Biol. 2012;14(6):725–734. doi: 10.1007/s11307-012-0549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.An M, Wijesinghe D, Andreev OA, Reshetnyak YK, Engelman DM. pH-(low)-insertion-peptide (pHLIP) translocation of membrane impermeable phalloidin toxin inhibits cancer cell proliferation. Proc Natl Acad Sci USA. 2010;107(47):20246–20250. doi: 10.1073/pnas.1014403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wijesinghe D, Engelman DM, Andreev OA, Reshetnyak YK. Tuning a polar molecule for selective cytoplasmic delivery by a pH (low) insertion peptide. Biochemistry. 2011;50(47):10215–10222. doi: 10.1021/bi2009773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roger et al. (2011) Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 123(4):e18–e209. [DOI] [PMC free article] [PubMed]
- 15.Fox K, et al. Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology ESC Committee for Practice Guidelines (CPG) Guidelines on the management of stable angina pectoris: Executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27(11):1341–1381. doi: 10.1093/eurheartj/ehl001. [DOI] [PubMed] [Google Scholar]
- 16.Cobbe SM, Poole-Wilson PA. The time of onset and severity of acidosis in myocardial ischaemia. J Mol Cell Cardiol. 1980;12(8):745–760. doi: 10.1016/0022-2828(80)90077-2. [DOI] [PubMed] [Google Scholar]
- 17.Hirche H, et al. Myocardial extracellular K+ and H+ increase and noradrenaline release as possible cause of early arrhythmias following acute coronary artery occlusion in pigs. J Mol Cell Cardiol. 1980;12(6):579–593. doi: 10.1016/0022-2828(80)90016-4. [DOI] [PubMed] [Google Scholar]
- 18.Cobbe SM, Poole-Wilson PA. Continuous coronary sinus and arterial pH monitoring during pacing-induced ischaemia in coronary artery disease. Br Heart J. 1982;47(4):369–374. doi: 10.1136/hrt.47.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galagudza MM, et al. Targeted drug delivery into reversibly injured myocardium with silica nanoparticles: Surface functionalization, natural biodistribution, and acute toxicity. Int J Nanomedicine. 2010;5:231–237. doi: 10.2147/ijn.s8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott RC, Crabbe D, Krynska B, Ansari R, Kiani MF. Aiming for the heart: Targeted delivery of drugs to diseased cardiac tissue. Expert Opin Drug Deliv. 2008;5(4):459–470. doi: 10.1517/17425247.5.4.459. [DOI] [PubMed] [Google Scholar]
- 21.Wang AZ, et al. Biofunctionalized targeted nanoparticles for therapeutic applications. Expert Opin Biol Ther. 2008;8(8):1063–1070. doi: 10.1517/14712598.8.8.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caride VJ, Zaret BL. Liposome accumulation in regions of experimental myocardial infarction. Science. 1977;198(4318):735–738. doi: 10.1126/science.910155. [DOI] [PubMed] [Google Scholar]
- 23.Palmer TN, Caride VJ, Caldecourt MA, Twickler J, Abdullah V. The mechanism of liposome accumulation in infarction. Biochim Biophys Acta. 1984;797(3):363–368. doi: 10.1016/0304-4165(84)90258-7. [DOI] [PubMed] [Google Scholar]
- 24.Torchilin VP, et al. Targeted accumulation of polyethylene glycol-coated immunoliposomes in infarcted rabbit myocardium. FASEB J. 1992;6(9):2716–2719. doi: 10.1096/fasebj.6.9.1612296. [DOI] [PubMed] [Google Scholar]
- 25.Vance JE, Steenbergen R. Metabolism and functions of phosphatidylserine. Prog Lipid Res. 2005;44(4):207–234. doi: 10.1016/j.plipres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Khaw BA, DaSilva J, Hartner WC. Cytoskeletal-antigen specific immunoliposome-targeted in vivo preservation of myocardial viability. J Control Release. 2007;120(1–2):35–40. doi: 10.1016/j.jconrel.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Khaw BA, Torchilin VP, Vural I, Narula J. Plug and seal: Prevention of hypoxic cardiocyte death by sealing membrane lesions with antimyosin-liposomes. Nat Med. 1995;1(11):1195–1198. doi: 10.1038/nm1195-1195. [DOI] [PubMed] [Google Scholar]
- 28.Scott RC, et al. Targeted delivery of antibody conjugated liposomal drug carriers to rat myocardial infarction. Biotechnol Bioeng. 2007;96(4):795–802. doi: 10.1002/bit.21233. [DOI] [PubMed] [Google Scholar]
- 29.Macholl S, et al. 2012. In vivo pH imaging with 99mTc-pHLIP. Mol Imaging Biol 14:725–734.
- 30.Zoonens M, Reshetnyak YK, Engelman DM. Bilayer interactions of pHLIP, a peptide that can deliver drugs and target tumors. Biophys J. 2008;95(1):225–235. doi: 10.1529/biophysj.107.124156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu Q, Lee L-Y. Acid-sensing ion channels and pain. Pharmaceuticals (Ott) 2010;3(5):1411–1425. doi: 10.3390/ph3051411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan HL, Longhurst JC, Eisenach JC, Chen SR. Role of protons in activation of cardiac sympathetic C-fibre afferents during ischaemia in cats. J Physiol. 1999;518(Pt 3):857–866. doi: 10.1111/j.1469-7793.1999.0857p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circ Res. 1999;84(8):921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- 34.Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci USA. 2001;98(2):711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99(5):501–509. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
- 36.Naves LA, McCleskey EW. An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res. 2005;38(11):1561–1569. doi: 10.1590/s0100-879x2005001100001. [DOI] [PubMed] [Google Scholar]
- 37.Gazit AZ, Avari JN, Balzer DT, Rhee EK. Electrocardiographic diagnosis of myocardial ischemia in children: Is a diagnostic electrocardiogram always diagnostic? Pediatrics. 2007;120(2):440–444. doi: 10.1542/peds.2007-0170. [DOI] [PubMed] [Google Scholar]
- 38.Guide for the Care and Use of Laboratory Animals. 1996. Committee on Care and Use of Laboratory Animals. (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85–23. [Google Scholar]
- 39.Anyukhovsky EP, et al. Expression of skeletal muscle sodium channel (Nav1.4) or connexin32 prevents reperfusion arrhythmias in murine heart. Cardiovasc Res. 2011;89(1):41–50. doi: 10.1093/cvr/cvq284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sosunov EA, Anyukhovsky EP, Rosen MR. Altered ventricular stretch contributes to initiation of cardiac memory. Heart Rhythm. 2008;5(1):106–113. doi: 10.1016/j.hrthm.2007.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.