Abstract
Batrachochytrium dendrobatidis, a pathogenic chytrid fungus implicated in worldwide amphibian declines, is considered an amphibian specialist. Identification of nonamphibian hosts could help explain the virulence, heterogeneous distribution, variable rates of spread, and persistence of B. dendrobatidis in freshwater ecosystems even after amphibian extirpations. Here, we test whether mosquitofish (Gambusia holbrooki) and crayfish (Procambarus spp. and Orconectes virilis), which are syntopic with many amphibian species, are possible hosts for B. dendrobatidis. Field surveys in Louisiana and Colorado revealed that zoosporangia occur within crayfish gastrointestinal tracts, that B. dendrobatidis prevalence in crayfish was up to 29%, and that crayfish presence in Colorado wetlands was a positive predictor of B. dendrobatidis infections in cooccurring amphibians. In experiments, crayfish, but not mosquitofish, became infected with B. dendrobatidis, maintained the infection for at least 12 wk, and transmitted B. dendrobatidis to amphibians. Exposure to water that previously held B. dendrobatidis also caused significant crayfish mortality and gill recession. These results indicate that there are nonamphibian hosts for B. dendrobatidis and suggest that B. dendrobatidis releases a chemical that can cause host pathology, even in the absence of infection. Managing these biological reservoirs for B. dendrobatidis and identifying this chemical might provide new hope for imperiled amphibians.
Keywords: alternative hosts, field correlation, vectors, Bd toxin
Although some pathogens are highly host-specific, those infecting multiple host species can profoundly affect disease dynamics by increasing pathogen persistence, virulence, and movement between host populations (1). Furthermore, when there are multiple hosts for a pathogen, some can serve as reservoir hosts. Reservoir hosts can sustain the parasite when particular hosts of interest are absent or temporarily resistant to infection and are often necessary for pathogens to drive other host populations or species extinct (2, 3).
The chytrid fungus Batrachochytrium dendrobatidis is an example of a parasite that putatively causes host extinctions. Indeed, it has been implicated in the declines of hundreds of amphibian species worldwide (4-10). B. dendrobatidis is able to persist without amphibian hosts (11, 12), which could prevent successful amphibian reintroductions (3). One possible mechanism for persistence is the presence of nonamphibian hosts of B. dendrobatidis. B. dendrobatidis is generally thought of as an amphibian specialist that consumes host keratin for sustenance (13), despite it commonly being maintained in the laboratory on nonkeratinized media, such as tryptone. Numerous vertebrate and invertebrate taxa possess keratin or keratin-like compounds in their gastrointestinal (GI) tracts (14). Hence, it is not surprising that previous researchers have hypothesized that there might be nonamphibian hosts or vectors of B. dendrobatidis (15, 16). However, this idea appeared to be temporarily abandoned after Rowley et al. (17) retracted their initial report of the detection of B. dendrobatidis on nonamphibian hosts (18). Recently, it was reported that B. dendrobatidis can be carried on algae (12), terrestrial reptiles (19), waterfowl (20), and nematodes (21), but there is currently no evidence that these carriers actually supported pathogen growth or transmission, which would be necessary to explain the long-term persistence of B. dendrobatidis in the absence of amphibians. Other studies have grown B. dendrobatidis on boiled snake skin (11, 22), sterilized bird feathers (23), and toe scales from waterfowl (20), but none of these studies demonstrated B. dendrobatidis growth on live hosts with functioning immune systems. Consequently, we lack studies that demonstrate B. dendrobatidis growth on living, nonamphibian hosts, transmission of B. dendrobatidis from these hosts to amphibians, and links between nonamphibian hosts and B. dendrobatidis prevalence in the field.
Here, we test whether mosquitofish (Gambusia holbrooki) and crayfish (Procambarus spp. and Orconectes virilis) are hosts for B. dendrobatidis by field-collecting each species, examining them for embedded zoosporangia, screening them for B. dendrobatidis using quantitative (q)PCR, and testing for associations between crayfish and B. dendrobatidis occurrence in nature. We selected these species because they cooccur with many amphibian species and have been widely introduced beyond their native ranges (24). We then attempted to experimentally infect mosquitofish and crayfish (Procambarus alleni) with B. dendrobatidis and determined whether these potential hosts could transmit B. dendrobatidis to amphibians.
Results and Discussion
We found B. dendrobatidis+ Procambarus spp. (P. alleni and P. clarkii) in three of the five southeastern Louisiana sites sampled in September 2011 and in one of two sites sampled in April 2012. Conservative estimates of average prevalence based on qPCR of swabs from the carapace and GI tract and light microscopy of the GI tracts were 17.3% and 10% for these two surveys, respectively (see Table S1 for information by site and mean B. dendrobatidis intensity data and SI Methods). The crayfish from all of the B. dendrobatidis+ Louisiana sites had distinct zoosporangia with discharge tubules in their GI tracts (Fig. 1A). During light microscopy, the zoosporangia could not be rinsed away and did not move independent of the GI tract when agitated with a probe (Fig. 1A), and histological sections verified that these zoosporangia were embedded in the GI tract of the crayfish (Fig. 1B). The zoosporangia grew colonially just below the GI epithelial surface (Fig. 1B), similar to their growth in frog skin. Furthermore, crayfish that were considered B. dendrobatidis+ based on light microscopy were also B. dendrobatidis+ based on qPCR. Despite B. dendrobatidis prevalence in crayfish being >17% in September, frogs (n = 11) collected at the same time from these sites were B. dendrobatidis− (see Table S1 for information by site). The fact that we did not detect B. dendrobatidis on the frogs is consistent with a multisite seasonal survey in southeastern Louisiana that showed that B. dendrobatidis prevalence on amphibian skin approaches zero in September, despite being high (∼45%) in the spring (Fig. S1 and SI Methods). These results suggest that crayfish could function as hosts for B. dendrobatidis during the time of the year when B. dendrobatidis prevalence in amphibians is low, supporting the hypothesis that crayfish are reservoir hosts for this pathogen.
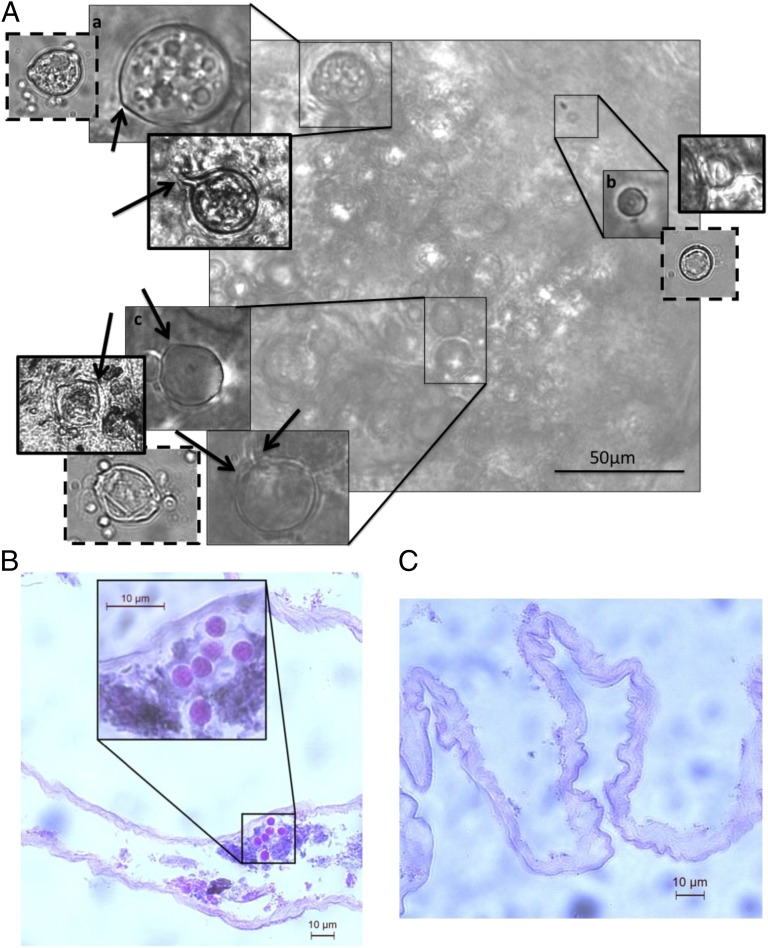
Fig. 1.
Light microscopy of laboratory-infected and wild-caught P. alleni (collected in September) (A), a histological section of a wild-caught Procambarus spp. intestinal tract embedded with developing B. dendrobatidis (collected in April) (B) and an uninfected intestinal tract (C). (A) The laboratory-infected P. alleni have thin solid borders, the wild-caught Procambarus spp. have thick solid borders, and the same life stages grown in culture have dashed borders. Light microscopy images show zoosporangia filled with developing zoospores (a), encysted zoospore beginning to form rhizoids (b), and empty zoosporangia (c); arrows point to potential zoospore discharge tubules on the zoosporangia. (B and C) Histology images are stained with hematoxylin and eosin, and B shows what appears to be developing zoosporangia that grew colonially just below the GI epithelial surface.
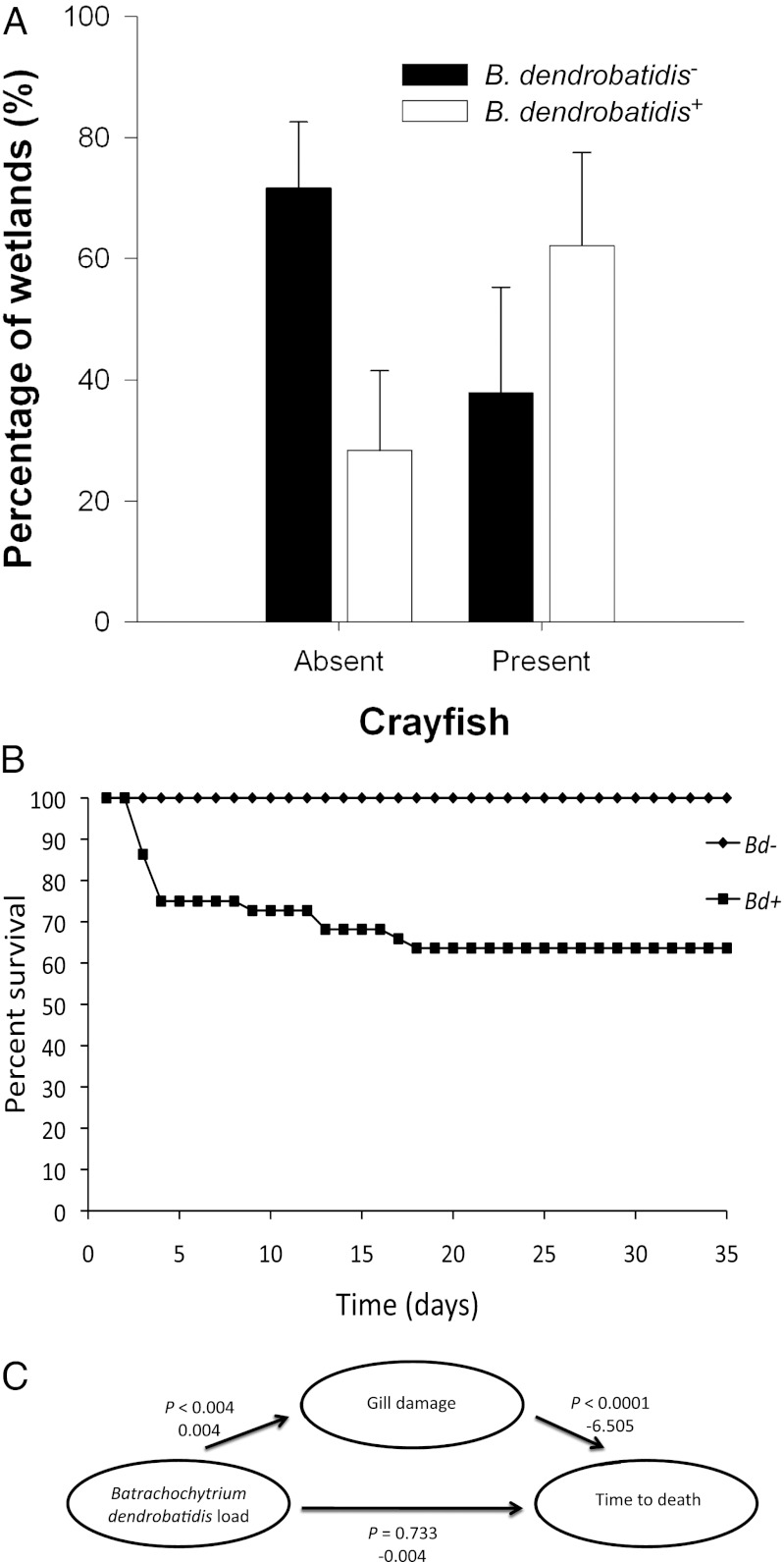
Field-collected crayfish (O. virilis) from two of three sites surveyed in Colorado in May 2012 also had embedded zoosporangia with discharge tubules visible in their GI tracts (Fig. S2). A conservative estimate of prevalence based on light microscopy was 20%, although sample inhibition prevented verification of B. dendrobatidis presence by qPCR (SI Text). To test whether Orconectes spp. (O. virilis and Orconectes immunis) presence was a positive predictor of B. dendrobatidis infections in amphibians, we sampled 97 wetlands in Colorado, swabbing 9,174 amphibians for B. dendrobatidis (representing five species; SI Methods). Amphibians were B. dendrobatidis+ at 40 wetlands, including six sites with positive results from more than one amphibian species (see Table S2 for frequency of positive results among species). The occurrence of Orconectes spp. was a significant positive predictor of B. dendrobatidis detection in one or more amphibian species (χ2 = 10.87; df = 1; P = 0.001; Fig. 2A). There was no evidence of overdispersion, and no other variables, including larval amphibian density, occurrence of bullfrogs (a known reservoir host for B. dendrobatidis), wetland area, or amphibian species richness, significantly improved model fit. When included as univariate predictors, each of these variables had ΔAICc (Akaike information criterion) values of >7.2 relative to the crayfish-only model, reinforcing the hypothesis of a positive association between crayfish and B. dendrobatidis infections.
Fig. 2.
Relationship between crayfish and amphibian B. dendrobatidis infections in the field and the effects of B. dendrobatidis on crayfish survival in the laboratory. (A) Percentage of wetlands with positive and negative B. dendrobatidis detections in the amphibian community as a function of crayfish (O. virilis) presence or absence (positive upper 95% confidence interval for a proportion). Ninety-seven wetlands were sampled with 40 yielding a positive detection of B. dendrobatidis in one or more amphibian species. All wetlands categorized as being negative for B. dendrobatidis had a minimum of 20 amphibians tested. (B) Percentage of survival through time of the crayfish P. alleni when exposed to B. dendrobatidis+ (Bd+) (n = 44) or B. dendrobatidis− (Bd−) inoculates (n = 21). (C) Path model suggesting that effect of B. dendrobatidis load on time to death was mediated by gill recession. Probability values and unstandardized path coefficients are provided next to each path.
To test whether B. dendrobatidis could use crayfish carapace and GI tract as a resource, B. dendrobatidis growth was quantified on agar alone, agar plus autoclaved crayfish carapace, and agar plus autoclaved crayfish GI tracts. B. dendrobatidis grew and reproduced for a minimum of 7 d (the duration of the experiment) on agar mixed with crayfish carapace or GI tracts but died within 3 d on the plates containing only agar (Fig. S3), verifying that B. dendrobatidis can be sustained on crayfish tissues in the absence of an immune response.
To test whether P. alleni and G. holbrooki could be infected with B. dendrobatidis, noninfected P. alleni and G. holbrooki were exposed to either B. dendrobatidis+ or B. dendrobatidis− water for 2 wk, after which they were transferred to new containers with B. dendrobatidis− artificial spring water (ASW) (25) and were given weekly water and container changes to ensure that we were not detecting the initial B. dendrobatidis inoculate. After 7 wk, animals were euthanized and swabbed externally and internally (GI tract), and B. dendrobatidis load (genome equivalents) on the swabs was determined using qPCR.
No G. holbrooki (n = 13 per treatment) were B. dendrobatidis+ nor did any die during the experiment. In contrast, 91% of B. dendrobatidis–exposed P. alleni (n = 44) had detectable B. dendrobatidis based on qPCR, whereas no control P. alleni were B. dendrobatidis+ (n = 21). By week 7, B. dendrobatidis–exposed P. alleni experienced 36% mortality compared with 0% mortality in the controls (χ21 = 15.53; P < 0.0001; Fig. 2B). Moreover, 100% of the dead and 84% of the live B. dendrobatidis–exposed P. alleni were B. dendrobatidis+ and the P. alleni that died had higher B. dendrobatidis loads at their time of death than the loads of P. alleni that lived with the infection (χ21 = 28.03; P < 0.001; mean log10 B. dendrobatidis intensity ± SE: dead external, 3.31 ± 0.33; dead internal, 2.90 ± 0.47; live external, 2.63 ± 0.33; live internal, 0.58 ± 0.39). Light microscopy of the GI tract of P. alleni revealed zoosporangia filled with zoospores, empty zoosporangia with discharge tubules, and encysting zoospores (Fig. 1A), demonstrating infection of the crayfish GI tract. No zoosporangia were found in control P. alleni (Fig. 1C for uninfected section of GI tract).
To examine whether P. alleni could transmit B. dendrobatidis to amphibians, we exposed uninfected tadpoles to either infected or uninfected P. alleni (three tadpoles per replicate crayfish). The frogs were collected as egg masses from a B. dendrobatidis–free pond and maintained in the laboratory under B. dendrobatidis–free conditions until the experiment began. B. dendrobatidis was successfully transmitted from infected P. alleni to tadpoles in 7 of 10 replicates (crayfish mean log10 external intensity ± SE: 2.07 ± 0.66; mean log10 intensity/tadpole mouthpart ± SE: 0.79 ± 0.07), whereas all 12 tadpoles in the four replicates with uninfected P. alleni were negative for B. dendrobatidis.
Examination of the gills of P. alleni from our initial infection experiment revealed that the B. dendrobatidis–exposed crayfish, especially those that died early in the experiment, had significantly more gill recession (mean distance between epithelium and gill tip ± SE: 1.15 ± 0.4 µm; n = 18; Fig. S4A) than did those that were not exposed to B. dendrobatidis (mean ± SE: 0.12 ± 0.12 µm; n = 7; Fig. S4B; see Fig. 2C for statistics). To test whether death alone or fouling of the crayfish between death and preservation could explain the gill recession, a group of P. alleni were euthanized by pithing and allowed to sit for 24 h (n = 5). These crayfish had no more gill recession than the crayfish that were not exposed to B. dendrobatidis (mean ± SE: 0.06 ± 0.007 µm; Fig. S4C), suggesting that death alone or fouling of the crayfish between death and preservation could not explain the gill recession. This suggests that gill recession contributed to B. dendrobatidis–induced mortality rather than mortality causing the gill recession. Indeed, a path model supports the hypothesis that B. dendrobatidis exposure indirectly lead to reduced survival time by causing gill recession that was a negative predictor of time of death (Fig. 2C). Gill damage has been associated with other crayfish parasitic infections (26), where it reduced gill functioning and oxygen intake resulting in acute mortality (27).
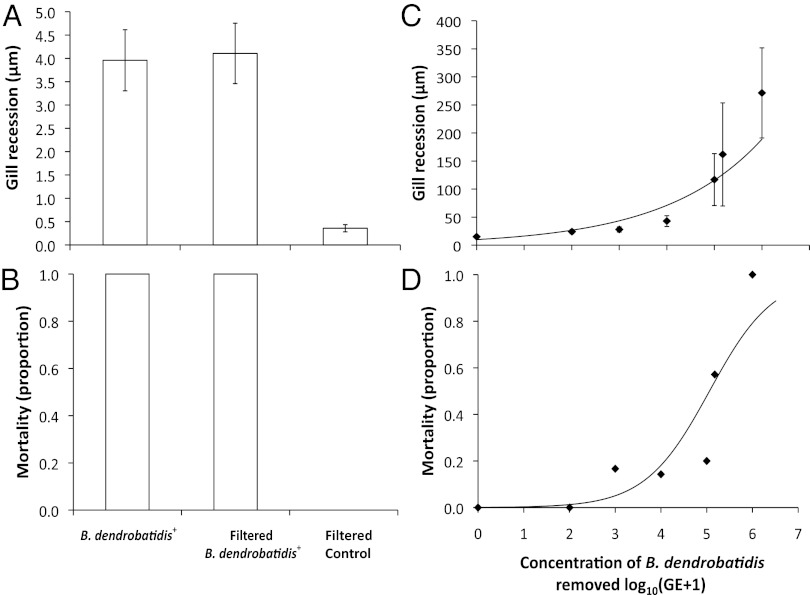
Although B. dendrobatidis exposure seemed to cause gill recession, B. dendrobatidis was not observed to infect the gills directly. Consequently, as Berger et al. (28) hypothesized in their seminal study discovering B. dendrobatidis, we postulated that B. dendrobatidis might be producing a factor that can cause pathology in the absence of actual infection; for instance, B. dendrobatidis produces proteolytic enzymes known to degrade host tissues (22, 29–31). To test this hypothesis, we exposed P. alleni to (i) an unfiltered B. dendrobatidis+ inoculum, (ii) a B. dendrobatidis+ inoculum where all of the zoospores and zoosporangia were removed with a 0.7 µm filter, or (iii) a control B. dendrobatidis− inoculum filtered through a 0.7 µm filter (n = 5 per treatment). We found that the filtered and unfiltered B. dendrobatidis+ inocula induced similar, elevated levels of gill recession compared with the B. dendrobatidis− inoculum (F2,11 = 17.28; P = 0.0004; Fig. 3A and Fig. S4D). Moreover, all of the crayfish exposed to the filtered and unfiltered B. dendrobatidis+ inocula died within 3 d, whereas all of the crayfish exposed to the filtered B. dendrobatidis− control inoculum survived until the end of the 4-d experiment (χ21 = 16.01; P = 0.0003; Fig. 3B). The higher mortality seen in this experiment (100%) compared with the infection experiment (36%) was probably because we exposed the crayfish to a filtered B. dendrobatidis inoculum that previously had more B. dendrobatidis zoospores than used in the infection experiment (1.2 × 105 and 1.2 × 103 zoospores per milliliter, respectively). We conducted a follow up dose–response experiment, exposing two separate populations of P. alleni to serially diluted, filtered B. dendrobatidis+ inocula (concentration of zoospores removed: 106, 1.5 × 105, 105, 104, 103, 102 zoospores per milliliter; n = 4, n = 10, n = 10, n = 11, n = 10, and n = 9, respectively) and a filtered B. dendrobatidis− control inoculum (n = 10). We found that the populations responded similarly and that the concentration of B. dendrobatidis filtered from the inoculum was associated positively with molting frequency (χ21 = 4.26; P = 0.03; Fig. S5), gill recession (F1,51 = 33.28; P < 0.001; Fig. 3C), and mortality (χ21 = 26.49; P < 0.0001; Fig. 3D; see SI Text for caveat on population-level differences). Molting might be an important stage for parasitic infections (32) because after molting, crayfish have a soft exoskeleton and might be immunosuppressed (33). These results indicate that B. dendrobatidis can induce pathology in the absence of direct host contact or infection. This could help explain rapid mortality of tadpoles (within 48 h) exposed to B. dendrobatidis (34) and amphibian pathology associated with B. dendrobatidis exposure without infections (e.g., refs. 35 and 36). Whether these pathology-inducing chemicals released by B. dendrobatidis are known proteases (22, 29–31) and are the cause of B. dendrobatidis–induced electrolyte imbalance and cardiac arrest in amphibians (37) remains to be tested.
Fig. 3.
Effects of an unfiltered B. dendrobatidis+ inoculum, a B. dendrobatidis+ inoculum where all of the zoospores and zoosporangia were removed, and a B. dendrobatidis− inoculum on P. alleni gill recession (distance between epithelium and gill tip) (A) and mortality (B). Dose–response relationship between B. dendrobatidis inocula with all of the zoospores and zoosporangia removed and P. alleni gill recession (C) and mortality (D). Values are mean ±1 SE. Shown is the best fit line in C and a logistic regression fit [y = exp(−7.1962833 + (1.4220071 × x)/(1 + exp(−7.1962833 + (1.4220071 × x))] in D.
Building upon the results of our initial infection experiment, we conducted a 12-wk study to evaluate whether P. alleni could maintain B. dendrobatidis infections long-term and thereby potentially function as reservoir hosts. At 7 wk, 89% (25/28) of B. dendrobatidis–exposed P. alleni were B. dendrobatidis+. At 12 wk, 64% (18/28) of B. dendrobatidis–exposed P. alleni had survived and 22% (4/18) of those survivors still had detectable B. dendrobatidis (mean log10 internal intensity ± SE: 1.79 ± 0.20; Fig. S6), indicating that some P. alleni cleared the infection, whereas others maintained the infection for at least 3 mo. Although there was a significant decrease in external B. dendrobatidis load between weeks 7 and 12 (χ21 = 18.53; P < 0.0001; Fig. S6), there was a significant increase in internal B. dendrobatidis loads over this same time period (χ21 = 6.37; P = 0.01; Fig. S6). Between weeks 7 and 12, control P. alleni gained weight, whereas P. alleni exposed to B. dendrobatidis lost weight (mean mass change between 7 and 12 wk ± SE; control: 11.56 ± 12.1%; B. dendrobatidis–exposed: −10.63% ± 14.4%; F1,12 = 7.97; P = 0.01), indicating a cost of infection even for the surviving individuals. Whereas the gill recession described above is likely the cause of the acute crayfish mortality, B. dendrobatidis infections of the GI tract might have contributed to the reduced growth rates of surviving P. alleni.
Overall, our results indicate that crayfish become infected with B. dendrobatidis in nature, can maintain these infections for months in the laboratory, and can transmit infections to amphibians. Furthermore, crayfish presence was a positive predictor of B. dendrobatidis occurrence in cooccurring amphibians among field sites in Colorado, even after considering competing factors such as host density or amphibian reservoir hosts (e.g., bullfrogs). Previous studies investigating potential nonamphibian hosts for B. dendrobatidis have not (i) tested live nonamphibian species with functioning immune systems, (ii) demonstrated B. dendrobatidis growth on nonamphibian species, or (iii) transmitted B. dendrobatidis from any nonamphibian host to amphibians. Our study is unique in demonstrating all three.
Mathematical models indicate that alternative hosts can allow for increased pathogen virulence and can cause host extinctions because the pathogen can persist in the remaining host species (2, 3). This might explain why B. dendrobatidis is so virulent, causes host extirpations, and can persist in local environments after amphibians have been extirpated. The abundance and distribution of alternative hosts might also help explain geographic variation in the distribution and rates of spread of B. dendrobatidis. For example, both crayfish and B. dendrobatidis–related amphibian declines are more common in stream than pond systems (38, 39). Additionally, crayfish infection with B. dendrobatidis might explain how crawfish frogs (Lithobates areolatus) obtained B. dendrobatidis infections while overwintering in crayfish burrows (40).
Although more work is needed to generalize these results and the role of crayfish in B. dendrobatidis epizootics in amphibians, alternative hosts might help elucidate the emergence of the global B. dendrobatidis pandemic. Crayfish are regularly moved among water bodies as fish bait (41), and crayfish are regularly transported nationally and internationally in the live food, aquaculture, and aquarium and pond trade, where crayfish escapes and releases are not uncommon (24). These different methods of live crayfish relocations could rapidly move B. dendrobatidis great distances and contribute to the global B. dendrobatidis pandemic. Most efforts to conserve and restore amphibian populations challenged by B. dendrobatidis have been unsuccessful, but managing alternative hosts offers a new and potentially more effective approach to managing B. dendrobatidis. Likewise, identifying the specific pathology-inducing chemical released by B. dendrobatidis might facilitate the development of new strategies to reduce the risk posed by this devastating pathogen.
Methods
General.
All crayfish (mean initial mass ± SE: 3.35 ± 0.75 g) in the laboratory studies were exposed to B. dendrobatidis isolate SRS 812 (isolated from Lithobates catesbeianus; see SI Methods for B. dendrobatidis culture and inoculation methodology) and were maintained individually in 1-L polyethylene containers filled with 500 mL of ASW (25) at 23 °C and on a 14:10 h light:dark cycle. All of the crayfish and tadpoles were fed organic spinach ad libitum and were checked daily for mortality. Zoospore densities in the B. dendrobatidis+ inoculums were estimated with a hemocytometer and were diluted with deionized water to the targeted concentrations in each experiment (Table S3).
Crayfish and mosquitofish were euthanized by freezing and MS222 overdose, respectively, were swabbed externally (30 swipes from the snout to tail) and internally (15 swipes of the entire length of the inside of the GI tract), and were preserved individually. In between each swab, gloves were cleaned with 10% bleach and rinsed with 1% Novaqua (neutralizes the bleach) and then water. To ensure that the GI tract was not contaminated with B. dendrobatidis from the exoskeleton or scales, they were removed with sterilized forceps by one experimenter and were swabbed by a second experimenter. B. dendrobatidis abundance on swabs was determined using qPCR following the methods of Hyatt et al. (42) (see SI Methods for qPCR methodology).
Crayfish Screening.
P. alleni and P. clarkii (9–20 crayfish per site from five sites in September 2011 and 10 crayfish per site from two sites in April 2012) were collected from southeastern Louisiana (Table S1). O. virilis (4–18 crayfish per site from three sites in May 2012) were collected from Colorado (Table S1). Crayfish were swabbed externally and internally (GI tract) as described above, and light microscopy, histology (see SI Methods), and qPCR were used to determine prevalence and abundance of B. dendrobatidis.
Colorado Field Surveys.
Between 2007 and 2010, 97 wetlands distributed across an 11 county region of Colorado were sampled to evaluate the importance of biotic and abiotic factors in predicting B. dendrobatidis occurrence on amphibians (Table S2). Standard methods (visual encounter surveys, dip-net sampling, and seine hauls; SI Methods) were used to characterize amphibian and invertebrate communities (43) over the course of two visits to each site. Larval, metamorphic, or adult amphibians were tested for B. dendrobatidis using nonlethal swabs followed by a qPCR assay (SI Methods). The goal was to detect B. dendrobatidis when present rather than to estimate prevalence; thus, species swabs were batch-pooled for each wetland and targeted a minimum of 20 swabbed individuals per site.
B.dendrobatidis Culture and Inoculation.
B. dendrobatidis inoculum was prepared by growing 1 mL of B. dendrobatidis stock (strain SRS 812 isolated from L. catesbeianus) on a 1% tryptone agar plate for 8 d at 23 °C. Each plate was flooded with 3 mL of ultrapure water to suspend the zoospores and the water from each plate was homogenized to generate the B. dendrobatidis+ inoculum. The B. dendrobatidis− inoculum was simultaneously prepared using the same method but no B. dendrobatidis was added to the agar plates (see Table S3 for zoospore concentrations).
Infection Experiment.
We collected G. holbrooki and P. alleni from a pond in Tampa, FL (28°06.759′N, 082°23.014′W) that is free of B. dendrobatidis. Each animal received 10 mL of either the B. dendrobatidis− inoculum (control; G. holbrooki: n = 13; P. alleni: n = 12) or the B. dendrobatidis+ inoculum (G. holbrooki: n = 13; P. alleni: n = 22). After 2 wk of exposure to B. dendrobatidis with no water changes, all animals were moved to new containers with fresh B. dendrobatidis− ASW for 5 more weeks and water and container changes occurred weekly. The animals were weighed at the end of the experiment.
The gills from each crayfish were removed and photographed (100× magnification). The greatest distance between the epithelium and the external surface of the gill was measured on three randomly selected gill filaments per crayfish using ImageJ software. A follow-up study was conducted to determine whether gill recession was an artifact of animal death and/or fouling of B. dendrobatidis–exposed animals (given that no control animals died during the experiment). Five P. alleni (collected from the same Tampa, FL population) were euthanized (pithed) and held for 24 h in the same conditions as the control animals in the experiment. The gills were removed and the distance between the epithelium and the external surface of the gill was then measured as described above.
B.dendrobatidis Culture Experiment.
B. dendrobatidis was cultured on agar plates containing either (n = 3 per treatment) autoclaved crayfish GI tract, autoclaved crayfish carapace, 1% tryptone (positive control), or agar alone (negative control) to test whether B. dendrobatidis is able to use these substrates for growth and reproduction (SI Methods). Each plate was inoculated with B. dendrobatidis (SI Methods), maintained at 23 °C for 7d, and monitored daily for zoospore activity. On day 7, each plate was flooded with 7 mL of ultrapure water to suspend all zoospores. A 150-µL aliquot was taken from each plate and the number of living zoospores was counted using a hemocytometer.
Transmission Experiment.
Three L. sphenocephalus egg masses were collected from a B. dendrobatidis–free pond (28°06.759′N, 82°23.014′W) and raised in the laboratory under B. dendrobatidis–free conditions until the tadpoles reached Gosner (44) stage 28 (SI Methods). Three tadpoles were haphazardly selected and placed in each of fourteen 1-L polyethylene cups filled with 750 mL of ASW. One P. alleni was added to each of these cups directly above the tadpoles (B. dendrobatidis+: n = 10; B. dendrobatidis−: n = 4; these crayfish were also part of the infection maintenance experiment and were verified 7 wk after exposure to be B. dendrobatidis+ or B. dendrobatidis− by external swabs) and crayfish and tadpoles were separated by a window screen to prevent predation. The crayfish were placed above the tadpoles to ensure that the crayfish feces dropped into the foraging arena of the tadpoles. After 5 d, the species were separated and the tadpoles were maintained in separate 1-L polyethylene cups filled with 750 mL of B. dendrobatidis− ASW for 10 d. Tadpoles were euthanized in MS222, and their mouthparts were removed and stored in 70% (vol/vol) ethanol for qPCR analysis. The P. alleni were maintained in B. dendrobatidis− ASW until 12 wk after the initial B. dendrobatidis exposure, at which time they were euthanized, weighed, and swabbed (these crayfish were used in the infection maintenance experiment as well).
Filtered B. dendrobatidis Experiment.
P. alleni housed in 500 mL of ASW were exposed to 15 mL of each of the following: (i) a B. dendrobatidis+ inoculum (1.2 × 105 zoospores/mL); (ii) a B. dendrobatidis+ inoculum (1.2 × 105 zoospores/mL) where all of the zoospores and zoosporangia were removed with a 0.7 µm filter (G6 Glass Fiber Filter; Fisher Scientific); or (iii) a control B. dendrobatidis− inoculum filtered through a 0.7-µm filter (n = 5). All inocula were prepared as described in SI Methods, and the crayfish were exposed to the filtered inocula 10 min after filtering was complete. Fifteen 20-µL aliquots of the filtered B. dendrobatidis+ inoculum were examined for B. dendrobatidis using a compound microscope and a hemocytometer, and none had any detectable zoospores or zoosporangia. The exposures lasted 4 d, after which, the crayfish were euthanized and gill recession was assessed as described above.
Filtered B. dendrobatidis Dose–Response Experiment.
This experiment was run in two temporal blocks where crayfish were exposed to serially diluted filtered B. dendrobatidis+ inocula or a filtered B. dendrobatidis− control inoculum. For block 1, P. alleni were purchased from The Marine Warehouse (Tampa, FL), and the exposure lasted for 22 d (concentration of zoospores removed: 1.5 × 105, 105, 104, 103, 102 zoospores per milliliter; n = 6, n = 6, n = 7, n = 6, and n = 6, respectively; B. dendrobatidis− control: n = 6). For block 2, P. alleni were collected from the same population used in the Filtered B. dendrobatidis experiment, and exposures lasted for 9 d (concentration of zoospores removed: 106, 105, 104, 103, 102 zoospores per milliliter; n = 4, n = 4, n = 4, n = 4, and n = 3, respectively; B. dendrobatidis− control: n = 4). For both blocks, the crayfish were exposed to the inocula 25 min after filtering was complete, and fifteen 20-µL aliquots of the filtered B. dendrobatidis+ inoculum were examined for B. dendrobatidis as described above, and none had any detectable zoospores or zoosporangia. Gill recession was assessed as described above.
Infection Maintenance Experiment.
P. alleni were housed and exposed to either B. dendrobatidis− or B. dendrobatidis+ inoculum (B. dendrobatidis−: n = 9; B. dendrobatidis+: n = 28) using the same methodology as described for the infection experiment, except that 7 wk after initial exposure, animals were swabbed externally (to verify infection), and 12 wk after initial exposure, animals were euthanized and swabbed internally and externally (as described above). After the week 7 swabbing, a subset of these P. alleni (B. dendrobatidis+: n = 10; B. dendrobatidis−: n = 4) were selected for use in the transmission trials.
Statistical Analysis.
For the Colorado survey, we used generalized linear models with a binomial response and a logit-link function to test whether crayfish presence (Orconectes spp.), bullfrog (L. catesbeianus) presence, larval amphibian density (summed among species; log10-transformed), wetland area (log10-transformed), and amphibian species richness were significant predictors of B. dendrobatidis occurrence in amphibians at each wetland. In the laboratory experiments, we tested for the effect of B. dendrobatidis exposure on crayfish survival using censored survival regression, using a Weibull distribution. Analysis of variance was used to determine whether B. dendrobatidis exposure affected weight change relative to the controls. We analyzed weight change between weeks 7 and 12. We conducted a path analysis to evaluate the level of support for the hypothesis that B. dendrobatidis exposure was indirectly related to crayfish death via gill recession [using the Lavaan package in R (45)]. We tested whether population and log10 concentration of filtered B. dendrobatidis inoculum affected crayfish mortality and molting compared with the filtered controls with Cox proportional hazards regression (function: coxph). We also tested whether log10 concentration of filtered B. dendrobatidis affected log10 gill recession with a linear regression model (function: lm). Statistical analyses were conducted in R statistical software (45).
Supplementary Material
Acknowledgments
We thank V. Vasquez for providing the B. dendrobatidis isolate used in these experiments, J. Kerby and J. Wood for helping to process B. dendrobatidis samples from Colorado field sites, and the field and laboratory assistants from the University of South Florida (S. Mellott, B. Austin, S. Lopez, and M. Manzi) and Colorado University (A. Peterson, A. Jensen, R. Adams, E. Daly, D. Herasimtschuk, E. Holldorf, R. Jadin, K. Dorsa, R. Parkhill, and L. Arellano). This work was supported by National Science Foundation Grant DEB 0516227, US Department of Agriculture Grants NRI 2006-01370 and 2009-35102-0543, US Environmental Protection Agency Science To Achieve Results Grant R833835, the Morris Animal Foundation, the National Fish and Wildlife Foundation Wildlife Links Program, the David and Lucile Packard Foundation, the Colorado Division of Wildlife, and the Colorado Wildlife Conservation Grant Program.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200592110/-/DCSupplemental.
References
- 1.Hess G. Disease in metapopulation models: Implications for conservation. Ecology. 1996;77(5):1617–1632. [Google Scholar]
- 2.de Castro F, Bolker B. Mechanisms of disease-induced extinction. Ecol Lett. 2005;8(1):117–126. [Google Scholar]
- 3.Mitchell KM, Churcher TS, Garner TWJ, Fisher MC. Persistence of the emerging pathogen Batrachochytrium dendrobatidis outside the amphibian host greatly increases the probability of host extinction. Proc Biol Sci. 2008;275(1632):329–334. doi: 10.1098/rspb.2007.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skerratt LF, et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4(2):125–134. [Google Scholar]
- 5.Wake DB, Vredenburg VT. Colloquium paper: Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA. 2008;105(Suppl 1):11466–11473. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohr JR, Raffel TR, Romansic JM, McCallum H, Hudson PJ. Evaluating the links between climate, disease spread, and amphibian declines. Proc Natl Acad Sci USA. 2008;105(45):17436–17441. doi: 10.1073/pnas.0806368105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lips KR, et al. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci USA. 2006;103(9):3165–3170. doi: 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng TL, Rovito SM, Wake DB, Vredenburg VT. Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci USA. 2011;108(23):9502–9507. doi: 10.1073/pnas.1105538108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briggs CJ, Knapp RA, Vredenburg VT. Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc Natl Acad Sci USA. 2010;107(21):9695–9700. doi: 10.1073/pnas.0912886107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Natl Acad Sci USA. 2010;107(21):9689–9694. doi: 10.1073/pnas.0914111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longcore JE, Pessier AP, Nichols DK. Batrachochytrium dendrobatidis gen et sp nov, a chytrid pathogenic to amphibians. Mycologia. 1999;91(2):219–227. [Google Scholar]
- 12.Johnson ML, Speare R. Survival of Batrachochytrium dendrobatidis in water: Quarantine and disease control implications. Emerg Infect Dis. 2003;9(8):922–925. doi: 10.3201/eid0908.030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voyles J, Rosenblum EB, Berger L. Interactions between Batrachochytrium dendrobatidis and its amphibian hosts: A review of pathogenesis and immunity. Microbes Infect. 2011;13(1):25–32. doi: 10.1016/j.micinf.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Schaffeld M, Marld J. Fish keratins. Methods Cell Biol. 2004;78:627–671. doi: 10.1016/s0091-679x(04)78022-x. [DOI] [PubMed] [Google Scholar]
- 15.Laurance WF, McDonald KR, Speare R. Epidemic disease and the catastrophic decline of Australian rain forest frogs. Conserv Biol. 1996;10(2):406–413. [Google Scholar]
- 16.Rachowicz LJ, et al. The novel and endemic pathogen hypotheses: Competing explanation for the origin of emerging infectious diseases. Conserv Biol. 2005;19(5):1441–1448. [Google Scholar]
- 17.Rowley JJL, et al. Experimental infection and repeat survey data indicate the amphibian chytrid Batrachochytrium dendrobatidis may not occur on freshwater crustaceans in northern Queensland, Australia. EcoHealth. 2007;4(1):31–36. [Google Scholar]
- 18.Rowley JJL, Alford RA, Skerratt LF. The amphibian chytrid Batrachochytrium dendrobatidis occurs on freshwater shrimp in rain forest streams in Northern Queensland, Australia. EcoHealth. 2006;3(1):49–52. [Google Scholar]
- 19.Kilburn VL, Ibáñez R, Green DM. Reptiles as potential vectors and hosts of the amphibian pathogen Batrachochytrium dendrobatidis in Panama. Dis Aquat Organ. 2011;97(2):127–134. doi: 10.3354/dao02409. [DOI] [PubMed] [Google Scholar]
- 20.Garmyn A, et al. Waterfowl: Potential environmental reservoirs of the chytrid fungus Batrachochytrium dendrobatidis. PLoS ONE. 2012;7(4):e35038. doi: 10.1371/journal.pone.0035038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapard EJ, Moss AS, San Francisco MJ. Batrachochytrium dendrobatidis can infect and cause mortality in the nematode Caenorhabditis elegans. Mycopathologia. 2012;173(2-3):121–126. doi: 10.1007/s11046-011-9470-2. [DOI] [PubMed] [Google Scholar]
- 22.Symonds EP, Trott DJ, Bird PS, Mills P. Growth characteristics and enzyme activity in Batrachochytrium dendrobatidis isolates. Mycopathologia. 2008;166(3):143–147. doi: 10.1007/s11046-008-9135-y. [DOI] [PubMed] [Google Scholar]
- 23.Johnson ML, Speare R. Possible modes of dissemination of the amphibian chytrid Batrachochytrium dendrobatidis in the environment. Dis Aquat Organ. 2005;65(3):181–186. doi: 10.3354/dao065181. [DOI] [PubMed] [Google Scholar]
- 24.Lodge D, Taylor C, Holdich D, Skurdal J. Nonindigenous crayfishes threaten North American freshwater biodiversity: Lessons from Europe. Fisheries (Bethesda, Md) 2000;25(8):7–20. [Google Scholar]
- 25.Cohen LM, Neimark H, Eveland LK. Schistosoma mansoni: Response of cercariae to a thermal gradient. J Parasitol. 1980;66(2):362–364. [PubMed] [Google Scholar]
- 26.Brown BL, Creed RP, Skelton J, Rollins MA, Farrell KJ. The fine line between mutualism and parasitism: Complex effects in a cleaning symbiosis demonstrated by multiple field experiments. Oecologia. 2012;170(1):199–207. doi: 10.1007/s00442-012-2280-5. [DOI] [PubMed] [Google Scholar]
- 27.Bielawski J. Ultrastructure and ion transport in gill epithelium of the crayfish, Astacus leptodactylus Esch. Protoplasma. 1971;73(2):177–190. doi: 10.1007/BF01275593. [DOI] [PubMed] [Google Scholar]
- 28.Berger L, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA. 1998;95(15):9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenblum EB, Stajich JE, Maddox N, Eisen MB. Global gene expression profiles for life stages of the deadly amphibian pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci USA. 2008;105(44):17034–17039. doi: 10.1073/pnas.0804173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joneson S, Stajich JE, Shiu SH, Rosenblum EB. Genomic transition to pathogenicity in chytrid fungi. PLoS Pathog. 2011;7(11):e1002338. doi: 10.1371/journal.ppat.1002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moss AS, Carty N, San Francisco MJ. Identification and partial characterization of an elastolytic protease in the amphibian pathogen Batrachochytrium dendrobatidis. Dis Aquat Organ. 2010;92(2-3):149–158. doi: 10.3354/dao02223. [DOI] [PubMed] [Google Scholar]
- 32.Keller TA. The effect of the branchiobdellid annelid Cambarincola fallax on the growth rate and condition of the crayfish Orconectes rusticus. J Freshwat Ecol. 1992;7(2):165–171. [Google Scholar]
- 33.Cerenius L, Bangyeekhun E, Keyser P, Söderhäll I, Söderhäll K. Host prophenoloxidase expression in freshwater crayfish is linked to increased resistance to the crayfish plague fungus, Aphanomyces astaci. Cell Microbiol. 2003;5(5):353–357. doi: 10.1046/j.1462-5822.2003.00282.x. [DOI] [PubMed] [Google Scholar]
- 34.Blaustein AR, et al. Interspecific variation in susceptibility of frog tadpoles to the pathogenic fungus Batracbochytrium dendrobatidis. Conserv Biol. 2005;19(5):1460–1468. [Google Scholar]
- 35.Luquet E, et al. Genetic erosion in wild populations makes resistance to a pathogen more costly. Evolution. 2012;66(6):1942–1952. doi: 10.1111/j.1558-5646.2011.01570.x. [DOI] [PubMed] [Google Scholar]
- 36.Romansic JM, et al. Individual and combined effects of multiple pathogens on Pacific treefrogs. Oecologia. 2011;166(4):1029–1041. doi: 10.1007/s00442-011-1932-1. [DOI] [PubMed] [Google Scholar]
- 37.Voyles J, et al. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science. 2009;326(5952):582–585. doi: 10.1126/science.1176765. [DOI] [PubMed] [Google Scholar]
- 38.Englund G, Krupa JJ. Habitat use by crayfish in stream pools: Influence of predators, depth and body size. Freshw Biol. 2000;43(1):75–83. [Google Scholar]
- 39.Burrowes PA, Joglar RL, Green DE. Potential causes for amphibian declines in Puerto Rico. Herpetologica. 2004;60(2):141–154. [Google Scholar]
- 40.Kinney VC, Heemeyer JL, Pessier AP, Lannoo MJ. Seasonal pattern of Batrachochytrium dendrobatidis infection and mortality in Lithobates areolatus: Affirmation of Vredenburg’s “10,000 zoospore rule”. PLoS ONE. 2011;6(3):e16708. doi: 10.1371/journal.pone.0016708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hobbs HH, Jass JP, Huner JV. A review of global crayfish introductions with particular emphasis on two North American species (Decapoda, Cambaridae) Crustaceana. 1989;56:299–316. [Google Scholar]
- 42.Hyatt AD, et al. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis Aquat Organ. 2007;73(3):175–192. doi: 10.3354/dao073175. [DOI] [PubMed] [Google Scholar]
- 43.Johnson PT, et al. Regional decline of an iconic amphibian associated with elevation, land-use change, and invasive species. Conserv Biol. 2011;25(3):556–566. doi: 10.1111/j.1523-1739.2010.01645.x. [DOI] [PubMed] [Google Scholar]
- 44.Gosner A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16(3):183–190. [Google Scholar]
- 45.R Development Core Team 2010. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria) Version 2.8.1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.