Abstract
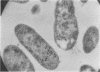
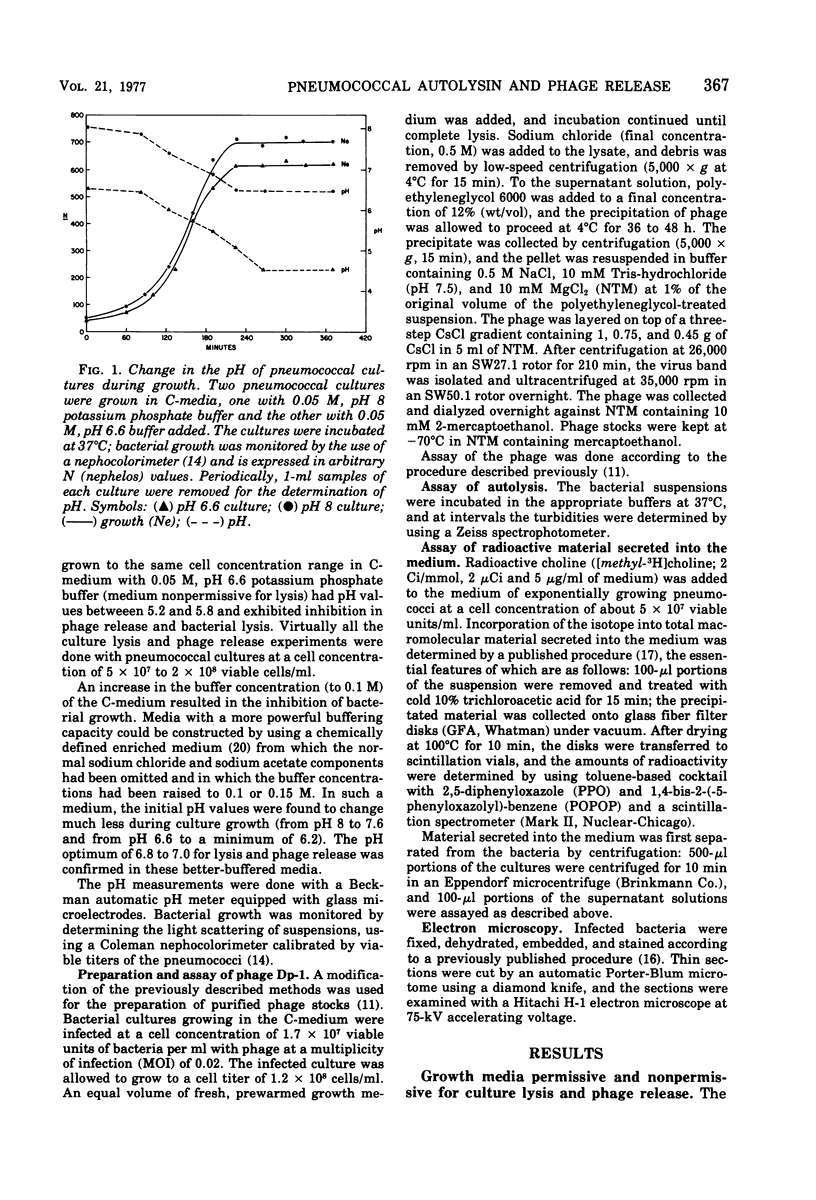
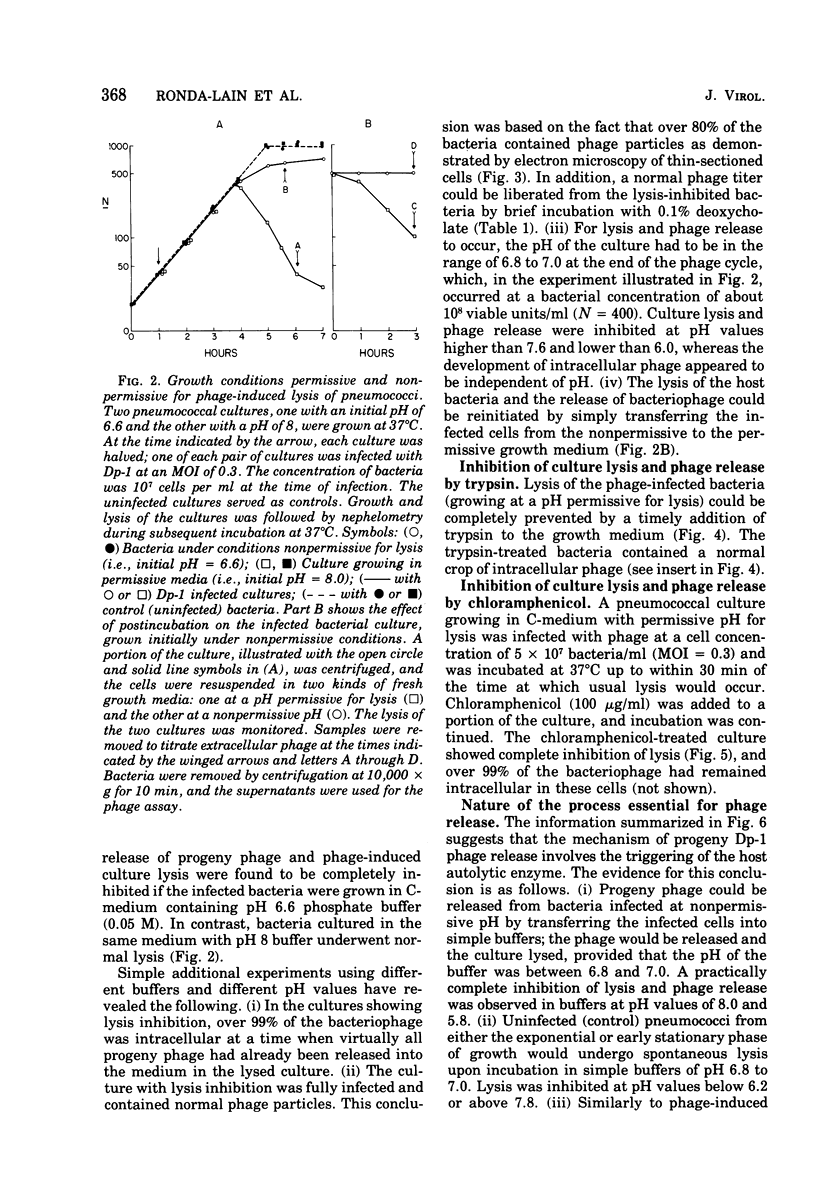
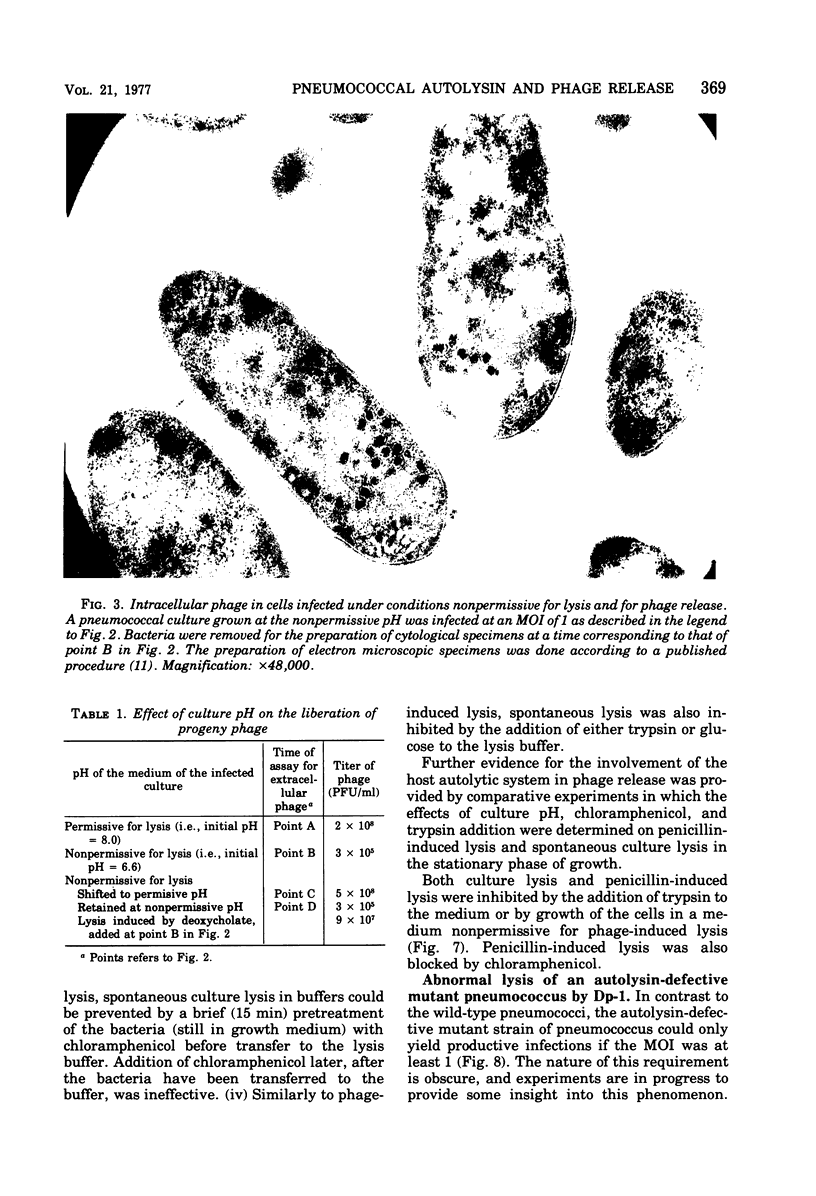
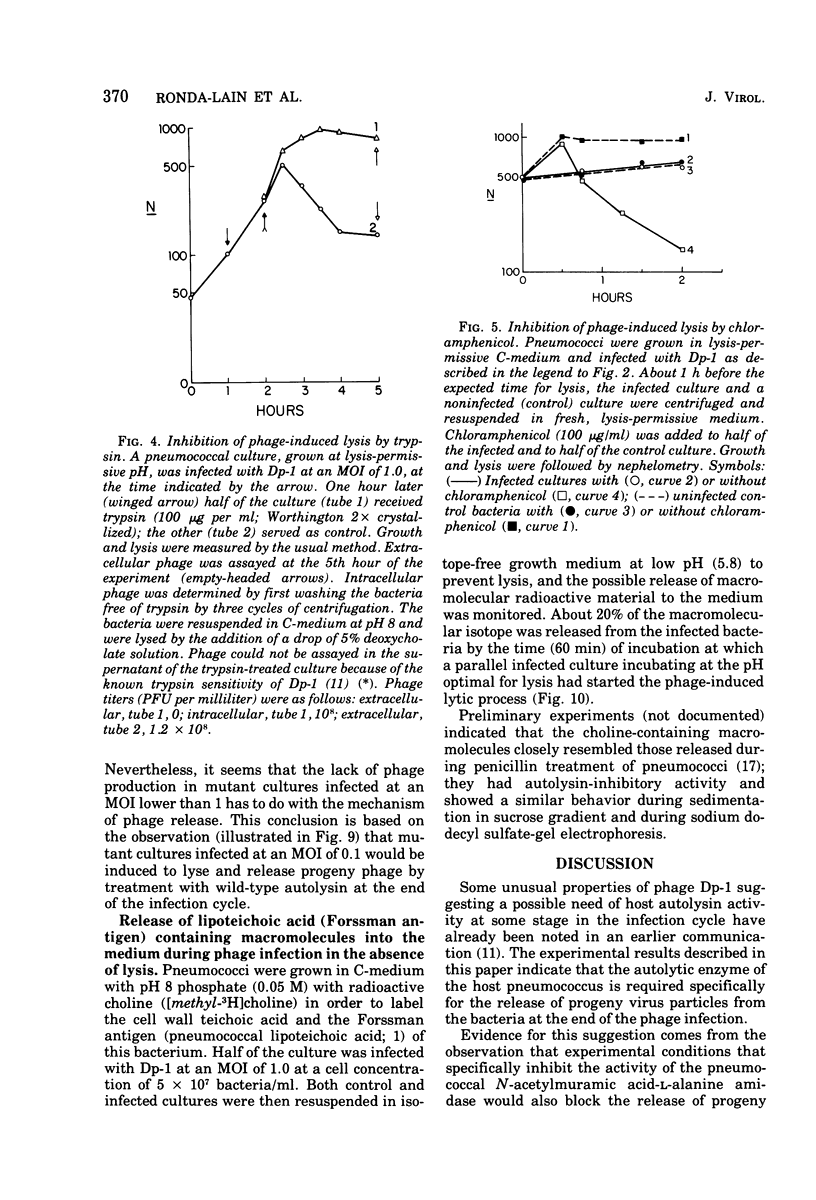
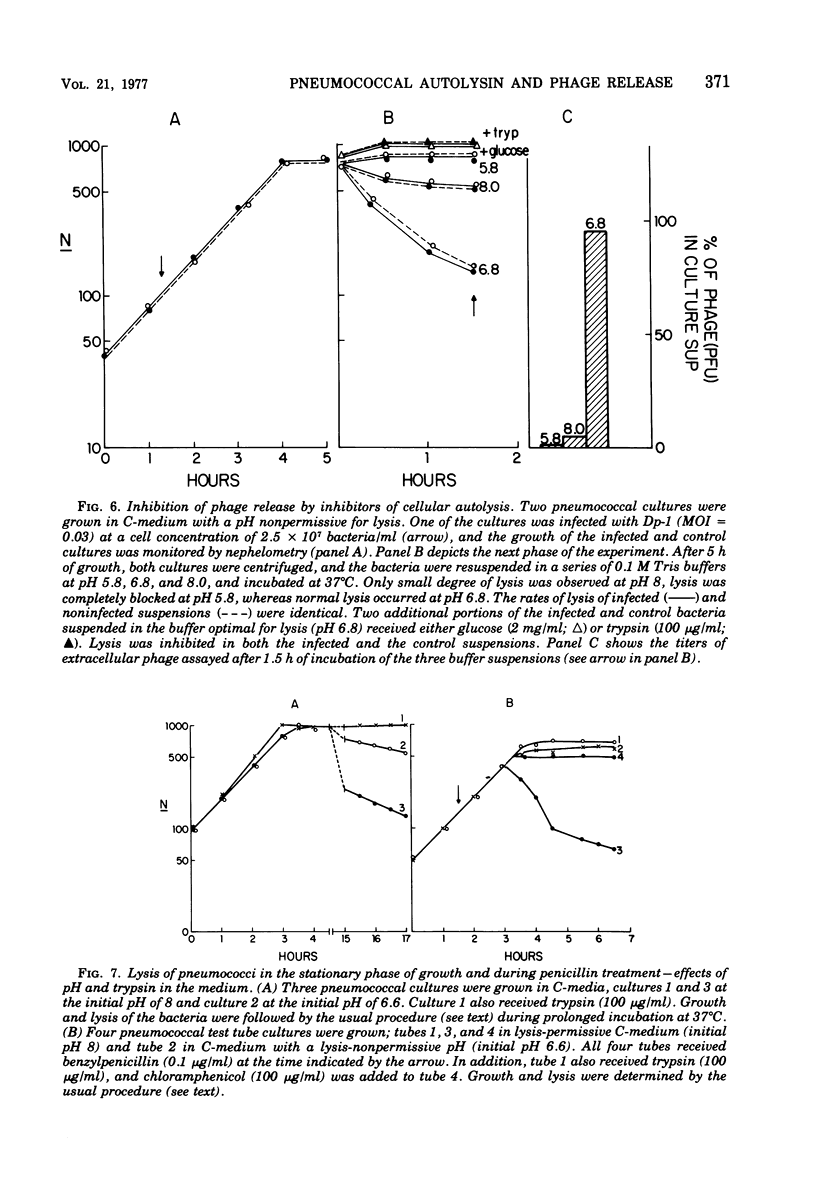
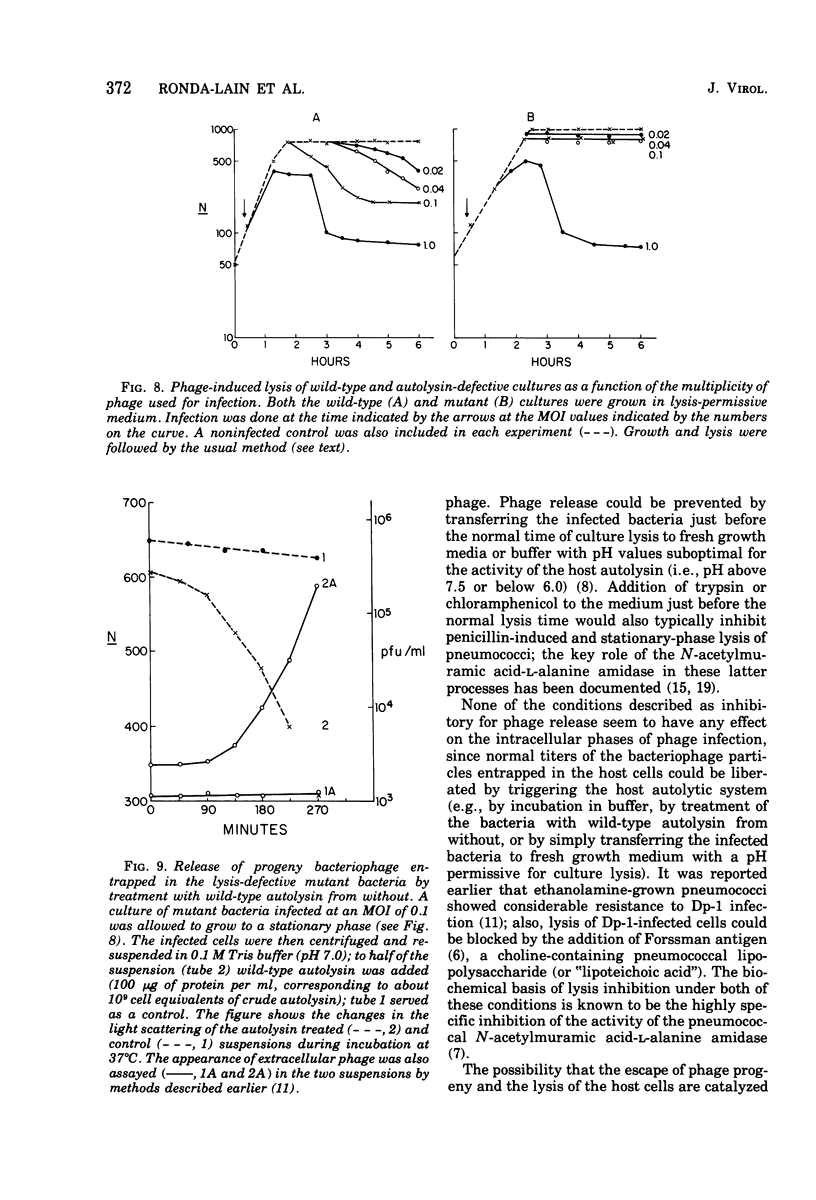
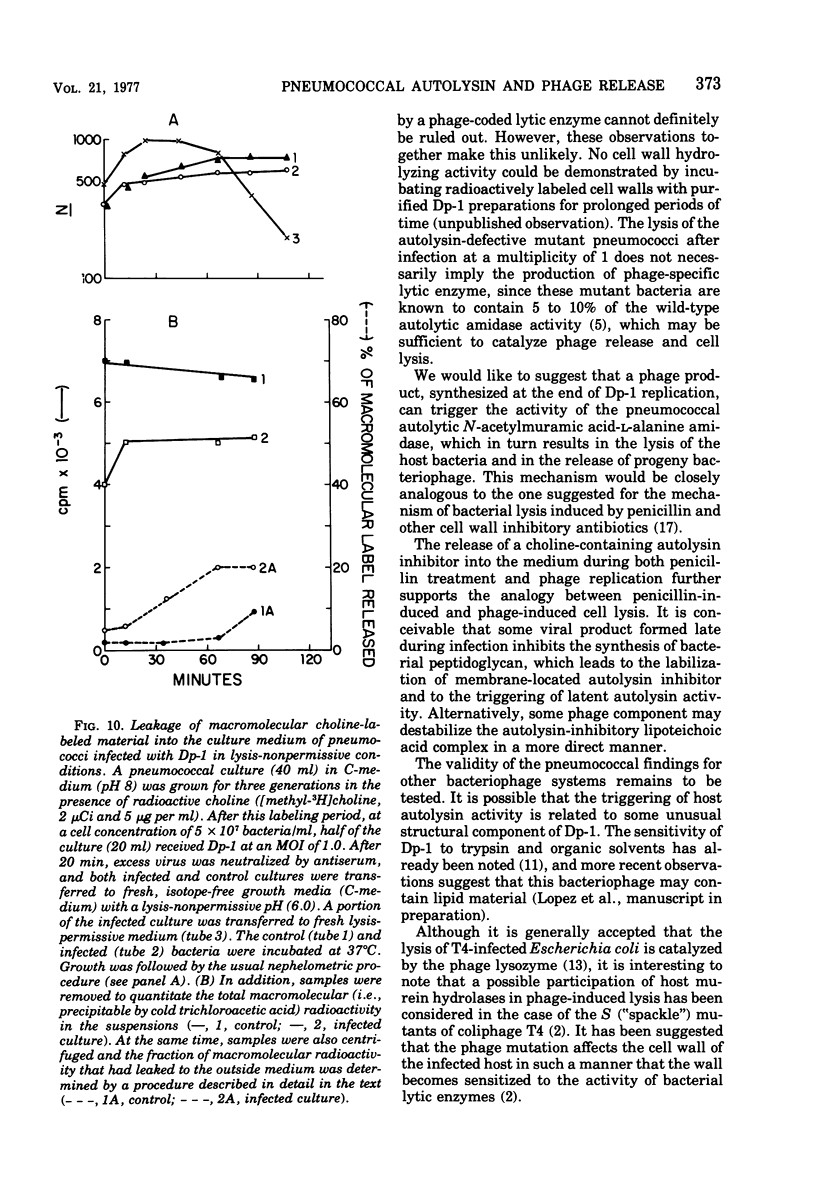
The pneumococcal bacteriophage Dp-1 seems to require the activity of the N-acetylmuramic acid-L-alanine amidase of the host bacterium for the liberation of phage progeny into the medium. This conclusion is based on a series of observations indicating that the exit of progeny phage particles is prevented by conditions that specifically inhibit the activity of the pneumococcal autolysin. These inhibitory conditions are as follows: (i) growth of the bacteria on ethanolamine-containing medium; (ii) growth of the cells at pH values that inhibit penicillin-induced lysis of pneumococcal cultures and lysis in the stationary phase of growth; (iii) addition of trypsin or the autolysin-inhibitory pneumococcal Forssman antigen (lipoteichoric acid) to the growth medium before lysis; (iv) infection of an autolysin-defective pneumococcal mutant at a multiplicity of infection less than 10 (treatment of such infected mutant bacteria with wild-type autolysin from without can liberate the entrapped progeny phage particles); (v) release of phage particles and culture lysis can also be inhibited by the addition of chloramphenicol to infected cultures just before the time at which lysis would normally occur. Bacteria infected with Dp-1 under conditions nonpermissive for culture lysis and phage release secrete into the growth medium a substantial portion of their cellular Forssman antigen in the form of a macromolecular complex that has autolysin-inhibitory activity. We suggest that a phage product may trigger the bacterial autolysin by a mechanism similar to that operating during treatment of pneumococci with penicillin (Tomasz and Waks, 1975).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briles E. B., Tomasz A. Pneumococcal Forssman antigen. A choline-containing lipoteichoic acid. J Biol Chem. 1973 Sep 25;248(18):6394–6397. [PubMed] [Google Scholar]
- Emrich J. Lysis of T4-infected bacteria in the absence of lysozyme. Virology. 1968 May;35(1):158–165. doi: 10.1016/0042-6822(68)90315-2. [DOI] [PubMed] [Google Scholar]
- Holtje J. V., Tomasz A. Biological effects of lipoteichoic acids. J Bacteriol. 1975 Nov;124(2):1023–1027. doi: 10.1128/jb.124.2.1023-1027.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Teichoic acid phosphorylcholine esterase. A novel enzyme activity in pneumococcus. J Biol Chem. 1974 Nov 10;249(21):7032–7034. [PubMed] [Google Scholar]
- Lacks S. Mutants of Diplococcus pneumoniae that lack deoxyribonucleases and other activities possibly pertinent to genetic transformation. J Bacteriol. 1970 Feb;101(2):373–383. doi: 10.1128/jb.101.2.373-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonnell M., Lain R., Tomasz A. "Diplophage": a bacteriophage of Diplococcus pneumoniae. Virology. 1975 Feb;63(2):577–582. doi: 10.1016/0042-6822(75)90329-3. [DOI] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Mukai F., Streisinger G., Miller B. The mechanism of lysis in phage T4-infected cells. Virology. 1967 Nov;33(3):398–404. doi: 10.1016/0042-6822(67)90115-8. [DOI] [PubMed] [Google Scholar]
- TOMASZ A., JAMIESON J. D., OTTOLENGHI E. THE FINE STRUCTURE OF DIPLOCOCCUS PNEUMONIAE. J Cell Biol. 1964 Aug;22:453–467. doi: 10.1083/jcb.22.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. Cellular metabolism in genetic transformation of pneumococci: requirement for protein synthesis during induction of competence. J Bacteriol. 1970 Mar;101(3):860–871. doi: 10.1128/jb.101.3.860-871.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Westphal M. Abnormal autolytic enzyme in a pneumococus with altered teichoic acid composition. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2627–2630. doi: 10.1073/pnas.68.11.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Westphal M., Briles E. B., Fletcher P. On the physiological functions of teichoic acids. J Supramol Struct. 1975;3(1):1–16. doi: 10.1002/jss.400030102. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Zanati E., Ziegler R. DNA uptake during genetic transformation and the growing zone of the cell envelope. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1848–1852. doi: 10.1073/pnas.68.8.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautner T. A., Spatz H. C. Transfection in B. subtilis. Curr Top Microbiol Immunol. 1973;62:61–88. doi: 10.1007/978-3-642-65772-6_3. [DOI] [PubMed] [Google Scholar]