Abstract
Rasal, belonging to the GAP1 subfamily of Ras GTPase-activating proteins (RasGAPs) with dual RasGAP/RapGAP specificity, is epigenetically silenced in several tumor types. Surprisingly, the isolated protein has GAP activity on Rap but not on Ras. Its membrane recruitment is regulated by interaction with calcium and lipids, which simultaneously induces its RasGAP activity through a yet unknown mechanism. Here we show that the interaction of Rasal with membranes induces Rasal RasGAP activity by spatial and conformational regulation, although it does not have any effect on its RapGAP activity. Not only is colocalization of Rasal and Ras in the membrane essential for RasGAP activation, but direct and Ca-dependent interaction between the tandem C2 domains of Rasal and lipids of the membrane is also required. Whereas the C2A domain binds specifically phosphatidylserine, the C2B domain interacts with several phosphoinositol lipids. Finally we show, that similar to the C2 domains of synaptotagmins, the Rasal tandem C2 domains are able to sense and induce membrane curvature by the insertion of hydrophobic loops into the membrane.
Ras is a GTP-binding protein (G protein in short) of the Ras subfamily. It is involved in a wide range of important cellular processes. When Ras binds GTP, it is in an active conformation (“on” state) and hence able to bind to and activate downstream effectors. The hydrolysis of GTP to GDP inactivates Ras (“off” state). GTPase-activating proteins (RasGAPs) are responsible for accelerating the intrinsically slow GTPase activity of Ras. Oncogenic Ras mutants incapable of hydrolyzing GTP are found in 20–30% of human tumors (1). Frequently mutated residues are the P-loop glycine and the catalytic Gln61 (2, 3). Mutations or posttranslational modifications of RasGAPs, leading to deregulation or inactivation of the proteins, also contribute to tumor formation in cells containing wild-type (WT) Ras. This is particularly evident in the case of the tumor suppressor neurofibromin, a RasGAP, which is mutated or deleted in neurofibromatosis type I (4, 5). Furthermore, the RasGAP family member Rasal (Ras-GTPase–activating-like protein) is epigenetically silenced by methylation in multiple tumors (6–10).
Rasal belongs to the Gap1 subfamily, which consists of Rasal, Capri, tetrakisphosphate binding protein (GAP1IP4BP) and GAP1m. Members of the Gap1 family contain two N-terminal C2 domains (C2A and C2B), a GAP domain, and a pleckstrin homology domain (PH) connected to a Bruton’s tyrosine kinase (Btk) motif. Rasal, Capri, and GAP1IP4BP are dual-specificity GAPs, able to stimulate the slow GTPase reaction of both Ras and Rap in vivo (11–13). Another interesting feature of Rasal and Capri is that their GAP activity is regulated by intracellular Ca2+ levels (14, 15). Their C2 domains are able to bind phospholipids at high Ca2+ concentrations, although their PH domains seem to be inactive for lipid binding (14). In contrast, GAP1m and GAP1IP4BP have inactive C2 domains for lipid interaction, but bind to membranes through their PH domain (16, 17). Thus, a receptor-mediated increase in intracellular calcium concentration recruits Rasal and Capri, which both reside as soluble proteins in the cytoplasm, to the plasma membrane. Association with the membrane increases RasGAP activity of Rasal and Capri by a process that is mechanistically unclear, in particular because isolated Rasal in solution shows RapGAP but almost no RasGAP activity (15).
C2 domains are around 130 residues in length and fold in an eight-stranded antiparallel β sandwich. Three loops, named calcium binding loops (CBL1, CBL2, and CBL3), are responsible for binding Ca2+ ions (18). Specifically, CBL1 and -2 contain aspartate residues that serve as bidentate ligands for either two or three Ca2+ ions. Binding of phospholipids to C2 domains is mediated by Ca2+, which interacts with the negatively charged head groups of the phospholipids, mostly phosphatidylserine (PS) (18). Residues situated in the CBLs determine the selectivity for certain lipids. Members of the GAP1 family possess two consecutive C2 domains, C2A and C2B, a situation frequently found in exocytosis-associated proteins, like Synaptotagmin I.
In this study we characterize the lipid binding specificity of the C2 domains of Rasal and show their capability to sense and/or induce membrane curvature. Furthermore, we unravel the mechanism of Rasal RasGAP activation by membrane binding. We show that, whereas colocalization of both Rasal and Ras in the membrane is required for the activity, additional binding of the C2 domains is necessary for full activation.
Results
Rasal Binds Phosphatidylserine and Phosphatidylinositol Phosphates.
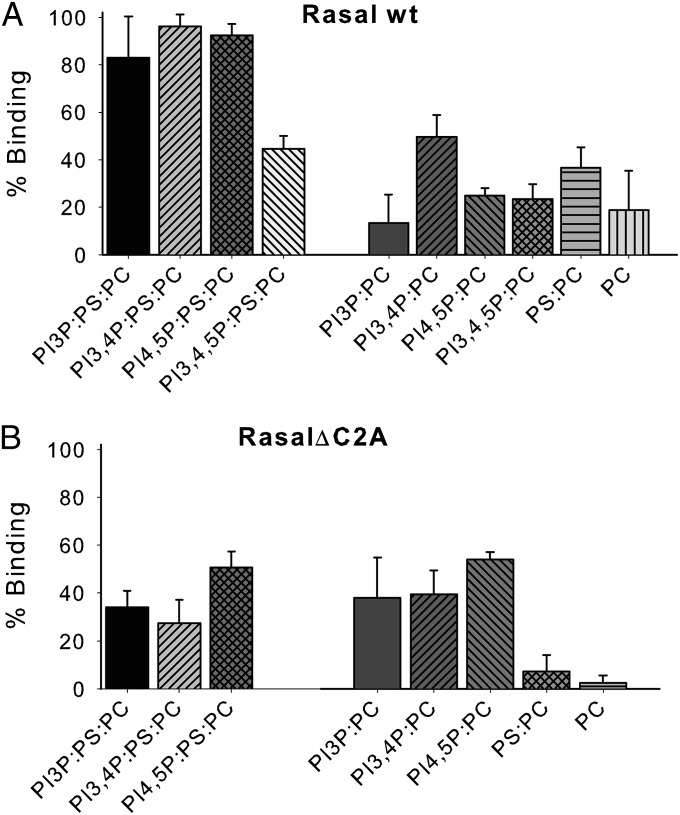
To study the effect of lipids on Rasal RasGAP activity, we first characterized the lipid binding specificity of Rasal C2 domains. Rasal–lipid vesicle interaction was measured by cosedimentation experiments (15, 19). Using similar methods, Walker et al. (15) had previously shown that Rasal C2 domains bind phosphatidylcholine (PC) and phosphatidylserine (PS), but do not bind phosphatidylinositol (PI) or phosphatidylethanolamine (PE). However, in our hands Rasal did not efficiently bind to PC or PC:PS (85:15 molar ratio) lipid vesicles (Fig. 1A). Rasal C2 domains (C2A–C2B) are quite homologous to the C2A–C2B domains of synaptotagmins, that bind PS and several phosphatidylinositol phosphates (PIPs) (20). Thus, we measured the binding of Rasal to lipid vesicles containing PC, PS, and different PIPs (PI3P; PI3,4P; PI4,5P; and PI3,4,5P), at a molar ratio similar to physiological membrane composition (PC:PS:PIP 75:15:10) in the presence of 1 mM Ca2+. This calcium concentration is high enough to ensure that the lipid binding is not limited by calcium concentration, but not too high to induce fusion of the vesicles in the condition used (SI Appendix, Fig. S7). Indeed, Rasal bound efficiently to vesicles containing PS and PI3P; PI3,4P; or PI4,5P although binding to PI3,4,5P containing liposomes was much weaker compared with the other phosphoinositides (Fig. 1A and SI Appendix, Fig. S1). When PS or PIP was removed, only 20–50% of Rasal was bound to the lipid vesicle (Fig. 1A). Thus, a combination of PS and PIPs is needed for efficient binding. This interaction depends on calcium, as the presence of EDTA inhibits the binding (SI Appendix, Fig. S2). To compare Rasal affinity for PS and PIP, Rasal binding to PS:PC (10:90) and PI3P:PC (10:90) at different total lipid concentration was measured (SI Appendix, Fig. S3), showing that Rasal possesses a similar affinity for both lipids. The resultant apparent dissociation constants (Kds) were 35 ± 23 and 29 ± 18 µM for PI3P and PS, respectively, which are in a similar range of other C2 domain affinities for lipids (21–24).
Fig. 1.
Rasal interacts with PS and PIPs. A total of 1 µM Rasal WT (A) or Rasal∆C2A (B) was incubated (30 min, 25 °C) with MLVs at 500 µM lipid concentration, in 50 mM Tris⋅HCl, 150 mM NaCl, 1 mM CaCl2. Percentage of binding was calculated by cosedimentation.
For synaptotagmins and other proteins with tandem C2A–C2B domains, it has been shown that C2B is responsible for binding to PIPs, mostly by a calcium-independent mechanism (25). To check if this is also the case for Rasal C2 domains, we measured the binding of Rasal∆C2A, which lacks the C2A domain, to PC:PS:PIP lipid vesicles (Fig. 1B and SI Appendix, Fig. S1). Similar to other C2A–C2B proteins, the absence of one C2 domain decreased the binding to vesicles (25, 26). Only 30–60% of total protein interacted with the vesicles containing PC:PS:PIP. Interestingly, these values did not decrease when PS was removed (PC:PIP vesicles), suggesting that the C2B domain binds preferentially to PIPs rather than to PS. ln contrast to synaptotagmin, binding of C2B to PIPs is calcium dependent, as it is inhibited by the presence of EDTA (SI Appendix, Fig. S2).
RasGAP Activation of Rasal by Colocalization of Ras and Rasal.
It was previously reported that isolated Rasal is not an active RasGAP in solution (15). Furthermore, in cells expressing Rasal, the RasGAP activity was detected only upon increasing the intracellular calcium concentration, which in turn is responsible for Rasal membrane localization. However, the mechanism of activating the RasGAP activity through Rasal lipid binding is unknown, in particular whether the activation is due to lipid or calcium binding. One possible mechanism would be the allosteric activation of the GAP domain by C2 domains binding to calcium and/or lipid. Thus, we measured the RasGTPase activity in the presence of recombinant soluble Rasal. As expected and previously reported, soluble Rasal was not able to activate the GTP hydrolysis of Ras. Furthermore, the addition of calcium (1 mM CaCl2) had no effect on the RasGAP activity (Fig. 2A), ruling out this ion as a direct (allosteric) RasGAP regulator.
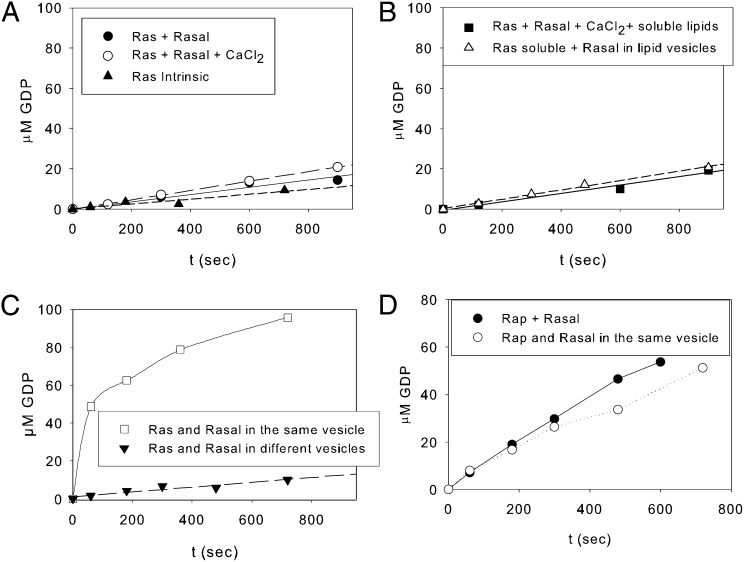
Fig. 2.
Rasal RasGAP function is activated by colocalization with Ras in lipid vesicles. GTPase activity of 100 µM Ras-GTP (A) in the absence of GAP or in the presence of 2.5 µM Rasal or 2.5 µM Rasal and 1 mM CaCl2. (B) In the presence of 2.5 µM Rasal, 1 mM CaCl2 and 100 µM soluble lipids (phosphatidylinositol 3-phosphate diC8 [PI(3)P diC8) and 1,2-dioctanoyl-sn-glycero-3-phospho-L-serine (PS diC8)] or in the presence of Rasal bound to PC:PS:PI3P (75:15:10 molar ratio) vesicles, 3 mM total lipid concentration and 1 mM CaCl2. (C) GTPase activity when 100 µM Ras-GTP and 2.5 µM Rasal are bound to the same vesicles (PC:DOGS-NTA:PS:PI3P at 52.5:30:10:7.5 molar ratio, 3 mM total lipid concentration) or when Ras and Rasal are bound to different vesicles. A total of 100 µM Ras-GTP bound to 3 mM PC:DOGS-NTA 50:50 vesicles and 2.5 µM Rasal bound to PC:PS:PI3P 65:30:15. (D) GTPase activity of 100 µM Rap-GTP catalyzed by 25 nM Rasal in solution and when both proteins are bound to the same vesicles (PC:DOGS-NTA:PS:PI3P at 52.5:30:10:7.5 molar ratio, 3 mM total lipid concentration).
To test a possible effect of anionic polar lipid head groups, we measured the GTPase activity in the presence of PS and PI3P with short aliphatic chains of eight carbons, which are soluble in aqueous buffers. Rasal was still inactive in their presence (Fig. 2B). Finally, we wanted to explore the possibility that Rasal needs the insertion of C2 domains in the lipid vesicle for allosteric activation. Therefore, we bound Rasal to lipid vesicles (PC:PS:PI3P 75:15:10) (SI Appendix, Fig. S4A) and measured GTP hydrolysis of soluble Ras. However, there was no activation of RasGAP function, indicating that Rasal is not appreciably activated by the presence of these lipids (Fig. 2B).
Because a direct and strong allosteric activation mechanism can be excluded, the most plausible possibility is that Rasal is not an active GAP due to very low affinity for Ras. In fact, fluorescence polarization experiments with Rasal homolog GAP1IP4BP and fluorescently labeled Ras·mGppNHp, a standard method to measure affinity between G proteins and its cognate GAP (27–29), gave no binding saturation signal, even at high GAP concentration (100 µM) (SI Appendix, Fig. S5), indicating a rather low binding affinity, although GAP1IP4BP is an active RasGAP in solution (11, 13). The colocalization of both proteins in the same membrane would induce close proximity and facilitate an effective interaction. In vivo, Ras is attached to cellular membranes due to lipidation of its C terminus. For Ras isoforms H- and N-Ras, this modification involves farnesylation of cysteine 181 and further processing of the C-terminal Caax box (30). To mimic Ras interaction with membranes, we used the C-terminal His6-tag of our Ras construct (Materials and Methods), which is expected to bind to vesicles containing Ni-NTA lipids, such as 1,2-dioleoyl-sn-glycero-3-{[N-(5-amino-1-carboxylpentyl)iminodiacetic acid]succinyl} (nickel salt) (Ni-NTA-DOGS). This method has been successfully used for several proteins (31, 32), including the small GTP binding protein Arf6 (33). When both proteins were bound to PC:DOGS-NTA:PS:PI3P (52.5:30:10:7.5) vesicles (SI Appendix, Fig. S4B), there was indeed a strong activation of the GTPase activity of Ras (Fig. 2C). As expected when both enzyme and substrate are attached to membrane vesicles, the reaction kinetics did not follow a normal monoexponential equation. The association rate constant decreases with time due to enzyme and substrate segregation at longer times as previously observed (33–35). To check whether it is sufficient to localize the proteins to membranes, or whether colocalization in the same membrane compartment and thus proximity is required, we measured the GTP hydrolysis when Rasal and Ras were bound to different vesicles. We thus preincubated Rasal with vesicles without DOGS-NTA, and RasGTP with vesicles without PS and PI3P. When both populations were mixed, Rasal did not show any RasGAP activity, as shown in Fig. 2C.
Members of the Gap1 family such as Rasal and GAP1IP4BP possess GAP activity for both Ras and Rap (11). Interestingly, it has been shown, using cells overexpressing Rasal and Rap (11), that RapGAP activity of Rasal in vivo appeared to be independent of its translocation to plasma membrane. To check the possible effect of Rasal and Rap colocalization in membranes using the system described above, we bound Rasal and Rap1B(1–180)-His6 to vesicles. As can be seen in Fig. 2D, it did not induce a relevant increase of Rasal RapGAP activity, arguing that for the latter activity, colocalization does not provide any advantage.
Allosteric Stimulation from Lipid Binding of C2 Domains.
It has been previously shown that the tandem C2 domains increase the affinity of GAP1IP4BP, a close homolog of Rasal, for Ras (13), although it is unclear whether this is due to their direct participation in Ras binding or to conformational changes in the GAP domain by the presence of C2AB. Although we have shown here for Rasal that colocalization seems to be essential for activity, it might still be possible that membrane binding of C2 domains and their correct orientation has an additional direct effect on RasGAP activity. To test for such a direct effect, it was necessary to have a system where both proteins colocalized in the same membrane but where the C2AB domains were unable to bind lipids. The single mutants D21A and D202A, that eliminate two of the aspartic acid residues implicated in calcium interaction, have previously been used (15) to inhibit lipid interaction. To more drastically affect calcium binding, we designed a double [D21A/D202A, here called Rasal(DD) mutant] and a quadruple mutant with two additional mutations of aspartic acids [D21A/D76R/D149R/D202A, here called Rasal(DDDD) mutant]. The mutations were picked based on the high sequence homology with synaptotagmin 1 (SI Appendix, Fig. S6A) and the crystallographic structure of its C2A domain bound to calcium (36).
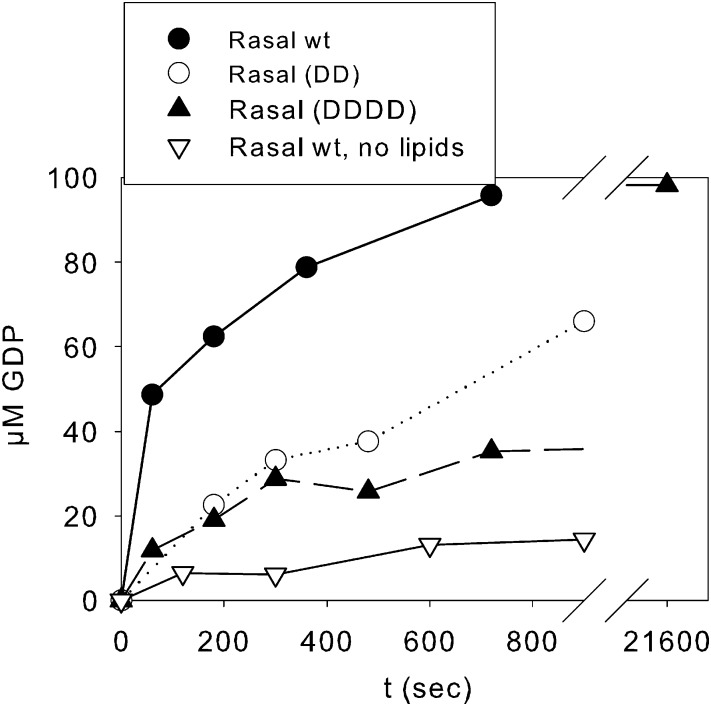
As expected, Rasal(DD) and Rasal(DDDD) mutants did not bind vesicles containing PS and PI3P (SI Appendix, Fig. S6B). To colocalize both mutants with Ras in lipid vesicles, we took advantage of the unexpected capability of Ni-NTA-DOGS to bind GAP domains. Rasal WT could bind PC:NTA-DOGS vesicles (SI Appendix, Fig. S6C), unless they did not contain PS or PI3P. This interaction was not mediated by the C2 domains, as Rasal(DD) and Rasal(DDDD) mutants, as well as RasalΔC2AB mutant, lacking the C2 domains, were able to bind these vesicles. GAP1IP4BP isolated GAP domain could also interact with the PC:NTA-DOGS vesicles (SI Appendix, Fig. S6C), leading us to the conclusion that the Ni-NTA-DOGS binding site is in the GAP domain. This binding did not interfere with GAP activity, as the GAP domain of the closely related GAP1IP4BP colocalized with Ras in PC:NTA-DOGS vesicles possessed the same RasGAP activity as that of the unbound protein with free Ras in solution (SI Appendix, Fig. S6D). Rasal(DD) and Rasal(DDDD) mutants of Rasal colocalized with Ras in PC:NTA-DOGS:PS:PI3P vesicles were much less active than Rasal WT under the same conditions (Fig. 3). The structure of the proteins appears to be unperturbed because the mutants remained active RapGAPs (SI Appendix, Fig. S6E). However, the extent of Ras activation by the mutants on lipid membranes was still higher compared with soluble components. This shows that for full activation, colocalization of Ras and Rasal is not sufficient and requires direct interaction of the C2AB domains to lipids.
Fig. 3.
Binding of C2 domains to lipid vesicles contributes to Rasal RasGAP activity. (A) RasGAP activity of Rasal WT, (DD) and (DDDD) (2.5 µM) colocalized in PC:DOGS-NTA:PS:PI3P 52.5:30:10:7.5 vesicles with 100 µM Ras-GTP. To compare, RasGAP activity of Rasal in the absence of lipids is also shown.
Rasal C2AB Domains Induce Curvature in Lipid Vesicles.
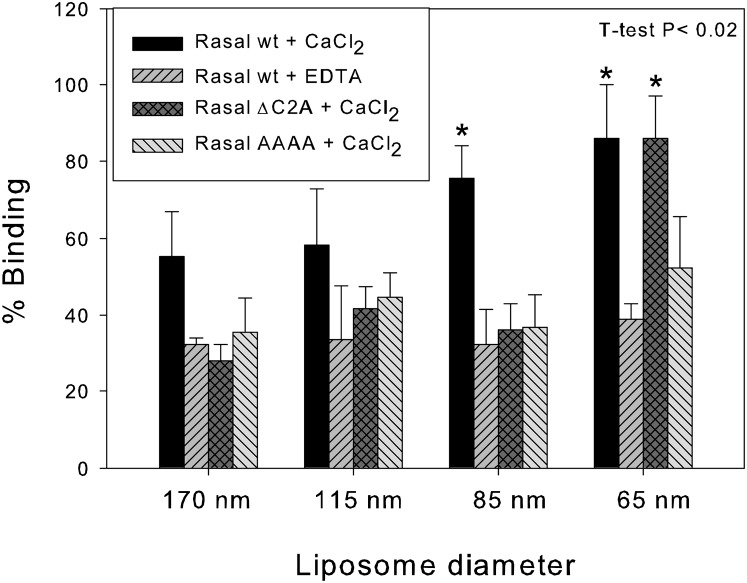
Rasal C2 domains have high sequence homology with synaptotagmins. These proteins are implicated in neurotransmitter exocytosis and are able to promote membrane fusion. Their interactions with membranes, mediated by calcium binding, promote the insertion of four hydrophobic loops (two per C2 domain) into the membrane. This insertion induces membrane curvature, which triggers membrane fusion (26). Interestingly, the hydrophobic residues able to induce membrane curvature are also conserved in Rasal (as V22, V78, L149, and V206) (SI Appendix, Fig. S6A). This opens the possibility of Rasal C2 domains also being “active domains,” able to sense and or induce membrane curvature and therefore to be implicated in membrane fusion or fission events. To test this possibility, we performed two kinds of experiments previously applied to synaptotagmin 1 (26). We first measured Rasal binding to liposomes of different diameters. If the C2 domains induce curvature due to the insertion of the hydrophobic loops, they should show a preference of binding to curved membranes. We prepared size-fractionated liposomes of ∼170, 115, 85, and 65 nm diameters (experimentally measured by dynamic light scattering) and checked that the calcium concentration used (1 mM) for Rasal binding did not induce vesicle fusion (SI Appendix, Fig. S7). Rasal WT in the presence of calcium showed a higher affinity for smaller vesicles (Fig. 4 and SI Appendix, Fig. S8A), with the highest affinities for liposomes of 85 and 65 nm. In the presence of EDTA, when the binding to liposomes is calcium independent, we did not observe any preference for smaller vesicles. Rasal∆C2A, which lacks two of the four hydrophobic residues, needs a bigger curvature to significantly increase its membrane binding. Thus, by decreasing the number of bulky hydrophobic residues a very low lipid packing is required to increase the probability of insertion. Finally, a Rasal mutant, called Rasal(AAAA), with the four bulky hydrophobic residues (V22, V78, L149, and V206) mutated to alanine, had no preference for curved membranes.
Fig. 4.
Rasal binding preference for curved vesicles. The binding of 1 µM Rasal WT, Rasal∆C2A, and Rasal(AAAA) to LUVs of different diameter (260 µM total lipid concentration), in the presence of 1 mM CaCl2 or 1 mM EDTA was measured by cosedimentation.
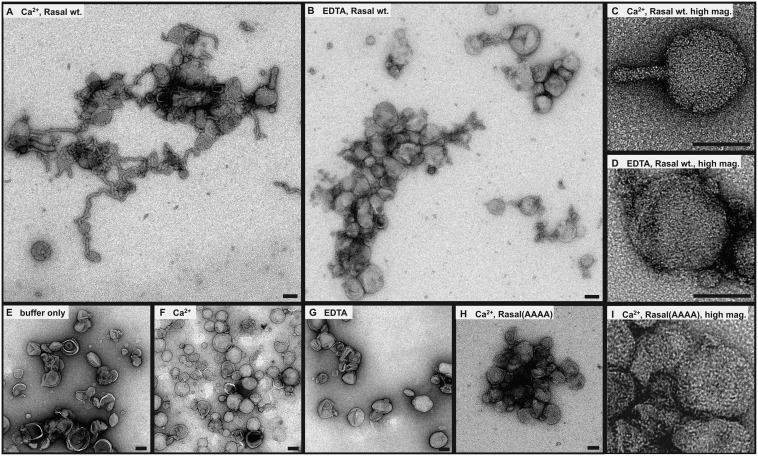
We went on to test the ability of Rasal to induce tubulation of Folch lipid liposomes (100 nm diameter) using electron microscopy (EM). These lipids have been used instead of PC:PS:PI3P liposomes before (26, 37), because the latter are unstable under EM conditions (26). Rasal is able to bind Folch lipid liposomes (SI Appendix, Fig. S8B), and EM reveals that the protein induces membrane tubulation (Fig. 5 A and C and SI Appendix, Figs. S9 and S10). Remarkably, the depletion of free Ca2+ by EDTA inhibits the tubulation, suggesting that it is induced by a Ca2+-mediated interaction of Rasal with the membrane (Fig. 5 B and D) (SI Appendix, Figs. S9 and S10). Control experiments in absence of protein showed that this effect is not due to the buffer conditions (Fig. 5 E–G). To check whether the Ca2+-dependent tubulation is induced by the insertion of hydrophobic residues of C2 domains, we performed the same experiments using Rasal(AAAA), which lacks the hydrophobic residues thought to be responsible for tubulation. Although the protein seems to localize to the liposomes (SI Appendix, Fig. S8B), and it causes their clustering, it does not induce tubulation (Fig. 5 H and I and SI Appendix, Figs. S9 and S10). This suggests that the tandem C2 domains and the conserved hydrophobic residues V22, V78, L149, and V206 are essential for changing the curvature of the membrane.
Fig. 5.
Ca2+-dependent tubulation of vesicles by Rasal. (A–H) Electron micrographs of negatively stained vesicles incubated with the indicated additives for 30 min at RT. (A and C) Vesicles tubulate in the presence of 1 mM Ca2+ and 10 µM Rasal WT. (B and D) Tubulation is prevented when EDTA is used to complex Ca2+ ions. (E–G) Control experiments showing that neither 1 mM Ca2+ nor 1 mM EDTA alone have an effect on the vesicles. (H and I) Mutation of the hydrophobic residues leads to vesicle clustering instead of tubulation in the presence of 1 mM Ca2+ and 10 µM protein. (Scale bars, 100 nm.)
Discussion
Rasal expression is down-regulated in several types of cancer, the consequence of which should be enhanced Ras activation in cancer cells containing WT Ras. However, whereas Rasal has a consistent RapGAP activity, its RasGAP activity, which is highly regulated by the interaction of its C2 domains with calcium and lipids, seems to be biologically at least as important. We thus set out to explore the molecular mechanism of this regulation and the contribution of the Rasal C2 domains. Here we show that the C2A domain binds PS, and the C2B domain interacts with several PIPs. This kind of selectivity for both types of negative lipids has also been described for synaptotagmins (22) and Rabphilin3A (38, 39), although only for Rabphilin3A a calcium-dependent PIP binding has been described (39). A comparison of Rasal C2B and Rabphilin3A C2A domain sequences suggests that Rasal C2B PIP specificity is provided by negative residues inside the CBL2 loop (see SI Appendix for a longer discussion).
The results presented here show that calcium and membrane interaction activates Rasal RasGAP activity by (i) temporal, (ii) spatial, and (iii) conformational regulation. The (i) temporal regulation is introduced by the necessity of a high intracellular calcium concentration (15) for Rasal membrane binding, which will allow calcium release-dependent Rasal-mediated Ras inactivation. The (ii) spatial regulation is controlled by the low affinity of Rasal for Ras. The colocalization of Ras and Rasal in close proximity in the same membrane would increase their local concentration, allowing their interaction only in spatially defined areas of the cell. Such colocalization would increase 1,000 times the effective protein concentration (40–42). Furthermore, the Rasal selectivity for PIPs could also be important for this spatial regulation although cell biology and “in vivo” experiments would be required to completely elucidate its physiological relevance. Rasal can bind PIPs as well as PS, and with similar affinity. Although PS is a more abundant lipid than PIPs, the later are enriched in specific membranes or domains. Different PIPs are distributed in different cellular compartments and could therefore control where Rasal will inactivate Ras. Walker et al. (15) showed that Rasal interacted with the plasma membrane. However, the authors did not check if Rasal also localized to other subcellular membranes. Walker et al. (15) also showed that Rasal did not associate with the plasma membrane after EGF receptor stimulation, inferring that the association was not controlled by PI3K activation and leading to the conclusion that Rasal did not bind PI3,4P. Although more experiments are needed to completely rule out a contribution of PI3,4P, it is a possibility that an increase of this lipid concentration is not required for the membrane binding of Rasal, as it is already highly controlled by calcium. This suggests that PI4,5P, a constitutive and abundant PIP (25), could interact with Rasal C2B domain in the plasma membrane. As it has been suggested before for GAP1m (43), its capability to associate with PIP2 would target RASAL out of lipid rafts, because PIP2 does not accumulate in rafts (44). As Ras has been shown to be absent from rafts after GTP loading, PIP2–Rasal interaction would target Rasal to activated Ras in the lipid-unordered phase. Furthermore, PI4,5P is enriched in membrane structures prone to be endocyted, which can be related to Rasal capability to sense and induce membrane curvature (see below). The (iii) conformational regulation is introduced by the fact that a complete Rasal RasGAP activation needs the interaction of C2AB domains with lipids in the membrane. It can be speculated that, as Rasal affinity for Ras is low, a favorable orientation for the interaction with Ras is achieved by the binding of the C2 domains to lipids. In addition, an allosteric activation similar to the one whereby GAP1IP4BP regulates RapGAP activity via the C2 domains (13) cannot be ruled out. Thus, in contrast to GAP1IP4BP, C2AB domains of Rasal are required for both RasGAP and RapGAP activities.
An interesting and intriguing observation is the capability of Rasal to sense and induce membrane curvature in vitro. Although cell biology studies are required to confirm such behavior in vivo, the results presented here open a unique avenue toward Rasal biology. It is tempting to speculate that Rasal is involved in Ca2+-mediated membrane fusion processes like endo- or exocytosis. In fact, all tandem C2 domain-containing proteins able to bind lipid membranes are involved in exocytosis. Most receptors implicated in Ras activation are endocytosed and eventually recycled (45). Endocytosis has been shown to be directly stimulated by interaction of activated Ras with the Rab5-GEF RIN1 (Ras interaction proteins 1) (46, 47). Furthermore, Ras directly initiates clathrin-independent endocytosis named macropinocytosis (48). Thus, Rasal could stabilize bud structures in these processes. As PI(4,5)P is necessary and enriched for endocytosis (dependent and independent) (45, 48), the presence of this lipid would increase Rasal accumulation and in turn Ras deactivation in a negative feedback loop. Even if Rasal is not directly implicated in budding, the C2AB domains could be used as membrane curvature sensors, driving Rasal to membrane regions of high curvature to turn off Ras-GTP. A similar kind of mechanism has been described before for ArfGAP1, a GTPase-activating protein of the small G protein Arf1. Arf1 interacts with coat protein I (COPI), a coat complex that promotes the budding of small transport vesicles between endoplasmic reticulum and the Golgi (49). ArfGAP1 is an active GAP only when it is bound to lipid membrane (50). It binds lipids by the ArfGAP1 lipid packing sensor (ALPS) motif, a high sensitive membrane interacting domain (51), and therefore it only binds to highly curved membranes, like transport vesicles and buds of this transport vesicle. Thus, it will inactivate Arf1 function as budding promoter only once the bud is formed (50). However, in contrast to ArfGAP1, membrane curvature does not increase the RasGAP (SI Appendix, Fig. S11) activity of Rasal. Its activity is rather tightly controlled by colocalization, C2 domain binding to lipids, and high calcium concentration.
Materials and Methods
Protein Expression and Purification.
Rasal WT and mutants were purified as previously described (13). A construct encoding N-Ras(1–179) with a C-terminal His6-tag was cloned in a ptac expression vector and expressed in Escherichia coli CK600K cells. Rap1B(1–180) with a C-terminal His-tag was cloned in pet-21d expression vector and expressed in BL21 DE3 Codon + RIL cells. These proteins were purified by Ni2+-NTA affinity chromatography followed by gel filtration chromatography. They are referred to as Ras and Rap throughout this study.
Vesicle Preparation.
Refer to SI Appendix for lipid description and further information. The multilamellar vesicles (MLVs) were prepared rehydrating in a buffer containing 150 mM NaCl, and 25 mM Tris⋅HCl (pH 7.5) a dried mixture of lipids. The suspension was then subjected to nine cycles of freezing and thawing and used directly for the experiments.
For the preparation of fixed-sized large unilamellar vesicles (LUVs), MLVs (500 µM lipids) of PC:PS:PI3P: rhodamine-PE at 87:7.5:5:0.5 molar ratio were passed 10 times through a 200 nm filter (Whatman) and later another 20 times through a 200-, 100-, 50-, or 30-nm filter, using the Avanti Mini-Extruder (Avanti Polar Lipids). The average hydrodynamic diameter of the liposomes shown in Fig. 4 was determined by dynamic light scattering, being 170, 115, 85, and 65 nm, respectively (SI Appendix, Fig. S7). The total concentration of the obtained LUVs was measured using the absorbance of rhodamine at 543 nm.
Cosedimentation Assays.
Vesicles (MLVs or LUVs) were incubated with the different proteins used in this work. Ca2+ or EDTA was added to a final concentration of 1 mM. After 30 min at room temperature the liposomes were centrifuged in a TLA-45 rotor at 125,000 × g for 30 min (MLVs) or 1 h 30 min (LUVs) at 10 °C. The pellets were resuspended in buffer, up to the same volume as the supernatant. Equal amounts of the supernatants and resuspended pellets volumes (usually 10 µL) were analyzed by SDS/PAGE. Proteins were subsequently visualized by Coomassie staining and quantified using ImageJ program. The percentage of binding was calculated as the pellet protein signal percentage of the total protein (pellet + supernatant).
GTP Hydrolysis.
Nucleotide exchange was performed according to the procedure described by ref. 52. Reverse-phase HPLC was used to monitor the kinetics of GTP hydrolysis as described before (53). The standard buffer for all experiments contained 25 mM Tris pH 7.5 and 100 mM NaCl. A total of 100 μM Ras-GTP and 2.5 μM Rasal were used. For more details about the different conditions used, refer to SI Appendix.
Electron Microscopy.
A total of 500 µM Folch lipid LUVs (100 nm diameter) were incubated with 10 µM Rasal WT or mutants for 30 min at room temperature, in the presence of 1 mM CaCl2 or EDTA. Samples were prepared for electron microscopy according to Ohi et al. (54) with minor changes (SI Appendix). Specimens were imaged at room temperature using a JEM1400 electron microscope (JEOL) equipped with a LaB6 electron source. The microscope was operated at an acceleration voltage of 120 kV. Images were taken at a magnification of either 10,400× or 53,800× and recorded on a 2,000 × 2,000 14 μm-per-pixel FastScan-F214 CCD camera (TVIPS).
Supplementary Material
Acknowledgments
We thank Carolin Kroerner for Rap1B(1-180)-His cloning and purification and Carsten Kötting for the generous gift of the ptac-Ras-His plasmid. S.R. is grateful to R. S. Goody for continuous support. This work was supported by an Alexander von Humboldt Foundation Research Fellowship (to B.S.), Deutsche Forschungsgemeinschaft Grant RA 1781/1-1 (to S.R.), Fonds der chemischen Industrie Grant 684052 (to E.B.), and the Max Planck Society (S.R. and E.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201658110/-/DCSupplemental.
References
- 1.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 2.Bos JL. ras oncogenes in human cancer: A review. Cancer Res. 1989;49(17):4682–4689. [PubMed] [Google Scholar]
- 3.Malumbres M, Barbacid M. RAS oncogenes: The first 30 years. Nat Rev Cancer. 2003;3(6):459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 4.Dasgupta B, Dugan LL, Gutmann DH. The neurofibromatosis 1 gene product neurofibromin regulates pituitary adenylate cyclase-activating polypeptide-mediated signaling in astrocytes. J Neurosci. 2003;23(26):8949–8954. doi: 10.1523/JNEUROSCI.23-26-08949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cichowski K, Jacks T. NF1 tumor suppressor gene function: Narrowing the GAP. Cell. 2001;104(4):593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 6.Kolfschoten IG, et al. A genetic screen identifies PITX1 as a suppressor of RAS activity and tumorigenicity. Cell. 2005;121(6):849–858. doi: 10.1016/j.cell.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Jin H, et al. Epigenetic silencing of a Ca(2+)-regulated Ras GTPase-activating protein RASAL defines a new mechanism of Ras activation in human cancers. Proc Natl Acad Sci USA. 2007;104(30):12353–12358. doi: 10.1073/pnas.0700153104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohta M, et al. Decreased expression of the RAS-GTPase activating protein RASAL1 is associated with colorectal tumor progression. Gastroenterology. 2009;136(1):206–216. doi: 10.1053/j.gastro.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 9.Seto M, et al. Reduced expression of RAS protein activator like-1 in gastric cancer. Int J Cancer. 2011;128(6):1293–1302. doi: 10.1002/ijc.25459. [DOI] [PubMed] [Google Scholar]
- 10.Bell A, Bell D, Weber RS, El-Naggar AK. CpG island methylation profiling in human salivary gland adenoid cystic carcinoma. Cancer. 2011;117(13):2898–2909. doi: 10.1002/cncr.25818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kupzig S, et al. GAP1 family members constitute bifunctional Ras and Rap GTPase-activating proteins. J Biol Chem. 2006;281(15):9891–9900. doi: 10.1074/jbc.M512802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kupzig S, Bouyoucef-Cherchalli D, Yarwood S, Sessions R, Cullen PJ. The ability of GAP1IP4BP to function as a Rap1 GTPase-activating protein (GAP) requires its Ras GAP-related domain and an arginine finger rather than an asparagine thumb. Mol Cell Biol. 2009;29(14):3929–3940. doi: 10.1128/MCB.00427-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sot B, et al. Unravelling the mechanism of dual-specificity GAPs. EMBO J. 2010;29(7):1205–1214. doi: 10.1038/emboj.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockyer PJ, Kupzig S, Cullen PJ. CAPRI regulates Ca(2+)-dependent inactivation of the Ras-MAPK pathway. Curr Biol. 2001;11(12):981–986. doi: 10.1016/s0960-9822(01)00261-5. [DOI] [PubMed] [Google Scholar]
- 15.Walker SA, et al. Identification of a Ras GTPase-activating protein regulated by receptor-mediated Ca2+ oscillations. EMBO J. 2004;23(8):1749–1760. doi: 10.1038/sj.emboj.7600197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cozier GE, et al. GAP1IP4BP contains a novel group I pleckstrin homology domain that directs constitutive plasma membrane association. J Biol Chem. 2000;275(36):28261–28268. doi: 10.1074/jbc.M000469200. [DOI] [PubMed] [Google Scholar]
- 17.Lockyer PJ, et al. Identification of the ras GTPase-activating protein GAP1(m) as a phosphatidylinositol-3,4,5-trisphosphate-binding protein in vivo. Curr Biol. 1999;9(5):265–268. doi: 10.1016/s0960-9822(99)80116-x. [DOI] [PubMed] [Google Scholar]
- 18.Rizo J, Südhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. J Biol Chem. 1998;273(26):15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- 19.Török Z, et al. Evidence for a lipochaperonin: Association of active protein-folding GroESL oligomers with lipids can stabilize membranes under heat shock conditions. Proc Natl Acad Sci USA. 1997;94(6):2192–2197. doi: 10.1073/pnas.94.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vrljic M, et al. Post-translational modifications and lipid binding profile of insect cell-expressed full-length mammalian synaptotagmin 1. Biochemistry. 2011;50(46):9998–10012. doi: 10.1021/bi200998y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerrero-Valero M, Marín-Vicente C, Gómez-Fernández JC, Corbalán-García S. The C2 domains of classical PKCs are specific PtdIns(4,5)P2-sensing domains with different affinities for membrane binding. J Mol Biol. 2007;371(3):608–621. doi: 10.1016/j.jmb.2007.05.086. [DOI] [PubMed] [Google Scholar]
- 22.Radhakrishnan A, Stein A, Jahn R, Fasshauer D. The Ca2+ affinity of synaptotagmin 1 is markedly increased by a specific interaction of its C2B domain with phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2009;284(38):25749–25760. doi: 10.1074/jbc.M109.042499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torrecillas A, Laynez J, Menéndez M, Corbalán-García S, Gómez-Fernández JC. Calorimetric study of the interaction of the C2 domains of classical protein kinase C isoenzymes with Ca2+ and phospholipids. Biochemistry. 2004;43(37):11727–11739. doi: 10.1021/bi0489659. [DOI] [PubMed] [Google Scholar]
- 24.van den Bogaart G, Meyenberg K, Diederichsen U, Jahn R. Phosphatidylinositol 4,5-bisphosphate increases Ca2+ affinity of synaptotagmin-1 by 40-fold. J Biol Chem. 2012;287(20):16447–16453. doi: 10.1074/jbc.M112.343418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9(2):99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 26.Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316(5828):1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- 27.Leonardy S, et al. Regulation of dynamic polarity switching in bacteria by a Ras-like G-protein and its cognate GAP. EMBO J. 2010;29(14):2276–2289. doi: 10.1038/emboj.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ismail SA, et al. Arl2-GTP and Arl3-GTP regulate a GDI-like transport system for farnesylated cargo. Nat Chem Biol. 2011;7(12):942–949. doi: 10.1038/nchembio.686. [DOI] [PubMed] [Google Scholar]
- 29.Veltel S, Gasper R, Eisenacher E, Wittinghofer A. The retinitis pigmentosa 2 gene product is a GTPase-activating protein for Arf-like 3. Nat Struct Mol Biol. 2008;15(4):373–380. doi: 10.1038/nsmb.1396. [DOI] [PubMed] [Google Scholar]
- 30.Brunsveld L, Waldmann H, Huster D. Membrane binding of lipidated Ras peptides and proteins—the structural point of view. Biochim Biophys Acta. 2009;1788(1):273–288. doi: 10.1016/j.bbamem.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Dietrich C, Schmitt L, Tampé R. Molecular organization of histidine-tagged biomolecules at self-assembled lipid interfaces using a novel class of chelator lipids. Proc Natl Acad Sci USA. 1995;92(20):9014–9018. doi: 10.1073/pnas.92.20.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh KJ, et al. A membrane-targeted BID BCL-2 homology 3 peptide is sufficient for high potency activation of BAX in vitro. J Biol Chem. 2006;281(48):36999–37008. doi: 10.1074/jbc.M602341200. [DOI] [PubMed] [Google Scholar]
- 33.Ismail SA, Vetter IR, Sot B, Wittinghofer A. The structure of an Arf-ArfGAP complex reveals a Ca2+ regulatory mechanism. Cell. 2010;141(5):812–821. doi: 10.1016/j.cell.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 34.Berry H. Monte carlo simulations of enzyme reactions in two dimensions: Fractal kinetics and spatial segregation. Biophys J. 2002;83(4):1891–1901. doi: 10.1016/S0006-3495(02)73953-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takakuwa Y, Nishino H, Ishibe Y, Ishibashi T. Properties and kinetics of membrane-bound enzymes when both the enzyme and substrate are components of the same microsomal membrane. Studies on lathosterol 5-desaturase. J Biol Chem. 1994;269(45):27889–27893. [PubMed] [Google Scholar]
- 36.Sutton RB, Davletov BA, Berghuis AM, Südhof TC, Sprang SR. Structure of the first C2 domain of synaptotagmin I: A novel Ca2+/phospholipid-binding fold. Cell. 1995;80(6):929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 37.Gerlach H, et al. HIV-1 Nef membrane association depends on charge, curvature, composition and sequence. Nat Chem Biol. 2010;6(1):46–53. doi: 10.1038/nchembio.268. [DOI] [PubMed] [Google Scholar]
- 38.Coudevylle N, Montaville P, Leonov A, Zweckstetter M, Becker S. Structural determinants for Ca2+ and phosphatidylinositol 4,5-bisphosphate binding by the C2A domain of rabphilin-3A. J Biol Chem. 2008;283(51):35918–35928. doi: 10.1074/jbc.M804094200. [DOI] [PubMed] [Google Scholar]
- 39.Montaville P, et al. The PIP2 binding mode of the C2 domains of rabphilin-3A. Protein Sci. 2008;17(6):1025–1034. doi: 10.1110/ps.073326608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kholodenko BN, Hoek JB, Westerhoff HV. Why cytoplasmic signalling proteins should be recruited to cell membranes. Trends Cell Biol. 2000;10(5):173–178. doi: 10.1016/s0962-8924(00)01741-4. [DOI] [PubMed] [Google Scholar]
- 41.Kuriyan J, Eisenberg D. The origin of protein interactions and allostery in colocalization. Nature. 2007;450(7172):983–990. doi: 10.1038/nature06524. [DOI] [PubMed] [Google Scholar]
- 42.Gureasko J, et al. Membrane-dependent signal integration by the Ras activator Son of sevenless. Nat Struct Mol Biol. 2008;15(5):452–461. doi: 10.1038/nsmb.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yarwood S, Bouyoucef-Cherchalli D, Cullen PJ, Kupzig S. The GAP1 family of GTPase-activating proteins: spatial and temporal regulators of small GTPase signalling. Biochem Soc Trans. 2006;34(Pt 5):846–850. doi: 10.1042/BST0340846. [DOI] [PubMed] [Google Scholar]
- 44.Hanzal-Bayer MF, Hancock JF. Lipid rafts and membrane traffic. FEBS Lett. 2007;581(11):2098–2104. doi: 10.1016/j.febslet.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 46.Han L, Colicelli J. A human protein selected for interference with Ras function interacts directly with Ras and competes with Raf1. Mol Cell Biol. 1995;15(3):1318–1323. doi: 10.1128/mcb.15.3.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tall GG, Barbieri MA, Stahl PD, Horazdovsky BF. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev Cell. 2001;1(1):73–82. doi: 10.1016/s1534-5807(01)00008-9. [DOI] [PubMed] [Google Scholar]
- 48.Donaldson JG, Porat-Shliom N, Cohen LA. Clathrin-independent endocytosis: A unique platform for cell signaling and PM remodeling. Cell Signal. 2009;21(1):1–6. doi: 10.1016/j.cellsig.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lippincott-Schwartz J, Cole NB, Donaldson JG. Building a secretory apparatus: Role of ARF1/COPI in Golgi biogenesis and maintenance. Histochem Cell Biol. 1998;109(5–6):449–462. doi: 10.1007/s004180050247. [DOI] [PubMed] [Google Scholar]
- 50.Antonny B, et al. Membrane curvature and the control of GTP hydrolysis in Arf1 during COPI vesicle formation. Biochem Soc Trans. 2005;33(Pt 4):619–622. doi: 10.1042/BST0330619. [DOI] [PubMed] [Google Scholar]
- 51.Bigay J, Casella JF, Drin G, Mesmin B, Antonny B. ArfGAP1 responds to membrane curvature through the folding of a lipid packing sensor motif. EMBO J. 2005;24(13):2244–2253. doi: 10.1038/sj.emboj.7600714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tucker J, et al. Expression of p21 proteins in Escherichia coli and stereochemistry of the nucleotide-binding site. EMBO J. 1986;5(6):1351–1358. doi: 10.1002/j.1460-2075.1986.tb04366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scrima A, Thomas C, Deaconescu D, Wittinghofer A. The Rap-RapGAP complex: GTP hydrolysis without catalytic glutamine and arginine residues. EMBO J. 2008;27(7):1145–1153. doi: 10.1038/emboj.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohi M, Li Y, Cheng Y, Walz T. Negative staining and image classification - powerful tools in modern electron microscopy. Biol Proced Online. 2004;6:23–34. doi: 10.1251/bpo70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.