Abstract
T-cell recognition of ligands is polyspecific. This translates into antiviral T-cell responses having a range of potency and specificity for viral ligands. How these ligand recognition patterns are established is not fully understood. Here, we show that an activation threshold regulates whether robust CD4 T-cell activation occurs following viral infection. The activation threshold was variable because of its dependence on the density of the viral peptide (p)MHC displayed on infected cells. Furthermore, the activation threshold was not observed to be a specific equilibrium affinity (KD) or half-life (t1/2) of the TCR–viral pMHC interaction, rather it correlated with the confinement time of TCR–pMHC interactions, i.e., the half-life (t1/2) of the interaction accounting for the effects of TCR–pMHC rebinding. One effect of a variable activation threshold is to allow high-density viral pMHC ligands to expand CD4 T cells with a variety of potency and peptide cross-reactivity patterns for the viral pMHC ligand, some of which are only poorly activated by infections that produce a lower density of the viral pMHC ligand. These results argue that antigen concentration is a key component in determining the pattern of KD, t1/2 and peptide cross-reactivity of the TCRs expressed on CD4 T cells responding to infection.
Keywords: kinetic proofreading T cell receptor
Upon infection, CD4 T cells scan antigen-presenting cells (APCs) for pathogen-derived peptides displayed on host MHC class II proteins (pMHCII). If a T-cell–APC encounter results in intracellular signals that exceed a threshold, naïve CD4 T cells are triggered to undergo clonal expansion and acquire effector cell functions that help eliminate pathogens (1). Thymic selection equips mature CD4 T cells with T-cell receptors (TCRs) that preferentially react with specific foreign antigens bound to MHCII molecules (2). Despite the focusing of CD4 T-cell reactivity toward unique ligands, mature CD4 T cells are polyspecific, capable of being activated by multiple, distinct pMHCII ligands (3, 4). T-cell polyreactivity can have both beneficial and detrimental effects during antiviral responses and may allow pathogen-derived peptides to activate autoimmune disease-inducing T cells (5–7). Thus, understanding how T-cell reactivity patterns develop has clear implications for vaccine strategies, as well as for understanding the origins of some immune and autoimmune-mediated diseases.
The biophysical parameters of TCR–pMHC interactions that regulate T-cell activation have been studied in vitro and in vivo (1, 8, 9). Two mechanisms allow pMHC ligands to have a stronger or weaker potency to induce T-cell activation. First, different peptides may have an enhanced or diminished ability to be processed, loaded onto to MHC, and presented on the surface of APC. This can result in peptides derived from the same protein being displayed at up to 250-fold different densities (10). Second, pMHC complexes may engage a TCR with different equilibrium affinities (KD) or binding kinetics. T cells expressing TCRs with a weaker KD or shorter half-life (t1/2) for the pMHC complex usually require a higher density of ligand to be activated than do T cells expressing TCRs with a stronger KD or longer t1/2 (1, 8, 11–19). We and others have also suggested, based on in vitro assays, that TCR–pMHC interactions with fast on-rates (kon) can have enhanced ligand potency because of the phenomenon of TCR–pMHC rebinding, the ability of a single TCR and pMHC complex to have multiple productive-binding events before diffusing away from each other (20, 21). The probability with which TCR–pMHC rebinding occurs is dependent on the kon of the interaction, with a single rebinding event having the effect of doubling the dwell time of an individual TCR–pMHC interaction. The TCR–pMHC confinement-time model suggests ligand potency is dependent upon an aggregation of half-lives (ta), taking into account the probability rebinding will occur. Whether the confinement-time model predicts the clonal expansion of T cells in vivo has not been tested.
The goal of the studies here was to identify factors that regulate antiviral CD4 T cells responses. We observed that a T-cell activation threshold determines whether robust antiviral CD4 T-cell expansion occurs. The threshold value was not set by a specific equilibrium affinity (KD) or half-life (t1/2) of TCR–viral pMHC interaction. Rather, it was dependent upon the density of the viral pMHC displayed on infected cells and correlated with the confinement time of TCR–pMHC interactions. One consequence of a variable activation threshold is that viral pMHC presented at a high density can robustly expand CD4 T cells with a variety of potency and peptide cross-reactivity patterns, some of which are very poorly represented in CD4 T-cell responses that are activated by the same viral pMHC presented at a lower density. These studies demonstrate that the density with which a viral pMHC is displayed can influence the pattern of KD, t1/2, and peptide cross-reactivity of the TCR expressed on CD4 T cells responding to infection.
Results
Vaccinia Viruses Expressing Peptides Fused to IAbβ Are Displayed on the Cell Surface of Infected APCs at a Higher Density than Peptides Fused to GFP.
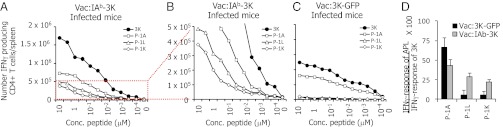
Pathogen challenge results in the expansion of T cells with a range of potencies and different patterns of cross-reactivity for viral epitopes (22). Because T-cell effector functions are often dependent upon ligand concentration, we hypothesized that the density in which a viral pMHC ligand is displayed will influence T-cell reactivity patterns following infection. To test this idea, we first constructed two sets of recombinant vaccinia viruses (Vac) that were expected to display, on infected APCs, different densities of viral pMHC complexes. One set expresses the foreign antigen peptide, 3K, or 3K altered peptide ligands (APLs) (peptides derived from the wild-type peptide sequence that carry individual amino acid substitutions) fused to green fluorescent protein (GFP) (Vac:3K–GFP). The other set expresses the same 3K or APL peptides fused to the carboxyl termini of the MHCII, IAbβ chain (Vac:IAb–3K) (Fig. S1 A and B). A comparison of in vitro T-cell activation experiments indicate that the 3K and 3K APL peptides are presented at approximately a 100-fold higher density on the surface of APCs infected with Vac:IAb–peptide viruses compared with Vac:peptide–GFP viruses (see Fig. S1 and SI Materials and Methods for explanation of the analysis).
When C57BL/6 mice were infected with Vac:3K–GFP, a limited 3K-reactive CD4 T-cell burst size was observed, making the peptide reactivity pattern of this T-cell response difficult to quantify. To overcome this limitation, we generated mice carrying the Vβ8.1 TCRβ chain of the IAb–3K–reactive B3K506 TCR as a transgene (506β mice). T cells in TCRβ mice express polyclonal TCRs and have been used extensively to study the dynamics of low frequency CD4 T-cell responses (23, 24).
Vac:IAb–3K Infections Induce Robust Expansion of CD4 T Cells with Cross-Reactivity Patterns That are Poorly Represented in Mice Infected with Vac:3K–GFP.
To identify peptide cross-reactivity patterns that arise in 506β mice following infections with Vac:3K–GFP and Vac:IAb–3K, ex vivo splenocytes were challenged to produce IFNγ in response to titrating amounts of soluble 3K, or 3K APLs that carry amino acid substitutions at the P-1 residue: P-1A, P-1L, and P-1K (Fig.1 and Fig. S2). 3K APLs carrying substitutions at the P-1 residue were analyzed because this residue is a TCRα chain contact in several TCR-IAb–3K cocrystal structures (25, 26), and 3K-reactive T cells can have different peptide fine specificities at this residue (27). Although the magnitude of response was greater in Vac:IAb–3K–infected mice, both Vac:3K–GFP and Vac:IAb–3K infection induced a strong IFNγ response directed at the 3K and P-1A peptides. In contrast, only Vac:IAb–3K–infected mice had a robust IFNγ response directed at the P-1L and P-1K peptides. When the IFNγ responses directed at the P-1A, P-1L, and P-1K peptides are compared with the response to the 3K peptide in the same mouse, Vac:3K–GFP infections expand a greater frequency of CD4 T cells that react with P-1A and underproduce ones that react with P-1L and P-1K, compared with mice infected with Vac:IAb–3K (Fig. 1D).
Fig. 1.
Vac:IAb–3K but not Vac:3K–GFP infection induces robust expansion of IFNγ-producing P-1L– and P-1K–reactive CD4 T cells. Mice (506β) were infected with Vac:IAb–3K (A and B), Vac:3K–GFP (C), or Vac:Neg (not shown). The scale of the y axis is the same in both B and C. Eight days postinfection, CD4 T cells from the mice were tested for the ability to produce IFNγ in response to titrating concentrations of soluble 3K (circles), P-1A (squares), P-1L (triangles), and P-1K (diamonds). Data are the average of six total mice per group. (D) Comparing the size of the APL response to the 3K response in the same mouse, Vac:3K–GFP mice expanded a greater percentage of P-1A–reactive IFNγ-producing CD4 T cells (P < 0.01) and a lesser percentage of P-1L– and P-1K–reactive IFNγ-producing CD4 T cells (P < 0.001) than do mice infected with Vac:IAb–3K.
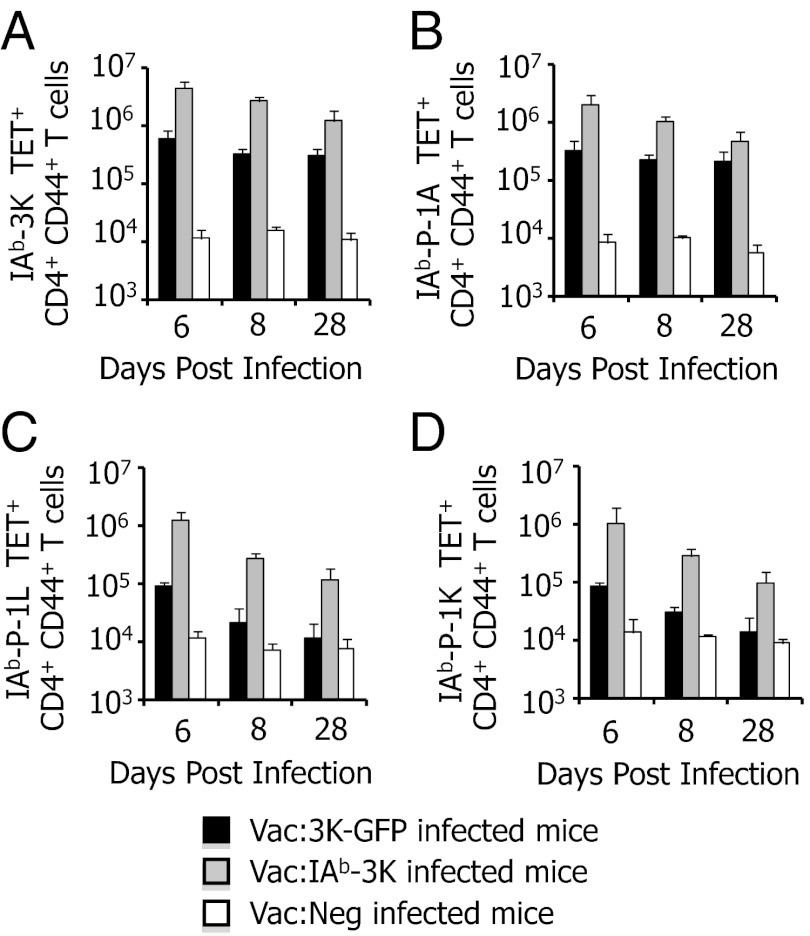
To determine whether Vac:3K–GFP infection caused 3K, P-1A, P-1L, or P-1K–reactive CD4 T cells to differentially accumulate in secondary lymphoid organs (SLO) other than the spleen, CD4 T cells from the mesenteric LN, cervical LN, bone marrow and peripheral blood were tested for the ability to be stained by IAb tetramers. Consistently, the greatest number of 3K, P-1A, P-1L, and P-1K tetramer-reactive CD4 T cells were found in the spleen, regardless of the time point (Figs. S3 and S4). Both Vac:3K–GFP– and Vac:IAb–3K–infected mice showed expanded populations of 3K and P-1A tetramer-reactive CD4 T cells on days 6, 8, and 28 postinfection in all SLO analyzed (Fig. 2 and Figs. S3 and S4). P-1L– and P-1K–reactive CD4 T cells were strongly expanded on days 6 and 8 postinfection with Vac:IAb–3K, and to a lesser extent withVac:3K–GFP. At 28 d postinfection, expanded populations of P-1L– and P-1K–reactive CD4 T cells were only found in Vac:IAb–3K–infected mice.
Fig. 2.
CD4 T-cell populations reactive to P-1L and P-1K in 506β mice are poorly expanded and not maintained in the spleens of mice infected with Vac:3K–GFP. Mice (506β) were infected with Vac:3K–GFP (black bars), Vac:IAb–3K (gray bars), or Vac:Neg (white bars). On days 6, 8, and 28 postinfection, 3K-reactive (A), P-1A–reactive (B), P-1L–reactive (C), and P-1K–reactive (D) CD4+ CD44+ T cells were quantified by staining with 1 μg/mL MHC tetramer. Data are the average of four (day 6), six (day 8), or five (day 28) total mice per group.
Vac:IAb–3K Infections Induce Robust Activation of Medium-Potency CD4 T Cells.
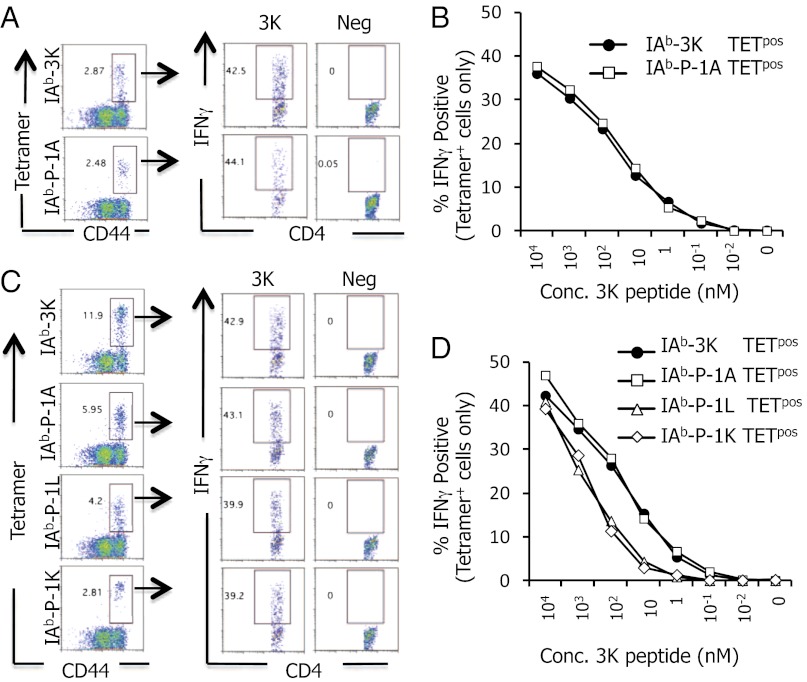
The findings above indicate that P-1L– and P-1K–reactive CD4 T cells are differentially expanded and maintained in 506β mice infected with Vac:IAb–3K versus Vac:3K–GFP. We hypothesized that the high density of IAb+3K presented on APC following Vac:IAb–3K infections was able to expand CD4 T cells with a lower potency for 3K, some of which cross-react with P-1L or P-1K. To test this idea, ex vivo IAb–3K, –P-1A, –P-1L, and –P-1K tetramer-positive CD4 T cells were challenged to produce IFNγ in response to titrating concentrations of 3K peptide (Fig. 3). IAb–3K and IAb–P-1A tetramer-positive cells, isolated from either Vac:IAb–3K– or Vac:3K–GFP–infected mice, produced IFNγ in response to similar concentrations of soluble 3K peptide (EC50 = 49–71 nM). In contrast, IAb–P-1L and IAb–P-1K tetramer-positive cells, isolated from Vac:IAb–3K–infected mice, were ∼10-fold less sensitive to soluble 3K peptide (EC50 = 540–630 nM) (Fig. 3 C and D and SI Materials and Methods). These data indicate that the P-1L and P-1K cross-reactive CD4 T cells are medium potency subsets of the 3K response and imply that the viral pMHC density is a key component for setting the threshold that regulates robust CD4 T-cell activation.
Fig. 3.
Repertoire of 3K-reactive CD4 T cells that cross-react with P-1L and P-1K have a weaker potency for soluble 3K peptide than do P-1A cross-reactive CD4 T cells. (A and B) IAb–3K and IAb–P-1A tetramer-positive CD4 T cells from 506β mice infected with Vac:3K–GFP were analyzed for the ability to produce IFNγ in response to 1 μg/mL soluble 3K peptide (A) or titrating amounts of soluble 3K peptide (B). (C and D) IAb–3K, –P-1A, –P-1L, and –P-1K tetramer-positive CD4 T cells from 506β mice infected with Vac:IAb–3K were analyzed for the ability to produce IFNγ in response to 1 μg/mL soluble 3K peptide (C) or titrating amounts of soluble 3K peptide (D). Data are the average of six total mice per group.
Ligand Potency and Density Regulates the Threshold for Robust Expansion of Antiviral CD4 T Cells.
To identify how the CD4 T-cell activation threshold is set, we analyzed the response of two monoclonal populations of CD4 T cells, B3K506 and B3K508, following infection in which viruses express different cross-reactive ligands. In vitro, soluble peptide versions of these ligands have different potencies to induce B3K508 and B3K506 CD4 T-cell proliferation. These potencies have been quantified based on the concentration of soluble peptide that induces half-maximal proliferation (EC50) (Table S1). For the activation of naïve B3K508 T cells, the 3K peptide has a strong potency, P5R has a medium potency and P8R has a weak potency. For the activation of naïve B3K506 T cells, the 3K, P5R, and P8R peptides all have a strong potency, whereas P-1A has a medium potency (20).
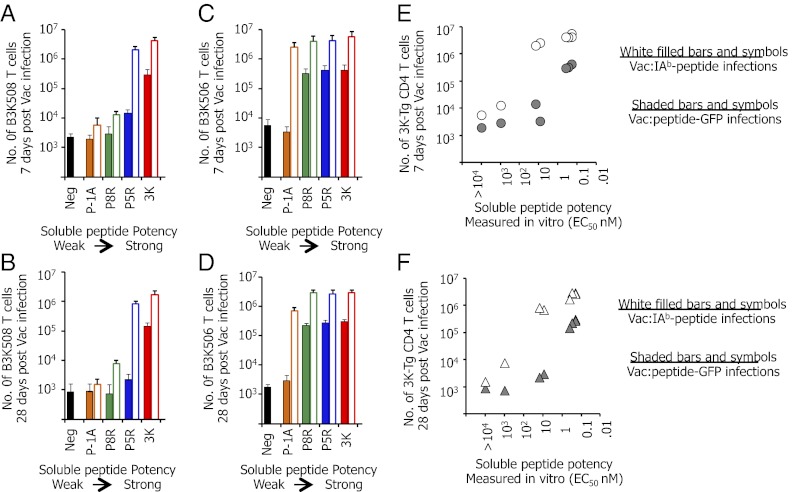
Naïve B3K508 or B3K506 CD4 T cells (1 × 105) were adoptively transferred into C57BL/6 mice and then recipient mice were virally infected. Because Vac-specific CD4 T cells are enriched in the spleens of mice infected with Vac (see above), we focused our experiments on T cells resident in this organ. Vac that carry medium potency 3K APLs fused to GFP induced limited accumulation of CD4 T cells 7 d postinfection that is largely absent on day 28. This is evidenced by the B3K 508 T cells responding to Vac:P5R–GFP (Fig. 4 A and B, blue solid bar) and B3K506 T cells responding to Vac:P-1A–GFP (Fig. 4 C and D, orange solid bar). In contrast, B3K508 and B3K506 CD4 T cells responding to Vac:peptide–GFP infections carrying stronger-potency ligands (in vitro EC50 values 10- to 30-fold greater) induced ∼100-fold greater expansion and maintenance (Fig. 4 A and B, red solid bar; and Fig. 4 C and D, green, blue, and red solid bars).
Fig. 4.
Infection of mice with Vac:IAb-linked peptides allows medium-potency ligands to induce massive CD4 T-cell expansion. A total of 1 × 105 CD45.1+ B3K508 (A and B) or B3K506 (C and D) T cells were transferred into C57BL/6 mice and infected with Vac:peptide–GFP (shaded bars) or Vac:IAb–peptide (white solid bars) viruses carrying 3K or APL peptides with different in vitro potencies. Recipient mice were analyzed on day 7 (A and C) or day 28 (B and D) postinfection for the number of B3K508 or B3K506 T cells present within the spleen. For B3K508 T cells, the 3K peptide has a strong potency, P5R has a medium potency, and P-1A and P8R have a weak potency. For B3K506 T cells, the 3K, P5, and P8R peptides all have a strong potency, whereas P-1A has a medium potency (Table S1). (E and F) Number of B3K508 and B3K506 T cells within the spleen of recipient mice on day 7 (E) or day 28 (F) postinfection with Vac:peptide–GFP (gray solid) or Vac:IAb–peptide (white filled) plotted against the soluble peptide potency [EC50 (nM)]. The soluble peptide potency is quantified as the concentration of the soluble peptide that induces half-maximal proliferation (EC50) of naïve CD4 T cells. Data are the average of six mice per group.
The limited B3K508 and B3K506 T-cell response following Vac infections that carry medium potency 3K APLs fused to GFP was not attributable to ignorance of the ligand. B3K508 CD4 T cells responding to Vac:P5R–GFP infections show an intermediate expression of CD69 (18 h postinfection), CD25, and inducible T-cell costimulator (ICOS) (48 h postinfection) (Fig. S5). Three days postinfection, CD4 T cells responding to Vac:P5R–GFP infections showed modest proliferation, although they were less capable of producing IFNγ compared with CD4 T cells responding to Vac:3K–GFP infections (Fig. S5). The poor ability to make IFNγ was observed on day 7 postinfection as well, and they did not become strong producers of Th2 or Th17 cytokines (Figs. S5 and S6). B3K508 CD4 T cells responding to Vac:P8R–GFP (a weak potency peptide) also showed a clear, albeit even more limited, response at early time points postinfection.
Importantly, analysis of the other monoclonal CD4 T-cell population, B3K506 CD4 T cells, using the identical experimental setup (for which 3K, P5R, and P8R are strong potency peptides) indicates that Vac:P5R–GFP and Vac:P8R–GFP infections can strongly activate CD4 T cells (Fig. 4 and Figs. S5 and S7). These data indicate that the P5R and P8R peptides are sufficiently presented following infections to drive a robust CD4 T-cell response, demonstrating that there is nothing defective with the Vac:P5R–GFP and Vac:P8R–GFP viruses. Similar to the B3K508 CD4 T cells responding to Vac:P5R–GFP infections, an intermediate activation profile was found for B3K506 CD4 T cells responding to Vac expressing the medium potency peptide P-1A fused to GFP (Figs. S5 and S7). Collectively, these data indicate that CD4 T cells responding to a low density of medium-potency ligands undergo a partial activation process that begins very early following infection.
In contrast to the limited activation induced by Vac:peptide–GFP infections, medium-potency ligands carried in Vac:IAb–peptide viruses induced an approximate 100- to 1,000-fold greater expansion (day 7) and maintenance (day 28) of naïve CD4 T cells (compare Fig. 4 A and B, empty and solid blue bars, and Fig. 4 C and D, orange bars; and Fig. S8). These robustly expanded medium potency CD4 T cells also became strong producers of TNFα and IFNγ (Fig. S9). The difference in the burst size and maintenance of CD4 T cells responding to strong potency viral ligands carried in Vac:IAb–peptide versus Vac:peptide–GFP, however, was approximately only 10-fold (Fig. 4 E and F). These data demonstrated that a sharp threshold regulates the robust activation of antiviral CD4 T cells that is dependent upon the potency of the ligand and the density in which it is presented.
TCR–pMHC Confinement Time Predicts the in Vivo Potency of CD4 T-Cell Recognition of Viral Ligands.
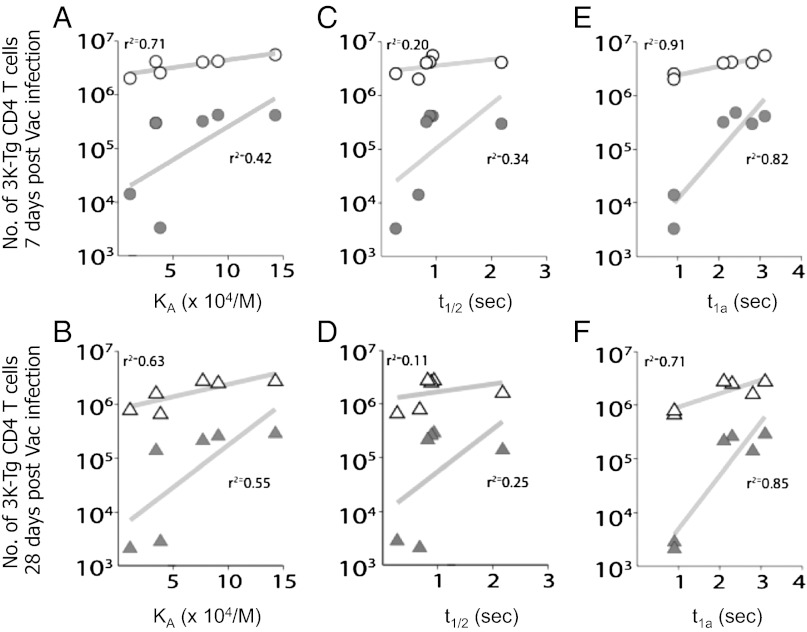
Models of T-cell activation suggest that the ligand potency aspect of the threshold could be set by the affinity (KD), the half-life (t1/2), or the confinement time (ta) of the TCR–pMHC interaction (1, 12–21, 28). To elucidate whether these parameters correlate with the antiviral CD4 T-cell responses, we compared the number of B3K508 and B3K506 T cells expanded by each virus on days 7 or 28 postinfection with the previously measured TCR–pMHC KD, t1/2, or calculated ta.
Several viral infections were identified in which neither the KD nor the t1/2 of the TCR–pMHC interaction predicted the size of the CD4 T-cell response (Fig. 5 A–D). For example, the B3K508 TCR has a similar KD for IAb+3K as does the B3K506 TCR for IAb+P-1A (Table S1), and yet B3K508 T cells responding to Vac:3K–GFP infection expand and are maintained at ∼100-fold greater than are B3K506 T cells responding to Vac:P-1A–GFP infections (Fig. 4). Additionally, the B3K508 TCR has a similar t1/2 when binding IAb+P5R as does the B3K506 TCR for IAb+3K, IAb+P5R, and IAb+P8R. However, B3K506 T cells responding to Vac:3K–GFP, Vac:P5R–GFP, and Vac:P8R–GFP infections expand and are maintained at a ∼30- to 100-fold greater level than are B3K508 T cells responding to Vac:P5R–GFP infections. These large differences in response are statistically significant and not likely attributable to experimental variability (two-sample t test, P < 0.0024 for all pairwise comparisons). In contrast, interactions with a similar calculated ta (Table S1) (20) lead to a similar response (P > 0.2 for these comparisons).
Fig. 5.
TCR–pMHC confinement time predicts the burst size and maintenance of CD4 T cells responding to ligands with different equilibrium affinity or half-life. The number of B3K506 or B3K508 CD4 T cells present in the spleen on day 7 (upper row) and day 28 (lower row) following infection with Vac:peptide–GFP (gray solid) or Vac:IAb–peptide (white filled) is shown with respect to the KA (A and B), t1/2 (C and D), and ta (E and F), with the rebinding threshold set at 45,000/(M × s). Linear regression analysis indicates that the ta of the TCR–pMHC interaction is a better predictor of burst size and maintenance than the KA or t1/2. r2 values are given in each graph.
To determine for the entire set of responses whether TCR–pMHC KD, t1/2, or calculated ta best predicts CD4 T-cell responses in vivo, we used two additional statistical analyses. The first method used a simple linear regression to determine how strongly the response correlates with the different parameters (Fig. 5). The second analysis used a mutual information metric. This analysis looks for relationships between the dependent and independent variables without a preconceived idea of what the relationship is supposed to be, unlike linear regression, which assumes the relationship is linear (see SI Materials and Methods for the complete analysis). For example, it would also allow for an increased ta to be toxic to the response past a certain threshold. However, the main motivation for using it was that the datasets appear to be nonlinear, principally around the threshold value. Instead of using a correlation coefficient, the analysis penalizes scatter in the data by quantifying the amount of information the KA, t1/2, or ta provide about the response. Using all methods, we found that the calculated ta best predicted the day 7 and day 28 response of B3K508 and B3K506 T cells responding to Vac infections, with the outlier analysis of T-cell responses grouped by a similar KD, t1/2, or ta proving the most direct evidence.
Discussion
Signaling thresholds control the fate of T cells (2, 8, 9, 28, 29). Having a signaling threshold ensures that the CD4 T cells that are activated are sufficiently potent to help orchestrate pathogen clearance, while avoiding cross-reactive autoimmune responses (1, 22, 24, 30). The polyclonal T-cell repertoire that is recruited into the immune response often carries a diversity of specificities and potencies for the invading pathogens (22). How the activation threshold set point relates to the biophysics of TCR–pMHC interactions and the reactivity pattern of CD4 T cells that are recruited into antiviral immunity is less well characterized.
Polyclonal CD4 T-cell responses to vaccinia infection are sensitive to ligand density. In vitro, Vac:IAb–3K infection resulted in a higher concentration of IAb+3K to be presented by APCs than Vac:3K–GFP infection. In vivo, Vac:IAb–3K infection induced a larger 3K-specific CD4 T-cell response than Vac:3K–GFP infection. Because APC presentation of pMHC in vivo has a half-life of display (31) and CD4 T-cell responses are sensitive to the duration of antigen presentation (31–34), the larger T-cell response following Vac:IAb–3K infection may result from a longer duration of antigen presentation, as well as the higher concentration of ligand presented during T-cell priming.
Qualitative changes to the T-cell response were also observed following Vac:IAb–3K versus Vac:3K–GFP infections. The 506β mice infected with Vac:IAb–3K expanded a large population of IFNγ-producing CD4 T cells that recognized the 3K variant ligands, P-1L and P-1K. In contrast, Vac:3K–GFP expanded CD4 T cells with these cross-reactivities very poorly. An increased precursor frequency of CD4 T cells in 506β mice for 3K and the 3K APLs may allow these lower potency, P-1L and P-1K cross-reactive CD4 T cells to be expanded following Vac:IAb–3K infections, whereas in a completely polyclonal population these cells would fail to be activated because of some aspect of T-cell competition (35). However, this need not be the case, because primary CD4 T-cell responses have a range of affinity, avidity or potency for the immunizing ligand (36–38). These data argue that high-density viral pMHC ligands allow CD4 T cells with lower potencies and, in some cases, different peptide cross-reactivity patterns to be recruited into the immune response.
The tight threshold that regulates whether B3K508 or B3K506 CD4 T cells undergo robust expansion is set by the potency of virally expressed peptide ligand and by the density in which it is presented. Changes in viral ligand density resulted in dramatically different CD4 T-cell responses to medium-potency ligands, indicating that the potency aspect of the threshold for robust CD4 T-cell activation is not a fixed value. This finding for vaccinia responses is similar to recent experiments showing that antigen dose and potency can regulate the transition of T-cell–APC interactions from brief serial encounters to longer, sustained interactions that allow full T-cell signaling to occur (39–42). The sharp potency threshold is also consistent with experiments suggesting an avidity threshold regulates CD4 T-cell expansion (24, 31, 43).
CD4 T cells responding to weak and medium potency viral ligands which did not pass the threshold were not ignorant as they up-regulated activation markers and underwent limited proliferation. CD4 T cells activated in vivo by low-potency ligands can differentiate into T helper 2 (Th2)-phenotype CD4 T cells (1, 44–46); however, we did not observe this differentiation pattern. This suggests that the antiviral inflammatory response can override the ability of low potency pMHC complexes to induce Th2 differentiation. On the opposite end of the spectrum, T cells responding to a high density of strong-potency ligands can have attenuated responses that skew toward being of lower avidity (18, 37, 47, 48). The magnitude of the CD4 T-cell responses studied here increased with the density and potency of the ligand displayed, indicating that the critical upper potency threshold was not surpassed even when the B3K506 CD4 T cells, which have a strong potency with IAb+3K (in vitro EC50 = 200 pM), were activated following Vac:1Ab–3K infections.
The B3K508 and B3K506 TCRs engage the series of virally expressed pMHC ligands with different binding kinetics. This allowed us to compare how different parameters of the TCR–pMHC interaction relate to the in vivo potency of ligands. For an individual clonotype (B3K508 or B3K506 T cells), responses track with both the KA and t1/2. The threshold for activation following Vac:peptide–GFP infections was approximately a KA of ∼4 × 104 (KD = ∼25 μM) and t1/2 ∼0.7 s. The responses of individual T-cell clonotypes track with both the KA and the t1/2 because the kon for each TCR binding the different 3K APLs does not significantly change. Thus, changes in the t1/2 resulted in the commensurate change in the KA. However, when the B3K508 and B3K506 T-cell responses were compared with each other, several outlier responses were observed with both the KA and t1/2. This likely results from differences in the on-rates of the B3K508 versus B3K506 TCRs for IAb+3K and the APLs. Outlier responses were not observed when T-cell responses were compared with the confinement time (ta). The confinement-time model predicts that the potency of a ligand can depend on both the half-life of the interaction (which controls the duration of each individual binding event) and the equilibrium affinity of the interaction (which influences the number of rebindings before complete disengagement). For the B3K508 TCR, the on-rate is slow and ta is determined by the half-life. For the B3K506 TCR, the on-rate is fast, rebinding can occur and ta is a sum of t1/2 before complete TCR–pMHC disengagement.
Recently, the 2D binding properties of TCR and pMHC have been directly measured using different techniques (49–51). In each system, both the kon and koff are strikingly faster than the rates measured in solution. In one report, in vitro ligand potency correlated with both kon and koff (50), whereas a second group found that the TCR–pMHC bond lifetime and the bond mechanical strength correlated with in vitro potency (51). Thus, there is still some discrepancy as to which 2D biophysical measurement best accounts for ligand potency. Because TCR–pMHC rebinding is proportional to kon, the observation that 2D kon are much faster than those measured in solution further emphasizes that TCR–pMHC rebinding can significantly contribute to ligand potency and, thus, to the reactivity pattern of antiviral CD4 T-cell responses as well.
Materials and Methods
C57BL/6 and C57BL/6.SJL mice were purchased from The Jackson Laboratory. Rag1−/− B3K506 and Rag1−/− B3K508 TCR Tg mice have been described previously (27). TCRβ Tg mice were established by expressing the rearranged B3K506 Vβ8.1 chain using the human CD2 promoter (52). All mice were maintained in a pathogen-free environment in accordance with institutional guidelines in the Animal Care Facility at the University of Massachusetts Medical School and experiments approved by the institute′s Institutional Animal Care and Use committee. Peptides were purchased from the Medical Research Center at National Jewish Medical Center. The 3K peptide is FEAQKAKANKAVD, numbered P-2 to P11; the P-1A peptide is FAAQKAKANKAVD.
Additional details are provided in SI Material and Methods.
Supplementary Material
Acknowledgments
We thank Andrew Mugler for helpful discussion on mutual information. This work was supported by Beckman Young Investigator and Searle Scholars Awards and National Institutes of Health (NIH) Grants RAI088495A and DK095077 (to E.S.H.). C.C.G. was supported by the Stichting voor Fundamenteel Onderzoek der Materie (FOM), Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO). E.S.H. is a member of the University of Massachusetts Medical School Diabetes and Endocrinology Research Center (NIH Grant DK32520).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. B.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208328110/-/DCSupplemental.
References
- 1.Corse E, Gottschalk RA, Allison JP. Strength of TCR-peptide/MHC interactions and in vivo T cell responses. J Immunol. 2011;186(9):5039–5045. doi: 10.4049/jimmunol.1003650. [DOI] [PubMed] [Google Scholar]
- 2.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 3.Wucherpfennig KW, et al. Polyspecificity of T cell and B cell receptor recognition. Semin Immunol. 2007;19(4):216–224. doi: 10.1016/j.smim.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisen HN, Chakraborty AK. Evolving concepts of specificity in immune reactions. Proc Natl Acad Sci USA. 2010;107(52):22373–22380. doi: 10.1073/pnas.1012051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosmrlj A, et al. Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature. 2010;465(7296):350–354. doi: 10.1038/nature08997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol Rev. 2010;235(1):244–266. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: Viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80(5):695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis MM, et al. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 9.Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol. 2012;13(2):121–128. doi: 10.1038/ni.2190. [DOI] [PubMed] [Google Scholar]
- 10.Velazquez C, DiPaolo R, Unanue ER. Quantitation of lysozyme peptides bound to class II MHC molecules indicates very large differences in levels of presentation. J Immunol. 2001;166(9):5488–5494. doi: 10.4049/jimmunol.166.9.5488. [DOI] [PubMed] [Google Scholar]
- 11.Sykulev Y, et al. Kinetics and affinity of reactions between an antigen-specific T cell receptor and peptide-MHC complexes. Immunity. 1994;1(1):15–22. doi: 10.1016/1074-7613(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 12.Matsui K, Boniface JJ, Steffner P, Reay PA, Davis MM. Kinetics of T-cell receptor binding to peptide/I-Ek complexes: Correlation of the dissociation rate with T-cell responsiveness. Proc Natl Acad Sci USA. 1994;91(26):12862–12866. doi: 10.1073/pnas.91.26.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kersh GJ, Kersh EN, Fremont DH, Allen PM. High- and low-potency ligands with similar affinities for the TCR: The importance of kinetics in TCR signaling. Immunity. 1998;9(6):817–826. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 14.Stefanová I, et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4(3):248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 15.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18(2):255–264. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 16.Tian S, Maile R, Collins EJ, Frelinger JA. CD8+ T cell activation is governed by TCR-peptide/MHC affinity, not dissociation rate. J Immunol. 2007;179(5):2952–2960. doi: 10.4049/jimmunol.179.5.2952. [DOI] [PubMed] [Google Scholar]
- 17.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci USA. 1995;92(11):5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalergis AM, et al. Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nat Immunol. 2001;2(3):229–234. doi: 10.1038/85286. [DOI] [PubMed] [Google Scholar]
- 19.Rosette C, et al. The impact of duration versus extent of TCR occupancy on T cell activation: A revision of the kinetic proofreading model. Immunity. 2001;15(1):59–70. doi: 10.1016/s1074-7613(01)00173-x. [DOI] [PubMed] [Google Scholar]
- 20.Govern CC, Paczosa MK, Chakraborty AK, Huseby ES. Fast on-rates allow short dwell time ligands to activate T cells. Proc Natl Acad Sci USA. 2010;107(19):8724–8729. doi: 10.1073/pnas.1000966107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aleksic M, et al. Dependence of T cell antigen recognition on T cell receptor-peptide MHC confinement time. Immunity. 2010;32(2):163–174. doi: 10.1016/j.immuni.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davenport MP, Price DA, McMichael AJ. The T cell repertoire in infection and vaccination: Implications for control of persistent viruses. Curr Opin Immunol. 2007;19(3):294–300. doi: 10.1016/j.coi.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Jorgensen JL, Esser U, Fazekas de St Groth B, Reay PA, Davis MM. Mapping T-cell receptor-peptide contacts by variant peptide immunization of single-chain transgenics. Nature. 1992;355(6357):224–230. doi: 10.1038/355224a0. [DOI] [PubMed] [Google Scholar]
- 24.Malherbe L, Hausl C, Teyton L, McHeyzer-Williams MG. Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties. Immunity. 2004;21(5):669–679. doi: 10.1016/j.immuni.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Dai S, et al. Crossreactive T Cells spotlight the germline rules for alphabeta T cell-receptor interactions with MHC molecules. Immunity. 2008;28(3):324–334. doi: 10.1016/j.immuni.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stadinski BD, et al. A role for differential variable gene pairing in creating T cell receptors specific for unique major histocompatibility ligands. Immunity. 2011;35(5):694–704. doi: 10.1016/j.immuni.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huseby ES, et al. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122(2):247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Krogsgaard M, Davis MM. How T cells ‘see’ antigen. Nat Immunol. 2005;6(3):239–245. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- 29.Palmer E, Naeher D. Affinity threshold for thymic selection through a T-cell receptor-co-receptor zipper. Nat Rev Immunol. 2009;9(3):207–213. doi: 10.1038/nri2469. [DOI] [PubMed] [Google Scholar]
- 30.Messaoudi I, Guevara Patiño JA, Dyall R, LeMaoult J, Nikolich-Zugich J. Direct link between mhc polymorphism, T cell avidity, and diversity in immune defense. Science. 2002;298(5599):1797–1800. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- 31.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005;201(10):1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bajénoff M, Wurtz O, Guerder S. Repeated antigen exposure is necessary for the differentiation, but not the initial proliferation, of naive CD4(+) T cells. J Immunol. 2002;168(4):1723–1729. doi: 10.4049/jimmunol.168.4.1723. [DOI] [PubMed] [Google Scholar]
- 33.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4(4):355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 34.Schrum AG, Turka LA. The proliferative capacity of individual naive CD4(+) T cells is amplified by prolonged T cell antigen receptor triggering. J Exp Med. 2002;196(6):793–803. doi: 10.1084/jem.20020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kedl RM, Kappler JW, Marrack P. Epitope dominance, competition and T cell affinity maturation. Curr Opin Immunol. 2003;15(1):120–127. doi: 10.1016/s0952-7915(02)00009-2. [DOI] [PubMed] [Google Scholar]
- 36.Busch DH, Pilip I, Pamer EG. Evolution of a complex T cell receptor repertoire during primary and recall bacterial infection. J Exp Med. 1998;188(1):61–70. doi: 10.1084/jem.188.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rees W, et al. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc Natl Acad Sci USA. 1999;96(17):9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10(4):485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 39.Hugues S, et al. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5(12):1235–1242. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- 40.Skokos D, et al. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat Immunol. 2007;8(8):835–844. doi: 10.1038/ni1490. [DOI] [PubMed] [Google Scholar]
- 41.Henrickson SE, et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9(3):282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kastenmüller W, Gerner MY, Germain RN. The in situ dynamics of dendritic cell interactions. Eur J Immunol. 2010;40(8):2103–2106. doi: 10.1002/eji.201040482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28(4):533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182(5):1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tao X, Grant C, Constant S, Bottomly K. Induction of IL-4-producing CD4+ T cells by antigenic peptides altered for TCR binding. J Immunol. 1997;158(9):4237–4244. [PubMed] [Google Scholar]
- 46.Rogers PR, Croft M. Peptide dose, affinity, and time of differentiation can contribute to the Th1/Th2 cytokine balance. J Immunol. 1999;163(3):1205–1213. [PubMed] [Google Scholar]
- 47.Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. The induction of virus-specific CTL as a function of increasing epitope expression: Responses rise steadily until excessively high levels of epitope are attained. J Immunol. 1999;163(7):3735–3745. [PubMed] [Google Scholar]
- 48.Corse E, Gottschalk RA, Krogsgaard M, Allison JP. Attenuated T cell responses to a high-potency ligand in vivo. PLoS Biol. 2010;8(9):8. doi: 10.1371/journal.pbio.1000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huppa JB, et al. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463(7283):963–967. doi: 10.1038/nature08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J, et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 2010;464(7290):932–936. doi: 10.1038/nature08944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robert P, et al. Kinetics and mechanics of two-dimensional interactions between T cell receptors and different activating ligands. Biophys J. 2012;102(2):248–257. doi: 10.1016/j.bpj.2011.11.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhumabekov T, Corbella P, Tolaini M, Kioussis D. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J Immunol Methods. 1995;185(1):133–140. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.