Abstract
We evaluated the response of the Earth land biomes to drought by correlating a drought index with three global indicators of vegetation activity and growth: vegetation indices from satellite imagery, tree-ring growth series, and Aboveground Net Primary Production (ANPP) records. Arid and humid biomes are both affected by drought, and we suggest that the persistence of the water deficit (i.e., the drought time-scale) could be playing a key role in determining the sensitivity of land biomes to drought. We found that arid biomes respond to drought at short time-scales; that is, there is a rapid vegetation reaction as soon as water deficits below normal conditions occur. This may be due to the fact that plant species of arid regions have mechanisms allowing them to rapidly adapt to changing water availability. Humid biomes also respond to drought at short time-scales, but in this case the physiological mechanisms likely differ from those operating in arid biomes, as plants usually have a poor adaptability to water shortage. On the contrary, semiarid and subhumid biomes respond to drought at long time-scales, probably because plants are able to withstand water deficits, but they lack the rapid response of arid biomes to drought. These results are consistent among three vegetation parameters analyzed and across different land biomes, showing that the response of vegetation to drought depends on characteristic drought time-scales for each biome. Understanding the dominant time-scales at which drought most influences vegetation might help assessing the resistance and resilience of vegetation and improving our knowledge of vegetation vulnerability to climate change.
Keywords: drought impacts, NDVI, drought adaptation, Standardized Precipitation Evapotranspiration Index, drought index
Drought is a natural phenomenon that occurs when water availability is significantly below normal levels over a long period and the supply cannot meet the existing demand. Drought is one of the main drivers of the reduction in Aboveground Net Primary Production (ANPP) (1), although land ecosystems differ in their sensitivity to drought (2). However, a general theory of the effects of drought on land vegetation is lacking and the subject of scientific debate (2–4).
Understanding the response of land vegetation to drought is a crucial challenge, as growth and CO2 uptake by plants are constrained to a large extent by drought (5). Its study is hindered by difficulties for drought quantification (6) and by the synergistic effects of temperature rise and drought on vegetation (7, 8). Differences in the physiological response of plant species to drought determine different levels of resistance and resilience to water deficits (9, 10) and ultimately influence the type of impact of a drought, differentiating those that slow growth (11) or reduce greenness (12), those that lead to loss of biomass (5), and those that result in plant mortality (8, 13).
The quantification of drought is a difficult task, as we usually identify a drought by its effects on different systems (agriculture, water resources, ecosystem), but there is not a unique physical variable we can measure to quantify drought intensity. Droughts are difficult to pinpoint in time and space, and it is very difficult to quantify their duration, magnitude, and spatial extent with a single variable or metric. Furthermore, the intrinsic multiscalar nature of drought introduces another element of uncertainty. In recent years the concept of drought time-scale has been widely used in drought studies (6, 14). The term refers to the time lag that typically exists between the starting of a water shortage and the identification of its consequences, for example by a decrease of the ANPP or an increase of tree mortality. Thus, the time-scales at which different plant species respond to drought may differ noticeably (11, 12, 15).
The response to water deficit among vegetation types is a crucial issue underlying geographic patterns of vegetation and a central concept to understanding the structure and dynamic of terrestrial ecosystems (2, 16). Nevertheless, the way by which the temporal variability of drought determines vegetation activity across the world biomes remains largely unknown because vegetation types have different characteristic response times (11, 15) and vulnerability (9, 10) to drought. Moreover, most studies considered the response of vegetation to climate by means of the simple anomaly of precipitation with respect to the average conditions. Such approach neglects the role of temperature and the drought time-scale at which the response of vegetation is highest. Both elements are essential to identify the response to climate variability and to understand the sensitivity of vegetation to drought.
In this study we focus on the analysis of drought impacts on vegetation by means of three vegetation parameters: (i) vegetation activity and greenness, (ii) tree radial growth, and (iii) ANPP. We stress the importance of considering the drought time-scale to understand drought impacts on a variety of vegetation types and biomes. For this purpose, we used the Standardized Precipitation Evapotranspiration Index (SPEI) (17), which is a site-specific drought indicator of deviations from the average water balance (precipitation minus potential evapotranspiration) (SI Appendix). Different SPEIs are obtained for different time-scales representing the cumulative water balance over the previous n months. The SPEI includes the role of temperature on drought severity by means of its influence on the atmospheric evaporative demand, hence improving the performance of previous drought indices based on precipitation data alone when determining the drought impacts on different hydrological and ecological systems (6, 18).
Results and Discussion
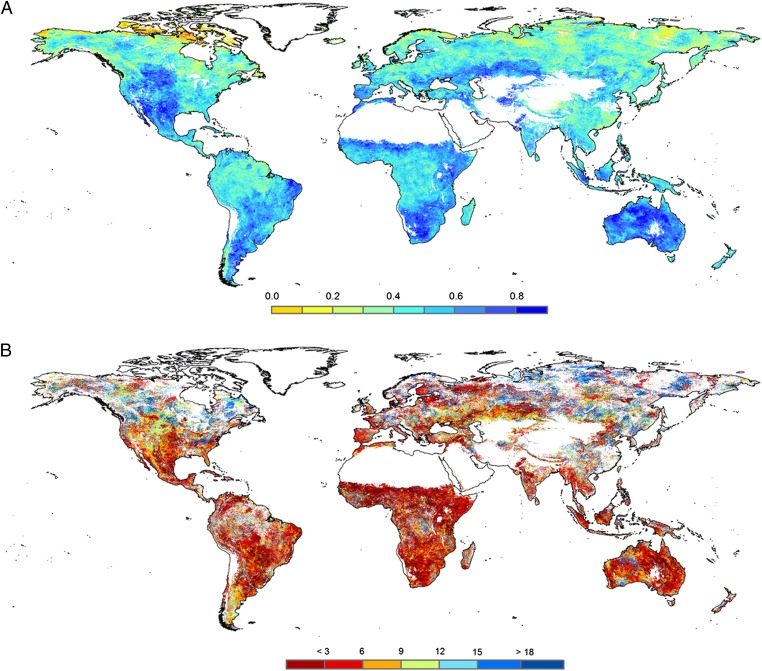
Considering an annual summary of the analysis of the Global Inventory Modeling and Mapping Studies–Normalized Difference Vegetation Index (GIMMS-NDVI) dataset, the vegetation activity correlates with drought in large areas of the world (Fig. 1A), although drought influence on NDVI changed markedly with season and among regions (SI Appendix, Figs. S1 and S2). Correlation between the SPEI and the GIMMS-NDVI data are particularly strong throughout large regions (e.g., eastern North America, the Mediterranean Basin, the Sahel). Overall, 72% of the vegetated land areas show significant correlation between the GIMMS-NDVI and the SPEI (SI Appendix, Fig. S3 and Table S2).
Fig. 1.
Geographical patterns of the association observed between drought and vegetation activity. (A) Spatial distribution of the correlations (Pearson coefficient, r) between SPEI and GIMMS-NDVI for the period 1981–2006. The values represent the maximum correlation recorded for each pixel, independently of the month of the year and the SPEI time-scale. (B) SPEI time-scales at which the maximum correlation between SPEI and GIMMS-NDVI is found. Areas with no significant correlations are depicted in white. Desert and ice areas are masked and not included in the analyses.
Tree-ring width data come predominantly from sites corresponding to mountain areas, temperate regions, and high latitudes of the Northern Hemisphere. Therefore, several forest types are not sampled, mainly in tropical and subtropical areas in which tree growth is not subject to seasonal variation and tree-rings are rarely formed, thereby limiting global spatial comparisons. Nevertheless, the high density of tree-ring series in North America, covering humid (mean annual water balance higher than 500 mm per year), subhumid (between 0 and 500 mm), semiarid (between 0 and –500 mm), and arid (lower than –500 mm) sites, shows that forests located in the semiarid and arid areas of central and southwest United States and Mexico have the highest correlations between the SPEI and tree-ring width (SI Appendix, Fig. S4). The same pattern is observed with the NDVI and the ANPP datasets, as the influence of the SPEI is lower in humid regions (including tropical rainforests and cool temperate areas of the northern hemisphere) than in semiarid and arid ones (SI Appendix, Fig. S5). This is consistent with other studies based on ANPP data (2, 19), as humid regions are characterized by a positive water balance and by vegetation having low water use efficiency (16, 19). Nevertheless, although vegetation activity in humid areas is less determined by drought than in arid ones, drought events also cause a marked reduction of vegetation activity and ANPP (16), as has been observed in the Amazon basin, particularly during the droughts of 2005 (20) and 2010 (21). Accordingly, the GIMMS-NDVI analysis showed that 78% of tropical and subtropical rainforests are characterized by significant correlation with the SPEI. This percentage was found to be even higher for the Moderate Resolution Imaging Spectroradiometer (MODIS) images obtained for the period 2001–2009 [90.7% for the Enhanced Vegetation Index (EVI), and 90.9% for the NDVI]. The percentage of surface area showing significant correlations was also high for boreal forests, cool temperate moist forests and rainforests (65.6% for the GIMMS-NDVI, and 85.5% and 84.4% for the MODIS-EVI and MODIS-NDVI datasets, respectively).
One of the main climate drivers of the geographical distribution of vegetation types is the water balance—that is, the difference between the annual precipitation and the atmospheric water demand (22). The water balance determines forest gradients and variations of forest biomass (23), but also the resistance of vegetation to drought explains the spatial distribution of vegetation in both humid (24) and dry environments (25). It is a reasonable hypothesis to think that not only the average water balance but also the characteristics related to the temporal variability (i.e., the frequency, severity, and duration of drought episodes) may play an important role in explaining the spatial distribution of vegetation types. Following the classification of world biomes by Holdridge (SI Appendix, Fig. S6), we found a relationship between the mean water balance in each biome and the average influence of droughts on the interannual variability of NDVI (Fig. 2A), tree growth (Fig. 2B), and ANPP (Fig. 2C). The drought influence was quantified by means of correlations between the SPEI series and the series of the three vegetation parameters. Thus, wet and moist forests of each region are always located in areas with a positive water balance, where the control of vegetation activity by drought is low, as indicated by low correlation with the SPEI. In cold regions, where temperature but not precipitation is the major constraint on plant development, there is little influence of drought on vegetation activity, resulting in low correlations too. In temperate, subtropical, and tropical regions, there are clear gradients of drought influence on vegetation activity as a function of the annual water balance, as revealed by large differences in the correlation with the SPEI. These areas contain dry biomes (including dry forests, scrublands, steppes) with very low ANPP (1, 2), which show the highest correlations with the SPEI.
Fig. 2.
(A) Relationships between the average SPEI/GIMMS-NDVI maximum Pearson correlation coefficients and the average annual water balance (in mm) across the world biomes. (B) Relationships between the average SPEI/tree-ring width correlations and the average annual water balance across the world biomes. (C) Relationships between the average SPEI/ANPP correlations and the average annual water balance across the world biomes. The biomes are grouped according to six eco-regions: subpolar, boreal, cool temperate, warm temperate, subtropical, and tropical. Colors represent the different biomes of each one of the six eco-regions in the A, B, and C plots. The symbols represent the different eco-regions in plots A, B, and C. Error bars represent ±1/2 SDs. The linear fits and their coefficients of determination are also shown in all graphs.
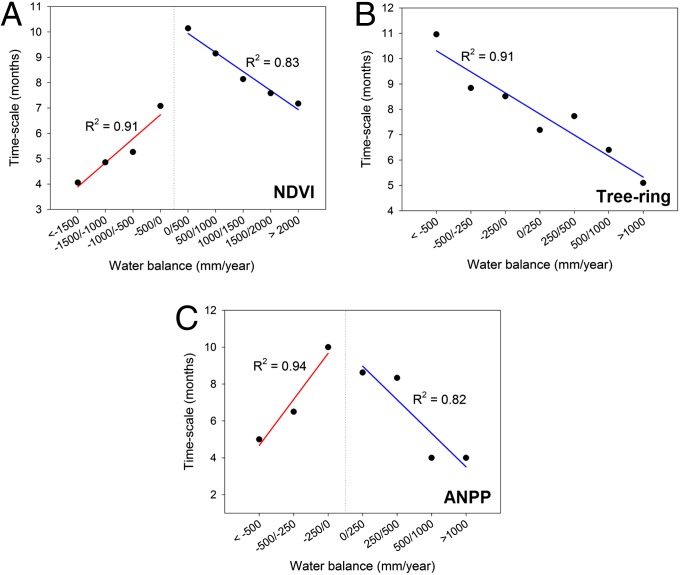
The time-scales at which droughts affect vegetation provide useful information to understand how biomes respond to drought. From analysis of the SPEI time-scales at which the maximum correlations are recorded, we found that vegetation activity responds predominantly to short drought time-scales (e.g., 2–4 mo; SI Appendix, Fig. S7), although spatial variability is high (Fig. 1B). Nevertheless, it is possible to identify general patterns, as the NDVI, for example, tends to respond to shorter drought time-scales in arid areas than in humid ones. This pattern is particularly evident in regions that include the most arid biomes. In warm temperate, subtropical, and tropical regions, the most arid biomes tend to respond at shorter time-scales than the humid ones (Fig. 3). This could be related to different mechanisms, which allow plants to reduce the damage caused by water deficits in arid areas (9). Generally, arid ecosystems respond in a highly plastic way to water availability (26), as plant species are adapted to water shortage (27) thanks to physiological, anatomical, and functional strategies that reduce water loss, respiration costs, photosynthetic activity, and growth rate (9). When areas with positive water balance are analyzed independently, it is found that correlations between SPEI and NDVI (Fig. 4A, blue), ANPP (Fig. 4B, blue), and tree growth (Fig. 4C) tend to occur at shorter time-scales as the average water balance increases. This suggests that the influence of drought time-scales is relevant to explain the temporal variability of vegetation parameters also in humid biomes.
Fig. 3.
(A) Relationships between the average SPEI time-scales at which the maximum SPEI/GIMMS-NDVI correlation is found and the average annual water balance across eco-regions considering separately negative and positive water balances. (B) Relationship between the average SPEI time-scale at which maximum SPEI/tree-ring correlation is found and the average annual water balance across eco-regions. (C) Relationship between the average SPEI time-scale at which maximum SPEI/ANPP correlation is found and the average annual water balance across eco-regions for negative and positive water balances. Error bars represent ±1/2 SDs. The linear fits and the coefficients of determination are also shown in all graphs. See corresponding colors in the legend of Fig. 2.
Fig. 4.
Average values of the time-scales (in months) at which the GIMMS-NDVI/SPEI (A), the tree-ring width/SPEI (B), and the ANPP/SPEI (C) maximum correlations are recorded, summarized for different ranges of the annual water balance. The linear fits and the corresponding coefficients of determination for negative and positive water balances are also shown.
In contrast with arid and humid regions, vegetation in semiarid and subhumid regions tends to respond to drought at longer time-scales. Vegetation of these regions is adapted to tolerate regularly periods of water deficit and has physiological mechanisms to cope with these conditions (9). Therefore, it is a reasonable hypothesis to consider that these plant communities must be exposed to sustained water deficits—that is, those registered by long time-scales of the SPEI—to be negatively affected by drought. Thus, in areas with water balance approaching zero, the highest correlations between SPEI and NDVI, tree-ring width, and ANPP occur at time-scales between 8–10 mo, but in the areas with the most positive water balance, the highest correlations between SPEI and vegetation parameters are found at shorter time-scales than in subhumid regions. There are relatively few tree-ring records available for wet tropical rainforests. However, the available data for humid boreal and cool temperate forests show a dominant response to drought at shorter time-scales than is generally recorded for semiarid and subhumid forests (Fig. 3). Boreal and cool temperate moist forests are thus highly sensitive to drought (28), an indicator that tree species dominating these forests do not tolerate water deficits (29). This may explain why droughts predominantly affect tree growth in these areas at short time-scales, as even a short period of water deficit could have negative consequences in vegetation activity and plant growth. Although tree-ring data are not available for the most humid areas of the world such as the tropical rainforests, the results derived from the NDVI suggest a similar pattern: a predominant effect of short-term droughts on vegetation activity (Fig. 3 and SI Appendix, Fig. S8). Previous studies identified a lagged response between drought, declining plant growth (30), and forest mortality (31) in similar humid forests. Using various drought time-scales, we have shown that this lag might be usually short, as demonstrated by the response of vegetation activity, forest growth, and the ANPP to very short drought time-scales.
Knowledge of the dominant time-scales at which drought influences vegetation could be critical for the early detection of vegetation damage, but it may also be useful for identifying response patterns that determine the resistance of diverse vegetation types and biomes to drought. Drought vulnerability, however, is related not only to the resistance of vegetation to water stress but also to how fast it recovers after the episode has ended—that is, by its resilience. Drought resilience depends on a variety of factors including the severity and duration of the water deficit, but also the vegetation type (32), the type and magnitude of the damage (33), the plant growth rates and competition between species (34), and even variations in environmental conditions recorded at small spatial scales (35). Although our analysis did not focus on the recovery times of vegetation after drought disturbance, the concept of drought time-scales also seems to constitute a promising tool for analyzing vegetation resilience to drought.
It is noteworthy that the highest influence of drought on vegetation identified in arid areas does not imply necessarily that plant communities from those areas are more vulnerable to drought than those dominant in humid biomes (3, 10). Thus, the short drought time-scales that mostly affect both arid and humid biomes are probably indicative of different types of impacts and different biophysical mechanisms. In arid and semiarid regions, drought impacts usually result in decreased vegetation activity (15) and plant growth (11), but rarely cause plant mortality or long-term damage, as plant communities commonly exhibit a strong resistance to water stress (36), as they contain species that are well adapted to water shortage through different mechanisms (9). This is in agreement with studies analyzing long-term trends of vegetation greenness in arid ecosystems that demonstrated the capacity of such ecosystems to recover the initial greenness values after severe and long-lasting droughts as soon as water is available (37). Nevertheless, although vegetation in arid regions is usually highly resistant to drought (3), when strong damages (e.g., tree mortality) occur during very extreme droughts, the recovery rates after the event has passed may be slow, as arid woody species have generally slow growth rates (38). Thus, unusual severe droughts, which correspond to long SPEI time-scales, can cause plant mortality (34) and even trigger desertification processes (35) in arid environments. Moreover, recurrent droughts can produce a progressive loss of resilience that affects negatively the ability of recovering the initial state (39), often leading to vegetation change.
In general, drought vulnerability is much larger in humid biomes than in arid ones (3, 24), although we found a lower response to drought in the former. This might be explained by the more complex relationship between drought and vegetation activity and plant growth in humid areas because they are characterized by water surplus. Consequently a negative SPEI there does not necessarily imply a water deficit because the water balance may still be positive, albeit lower than usual. Moreover, in humid sites other factors including phenological aspects such as the period of active leaf flushing and vapor pressure deficit may influence the effect of drought on plants (40). In humid regions, drought impacts are most probably linked to damages to plant tissues that result in loss of foliar biomass (29, 31), given the general poor tolerance of plants to water stress (3, 10), but the fast growth rates characteristic of plants of humid regions could allow vegetation to recover its prior state in a short period as soon as the drought has ended. However, in humid areas, long-lasting or recurrent droughts may also be too intense to allow for a fast vegetation recovery, and this could help explain some recent plant mortality episodes in humid forests around the world after severe drought events (7, 20, 29).
Our results concerning the time-scales of drought are similar irrespective of the data sources used: NDVI from National Oceanic and Atmospheric Administration-Advanced Very High Resolution Radiometer and MODIS images, EVI from MODIS images, a vast dataset of tree-ring growth series, and ANPP series across the world. Therefore, our results should be considered robust and unlikely to be explained by alternative causes, such as (i) possible residual noise in the GIMMS dataset, (ii) the saturation of the NDVI at high values of leaf area index, (iii) the low temporal coverage of the MODIS dataset, (iv) the low spatial representativeness of the available ANPP series, and (v) the lack of adequate coverage of dry and very humid regions by the tree-ring growth dataset. Despite the uncertainties present in each dataset, all of them point toward the same conclusions, and taking into account their complementary nature, this further enhances the robustness of our findings.
Overall, our results provide extensive evaluation of the impact of droughts on global vegetation activity and plant growth. They are particularly relevant within the changing climate framework because the degree to which ecosystems respond to limited water indicates how responsive they may be to future changes in precipitation and temperature. Therefore, the assessment of drought impacts on vegetation parameters may improve the accuracy of projections of vegetation shifts under global change scenarios. Global warming will almost certainly continue in the future (41), which would imply more land areas vulnerable to drought stress, including humid areas such as temperate, mountain, boreal, and wet tropical forests. Vegetation in these areas is already subject to increased drought stress leading to local and regional die-off events because of warming-induced drought stress (7, 29, 31). Although with increased aridity a reduction in vegetation activity might be partially compensated for by rising atmospheric CO2 concentrations, this mechanism will not enhance production under drought conditions because plant physiological processes are highly constrained by water deficits, independently of the atmospheric CO2 concentration (42). Increasing drought severity in humid areas may have unpredictable consequences for the biosphere and the global carbon cycle, because the main terrestrial carbon pool is stored in the humid world biomes (43).
In conclusion, we show that vegetation responds to drought at different characteristic time-scales across regions and biomes. Vegetation of both arid and humid biomes respond mostly at short drought time-scales (i.e., a fast reaction of several vegetation parameters is found as soon as relative water deficit occurs), but the mechanisms that drive this response are most likely very different. These mechanisms affect the resistance and resilience of vegetation to drought stress, conditioning their vulnerability to drought. Understanding the relationship between these mechanisms and the characteristics of droughts (for example, as determined by the drought time-scale) is crucial for improving our knowledge of vegetation vulnerability to climate fluctuations and climate change. As expected from current climate change scenarios, the water balance will become more negative in most areas of the world as a consequence of warming processes, which will probably reinforce drought severity worldwide (44).
Methods
To quantify drought severity we used monthly data of the SPEI at a spatial resolution of 0.5° and time-scales ranging from 1 to 24 mo obtained from the SPEIbase (45) (http://sac.csic.es/spei/download.html, SI Appendix). We used three different datasets of vegetation parameters, which provide information on ANPP, leaf photosynthetic activity, and tree radial growth across the world. First, we collected long-term ANPP series from the scientific literature using the published tabular data or by digitizing figures. A total set of 40 series that contain a minimum of 10 y were collected (SI Appendix, Table S1). The series cover different biomes and vegetation types. The second dataset was based on annual tree-ring width data, obtained from the International Tree-Ring Data Bank (www.ncdc.noaa.gov/paleo/treering.html). From the entire dataset, we selected the tree-ring width series with at least 25 y of data within the period 1945–2009. A total number of 1,846 site chronologies were selected and analyzed (SI Appendix). Finally, we included time series of vegetation indices obtained from long-term satellite imagery. We used the NOAA GIMMS-NDVI (46) from July 1981 to December 2006, at a resolution of 0.1°, available from the Global Land Cover Facility (www.glcf.umd.edu/data/gimms). Vegetation indices from the MODIS were also used to replicate the GIMMS-NDVI for the period 2001–2009. Monthly composites of the EVI (47) and the NDVI at a spatial resolution of 5.6 km from the MOD13A2 dataset were obtained from NASA (https://lpdaac.usgs.gov). To characterize the spatial distribution of the world biomes, we used the Holdridge classification (48) from the United Nations Environment Program–Division of Early Warning and Assessment/Global Resource Information Database–Geneva (www.grid.unep.ch) at a spatial resolution of 0.5°. The Global Land Cover Map (http://ionia1.esrin.esa.int/) was used with the purpose of masking the urban areas and irrigated lands.
The 0.5° SPEI data series were interpolated to 8 km for 1981–2006 to match the spatial resolution of the GIMMS-NDVI and to 5.6 km for the 2001–2009 to match the MODIS vegetation indices. The biweekly GIMMS-NDVI series were monthly composited according to the maximum monthly value to avoid different sources of noise. Taking into account the Gaussian shape of the monthly NDVI distributions (49), the 1981–2006 GIMMS-NDVI and the 2001–2009 MODIS EVI and NDVI series were standardized, according to the average and the SDs of the monthly series obtained for each NDVI pixel. In addition, annual ANPP and tree-ring growth series were also standardized before applying the analysis.
The impact of the SPEI interannual variability on vegetation activity, tree growth, and ANPP was assessed by means of parametric correlations using the Pearson coefficient for the entire period of available data, and considering a significance threshold of α < 0.05. Twelve series of the GIMMS-NDVI (one per month) were obtained per pixel, and each one was correlated (Pearson coefficient) to the monthly 1- to 24-mo SPEI series of the pixel for the period 1981–2006. For each grid cell, we obtained 288 correlation values (24 for each month of the year). To eliminate the influence of phenology on the results, the monthly correlations were summarized seasonally and annually. For this purpose, the highest correlation found in each season was retained and also the SPEI time-scale at which the maximum seasonal correlation was obtained. After that, seasonal results were summarized annually following the same approach. The same methodology was applied to the MODIS datasets, ANPP, and tree-ring series (SI Appendix).
Maximum annual and seasonal correlations between the GIMMS and MODIS vegetation indices and the SPEI as well as maximum annual correlations between tree-ring width and ANPP records and the SPEI were summarized according to the Holdridge classification by means of the calculation of the average correlation and average maximum SPEI time-scale for the different biomes. For this purpose, the average aridity conditions in each biome were quantified using precipitation and potential evapotranspiration data taken from the CRU TS3.0 dataset (SI Appendix).
Supplementary Material
Acknowledgments
We thank to the two anonymous reviewers for their helpful comments, which have noticeably improved the final manuscript. We also thank the GIMMS and the MODIS science team of the National Aeronautics and Space Administration for providing the remote sensing data. We would also like to thank the Climate Research Unit of the University of East Anglia (United Kingdom) for providing the global land temperature and precipitation dataset used to obtain the SPEIbase and the contributors of the International Tree-Ring Data Bank for providing the tree-ring records used in this study. This work was supported by projects financed by the Spanish Commission of Science and Technology (CGL2011-27574-CO2-02, CGL2011-27536, and CGL2011-26654) and the Aragón Government. C.G. and R.T. were supported by Project PTDC/AAC-CLI/103361/2008 funded by the Portuguese Foundation for Science and Technology. J.J.C. thanks the support of ARAID, and A.S-L. was supported by a postdoctoral fellowship from the Generalitat de Catalunya (2009 BP-A 00035).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207068110/-/DCSupplemental.
References
- 1.Webb WL, Lauenroth WK, Szarek SR, Kinerson RS. Primary production and abiotic controls in forests, grasslands, and desert ecosystems in the United States. Ecology. 1983;64(1):134–151. [Google Scholar]
- 2.Knapp AK, Smith MD. Variation among biomes in temporal dynamics of aboveground primary production. Science. 2001;291(5503):481–484. doi: 10.1126/science.291.5503.481. [DOI] [PubMed] [Google Scholar]
- 3.Maherali H, Pockman WT, Jackson RB. Adaptative variation in the vulnerability of woody plants to xylem cavitation. Ecology. 2004;85(8):2184–2199. [Google Scholar]
- 4.Samanta A, et al. Amazon forests did not green-up during the 2005 drought. Geophys Res Lett. 2010;37(5):L05401. doi: 10.1029/2009GL042154. [DOI] [Google Scholar]
- 5.Ciais Ph, et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature. 2005;437(7058):529–533. doi: 10.1038/nature03972. [DOI] [PubMed] [Google Scholar]
- 6.Vicente-Serrano SM, Beguería S, López-Moreno JI. Comment on “Characteristics and trends in various forms of the Palmer Drought Severity Index (PDSI) during 1900-2008” by A. Dai. Journal of Geophysical Research-Atmosphere. 2011;116(19):D19112. doi: 10.1029/2011JD016410. [DOI] [Google Scholar]
- 7.Breshears DD, et al. Regional vegetation die-off in response to global-change-type drought. Proc Natl Acad Sci USA. 2005;102(42):15144–15148. doi: 10.1073/pnas.0505734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen CD, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manage. 2010;259(4):660–684. [Google Scholar]
- 9.Chaves MM, Maroco JP, Pereira JS. Understanding plant responses to drought—From genes to the whole plant. Funct Plant Biol. 2003;30(3):239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- 10.McDowell N, et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008;178(4):719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- 11.Pasho E, Camarero JJ, de Luis M, Vicente-Serrano SM. Impacts of drought at different time scales on forest growth across a wide climatic gradient in north-eastern Spain. Agric For Meteorol. 2011;151(12):1800–1811. [Google Scholar]
- 12.Ji L, Peters AJ. Assessing vegetation response to drought in the northern Great Plains using vegetation and drought indices. Remote Sens Environ. 2003;87(1):85–98. [Google Scholar]
- 13.Adams HD, et al. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc Natl Acad Sci USA. 2009;106(17):7063–7066. doi: 10.1073/pnas.0901438106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKee TBN, Doesken J, Kleist J. Eighth Conference on Applied Climatology. Anaheim, CA: American Meteorological Society; 1993. The relationship of drought frequency and duration to time scales; pp. 179–184. [Google Scholar]
- 15.Vicente-Serrano SM. Evaluating the impact of drought using remote sensing in a Mediterranean, semi-arid region. Nat Hazards. 2007;40(1):173–208. [Google Scholar]
- 16.Huxman TE, et al. Convergence across biomes to a common rain-use efficiency. Nature. 2004;429(6992):651–654. doi: 10.1038/nature02561. [DOI] [PubMed] [Google Scholar]
- 17.Vicente-Serrano SM, Beguería S, López-Moreno JI. A multi-scalar drought index sensitive to global warming: The Standardized Precipitation Evapotranspiration Index – SPEI. J Clim. 2010;23(17):1696–1718. [Google Scholar]
- 18.Vicente-Serrano SM, et al. Performance of drought indices for ecological, agricultural and hydrological applications. Earth Interact. 2012;16(10):1–27. [Google Scholar]
- 19.Schuur EAG. Productivity and global climate revisited: The sensitivity of tropical forest growth to precipitation. Ecology. 2003;84(5):1165–1170. [Google Scholar]
- 20.Phillips OL, et al. Drought sensitivity of the Amazon rainforest. Science. 2009;323(5919):1344–1347. doi: 10.1126/science.1164033. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, et al. Widespread decline in greenness of Amazonian vegetation due to the 2010 drought. Geophys Res Lett. 2011;38(7):L07402. doi: 10.1029/2011GL046824. [DOI] [Google Scholar]
- 22.Stephenson NL. Climatic control of vegetation distribution: The role of the water balance. Am Nat. 1990;135(5):649–670. [Google Scholar]
- 23.Stegen JC, et al. Variation in above-ground forest biomass across broad climatic gradients. Glob Ecol Biogeogr. 2011;20(5):744–754. [Google Scholar]
- 24.Engelbrecht BMJ, et al. Drought sensitivity shapes species distribution patterns in tropical forests. Nature. 2007;447(7140):80–82. doi: 10.1038/nature05747. [DOI] [PubMed] [Google Scholar]
- 25.Pockman WT, Sperry JS. Vulnerability to xylem cavitation and the distribution of Sonoran Desert vegetation. Am J Bot. 2000;87(9):1287–1299. [PubMed] [Google Scholar]
- 26.Schwinning S, Sala OE. Hierarchy of responses to resource pulses in arid and semi-arid ecosystems. Oecologia. 2004;141(2):211–220. doi: 10.1007/s00442-004-1520-8. [DOI] [PubMed] [Google Scholar]
- 27.Lundholm B. Adaptations in arid ecosystems. In: Rapp A, Le Houerou NH, Lundholm B, editors. Can Desert Encroachment Be Stopped? Ecological Bull. No. 24. Sweden: United Nations Environmental Programme, Stockholm; 1976. [Google Scholar]
- 28.Barber VA, Juday GP, Finney BP. Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature. 2000;405(6787):668–673. doi: 10.1038/35015049. [DOI] [PubMed] [Google Scholar]
- 29.Anderegg WRL, et al. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc Natl Acad Sci USA. 2012;109(1):233–237. doi: 10.1073/pnas.1107891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bréda N, Huc R, Granier A, Dreyer E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann For Sci. 2006;63(16):625–644. [Google Scholar]
- 31.Phillips OL, et al. Drought-mortality relationships for tropical forests. New Phytol. 2010;187(3):631–646. doi: 10.1111/j.1469-8137.2010.03359.x. [DOI] [PubMed] [Google Scholar]
- 32.del Cacho M, Lloret F. Resilience of Mediterranean shrubland to a severe drought episode: The role of seed bank and seedling emergence. Plant Biol (Stuttg) 2012;14(3):458–466. doi: 10.1111/j.1438-8677.2011.00523.x. [DOI] [PubMed] [Google Scholar]
- 33.Lapenis A, Shvidenko A, Shepaschenko D, Nilsson S, Aiyyer A. Acclimatation of Russian forests to recent changes in climate. Glob Change Biol. 2005;11(12):2090–2102. doi: 10.1111/j.1365-2486.2005.001069.x. [DOI] [PubMed] [Google Scholar]
- 34.Galiano L, Martínez-Vilalta J, Lloret F. Drought-induced multifactor decline of Scots Pine in the Pyrenees and potential vegetation change by the expansion of co-occurring oak species. Ecosystems (N Y) 2010;13(7):978–991. [Google Scholar]
- 35.Vicente-Serrano SM, Zouber A, Lasanta T, Pueyo Y. Dryness is accelerating degradation of vulnerable shrublands in semiarid Mediterranean environments. Ecol Monogr. 2012;82(4):407–428. [Google Scholar]
- 36.Craine JM, et al. Global diversity of drought tolerance and grassland climate-change resilience. Nature Climate Change. 2012. 10.1038/nclimate1634. [Google Scholar]
- 37.Heumann BW, Seaquist JW, Eklundh L, Jönsson P. AVHRR derived phenological change in the Sahel and Soudan, Africa, 1982-2005. Remote Sens Environ. 2007;108(4):385–392. [Google Scholar]
- 38.Bonet A. Secondary succession of semiarid Mediterranean old-fields in south-eastern Spain: Insights for conservation and restoration of degraded lands. J Arid Environ. 2004;56(2):213–233. [Google Scholar]
- 39.Lloret F, Siscart D, Dalmases C. Canopy recovery after drought dieback in holm-oak Mediterranean forests of Catalonia (NE Spain) Glob Change Biol. 2004;10(12):2092–2099. [Google Scholar]
- 40.Brando PM, et al. Seasonal and interannual variability of climate and vegetation indices across the Amazon. Proc Natl Acad Sci USA. 2010;107(33):14685–14690. doi: 10.1073/pnas.0908741107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solomon S, et al. Climate Change 2007: The Physical Science Basis. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 42.Smith SD, et al. Elevated CO2 increases productivity and invasive species success in an arid ecosystem. Nature. 2000;408(6808):79–82. doi: 10.1038/35040544. [DOI] [PubMed] [Google Scholar]
- 43.Nemani RR, et al. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science. 2003;300(5625):1560–1563. doi: 10.1126/science.1082750. [DOI] [PubMed] [Google Scholar]
- 44.Dai A. Increasing drought under global warming in observations and models. Nature Climate Change. 2012. 10.1038/nclimate1633. [Google Scholar]
- 45.Beguería S, Vicente-Serrano SM, Angulo M. A multi-scalar global drought data set: The SPEIbase: A new gridded product for the analysis of drought variability and impacts. Bull Am Meteorol Soc. 2010;91(10):1351–1354. [Google Scholar]
- 46.Tucker CJ, et al. An extended AVHRR 8-km NDVI data set compatible with MODIS and SPOT Vegetation NDVI Data. Int J Remote Sens. 2005;26(20):4485–5598. [Google Scholar]
- 47.Huete A, et al. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens Environ. 2002;83(1-2):195–213. [Google Scholar]
- 48.Holdridge LR. Determination of world plant formations from simple climatic data. Science. 1947;105(2727):367–368. doi: 10.1126/science.105.2727.367. [DOI] [PubMed] [Google Scholar]
- 49.Peters AJ, et al. Drought monitoring with NDVI-based Standardized Vegetation Index. Photogramm Eng Remote Sensing. 2002;68(1):71–75. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.