Abstract
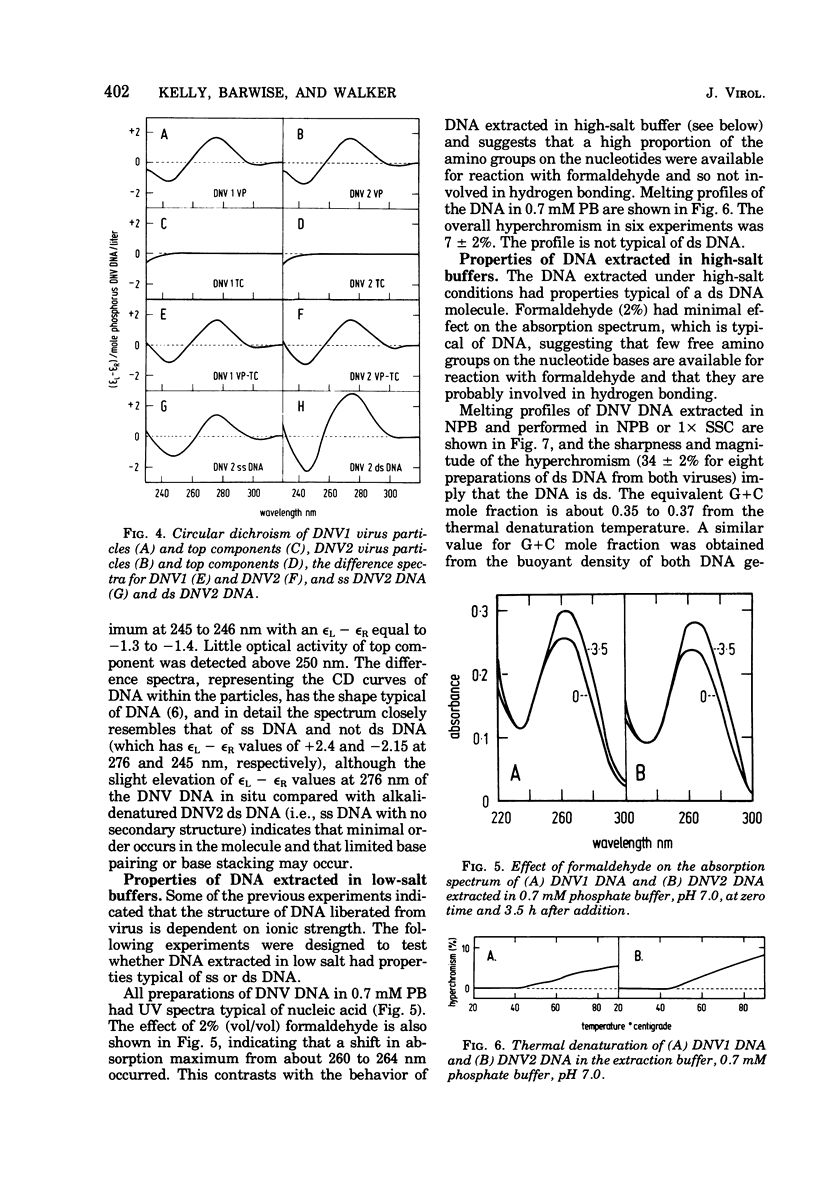
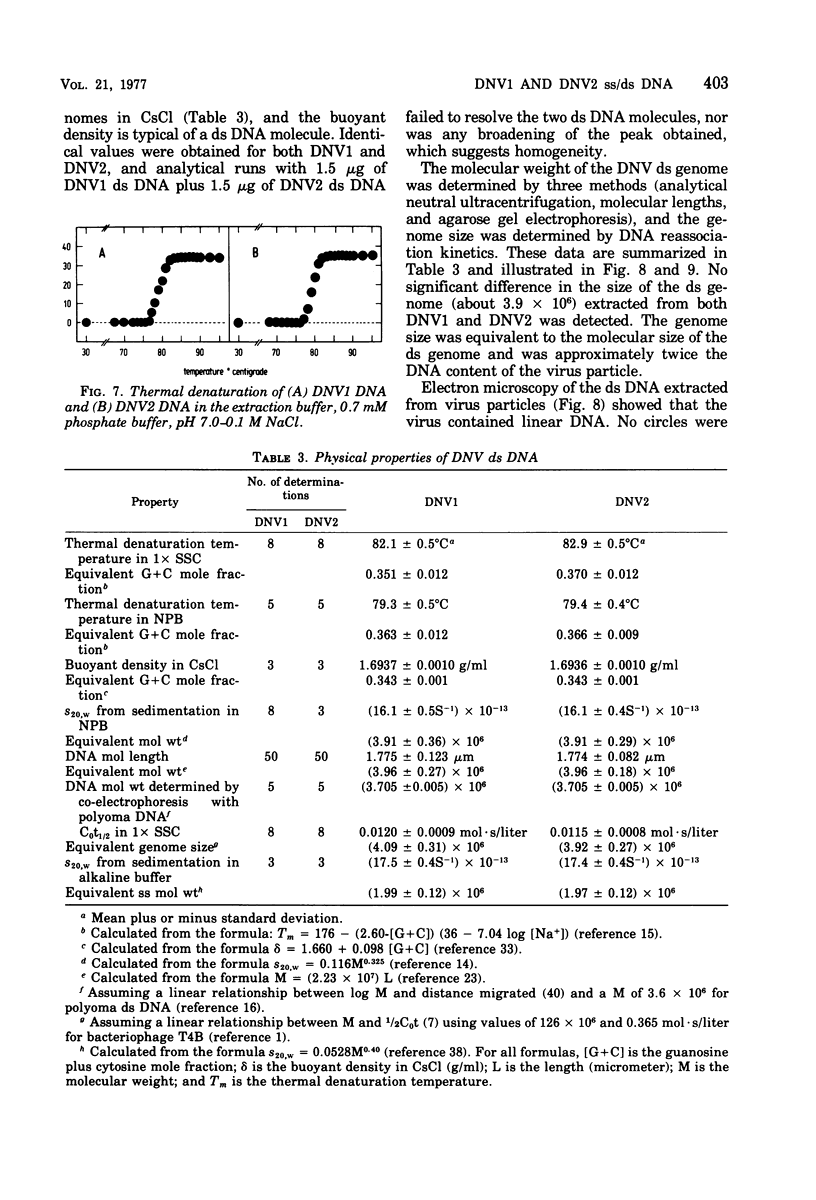
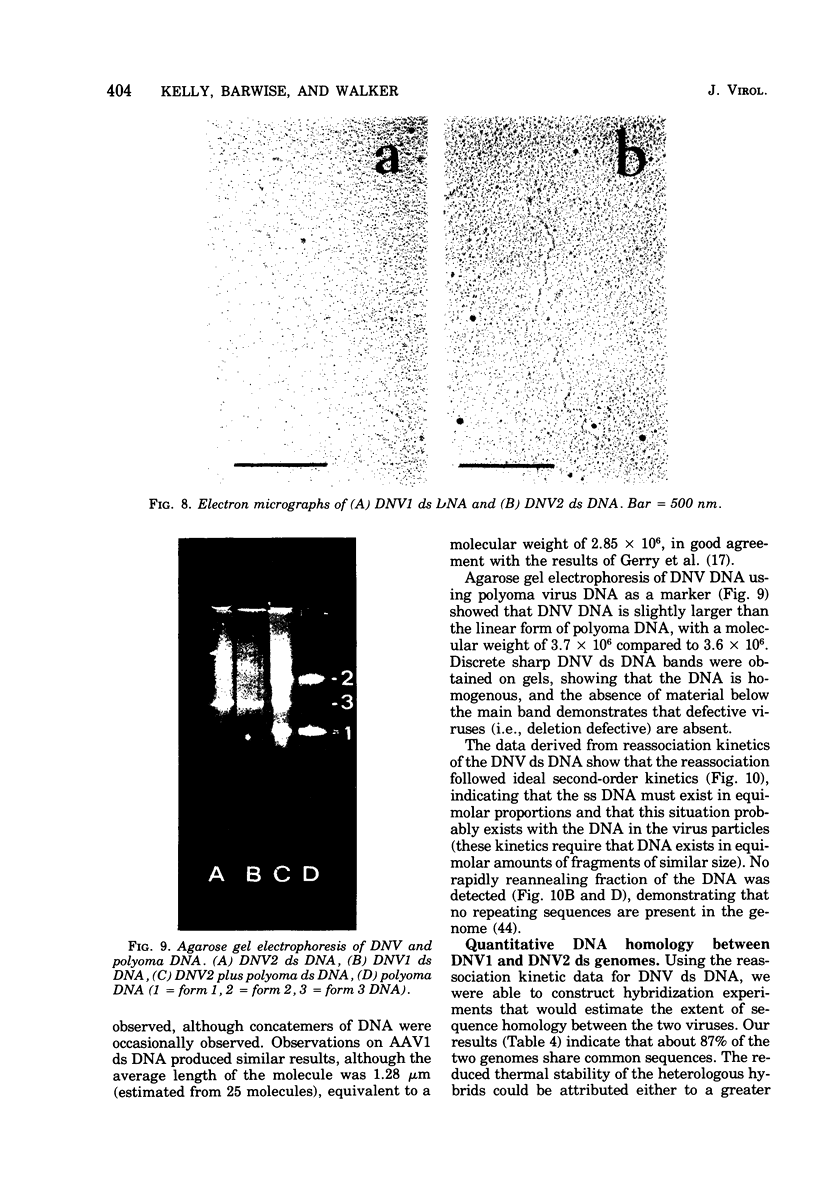
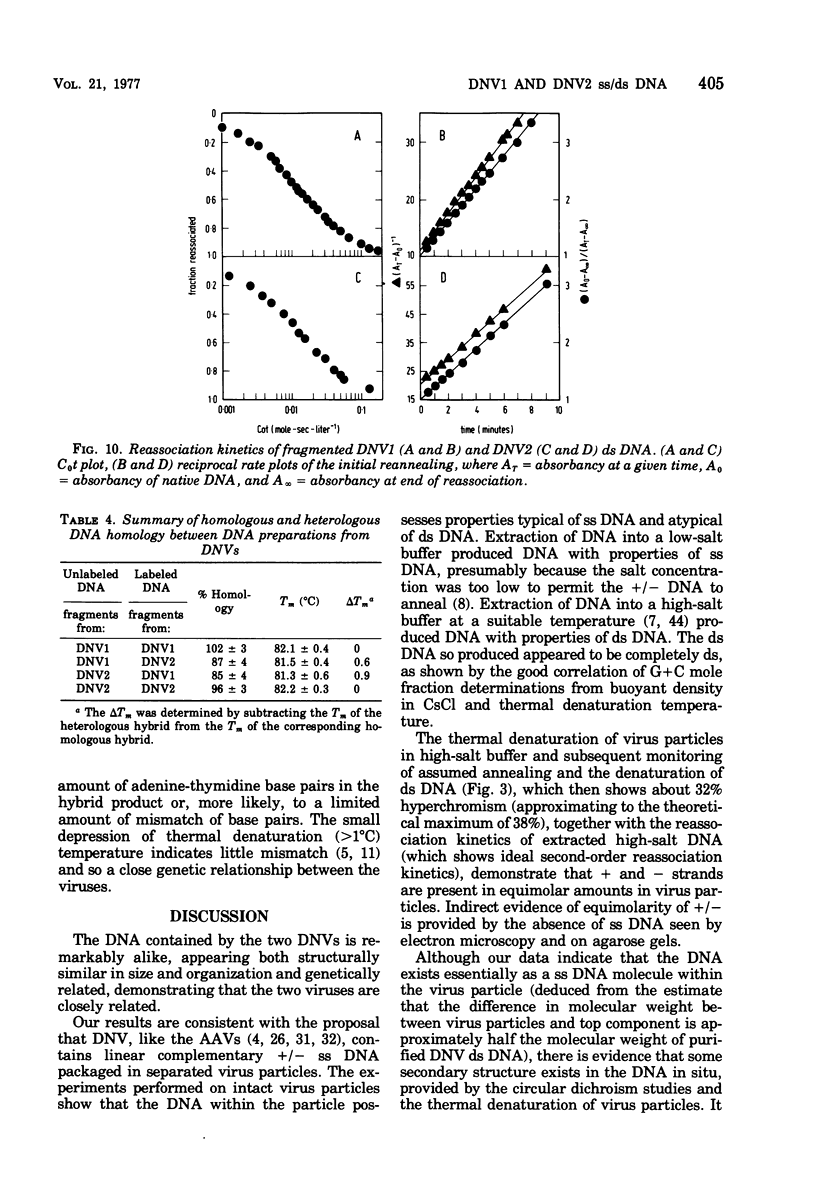
The DNA contained by particles of densonucleosis viruses 1 and 2 were analyzed within the particle, and properties of DNA extracted from these particles were determined. The DNA appears to exist as a single-stranded molecule with limited secondary structure within particles, as assessed by spectral changes induced by formaldehyde, melting profiles, and circular dichroism studies. The single-stranded DNA had an apparent molecular weight of 1.9 X 10(6) to 2.2 X 10(6) as assessed by differences in the molecular weight of virus particles and top component and percentage of nucleic acid. DNA extracted from virus particles in low-salt buffers possessed properties typical of a single-stranded molecule. Double-stranded DNA could be extracted from virus particles under appropriate high salt and elevated temperature. The linear double-stranded DNA extracted from both viruses had a molecular weight of about 3.9 X 10(6) to 4.1 ZX 10(6) determined by neutral sedimentation and electron microscopy and an equivalent genome size determined by reassociation kinetics. About 87% of the DNA was homologous between the two viruses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery R. J., Kelly D. C. Bacteriophage T4 and T7 DNA: a study of their redundancy by renaturation kinetics. Intervirology. 1974;3(4):277–280. doi: 10.1159/000149764. [DOI] [PubMed] [Google Scholar]
- BRAHMS J., MOMMAERTS W. F. A STUDY OF CONFORMATION OF NUCLEIC ACIDS IN SOLUTION BY MEANS OF CIRCULAR DICHROISM. J Mol Biol. 1964 Oct;10:73–88. doi: 10.1016/s0022-2836(64)80029-2. [DOI] [PubMed] [Google Scholar]
- Bachmann P. A., Hoggan M. D., Melnick J. L., Pereira H. G., Vago C. Parvoviridae. Intervirology. 1975;5(1-2):83–92. doi: 10.1159/000149884. [DOI] [PubMed] [Google Scholar]
- Barwise A. H., Walker I. O. Studies on the DNA of a virus from Galleria mellonella. FEBS Lett. 1970 Jan 15;6(1):13–16. doi: 10.1016/0014-5793(70)80028-x. [DOI] [PubMed] [Google Scholar]
- Berns K. I. Molecular biology of the adeno-associated viruses. Curr Top Microbiol Immunol. 1974;65:1–20. doi: 10.1007/978-3-642-65875-4_1. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Brenner D. J., Neufeld B. R., Britten R. J. Reduction in the rate of DNA reassociation by sequence divergence. J Mol Biol. 1973 Dec 5;81(2):123–135. doi: 10.1016/0022-2836(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Cowie D. B., Avery R. J., Champe S. P. DNA homology among the T-even bacteriophages. Virology. 1971 Jul;45(1):30–37. doi: 10.1016/0042-6822(71)90109-7. [DOI] [PubMed] [Google Scholar]
- Crawford L. V. A minute virus of mice. Virology. 1966 Aug;29(4):605–612. doi: 10.1016/0042-6822(66)90284-4. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Follett E. A., Burdon M. G., McGeoch D. J. The DNA of a minute virus of mice. J Gen Virol. 1969 Jan;4(1):37–46. doi: 10.1099/0022-1317-4-1-37. [DOI] [PubMed] [Google Scholar]
- Eigner J., Doty P. The native, denatured and renatured states of deoxyribonucleic acid. J Mol Biol. 1965 Jul;12(3):549–580. doi: 10.1016/s0022-2836(65)80312-6. [DOI] [PubMed] [Google Scholar]
- Frank-Kamenetskii F. Simplification of the empirical relationship between melting temperature of DNA, its GC content and concentration of sodium ions in solution. Biopolymers. 1971;10(12):2623–2624. doi: 10.1002/bip.360101223. [DOI] [PubMed] [Google Scholar]
- Fried M., Griffin B. E., Lund E., Robberson D. L. Polyoma virus--a study of wild-type, mutant and defective DNAs. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):45–52. doi: 10.1101/sqb.1974.039.01.009. [DOI] [PubMed] [Google Scholar]
- Gerry H. W., Kelly T. J., Jr, Berns K. I. Arrangement of nucleotide sequences in adeno-associated virus DNA. J Mol Biol. 1973 Sep 15;79(2):207–225. doi: 10.1016/0022-2836(73)90001-6. [DOI] [PubMed] [Google Scholar]
- Kelly D. C., Elliott R. M. Polyamines contained by two densonucleosis viruses. J Virol. 1977 Jan;21(1):408–410. doi: 10.1128/jvi.21.1.408-410.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. C., Vance D. E. The lipid content of two iridescent viruses. J Gen Virol. 1973 Nov;21(2):417–423. doi: 10.1099/0022-1317-21-2-417. [DOI] [PubMed] [Google Scholar]
- Kurstak E. Small DNA densonucleosis virus (DNV). Adv Virus Res. 1972;17:207–241. doi: 10.1016/s0065-3527(08)60751-4. [DOI] [PubMed] [Google Scholar]
- Kurstak E., Vernoux J. P., Niveleau A., Onji P. A. Visualisation du DNA du virus de la densonucléose (VDN) à chaînes monocaténaires complémentaires de polarités inverses plus et moins. C R Acad Sci Hebd Seances Acad Sci D. 1971 Feb 1;272(5):762–765. [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Maestre M. F., Tinoco I., Jr Optical rotatory dispersion of viruses. J Mol Biol. 1967 Feb 14;23(3):323–335. doi: 10.1016/s0022-2836(67)80108-6. [DOI] [PubMed] [Google Scholar]
- Mayor H. D., Torikai K., Melnick J. L., Mandel M. Plus and minus single-stranded DNA separately encapsidated in adeno-associated satellite virions. Science. 1969 Dec 5;166(3910):1280–1282. doi: 10.1126/science.166.3910.1280. [DOI] [PubMed] [Google Scholar]
- PEACOCKE A. R., WALKER I. O. The thermal denaturation of sodium deoxyribonucleate. III. Light-scattering studies. J Mol Biol. 1962 Nov;5:564–569. doi: 10.1016/s0022-2836(62)80130-2. [DOI] [PubMed] [Google Scholar]
- Rose J. A., Berns K. I., Hoggan M. D., Koczot F. J. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):863–869. doi: 10.1073/pnas.64.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Shoulder A., Darby G., Minson T. RNA-RNA hybridisation using 125I-labelled RNA from tobacco necrosis virus and its satellite. Nature. 1974 Oct 25;251(5477):733–735. doi: 10.1038/251733a0. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Ogino T., Baba K. Estimation of relative molecular length of DNA by electrophoresis in agarose gel. Biochim Biophys Acta. 1969 Jan 21;174(1):183–187. doi: 10.1016/0005-2787(69)90241-x. [DOI] [PubMed] [Google Scholar]
- Truffaut N., Berger G., Niveleau A., May P., Bergoin M., Vago C. Recherches sur l'acide nucléique du virus de la densonucléose du lepidoptère Galleria mellonella L. Arch Gesamte Virusforsch. 1967;21(3):469–474. [PubMed] [Google Scholar]
- Vernoux J. P., Kurstak E. Etude biophysique de l'acide désoxyrubonucléique du Virus de la densonucléose (VDN). I. Purification du VDN et ses propriétés en relation directe avec la structure de l'acide nucléique encapsidé. Arch Gesamte Virusforsch. 1972;39(1):190–195. [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- YPHANSTIS D. A. Rapid determination of molecular weights of peptides and preteins. Ann N Y Acad Sci. 1960 Aug 31;88:586–601. doi: 10.1111/j.1749-6632.1960.tb20055.x. [DOI] [PubMed] [Google Scholar]