Abstract
Despite different aetiologies, age-related macular degeneration and most inherited retinal disorders culminate in the same final common pathway, the loss of photoreceptors. There are few treatments and none reverse the loss of vision. Photoreceptor replacement by transplantation is proposed as a broad treatment strategy applicable to all degenerations. Recently, we demonstrated restoration of vision following rod-photoreceptor transplantation into a mouse model of stationary night-blindness, raising the critical question of whether photoreceptor replacement is equally effective in different types and stages of degeneration. We present a comprehensive assessment of rod-photoreceptor transplantation across six murine models of inherited photoreceptor degeneration. Transplantation is feasible in all models examined but disease type has a major impact on outcome, as assessed both by the morphology and number of integrated rod-photoreceptors. Integration can increase (Prph2+/Δ307), decrease (Crb1rd8/rd8, Gnat1−/−, Rho−/−), or remain constant (PDE6βrd1/rd1, Prph2rd2/rd2) with disease progression, depending upon the gene defect, with no correlation with severity. Robust integration is possible even in late-stage disease. Glial scarring and outer limiting membrane integrity, features that change with degeneration, significantly affect transplanted photoreceptor integration. Combined breakdown of these barriers markedly increases integration in a model with an intact outer limiting membrane, strong gliotic response, and otherwise poor transplantation outcome (Rho−/−), leading to an eightfold increase in integration and restoration of visual function. Thus, it is possible to achieve robust integration across a broad range of inherited retinopathies. Moreover, transplantation outcome can be improved by administering appropriate, tailored manipulations of the recipient environment.
Keywords: gliosis, retinal degeneration, stem cells
Retinal degeneration is a major cause of untreatable blindness. Despite very different aetiologies, degenerative retinopathies culminate in the same final common pathway, the loss of photoreceptors and vision. Because photoreceptors are terminally differentiated neurons, they cannot regenerate and once lost are not replaced. Although recently there has been considerable progress in strategies such as gene supplementation therapy (1), when cell death has already occurred, cell replacement therapies offer a complementary and potentially generic approach.
We have previously demonstrated proof-of-concept for rod (2) and cone (3) photoreceptor transplantation. Most recently, we have shown that transplanted postmitotic rod-photoreceptor precursor cells, identified by their expression of GFP driven by the promoter for the rod-specific transcription factor, Nrl (4), can migrate into the retina of a model of stationary night-blindness in numbers sufficient to restore rod-mediated vision (5). Recent advances in stem cell technology have also demonstrated the potential to generate suitable populations of donor cells (6–8). These advances provide strong justification for the development of photoreceptor replacement as a treatment for degenerative disease. Until now, photoreceptor transplantation has been assessed in normal animals and a few isolated models of degeneration at single points within the degenerative process (2, 3, 5, 9–11). To determine the feasibility of photoreceptor replacement therapy as a generic treatment for retinal disease, transplantation must be assessed in different models and at different stages of retinal degeneration, both to determine the potential breadth of application and to identify therapeutic time windows.
The degenerating retinal environment is very different from that of the normal retina and may have an adverse effect on the ability of cells to migrate from the site of transplantation into the recipient outer nuclear layer (ONL). CNS injury and degeneration are often associated with a series of events that culminate in reactive gliosis and the formation of a glial scar. This scar acts as a reservoir of inhibitory extracellular matrix molecules, such as chondroitin sulfate proteoglycans (CSPGs), which prevent axonal and cell migration and regeneration (12). In the retina, photoreceptor death can induce reactive gliosis in Müller glia, which may present a physical barrier to transplanted cell migration (13, 14). The integrity of the ONL is also lost with the death of photoreceptors. As they die, so the interphotoreceptor matrix (IPM) is reduced and normal barrier functions are compromised. The outer limiting membrane (OLM), located at the outer edge of the ONL, is a diffusion barrier comprised of a series of zonula-adherens junctions formed between photoreceptors and Müller glia. We have previously shown that disruption of the OLM enhances photoreceptor integration in wild-type murine recipients (10, 15). Although there are reports of OLM disruption in the degenerating retina (16, 17), no study to date has assessed the role of OLM integrity in transplantation outcome in the degenerating retina. Here, we present a unique comprehensive assessment of photoreceptor transplantation efficiency across several mouse models of inherited retinal degeneration, assessing the impact of degeneration, OLM integrity, and gliosis on transplanted cell integration.
Results
We chose six clinically relevant murine models of inherited retinal disease that represent a range of degeneration speeds: four models of Retinitis pigmentosa (RP) (Prph2+/Δ307, Prph2rd2/rd2, Rho−/−, PDE6βrd1/rd1), a model of Lebers congenital amaurosis (Crb1rd8/rd8), and a model of stationary night-blindness (Gnat1−/−). Each model undergoes progressive loss of photoreceptors over a period ranging from ∼10% loss over 12 mo (Gnat1−/−) to near complete loss of rods within 3 wk (PDE6βrd1) (Table S1).
Transplanted Rod-Photoreceptors Integrate with Different Efficiency in Different Models of RP.
We first examined the number of transplanted Nrl.GFP+ve rod-photoreceptor precursors integrating into each of the models at a time when the recipient retina is mature (6–8 wk) and compared these to age-matched wild-type controls. At 3 wk posttransplantation, integration into adult Gnat1−/−, Prph2+/Δ307, Prph2rd2/rd2, and PDE6β rd1/rd1 recipients was similar to wild-type (Fig. 1A; left axis, gray box plot). Conversely, integration was significantly higher in the Crb1rd8/rd8 and significantly lower into the Rho−/− mouse. The differences in disease severity mean that at 6–8 wk of age the recipients were at very different stages of their degeneration (Fig. 1A, right axis, black plots, and Table S1), yet we found that even models at mid- (Crb1rd8/rd8; Prph2rd2/rd2) and late- (PDE6β rd1/rd1) stage degeneration showed levels of integration comparable to wild-type.
Fig. 1.
Photoreceptor integration is dependent upon recipient disease type. (A, Left axis, box plots) number of integrated rods 3 wk after transplantation into 6- to 8-wk-old models of retinal degeneration, compared with wild-type controls. n = number of eyes. Black bars: statistical significance (ANOVA with Tukey’s correction). Right axis (black dots), recipient ONL thickness at 6–8 wk (n = 3 per model). Black asterisks: statistical significance. (B) Representative images of integrated cells in each model. (Scale bar, 25 μm.) Dotted line denotes boundary of ONL/INL, dashed line denotes boundary of ONL. ONL, outer nuclear layer; INL, inner nuclear layer.
Ability of Transplanted Rod-Photoreceptors to Assume Normal Morphology Is Significantly Affected by the Recipient Environment.
Photoreceptor survival and function are critically dependent upon the correct formation and maintenance of synapses and inner/outer segments; both are prerequisites for effective photoreceptor transplantation therapy. The ability of endogenous rods to elaborate segments differs dramatically (Fig. S1); those in the Gnat1−/− mouse form long segments very similar to wild-type, but those formed by rods in the PDE6βrd1/rd1 models, if present, are extremely short. Such structural pathologies are likely to present very different recipient environments that may affect the maturation of transplanted cells. We therefore examined the ability of transplanted rod precursors to form segments and synapses within the different degenerating retinae (Fig. 2 and Table S2).
Fig. 2.
Morphology of integrated rods is influenced by recipient retinal environment. (A and B) Percentage of integrated rods that develop outer segments (A) and presynaptic-like structures (B) (n = 3 or more per model; ANOVA with Tukey’s correction). (C and D) Typical morphology, outer-segment length (C) and presynaptic-bouton formation (D) of integrated cells. (C) Integrated cells expressed the rod-specific transcription factor Nrl (green), rod α-transducin (C, ii), peripherin-2 (C, v), rhodopsin (C, vi), or β-PDE (C, vii) (red), as appropriate; such markers are absent in the respective endogenous photoreceptors. (D) Most (arrowheads) but not all colocalized with RIBEYE (red). Dotted line in vii denotes ONL/INL boundary. (Scale bar, 25 μm.)
We assessed both the number of integrated rods with segments and their morphological quality (Fig. 2A). In all models we found the integrated wild-type rods showed correct expression of the protein missing in the disease model (Fig. 2C). However, overall, the morphology and frequency of segment formation by integrated rods correlated with the ability of the endogenous photoreceptors to form outer segments. Over 70% of rods integrated within the ONL of wild-type, Gnat1−/−, and Crb1rd8/rd8 recipients developed segments and adopted typical rod-like morphologies with long segments (Fig. 2 C, i–iii) like those of the endogenous rods in these models. In contrast, only a fifth of integrated rods found within the Rho−/− recipient ONL developed segments (Fig. 2A) and these were short (Fig. 2 C, vi). At 3 wk of age, the ONL of the PDE6βrd1/rd1 retina is reduced to a single layer of cones; despite this, significant numbers of rods were found within the remaining ONL. Gross morphology was markedly different to normal rods, with enlarged cell bodies and multiple processes (Fig. 2 C, vii), but some developed projections orientated toward the retinal pigment epitheliumthat colocalized with β- phosphodiesterase (PDE) (Fig. 2 A–C, vii), indicative of rudimentary segments. Approximately half of rods integrated within Prph2+/Δ307 and Prph2rd2/rd2 recipients developed segments, although those formed by cells transplanted into Prph2rd2/rd2 recipients were shorter than those formed in wild-type recipients. Of note, in models where endogenous segment formation was poor, the segments formed by transplanted cells were longer than those formed by endogenous photoreceptors (Table S2).
Spherule presynaptic-like structures, typical of rod photoreceptors, were formed by over 65% of integrated rods in wild-type, Gnat1−/−, and Crb1rd8/rd8 recipients (Fig. 2 B and D, i–iii). Significantly fewer presynaptic-like structures were observed following transplantation in Prph2rd2/rd2 and Rho−/− recipients (Fig. 2 B and D, v and vi). Most severely affected were cells transplanted into PDE6βrd1/rd1 recipients, where only a third of integrated rods possessed processes that terminated in bouton-like structures (Fig. 2 B and D, vi). A qualitative assessment of all models indicated that these structures typically colocalized with the ribbon synapse protein RIBEYE (Fig. 2D). Thus, the cytoarchitecture of the recipient retina influences the ability of transplanted rod precursors to assume mature rod-photoreceptor morphology, although all environments tested are able to support segment and synapse formation to some degree.
Disease Progression Has a Major Impact on Transplanted Photoreceptor Integration Efficiency.
We next sought to determine how disease progression affects transplanted rod precursor integration and for how long the degenerative recipient retina remains permissive to transplantation. Cells were transplanted into each model at three stages of degeneration: early, mid, and late (Fig. 3A and Table S1). The number of transplanted rod precursors integrating into wild-type recipients remained constant across all timepoints examined (Fig. 3 A, i). Conversely, integration efficiency decreased in the Gnat1−/− model as disease progressed (Fig. 3 A, ii) and was already markedly lower in the Rho−/− model than in any other model and continued to decline steeply over time (Fig. 3 A, vi). Integration into the Crb1rd8/rd8 mouse presented a bimodal pattern, first increasing then decreasing sharply when transplanted into late-stage recipients (Fig. 3 A, iii). Unexpectedly, integration significantly increased with disease in the Prph2+/Δ307model (Fig. 3 A, iv) and remained constant in Prph2rds/rds and PDE6βrd1/rd1, despite significant endogenous photoreceptor loss. Thus, very different trends in integration were observed in the different models of retinal degeneration as disease progressed (Fig. 3A, blue lines). These data suggest that the recipient microenvironment plays a major role in determining photoreceptor transplantation success and that different factors may be important in each model. We next examined aspects of the microenvironment of each of the disease models, specifically disease severity, OLM integrity, and glial scarring, to try to identify factors that could account for the differences observed in integration efficiency.
Fig. 3.
Disease progression significantly but differentially affects photoreceptor transplantation efficacy according to disease type. (A) Black: impact of disease progression upon transplantation outcome, compared with wild-type. n = number of eyes examined. ANOVA with Tukey’s correction. Blue: linear regression denotes integration trend. Note that changes in Crb1rd8/rd8 retinae were bimodal (shown as dashed line). (B) Gliosis in early and late degeneration, as assessed by CSPG (green) and GFAP (red) expression. (C) OLM integrity in early and late degeneration, as assessed by ZO-1 (red) expression. Disturbances in OLM integrity indicated by white arrows. (Scale bar, 50 µm.) (D) Trend correlations (nonquantitative) for integration (black), OLM integrity (red), and gliosis (green).
Rod-Photoreceptor Transplantation Success Is Independent of ONL Thickness and Rate of Degeneration.
Measurement of ONL thickness at each stage highlighted the different rates of endogenous photoreceptor loss in each model (Fig. S2A), but there was no correlation between the rate of degeneration and integration efficiency. For example, in the Gnat1−/− degeneration is largely stationary after 3 mo of age (Fig. S2A, black squares), yet integration declines over time (Fig. 3 A, i and D, i). Conversely, a rapid rate of degeneration in PDE6βrd1/rd1 recipients (Fig. S2A, white squares) was accompanied by little change in integration (Fig. 3 A, vii and D, vii). It has previously been suggested that changes in recipient ONL cell density may influence transplant outcome (18). However, few changes in cell density were observed either between early- and late-stage degeneration or between models (Fig. S2B) and none correlated with the different trends in integration. Finally, we examined whether there is a threshold or minimum ONL thickness that is required for integration success. The mean ONL thickness of each model at each degenerative stage was plotted against the corresponding mean integration (Fig. S3); we found no correlation highlighting the finding that successful photoreceptor transplantation can be achieved even in a thinned ONL. Notably, integration above or similar to wild-type was observed in some (Prph2+/Δ307, Prph2rds/rds, PDE6βrd1), but not all models at late-stage degeneration. Taken together, these data show that neither the recipient ONL cytoarchitecture, nor the rate of endogenous photoreceptor loss, are limiting factors for transplanted rod precursor integration.
OLM Integrity and Glial Scarring Affect Photoreceptor Transplantation Success.
It has been reported that both OLM integrity (10, 15) and glial scarring (13), particularly CSPG deposition (11, 14, 19), can affect transplantation into the retina. We analyzed changes in both factors between early- and late-stage degeneration in each of the models using immunohistochemistry (Fig. 3 B and C), Western blot (Fig. S1A), and ultrastructural analysis (Fig. S1 B and C) to ascertain if either factor influences the ability of transplanted photoreceptors to integrate.
In wild-type recipients, integration remained constant with age (Fig. 3 A, i and D, i, black trend line). As expected in the absence of degeneration, no glial scarring was observed (Fig. 3 B, i and D, i, green trend line, and Fig. S1 A, i): GFAP expression was minimal and CSPGs were sparsely distributed throughout the IPM at all stages examined (Fig. 3 B, i). Similarly, there were no changes in OLM integrity (Fig. 3 C, i and D, i, red trend line): ZO-1 expression appeared as a continuous unbroken line (Fig. 3 C, i) and at the ultrastructural level, neatly aligned adherens junctions of normal appearance were observed between Müller glial and photoreceptors (Fig. S1 B, i). In Gnat1−/− recipients, despite undergoing only mild degeneration, integration decreased modestly but significantly (Fig. 3 A, ii and D, ii). The OLM remained intact throughout (Fig. 3 C, ii and D, ii, and Fig. S1 B, ii) and there was little change in CSPG deposition (Fig. 3 B, ii). However, GFAP, which may be inhibitory to integration (13), increased by the latest stage examined (Fig. 3 B, ii and D, ii, and Fig. S1 A, ii). Integration also decreased with degeneration in Rho−/− recipients (Fig. 3 A, vi and D, vi), although the initial levels were much lower and the subsequent decline more pronounced. Despite rapid degeneration, OLM integrity was maintained even in late-stage degeneration [in contrast to previous reports (16)] (Fig. 3 C, vi, and Fig. S1 B, vi). However, degeneration is associated with a strong glial response, including significant up-regulation of GFAP (Fig. 3 B, vi, and Fig. S1 A, vi) and CSPG condensation at the edge of the ONL. A bimodal pattern of integration was observed in Crb1rd8/rd8 recipients (Fig. 3 A, iii and D, iii) (see also ref. 10), whereby increasing disruption of the OLM [permitting increased integration (10, 15)] appears to be offset by a delayed but significant increase in glial scarring. CRB1 is an essential component of the OLM adherens junctional complex (17). Accordingly, significant disturbances in OLM integrity were observed (Fig. 3 C, iii and Fig. S1 B, iii and C, iii). GFAP expression was limited in Crb1rd8/rd8 in early degeneration but increased significantly by late-stage (Fig. 3 B, iii, and Fig. S1 A, iii) together with moderate CSPG condensation (Fig. 3 B, iii). Strikingly, integration into the Prph2+/Δ307 recipient increased with disease progression (Fig. 3 A, iv and D, iv). In this model, the OLM undergoes some remodeling where cell death was apparent (Fig. 3 C, iv, and Fig. S1 B, asterisks, and C, iv), although this did not change with degeneration. However, there was a very marked reduction in glial scarring: extensive GFAP expression was observed throughout the retina in early degeneration, but decreased, particularly within the ONL, by late degeneration (Fig. 3 B, iv, and Fig. S1 A, iv). CSPG expression also decreased (Fig. 3 B, iv). Integration efficiency was similar in the Prph2rds/rds recipient regardless of the stage at which cells transplanted (Fig. 3A, v and D, v). Some disorganization of the OLM was observed, although this was similar at both early- and late-stage degeneration (Fig. 3 D, v). Interestingly, despite an increase in GFAP expression (Fig. 3 B, v and Fig. S1 A, v), CSPGs at the outer edge of the ONL decreased in end stage disease (Fig. 3 B, v). In PDE6βrd1/rd1 recipients, integration efficiency was surprisingly unaffected by disease progression (Fig. 3 A, vii and D, vii). This model demonstrated significant glial scarring (Fig. 3 B, vii, and Fig. S1 A, vii), yet also underwent an increase in disturbances in OLM integrity in late degeneration (Fig. 3 C, vii, and Fig. S1 B, vii and C, vii). Of note, ultrastructural analysis revealed that although adherens junctions were present, they were larger in size and fewer in number than in wild-type and the majority were formed between Müller glial cells, rather than Müller glia and photoreceptors, indicating a significant degree of remodeling.
These data demonstrate that despite sharing the same final common pathway of photoreceptor loss, different models and stages of retinal degeneration present very different microenvironments through which donor cells must migrate, leading to strikingly different outcomes in photoreceptor transplantation efficacy. Specifically, the extent of glial scarring and changes to OLM integrity appear important in determining transplantation outcome in different types of retinal degeneration.
Manipulation of the Microenvironment in the Degenerating Rho−/− Retina Increases Integration and Permits Restoration of Visual Function.
The observed correlations between glial scarring, OLM integrity, and transplant efficacy indicated it may be possible to improve integration by administering a tailored modulation of the microenvironment for a given disease type and treatment timepoint. To determine whether glial scarring and OLM integrity are indeed responsible for impeding integration in the degenerating recipient retina, we manipulated these factors pharmacologically and assessed their impact on transplanted rod-photoreceptor integration. We chose the Rho−/− mouse, the model with the poorest transplantation outcome, which also presented with an intact OLM together with a strong glial response.
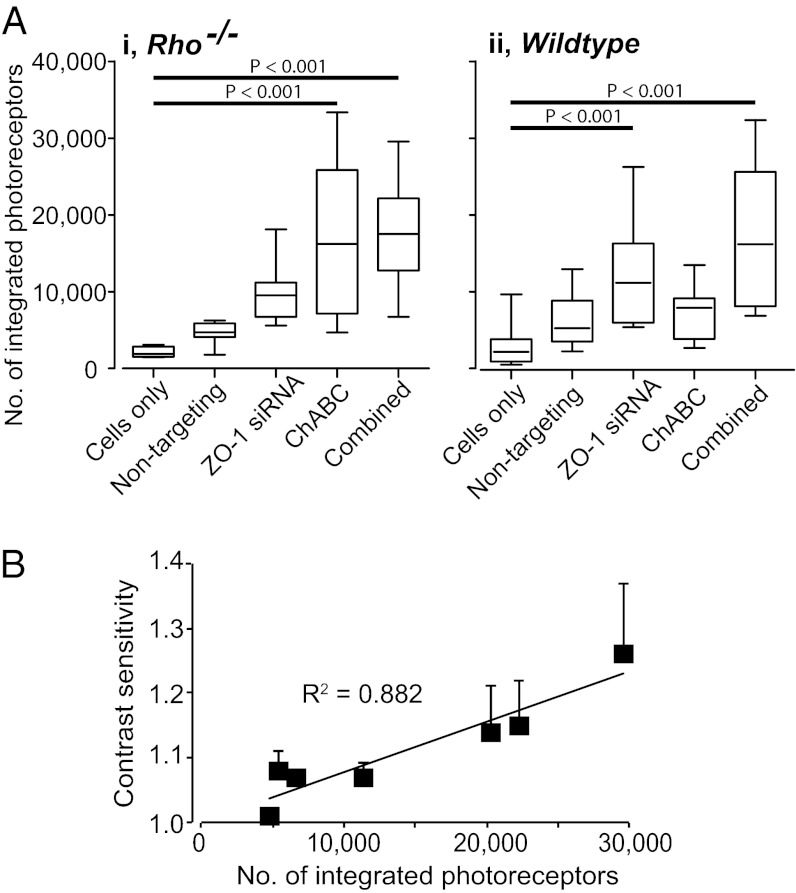
We used siRNAs targeted against ZO-1 to provide a reversible disruption of the OLM, a strategy we have previously shown to increase photoreceptor integration (10). Rho−/− and wild-type mice received either siRNA targeting ZO-1, a proven nontargeting control siRNA, or no injection 48 h before transplantation of Nrl.GFP+ve–rod-precursors to the same region. The number of integrated rods was markedly increased in ZO-1 siRNA-treated Rho−/− retinae compared with eyes receiving nontargeting siRNA or no pretreatment (4.2-fold and 2.3-fold increases, respectively) (Fig. 4A). Although the overall number of cells integrating into the wild-type mouse was higher than in the Rho−/−, a similar pattern was observed (4.4-fold and 2.2-fold increases, respectively). We next used chondroitinase ABC (ChABC) to enzymatically digest CSPGs (11, 14, 19) and combined this with transplantation of Nrl.GFP+ve–rod-precursors in Rho−/− and wild-type recipients. In Rho−/− mice, ChABC treatment lead to a highly significant increase in integration compared with controls (eightfold increase) (Fig. 4 A, i). In wild-type recipients, where there is no gliosis and CSPG expression is diffuse, the effect although present was less marked (2.5-fold increase) (Fig. 4 A, ii). We next examined the impact of combining the two manipulations. Wild-type and Rho−/− recipients received ZO-1 siRNA 48 h before coadministration of Nrl.GFP+ve–rod-precursors and ChABC. Combined treatment led to significant increases in transplanted cell integration in both wild-type and Rho−/− retinae (Fig. 4A, and Fig. S4). Thus, both the OLM and CSPG deposition impede the integration of transplanted photoreceptors into the degenerating retina but integration can be improved by targeted disruption of these barriers.
Fig. 4.
Manipulation of OLM and gliosis significantly increases integration and permits restoration of visual function. (A) impact of OLM disruption (using ZO-1 siRNA) and CSPG degradation (using ChABC), singularly and combined, on transplantation outcome in Rho−/− and wild-type recipients. n ≥ 7 per condition; ANOVA with Tukey’s correction. (B) Contrast sensitivity against number of integrated rod-photoreceptors in subset (n = 7) of Rho−/− recipients that underwent optomotor testing 3–4 wk after receiving combined treatment.
Finally, we sought to determine if the increase in integration achieved using combined treatment was sufficient to restore visual function, as assessed by optokinetic head-tracking behavior in Rho−/− mice (Fig. 4B, and SI Materials and Methods) (5, 20). Seven of the Rho−/− mice receiving combined treatment did so only in one eye and either no injection or cells only in the contralateral eye, before being tested 3–4 wk posttransplantation. No consistent head-tracking behavior was observed upon presentation of scotopic stimuli to control eyes. In contrast, head-tracking was seen following stimulation of six of seven eyes receiving the combined treatment. Histological assessment revealed a positive correlation between improvement in contrast sensitivity and the number of integrated rods (Fig. 4B), as shown previously when testing optomotor head-tracking responses in transplanted Gnat1−/− mice (5). Of note, greater numbers of integrated cells were required in Rho−/− recipients to generate contrast sensitivities equivalent to those recorded in Gnat1−/− recipients.
Discussion
Photoreceptor transplantation has the potential to restore vision following retinal degeneration (2, 5). While it could potentially be applied to a wide range of retinal degenerations, there have been no systematic assessments of efficacy across a spectrum of retinal dystrophies. Here we show that it is possible to achieve robust integration even in severely degenerate retinae and in a variety of murine models with very different aetiologies. In contrast to what might be expected, neither the rate nor extent of degeneration affected photoreceptor integration, indicating that photoreceptor replacement therapy may be an effective therapeutic strategy for severe retinal degeneration. Indeed, a more complex pattern was observed where integration increased, decreased, or remained constant with disease progression, and opposing trends were observed even in models with similar rates of degeneration. We show that the aetiology specific to each gene defect impacts both on the number and the morphology of the integrated rods within a given disease model. We also demonstrate that two characteristics of retinal degeneration, glial scarring and changes in OLM integrity, significantly affect transplantation outcome. Broadly, integration decreases in those models in which OLM integrity is maintained, but in which gliosis increases with disease progression (Gnat1−/−; Rho−/−). Integration remains constant in models in which the OLM is disrupted, but gliosis increases (PDE6βrd1/rd1). Finally, integration increases in the model in which the OLM undergoes remodeling and gliosis decreases with disease progression (Prph2+/Δ307). Importantly, it is possible to manipulate these barriers and increase transplanted photoreceptor integration to levels sufficient to restore visual function.
There are notable differences in the pattern of gliosis in the different models; GFAP+ve fibers were seen extending throughout the ONL in those models in which integration efficiency declined with degeneration (Gnat1−/−; Rho−/−), but the most poorly performing model (Rho−/−) also displayed significant CSPG deposition. Conversely, in the Prph2rd2/rd2, in which integration efficiency remained similar at different stages of degeneration, CSPG deposition decreased over time. In the Prph2+/Δ307 model, we observed a striking decrease in GFAP expression in the ONL, although such regional changes were not reflected in the global changes in GFAP expression. Thus, the specific characteristics of the glial response may be as important as its absolute magnitude.
Integration efficiency is typically higher in models of degeneration in which OLM integrity is compromised than in those in which it remains intact. Of all of the models studied, the Crb1rd8/rd8 mouse, which has a profoundly disrupted OLM (10, 17), had the highest levels of integration. Of note, we observed significant differences in OLM adherens junction composition in the different models. In wild-type mice, these junctions form between photoreceptors and Müller glia. However, in the Prph2+/Δ307, Prph2rd2/rd2, and PDE6βrd1/rd1 models, many formed directly between Müller glia, indicating significant OLM remodeling. This was supported by the presence of photoreceptors mislocalized within the IPM. In these models, integration efficiency increased or remained constant, presumably because of, at least in part, the continued changes in OLM integrity. Surprisingly, despite significant endogenous rod cell death in the Rho−/− mouse, the adherens junctions retain the typical photoreceptor-Müller glia association.
Although important, it is unlikely that OLM integrity and glial scar formation solely govern transplantation outcome within the degenerate retina; many more factors are likely to be involved. Here, our assessment of glial scarring focuses primarily on the up-regulation of GFAP and deposition of CSPG, and the application of ChABC leads to the breakdown of only some CSPGs. However, gliosis has many attributes, including Müller cell hypertrophy and proliferation and microglia accumulation. The biological roles of all these changes are unclear and their impact on cell transplantation has not been addressed. Others have shown that CSPG degradation, either by ChABC (11, 14, 19) or by endogenous enzymes (21), enhances the integration of transplanted cells. However, there are conflicting reports of the role of GFAP: one study reported an increase in transplanted cell integration in the GFAP−/−/Vim−/− mouse (13), suggesting that GFAP might be inhibitory to migration; others have reported enhanced integration around sites of GFAP up-regulation (22). Further work is needed to ascertain the exact role of intermediate filament assembly in transplantation outcome.
Recently, we have shown that it is possible to restore vision in the Gnat1−/− mouse by rod-photoreceptor transplantation (5). In this model, the retinal cytoarchitecture is well preserved and the integrating cells displayed morphologies almost indistinguishable from wild-type rods. However, as we have shown here, transplanted photoreceptor morphologies are profoundly affected by the recipient cytoarchitecture. Although the numbers of cells integrating within the PDE6βrd1/rd1 and Gnat1−/− models were similar, in PDE6βrd1/rd1 mice integrated photoreceptors often had multiple processes, with few synaptic-like structures or segments. Similarly, photoreceptors integrated within the Rho−/− mouse developed only short outer segments (present study and refs. 2 and 14). The failure to elaborate outer segments does not necessarily prevent a photoreceptor from functioning: outer segments fail to form in the Prph2rd2/rd2 mouse, yet these animals retain some residual function for several weeks postbirth (23). However, it is likely that a greater number of integrated cells will be required to restore visual function in these recipients than in recipients with more normal outer-segment morphology. Accordingly, although we observed restoration of optokinetic head-tracking in some Rho-/− mice following transplantation combined with OLM disruption and CSPG degradation, more integrated cells were required to generate contrast thresholds equivalent to those we reported recently for Gnat1−/− recipients (5). This finding highlights the need to find additional ways to achieve high levels of integration in advanced degeneration.
Materials and Methods
Full methods are available in SI Materials and Methods. Single subretinal transplantations of 200,000 live P6-8 Nrl.GFP+ve–rod-photoreceptors were given to wild-type (C57BL/6J) mice and models of inherited retinal degeneration at early-, mid-, and late-stages of degeneration (Results). Cell integration was assessed 3–4 wk posttransplantation. Gliosis was manipulated using ChABC (19) and OLM integrity was manipulated using siRNAs directed against ZO-1 (10). Optomotor responses were recorded as previously described (5). See Table S3 for immunohistochemistry.
Supplementary Material
Acknowledgments
We thank A. Eddaoudi for technical assistance. This work was supported by grants from the British Retinitis Pigmentosa Society (GR566), Wellcome Trust (082217; 086128), Royal Society (RG080398), and Medical Research Council UK (G03000341; G0901550 mr/j004553/1). R.A.P. is a Royal Society University Research Fellow; J.C.S. is supported by Great Ormond Street Hospital Children’s Charity; and R.R.A. is partially funded by the Department of Health's National Institute for Health Research Biomedical Research Centre, Moorfields Eye Hospital, and the Miller's Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.A.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212677110/-/DCSupplemental.
References
- 1.Bainbridge JW, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 2.MacLaren RE, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 3.Lakowski J, et al. Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells. Hum Mol Genet. 2010;19(23):4545–4559. doi: 10.1093/hmg/ddq378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akimoto M, et al. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc Natl Acad Sci USA. 2006;103(10):3890–3895. doi: 10.1073/pnas.0508214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson RA, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485(7396):99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eiraku M, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472(7341):51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 7.Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc. 2009;4(6):811–824. doi: 10.1038/nprot.2009.51. [DOI] [PubMed] [Google Scholar]
- 8.West EL, et al. Defining the integration capacity of embryonic stem cell-derived photoreceptor precursors. Stem Cells. 2012;30(7):1424–1435. doi: 10.1002/stem.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4(1):73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson RA, et al. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant. 2010;19(4):487–503. doi: 10.3727/096368909X486057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singhal S, et al. Chondroitin sulfate proteoglycans and microglia prevent migration and integration of grafted Müller stem cells into degenerating retina. Stem Cells. 2008;26(4):1074–1082. doi: 10.1634/stemcells.2007-0898. [DOI] [PubMed] [Google Scholar]
- 12.Fawcett J. Molecular control of brain plasticity and repair. Prog Brain Res. 2009;175:501–509. doi: 10.1016/S0079-6123(09)17534-9. [DOI] [PubMed] [Google Scholar]
- 13.Kinouchi R, et al. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat Neurosci. 2003;6(8):863–868. doi: 10.1038/nn1088. [DOI] [PubMed] [Google Scholar]
- 14.Ma J, Kabiel M, Tucker BA, Ge J, Young MJ. Combining chondroitinase ABC and growth factors promotes the integration of murine retinal progenitor cells transplanted into Rho(-/-) mice. Mol Vis. 2011;17:1759–1770. [PMC free article] [PubMed] [Google Scholar]
- 15.West EL, et al. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res. 2008;86(4):601–611. doi: 10.1016/j.exer.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell M, et al. Aberrant retinal tight junction and adherens junction protein expression in an animal model of autosomal dominant Retinitis pigmentosa: The Rho(-/-) mouse. Exp Eye Res. 2006;83(3):484–492. doi: 10.1016/j.exer.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 17.Mehalow AK, et al. CRB1 is essential for external limiting membrane integrity and photoreceptor morphogenesis in the mammalian retina. Hum Mol Genet. 2003;12(17):2179–2189. doi: 10.1093/hmg/ddg232. [DOI] [PubMed] [Google Scholar]
- 18.Yao J, et al. XIAP therapy increases survival of transplanted rod precursors in a degenerating host retina. Invest Ophthalmol Vis Sci. 2011;52(3):1567–1572. doi: 10.1167/iovs.10-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki T, et al. Chondroitinase ABC treatment enhances synaptogenesis between transplant and host neurons in model of retinal degeneration. Cell Transplant. 2007;16(5):493–503. doi: 10.3727/000000007783464966. [DOI] [PubMed] [Google Scholar]
- 20.Umino Y, Solessio E, Barlow RB. Speed, spatial, and temporal tuning of rod and cone vision in mouse. J Neurosci. 2008;28(1):189–198. doi: 10.1523/JNEUROSCI.3551-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Klassen HJ, Tucker BA, Perez MT, Young MJ. CNS progenitor cells promote a permissive environment for neurite outgrowth via a matrix metalloproteinase-2-dependent mechanism. J Neurosci. 2007;27(17):4499–4506. doi: 10.1523/JNEUROSCI.0200-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishida A, et al. Incorporation and differentiation of hippocampus-derived neural stem cells transplanted in injured adult rat retina. Invest Ophthalmol Vis Sci. 2000;41(13):4268–4274. [PubMed] [Google Scholar]
- 23.Reuter JH, Sanyal S. Development and degeneration of retina in rds mutant mice: The electroretinogram. Neurosci Lett. 1984;48(2):231–237. doi: 10.1016/0304-3940(84)90024-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.