Abstract
Aldehyde- and ketone-functionalized proteins are appealing substrates for the development of chemically modified biotherapeutics and protein-based materials. Their reactive carbonyl groups are typically conjugated with α-effect nucleophiles, such as substituted hydrazines and alkoxyamines, to generate hydrazones and oximes, respectively. However, the resulting C=N linkages are susceptible to hydrolysis under physiologically relevant conditions, which limits the utility of such conjugates in biological systems. Here we introduce a Pictet-Spengler ligation that is based on the classic Pictet-Spengler reaction of aldehydes and tryptamine nucleophiles. The ligation exploits the bioorthogonal reaction of aldehydes and alkoxyamines to form an intermediate oxyiminium ion; this intermediate undergoes intramolecular C–C bond formation with an indole nucleophile to form an oxacarboline product that is hydrolytically stable. We used the reaction for site-specific chemical modification of glyoxyl- and formylglycine-functionalized proteins, including an aldehyde-tagged variant of the therapeutic monoclonal antibody Herceptin. In conjunction with techniques for site-specific introduction of aldehydes into proteins, the Pictet-Spengler ligation offers a means to generate stable bioconjugates for medical and materials applications.
Keywords: bioorthogonal chemistry, protein conjugation, reaction methodology
Reaction methodology for protein modification has been an active area of research for decades. Early strategies focused on global modification of native amino acids, providing access to heterogeneously modified products (1). However, a variety of applications necessitate site-specific modification of proteins: biophysical studies requiring knowledge of the site of attachment of a reporter molecule (2), preparation of protein microarrays and functional materials requiring immobilization in a specific orientation (3), and conjugation of protein drugs with poly(ethylene glycol) or cytotoxic molecules, where the site of chemical modification affects the pharmacokinetic and therapeutic properties of the resulting biologic (4, 5). Therefore, in recent years, the field has refocused on methods to achieve site-specific protein modification, typically by introduction of a nonnative functional group exhibiting bioorthogonal reactivity (6, 7).
Aldehydes and ketones are popular choices as chemical handles for site-specific protein modification. Their unique reactivity as mild electrophiles enables selective conjugation with α-effect nucleophiles such as substituted hydrazines and alkoxyamines, which generate hydrazone and oxime-ligated products, respectively (8). Several chemical, enzymatic, and genetic methods have been developed to introduce aldehydes and ketones into proteins site specifically. These include periodate oxidation of N-terminal serine or threonine residues (9), pyridoxal phosphate-mediated N-terminal transamination to yield an α-ketoamide or glyoxamide (10–13), addition of ketone-containing small molecules to protein C-terminal thioesters generated by expressed protein ligation (14), genetically encoded incorporation of unnatural amino acids containing ketones via amber stop codon suppression (15–18), genetic encoding of peptide tags that direct enzymatic ligation of aldehyde- or ketone-bearing small molecules (19, 20), and genetic encoding of a site for modification by the formylglycine-generating enzyme (FGE), the “aldehyde tag” method developed in our laboratory (21–25).
The diversity of methods for introducing reactive carbonyl groups into proteins stands in contrast to the limited number of reactions that have been adopted for their chemical modification. Reductive amination has found some use, mainly with glycoprotein substrates in which aldehydes were introduced by glycan oxidation (26). But the vast majority of examples use the hydrazone and oxime-forming reactions mentioned previously because of their bioorthogonality, operational simplicity (i.e., no auxiliary reagents are required), and good yields under mild aqueous conditions. However, the resulting C=N bonds are susceptible to hydrolysis (27), undermining the use of such conjugates in situations in which long-term stability is required. The oxime has been identified as the most hydrolytically stable C=N linkage, but it is still thermodynamically unstable to hydrolysis under dilute conditions, decomposing via an acid-catalyzed process (28). Many researchers have found that oxime conjugates that are kept under ideal storage conditions—low temperature, high concentration, and neutral or high pH—are kinetically stable and are therefore suitable for short-term laboratory studies (23, 25, 29). However, biological applications requiring extended persistence of the conjugate at physiological temperatures and low concentrations necessitate a significantly more stable covalent linkage than the oxime provides.
The ideal bioconjugation reaction would form a stable C–C bond with protein aldehydes and ketones. A few such reactions have been reported, but they are limited by slow reaction kinetics (30) or the need for organic cosolvents (31, 32). A C–C bond-forming transformation possessing the kind of generality and operational simplicity that led to the widespread adoption of oxime bioconjugation has not yet been reported. Here we describe the development of the Pictet-Spengler ligation, a C–C bond-forming reaction that capitalizes on the bioorthogonality of oxime formation in an intermediate step. We used this reaction to prepare hydrolytically stable conjugates with glyoxyl- and formylglycine-modified proteins, including a monoclonal antibody.
Results and Discussion
Design and Synthesis of Pictet-Spengler Ligation Reagents.
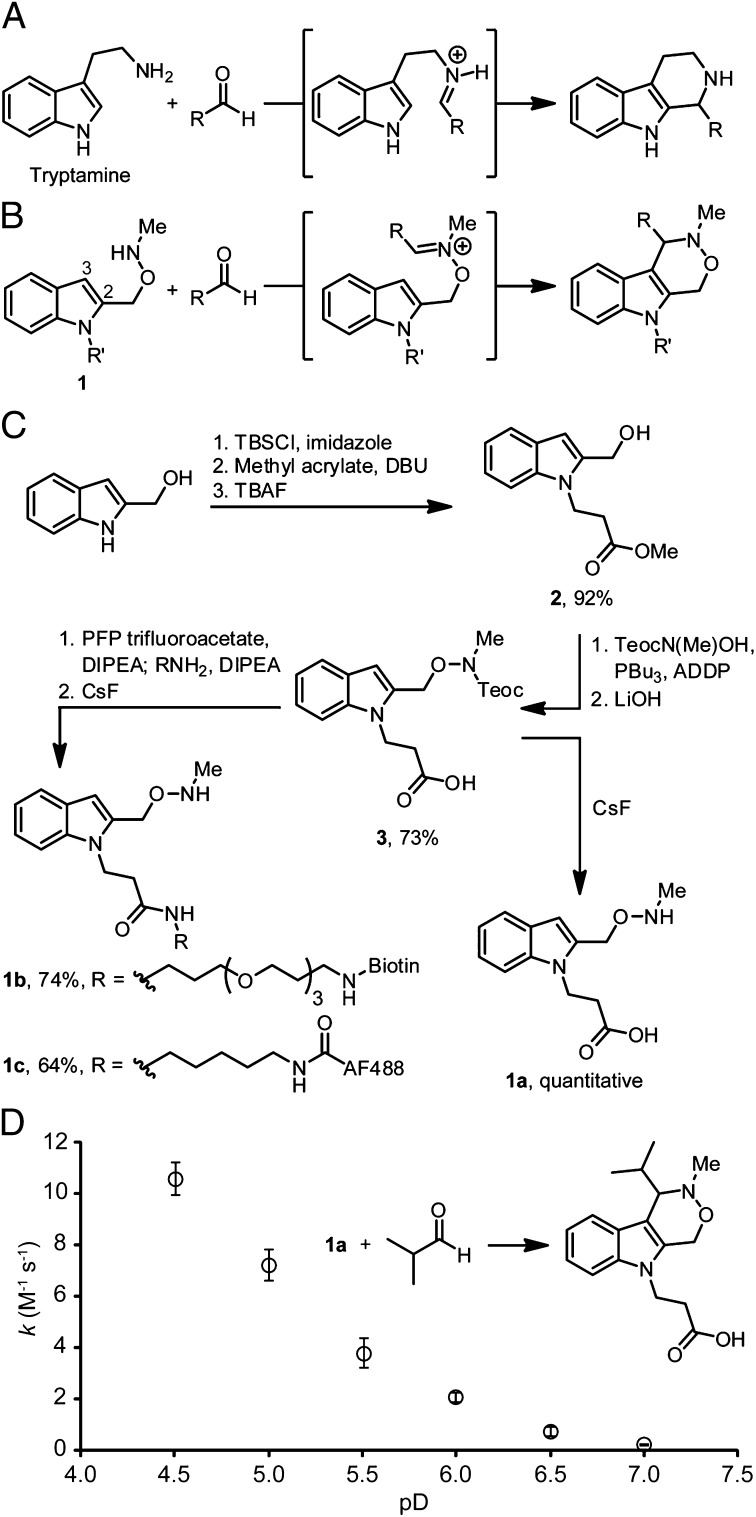
For the past century, the Pictet-Spengler reaction has played an important role in the synthesis of indole alkaloid natural products (33). We hypothesized that the transformation (Fig. 1A), which forms a C–C bond between tryptamine and an aldehyde or a ketone, could be adapted for the purpose of irreversible bioconjugation. The canonical Pictet-Spengler reaction has previously been used in this context (30); however, the reaction is slow under protein-compatible conditions, proceeding with a second-order rate constant of ∼10−4 M−1⋅s−1 at pH 4–5 (34). These slow reaction kinetics necessitate high concentrations (e.g., 50 mM) of the derivatizing reagent to achieve good yields of modified protein, which can be problematic from the standpoints of reagent cost, off-target reactivity, purification of the resulting conjugate, and toxicity if applied to protein labeling on live cells.
Fig. 1.
Design and evaluation of the Pictet-Spengler ligation. (A) The Pictet-Spengler reaction. (B) The Pictet-Spengler ligation. (C) Synthesis of aldehyde- and ketone-reactive indoles used in this study. (D) Second-order rate constants for the reaction of 1a with isobutyraldehyde in D2O solutions containing 100 mM deuterated acetate (pD ≤ 5.5) or phosphate (pD ≥ 6.0) buffers. Error bars represent SD of at least three replicate experiments. ADDP, 1,1′-(azodicarbonyl)dipiperidine; DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; DIPEA, diisopropylethylamine; PFP, pentafluorophenyl; TBAF, tetrabutylammonium fluoride; TBS, tert-butyldimethylsilyl; Teoc, 2-(trimethylsilyl)ethoxycarbonyl.
In our design of the Pictet-Spengler ligation (Fig. 1B), we were guided by a thorough kinetic study of the Pictet-Spengler reaction indicating that formation of an iminium ion intermediate is partially rate-limiting (34). Taking note of the rapid rates at which α-effect amines condense with aldehydes and ketones (35), we hypothesized that the rate of iminium ion formation could be enhanced by replacing the aliphatic amine of tryptamine with an aminooxy moiety. To further increase the rate of the reaction, we moved the aminooxy substituent to the 2-position of the indole, allowing the more nucleophilic 3-position to engage in electrophilic substitution. Indoles that are substituted with aliphatic amines at the 2-position are known to engage in “iso-Pictet-Spengler” reactions in organic solvents (36, 37). Finally, we methylated the aminooxy functionality to provide a reactive oxyiminium ion intermediate that would facilitate rapid C–C bond formation via intramolecular electrophilic substitution. Pictet-Spengler reactions of N-alkoxytryptamines to afford products with exocyclic aminooxy functionality are known (38–40), but to the best of our knowledge neither their kinetics nor their behavior in aqueous media has been studied. With these precedents, we expected aminooxy-functionalized indoles 1 to engage in a fast Pictet-Spengler type reaction (Fig. 1B).
We prepared model indole 1a in a short, high-yielding synthesis (Fig. 1C). First, an ester was installed as a masked functionalization handle by protection of indole-2-methanol with TBSCl followed by a 1,8-diazabicyclo[5.4.0]undec-7-ene–catalyzed aza-Michael addition to methyl acrylate (41). Following deprotection of the hydroxyl group to yield indole 2, the aminooxy moiety was installed by reaction with 2-(trimethylsilyl)ethoxycarbonyl (Teoc)-protected N-methylhydroxylamine under modified Mitsunobu conditions (42, 43). Saponification of the resulting product yielded compound 3, which was cleanly deprotected with CsF to afford indole 1a in 66% yield over six steps.
Reactivity of Model Indole 1a and Hydrolytic Stability of Products.
To validate the Pictet-Spengler ligation, we treated indole 1a with either isobutyraldehyde or acetone in methanolic ammonium acetate solutions at pH 4.5. Both reactions proceeded very cleanly in less than 1 h to afford the desired dihydro-β-oxa-γ-carboline (hereafter referred to as oxacarboline) products (SI Appendix, Fig. S1). Analysis of the rate of the reaction of 1a with isobutyraldehyde in D2O by 1H NMR spectroscopy revealed a rate law that is first-order in the concentrations of 1a and isobutyraldehyde at pD 7.0 (pD = pH meter reading + 0.41; SI Appendix, Fig. S2 and Table S1), and a pD-rate constant profile characteristic of aminooxy compounds under acidic conditions (Fig. 1D) (44). These results show that our rate-enhancement strategies were successful, as the Pictet-Spengler ligation is 4–5 orders of magnitude faster than the canonical Pictet-Spengler reaction in aqueous media.*
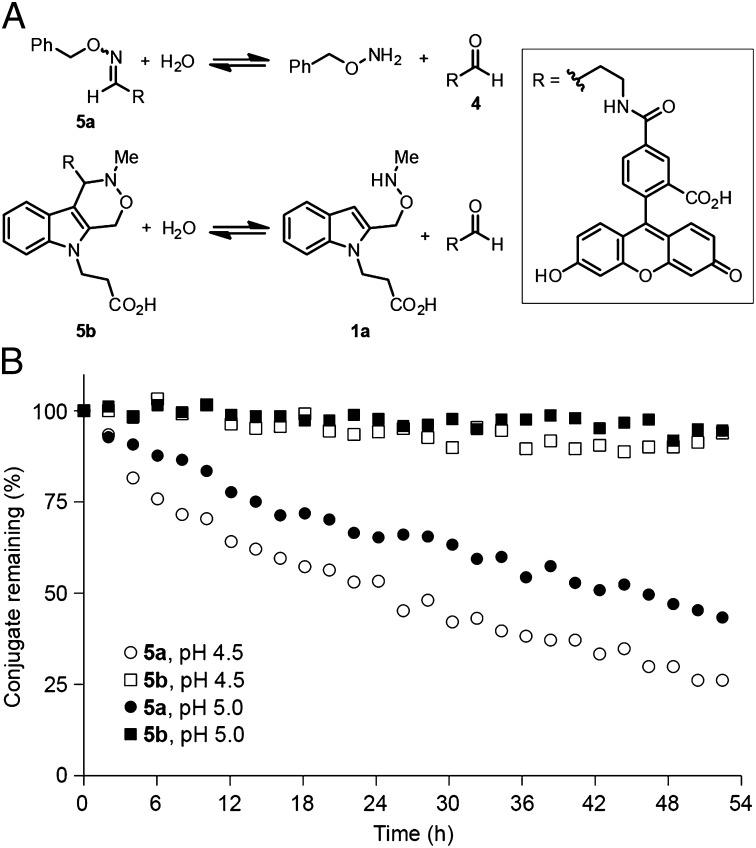
Next, we compared the hydrolytic stability of the oxacarboline generated by the Pictet-Spengler ligation with that of a model oxime. We treated an aldehyde-derivatized fluorescein 4 (Fig. 2A) with benzylalkoxyamine or 1a to generate conjugates 5a and 5b, respectively. Buffered solutions at pH 4.5 or 5.0 containing 1 μM 5a or 5b were incubated at room temperature and analyzed by liquid chromatography. Over the course of 2 d, the majority of oxime 5a hydrolyzed, whereas more than 90% of oxacarboline 5b remained intact (Fig. 2B); no products other than those arising from hydrolysis were detected (SI Appendix, Fig. S4). Previous work has shown that oxime hydrolysis occurs on a similar timescale in the presence of excess formaldehyde used as a trap to drive the reaction toward hydrolysis of the conjugate (28). Notably, our results indicate that oxime hydrolysis can occur to an appreciable extent in aqueous solution, even in the absence of a trap, underscoring the need for irreversible bioconjugation reactions. These model experiments establish that the Pictet-Spengler ligation proceeds rapidly under acidic conditions to yield a hydrolytically stable product.
Fig. 2.
Hydrolytic stability of a model oxime and oxacarboline. (A) Scheme showing hydrolysis of indoles 5a and 5b. (B) Liquid chromatography data showing hydrolysis of 1 μM 5a and 5b at room temperature over 2 d.
Scope of the Reaction on Model Proteins.
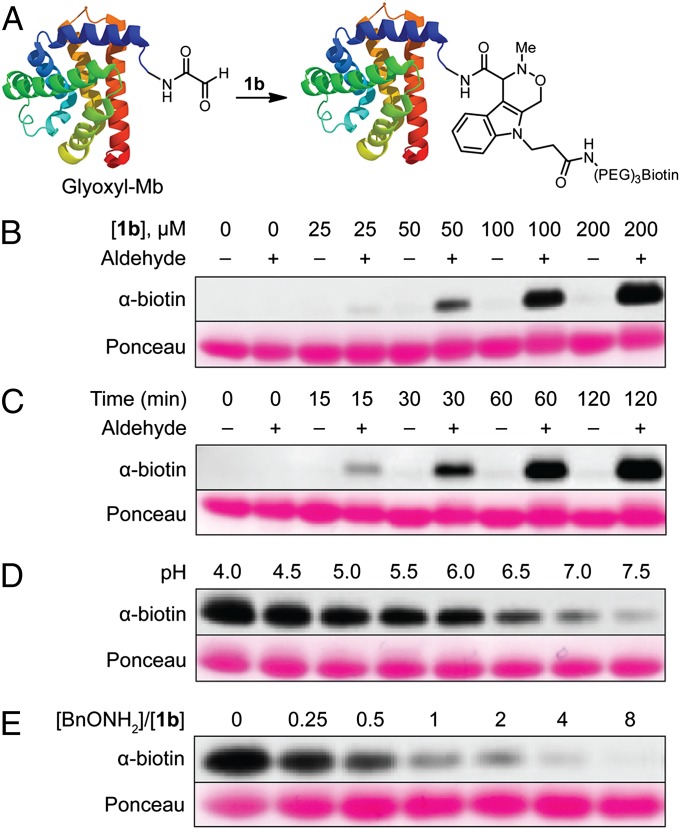
We next evaluated the Pictet-Spengler ligation as a means to label aldehyde-functionalized proteins. To facilitate the detection and manipulation of labeled proteins, we prepared biotinylated indole 1b by coupling indole 3 with amino-poly(ethylene glycol)-functionalized biotin, followed by deprotection of the Teoc group with CsF. As a protein substrate for reaction with 1b, we generated horse heart myoglobin with an N-terminal glyoxyl moiety (glyoxyl-Mb) by pyridoxal phosphate-mediated transamination (Fig. 3A) (10). In glyoxyl-Mb labeling experiments, conjugated product was detected by SDS/PAGE and Western blotting with a FITC-conjugated α-biotin antibody after quenching excess labeling reagent with benzaldehyde. First, we established that labeling occurs in a concentration- and time-dependent manner (Fig. 3 B and C, respectively). Importantly, control samples of Mb that were not aldehyde-functionalized showed negligible labeling. We next studied the pH-dependence of the reaction, observing a greater extent of biotinylation at more acidic pH (Fig. 3D), as was also observed in kinetic studies of indole 1a. Finally, we found that cotreatment with benzylalkoxyamine as an aldehyde scavenger resulted in diminished biotinylation (Fig. 3E). A similar series of experiments using commercial aminooxy-biotin as a labeling reagent showed the same qualitative trends in labeling (SI Appendix, Fig. S5). Collectively, these results establish that indole 1b specifically labels the aldehyde functionality in transaminated myoglobin, and, more generally, behaves like a typical aminooxy reagent.†
Fig. 3.
Optimization of the Pictet-Spengler ligation on glyoxyl-Mb. (A) General scheme for biotinylation of glyoxyl-Mb. Indole 1b exhibits (B) concentration-dependent, (C) time-dependent, and (D) pH-dependent labeling of glyoxyl-Mb. Additionally, (E) biotinylation can be diminished by cotreatment with BnONH2. Mb (– aldehyde) or glyoxyl-Mb (+ aldehyde) was treated with (B) 0–200 μM 1b for 3 h at pH 4.0, (C) 250 μM 1b for 0–2 h at pH 4.0, (D) 250 μM 1b for 3 h at pH 4.0–7.5, or (E) 100 μM 1b for 3 h at pH 4.5 in the presence of 0–800 μM BnONH2. All reactions were run at 37 °C and quenched with 10 mM benzaldehyde before resolution by SDS/PAGE. Biotinylation was assessed with an FITC-conjugated α-biotin antibody and total protein loading with Ponceau S.
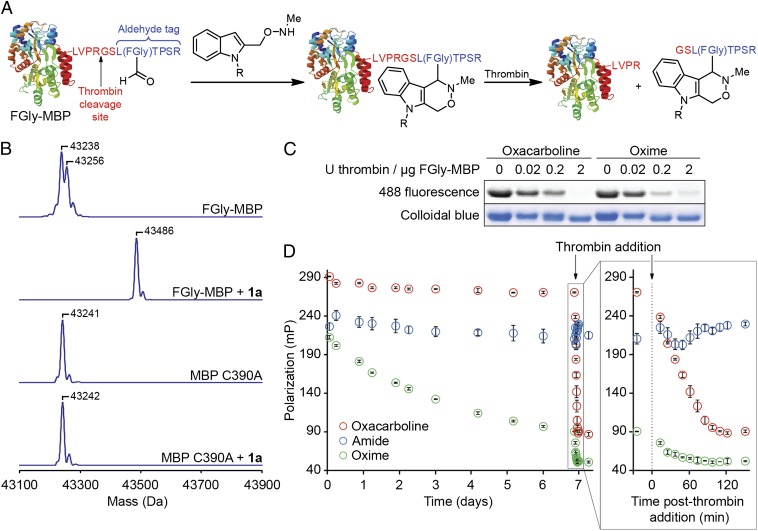
We next studied the reaction of 1a with formylglycine-functionalized maltose-binding protein (FGly-MBP), prepared using the genetically encoded aldehyde tag method (Fig. 4A) (24). Briefly, the 6-residue peptide sequence LCTPSR was engineered at the C terminus of MBP, constituting residues 389–394 in the recombinant protein. We also included a thrombin cleavage site N-terminal to the aldehyde tag sequence. Coexpression of the protein alongside the Mycobacterium tuberculosis FGE in Escherichia coli resulted in oxidation of Cys390 to FGly (21). As a control, we also expressed the C390A mutant, which is not a substrate for FGE and lacks the FGly aldehyde. Incubation of FGly-MBP with 1 mM indole 1a at 37 °C for 12 h resulted in quantitative conversion to the desired singly modified adduct, as judged by electrospray ionization mass spectrometry (ESI-MS), whereas the C390A mutant showed no reaction (Fig. 4B). Additionally, when an FGly-MBP conjugate of 1a was digested with trypsin, we were able to identify the C-terminal 8-residue tryptic peptide containing the desired adduct by high-resolution ESI-MS (SI Appendix, Fig. S6A). MS/MS fragmentation of the tryptic peptide by electron-transfer dissociation provided direct evidence for modification of the FGly residue (SI Appendix, Fig. S6B). Reaction of FGly-MBP with tryptophan methyl ester under identical conditions resulted in only minimal (<3%) conversion to the modified product (SI Appendix, Fig. S7), confirming that the Pictet-Spengler ligation proceeds much more quickly than the canonical Pictet-Spengler reaction on proteins.
Fig. 4.
Modification of FGly-MBP by the Pictet-Spengler ligation. (A) Scheme depicting Pictet-Spengler ligation with FGly-MBP followed by thrombin-catalyzed cleavage of a C-terminal 8-mer peptide containing the oxacarboline. (B) Deconvoluted mass spectra of Pictet-Spengler ligations. FGly-MBP and MBP C390A were incubated with 1 mM 1a at pH 5.0 for 12 h at 37 °C before analysis by ESI-MS. Expected masses (Da): FGly-MBP, 43256, and 43238 (M – H2O); FGly-MBP + 1a, 43486; MBP C390A, 43242. (C) Thrombin-catalyzed cleavage of FGly-MBP conjugates. (D) Fluorescence polarization analysis of AF488-MBP conjugate hydrolysis; (Inset) polarization of solutions immediately following thrombin addition. Solutions containing 100 nM AF488 conjugate were incubated in PBS (pH 7.2) at 37 °C for 1 wk before thrombin addition.
To confirm that labeling occurred only at the FGly residue, we exploited the thrombin cleavage site engineered directly upstream of the aldehyde tag sequence. First, we prepared indole 1c by coupling 3 with Alexa Fluor 488 (AF488) cadaverine followed by deprotection with CsF. Next, we prepared oxacarboline- or oxime-linked AF488 conjugates of FGly-MBP by treatment with either 1c or AF488 hydroxylamine, incubated the conjugates with various amounts of thrombin for 1 h, and then analyzed the products by SDS/PAGE. The intensity of in-gel fluorescence from the FGly-MBP band decreased at higher thrombin concentrations, consistent with labeling exclusively within the cleaved C-terminal 8-residue peptide (Fig. 4C). Notably, the oxime- and oxacarboline-linked AF488-MBP conjugates displayed qualitatively similar behavior, indicating that, relative to the oxime, the larger oxacarboline moiety did not inhibit the protein’s ability to serve as a substrate for thrombin. These experiments establish that the Pictet-Spengler ligation exclusively labels the FGly residue on the aldehyde-tagged protein.
Hydrolytic Stability of the Oxacarboline Linkage on a Protein.
Next, we assayed the hydrolytic stability of the oxacarboline linkage on FGly-MBP. Fluorescence polarization is a technique that yields information about the tumbling rate of a fluorophore in solution: macromolecule-conjugated fluorophores tumble slowly and exhibit high polarization values, whereas small-molecule fluorophores exhibit low polarization values. Thus, fluorescence polarization is ideally suited to monitor cleavage of protein-fluorophore conjugates (46). A solution of FGly-MBP was treated with 1c, AF488 hydroxylamine, or a lysine-reactive AF488-sulfodichlorophenol ester to make oxacarboline-, oxime-, or amide-linked AF488-MBP, respectively (SI Appendix, Fig. S8). The samples were then diluted to 100 nM in AF488 conjugate and incubated at 37 °C. The fluorescence polarization was monitored for 1 wk (Fig. 4D). The oxime conjugate exhibited a steady drop in polarization, indicating nearly complete hydrolysis of the conjugate over the course of 1 wk. In contrast, the oxacarboline and amide conjugates showed only a minimal change in polarization. To confirm that the oxacarboline-linked AF488 conjugate was still intact after 1 wk, we added thrombin to the samples, which resulted in an immediate decrease in polarization as the C-terminal peptide containing the fluorophore was cleaved from the rest of the protein. The polarization of the resulting solutions containing mixtures of free and peptide-linked AF488 matched the polarization of independently prepared solutions of the free fluorophores. The signal from the amide-linked AF488 conjugate remained stable (no lysine residues are present downstream of the thrombin cleavage site), indicating that the decrease in polarization was not an artifact of thrombin addition.
Application of the Pictet-Spengler Ligation to Site-Specific Modification of a Monoclonal Antibody.
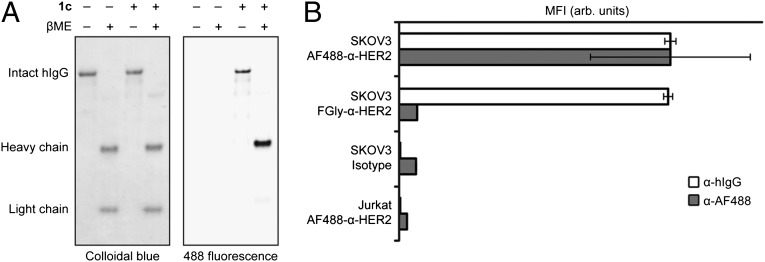
To showcase the utility of the Pictet-Spengler ligation in preparation of antibody conjugates, we used an α-HER2 human IgG modified with an aldehyde tag sequence at the C terminus of each of its two heavy chains (abbreviated FGly-α-HER2). The parent antibody is a variant of the clinically approved drug Herceptin (47) and of T-DM1, an antibody-drug conjugate based on Herceptin that is presently in late-stage clinical evaluation (48). FGly-α-HER2 was prepared as previously described (24) and then labeled with indole 1c at pH 4.5 for 12 h; the resulting conjugate (AF488-α-HER2) was cleanly modified on the heavy chain (Fig. 5A) with an average of 1.0 ± 0.13 fluorophores per hIgG (SI Appendix, Fig. S9). We next assessed binding of this antibody conjugate to the ovarian adenocarcinoma cell line SKOV3, which overexpresses HER2, by flow cytometry. SKOV3 cells were treated with AF488-α-HER2 or FGly-α-HER2, followed by a DyLight 649-conjugated α-hIgG secondary antibody to measure total hIgG binding. We found no difference in binding between AF488-α-HER2 and FGly-α-HER2 (Fig. 5B), suggesting that neither the Pictet-Spengler ligation reaction conditions nor the presence of the oxacarboline moiety negatively impacts the antibody’s affinity for HER2. Incubation of the labeled cells with a rabbit α-AF488 secondary antibody followed by a FITC-conjugated α-rabbit tertiary antibody resulted in increased fluorescence on cells treated with AF488-α-HER2 but not with FGly-α-HER2 (Fig. 5B). This result confirms that the AF488 cargo was successfully delivered to the cell surface by AF488-α-HER2. As expected, an isotype control hIgG showed no significant binding to SKOV3 cells; furthermore, the AF488-α-HER2 conjugate had no affinity for Jurkat T cells, which do not express HER2. Overall, these experiments show that the Pictet-Spengler ligation can be used to prepare a site-specifically labeled monoclonal antibody without compromising binding activity.
Fig. 5.
Characterization of FGly-α-HER2 modified by the Pictet-Spengler ligation. (A) Reducing and nonreducing SDS/PAGE analysis of FGly-α-HER2 and AF488-α-HER2. (B) Median fluorescence intensity of SKOV3 and Jurkat cell populations treated with human antibodies. Cells were treated with AF488-α-HER2, FGly-α-HER2, or human isotype control and then fluorescently labeled with α-hIgG and α-AF488 antibodies. Error bars represent SD of three replicate experiments. βME, beta-mercaptoethanol.
Conclusion
The Pictet-Spengler ligation possesses the selectivity, kinetics, and operational simplicity that originally popularized traditional oxime and hydrazone protein conjugation reactions. However, its oxacarboline product enables the persistence of bioconjugates in hydrolytically demanding environments where C=N linkages currently fail. We demonstrated the generality of the method using a variety of aldehyde-functionalized proteins, including a therapeutically relevant human IgG. Model reactions suggest that ketones are potential substrates as well and suggest a future direction to explore with respect to bioconjugation. We focused here on the use of the Pictet-Spengler ligation for modification of purified proteins, but applications extend to other biomolecules that are amenable to functionalization with reactive carbonyl groups. Methods for metabolic (49–51), enzymatic (52), and chemical (53, 54) functionalization of glycans with ketone and aldehyde groups are well-established and are finding use in proteomic analyses of glycosylated proteins. The Pictet-Spengler ligation may enhance the performance of these methods as well as others that seek to detect or manipulate carbonyl groups using bioorthogonal chemistry (55, 56).
Materials and Methods
Details concerning the synthesis and characterization of all new compounds can be found in SI Appendix, SI Materials and Methods. Also included are details of small molecule kinetics and hydrolysis experiments. Protocols for protein conjugations and characterization of FGly-MBP and FGly-α-HER2 conjugates are also included in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Jason Hudak for preparing FGly-MBP, Tony Lavarone and David King for technical assistance, Krishnan Palaniappan and Leah Witus for helpful discussions, Benjamin Swarts for a critical reading of the manuscript, and the laboratory of Prof. James Berger (University of California at Berkeley) for use of instrumentation. P.A., J.v.d.W., and E.M.S. were supported by a National Science Foundation Graduate Research Fellowship, a Nora Baart Foundation fellowship, and an American Chemical Society Organic Division graduate fellowship, respectively. This work was supported by National Institutes of Health Grant GM59907 (to C.R.B.).
Footnotes
Conflict of interest statement: C.R.B. is a cofounder and member of the Scientific Advisory Board of Redwood Bioscience.
This article is a PNAS Direct Submission.
*In an attempt to further increase the rate of the reaction, we also synthesized a variant of 1a with a methoxy group at the 5-position of the indole (SI Appendix, Fig. S3A). Kinetic experiments at pD 4.5 and 6.0 showed that the methoxylated indole reacts with isobutyraldehyde about 30% faster than 1a, depending on the acidity of the solution (SI Appendix, Table S2). Given the modest increase in reaction rate, we pursued further studies with unsubstituted indole 1a.
†We also explored whether the Pictet-Spengler ligation could be accelerated by aniline catalysis (45). Aniline did not increase the rate of the reaction of 1b with glyoxyl-Mb at pH 4.5, and at higher pH (5.5 or 6.5) aniline was found to inhibit the reaction (SI Appendix, Fig. S5E), which was consistent with previous observations (23, 24).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213186110/-/DCSupplemental.
References
- 1.Glazer AN. Specific chemical modification of proteins. Annu Rev Biochem. 1970;39:101–130. doi: 10.1146/annurev.bi.39.070170.000533. [DOI] [PubMed] [Google Scholar]
- 2.Michalet X, Weiss S, Jäger M. Single-molecule fluorescence studies of protein folding and conformational dynamics. Chem Rev. 2006;106(5):1785–1813. doi: 10.1021/cr0404343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong LS, Khan F, Micklefield J. Selective covalent protein immobilization: Strategies and applications. Chem Rev. 2009;109(9):4025–4053. doi: 10.1021/cr8004668. [DOI] [PubMed] [Google Scholar]
- 4.Shen B-Q, et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol. 2012;30(2):184–189. doi: 10.1038/nbt.2108. [DOI] [PubMed] [Google Scholar]
- 5.Cho H, et al. Optimized clinical performance of growth hormone with an expanded genetic code. Proc Natl Acad Sci USA. 2011;108(22):9060–9065. doi: 10.1073/pnas.1100387108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sletten EM, Bertozzi CR. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew Chem Int Ed Engl. 2009;48(38):6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephanopoulos N, Francis MB. Choosing an effective protein bioconjugation strategy. Nat Chem Biol. 2011;7(12):876–884. doi: 10.1038/nchembio.720. [DOI] [PubMed] [Google Scholar]
- 8.Jencks WP. Simple carbonyl group reactions. Prog Phys Org Chem. 1964;2:63–128. [Google Scholar]
- 9.Geoghegan KF, Stroh JG. Site-directed conjugation of nonpeptide groups to peptides and proteins via periodate oxidation of a 2-amino alcohol. Application to modification at N-terminal serine. Bioconjug Chem. 1992;3(2):138–146. doi: 10.1021/bc00014a008. [DOI] [PubMed] [Google Scholar]
- 10.Gilmore JM, Scheck RA, Esser-Kahn AP, Joshi NS, Francis MB. N-terminal protein modification through a biomimetic transamination reaction. Angew Chem Int Ed Engl. 2006;45(32):5307–5311. doi: 10.1002/anie.200600368. [DOI] [PubMed] [Google Scholar]
- 11.Scheck RA, Dedeo MT, Iavarone AT, Francis MB. Optimization of a biomimetic transamination reaction. J Am Chem Soc. 2008;130(35):11762–11770. doi: 10.1021/ja802495w. [DOI] [PubMed] [Google Scholar]
- 12.Witus LS, et al. Identification of highly reactive sequences for PLP-mediated bioconjugation using a combinatorial peptide library. J Am Chem Soc. 2010;132(47):16812–16817. doi: 10.1021/ja105429n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witus LS, Francis M. Site-specific protein bioconjugation via a pyridoxal 5′-phosphate-mediated N-terminal transamination reaction. Curr Protoc Chem Biol. 2009 doi: 10.1002/9780470559277.ch100018/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esser-Kahn AP, Francis MB. Protein-cross-linked polymeric materials through site-selective bioconjugation. Angew Chem Int Ed Engl. 2008;47(20):3751–3754. doi: 10.1002/anie.200705564. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Zhang Z, Brock A, Schultz PG. Addition of the keto functional group to the genetic code of Escherichia coli. Proc Natl Acad Sci USA. 2003;100(1):56–61. doi: 10.1073/pnas.0234824100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutchins BM, et al. Selective formation of covalent protein heterodimers with an unnatural amino acid. Chem Biol. 2011;18(3):299–303. doi: 10.1016/j.chembiol.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim CH, et al. Synthesis of bispecific antibodies using genetically encoded unnatural amino acids. J Am Chem Soc. 2012;134(24):9918–9921. doi: 10.1021/ja303904e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y, et al. Genetic incorporation of an aliphatic keto-containing amino acid into proteins for their site-specific modifications. Bioorg Med Chem Lett. 2010;20(3):878–880. doi: 10.1016/j.bmcl.2009.12.077. [DOI] [PubMed] [Google Scholar]
- 19.Rashidian M, Song JM, Pricer RE, Distefano MD. Chemoenzymatic reversible immobilization and labeling of proteins without prior purification. J Am Chem Soc. 2012;134(20):8455–8467. doi: 10.1021/ja211308s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen I, Howarth M, Lin W, Ting AY. Site-specific labeling of cell surface proteins with biophysical probes using biotin ligase. Nat Methods. 2005;2(2):99–104. doi: 10.1038/nmeth735. [DOI] [PubMed] [Google Scholar]
- 21.Carrico IS, Carlson BL, Bertozzi CR. Introducing genetically encoded aldehydes into proteins. Nat Chem Biol. 2007;3(6):321–322. doi: 10.1038/nchembio878. [DOI] [PubMed] [Google Scholar]
- 22.Wu P, et al. Site-specific chemical modification of recombinant proteins produced in mammalian cells by using the genetically encoded aldehyde tag. Proc Natl Acad Sci USA. 2009;106(9):3000–3005. doi: 10.1073/pnas.0807820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudak JE, Yu HH, Bertozzi CR. Protein glycoengineering enabled by the versatile synthesis of aminooxy glycans and the genetically encoded aldehyde tag. J Am Chem Soc. 2011;133(40):16127–16135. doi: 10.1021/ja206023e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudak JE, et al. Synthesis of heterobifunctional protein fusions using copper-free click chemistry and the aldehyde tag. Angew Chem Int Ed Engl. 2012;51(17):4161–4165. doi: 10.1002/anie.201108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi X, et al. Quantitative fluorescence labeling of aldehyde-tagged proteins for single-molecule imaging. Nat Methods. 2012;9(5):499–503. doi: 10.1038/nmeth.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakane PK, Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- 27.Mueller BM, Wrasidlo WA, Reisfeld RA. Antibody conjugates with morpholinodoxorubicin and acid-cleavable linkers. Bioconjug Chem. 1990;1(5):325–330. doi: 10.1021/bc00005a005. [DOI] [PubMed] [Google Scholar]
- 28.Kalia J, Raines RT. Hydrolytic stability of hydrazones and oximes. Angew Chem Int Ed Engl. 2008;47(39):7523–7526. doi: 10.1002/anie.200802651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi L, et al. A highly efficient strategy for modification of proteins at the C terminus. Angew Chem Int Ed Engl. 2010;49(49):9417–9421. doi: 10.1002/anie.201003834. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki T, Kodama K, Suzuki H, Fukuzawa S, Tachibana K. N-terminal labeling of proteins by the Pictet-Spengler reaction. Bioorg Med Chem Lett. 2008;18(16):4550–4553. doi: 10.1016/j.bmcl.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 31.Alam J, Keller TH, Loh T-P. Functionalization of peptides and proteins by Mukaiyama aldol reaction. J Am Chem Soc. 2010;132(28):9546–9548. doi: 10.1021/ja102733a. [DOI] [PubMed] [Google Scholar]
- 32.Alam J, Keller TH, Loh T-P. Indium mediated allylation in peptide and protein functionalization. Chem Commun. 2011;47(32):9066–9068. doi: 10.1039/c1cc12926k. [DOI] [PubMed] [Google Scholar]
- 33.Stöckigt J, Antonchick AP, Wu F, Waldmann H. The Pictet-Spengler reaction in nature and in organic chemistry. Angew Chem Int Ed Engl. 2011;50(37):8538–8564. doi: 10.1002/anie.201008071. [DOI] [PubMed] [Google Scholar]
- 34.Maresh JJ, et al. Strictosidine synthase: Mechanism of a Pictet-Spengler catalyzing enzyme. J Am Chem Soc. 2008;130(2):710–723. doi: 10.1021/ja077190z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jencks WP, Carriuolo J. Reactivity of nucleophilic reagents toward esters. J Am Chem Soc. 1960;82:1778–1786. [Google Scholar]
- 36.Molina P, Alcantara J, Lopez-Leonardo C. Regiospecific preparation of γ-carbolines and pyrimido[3, 4-a]indole derivatives by intramolecular ring-closure of heterocumulene-substituted indoles. Tetrahedron. 1996;52:5833–5844. [Google Scholar]
- 37.Lee Y, Klausen RS, Jacobsen EN. Thiourea-catalyzed enantioselective iso-Pictet-Spengler reactions. Org Lett. 2011;13(20):5564–5567. doi: 10.1021/ol202300t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plate R, Van Hout RHM, Behm H, Ottenheijm HCJ. Synthesis of 2-hydroxy-3-(ethoxycarbonyl)-1,2,3,4-tetrahydro-β-carbolines from N-hydroxytryptophans. An approach to the eudistomin series. J Org Chem. 1987;52:555–560. [Google Scholar]
- 39.Hermkens PHH, et al. Syntheses of 1,3-disubstituted N-oxy-β-carbolines by the Pictet-Spengler reactions of N-oxy-tryptophan and -tryptamine derivatives. Tetrahedron. 1990;46:833–846. [Google Scholar]
- 40.Kirkup MP, Shankar BB, McCombie S, Ganguly AK, McPhail AT. A concise route to the oxathiazepine containing eudistomin skeleton and some carba-analogs. Tetrahedron Lett. 1989;30:6809–6812. [Google Scholar]
- 41.Yeom C-E, Kim MJ, Kim BM. 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)-promoted efficient and versatile aza-michael addition. Tetrahedron. 2007;63:904–909. [Google Scholar]
- 42.Ishikawa T, et al. Novel [2,3]-sigmatropic rearrangement for carbon—nitrogen bond formation. J Am Chem Soc. 2001;123(31):7734–7735. doi: 10.1021/ja011128z. [DOI] [PubMed] [Google Scholar]
- 43.Tsunoda T, Yamamiya Y, Itô S. 1,1'-(azodicarbonyl)dipiperidine-tributylphosphine, a new reagent system for Mitsunobu reaction. Tetrahedron Lett. 1993;34:1639–1642. [Google Scholar]
- 44.Jencks WP. Studies on the mechanism of oxime and semicarbazone formation. J Am Chem Soc. 1959;81:475–481. [Google Scholar]
- 45.Dirksen A, Hackeng TM, Dawson PE. Nucleophilic catalysis of oxime ligation. Angew Chem Int Ed Engl. 2006;45(45):7581–7584. doi: 10.1002/anie.200602877. [DOI] [PubMed] [Google Scholar]
- 46.Jameson DM, Ross JA. Fluorescence polarization/anisotropy in diagnostics and imaging. Chem Rev. 2010;110(5):2685–2708. doi: 10.1021/cr900267p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ménard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22(42):6570–6578. doi: 10.1038/sj.onc.1206779. [DOI] [PubMed] [Google Scholar]
- 48.Krop IE, et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2012;30(26):3234–3241. doi: 10.1200/JCO.2011.40.5902. [DOI] [PubMed] [Google Scholar]
- 49.Mahal LK, Yarema KJ, Bertozzi CR. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science. 1997;276(5315):1125–1128. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- 50.Sadamoto R, et al. Control of bacteria adhesion by cell-wall engineering. J Am Chem Soc. 2004;126(12):3755–3761. doi: 10.1021/ja039391i. [DOI] [PubMed] [Google Scholar]
- 51.Hang HC, Bertozzi CR. Ketone isosteres of 2-N-acetamidosugars as substrates for metabolic cell surface engineering. J Am Chem Soc. 2001;123(6):1242–1243. doi: 10.1021/ja002962b. [DOI] [PubMed] [Google Scholar]
- 52.Tai H-C, Khidekel N, Ficarro SB, Peters EC, Hsieh-Wilson LC. Parallel identification of O-GlcNAc-modified proteins from cell lysates. J Am Chem Soc. 2004;126(34):10500–10501. doi: 10.1021/ja047872b. [DOI] [PubMed] [Google Scholar]
- 53.O’Shannessy DJ, Voorstad PJ, Quarles RH. Quantitation of glycoproteins on electroblots using the biotin-streptavidin complex. Anal Biochem. 1987;163(1):204–209. doi: 10.1016/0003-2697(87)90114-x. [DOI] [PubMed] [Google Scholar]
- 54.Zeng Y, Ramya TNC, Dirksen A, Dawson PE, Paulson JC. High-efficiency labeling of sialylated glycoproteins on living cells. Nat Methods. 2009;6(3):207–209. doi: 10.1038/nmeth.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith CD, et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci USA. 1991;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nyström T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005;24(7):1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.