The ribosome plays a universally conserved role in catalyzing the translation of all mRNAs in every cell across all kingdoms of life. It is therefore not surprising that the ribosome is considered one of the most complex and elaborately formed “molecular machines” in the cell, whose biogenesis is extraordinarily orchestrated, requiring all three RNA polymerases and more than 150 nonribosomal factors (1). Indeed, ribosomes make up much of the cell’s mass, and the eukaryotic ribosome is comprised of four RNA species and 79 ribosomal proteins (RPs). For decades, the dogma has been that, although the ribosome plays a critical function in translating the genomic code, it is largely a “back-stage” participant in gene regulation. This view has been reinforced by the widespread belief, early in the advent of the molecular era, that transcription rather than translation is the major molecular rheostat of gene expression. On the contrary, recent emerging evidence now reveals tremendous variation between expression of the transcriptome and proteome and that the cellular abundance of proteins may be predominantly controlled at the level of translational control (2). By extension, an interesting emerging question is whether the ribosome exerts a previously unappreciated regulatory function or specificity in translational control (3). A PNAS study by Lee et al. contributes to the growing realization of a surprising and emerging role for the ribosome as a “front-stage” participant in gene regulation (4). Moreover, their studies strongly suggest that much can be learned with respect to specialized mechanisms for cellular mRNA translation through the study of viral gene expression.
It has long been known that the mysterious lives of viruses may teach us important and critical lessons about mechanisms governing mRNA translation. This is because viral mRNA translational control is exquisite, and viruses often usurp the host translational machinery as an important means for self-propagation (5). One of the most striking mechanisms is the ability of certain viruses to shut down the cellular host general or cap-dependent translation, allowing for translation of their own viral mRNAs via a cap-independent mechanism. This is achieved through unique cis-acting translational regulatory elements known as internal ribosome entry sites (IRESes) that recruit ribosomes to specific viral mRNAs either directly or through a more limited number of initiation factors (6). Although IRESes were initially identified in viral mRNAs, there has been a subsequent growing appreciation that certain cellular mRNAs also harbor IRES elements in their 5′ UTRs that direct translation initiation, thereby greatly expanding our knowledge of translational regulatory specificity in eukaryotic cells (7). Moreover, it has also been recognized that ribosome-mediated specificity plays an important role in IRES-dependent translation of viral mRNAs. The most notable examples include a role for rRNA modifications (8, 9), as well as a specific requirement for a single RP belonging to the small ribosome subunit, RPS25, in facilitating direct interactions of the ribosome with viral IRES elements (10, 11), thereby promoting a specialized form of translational control.
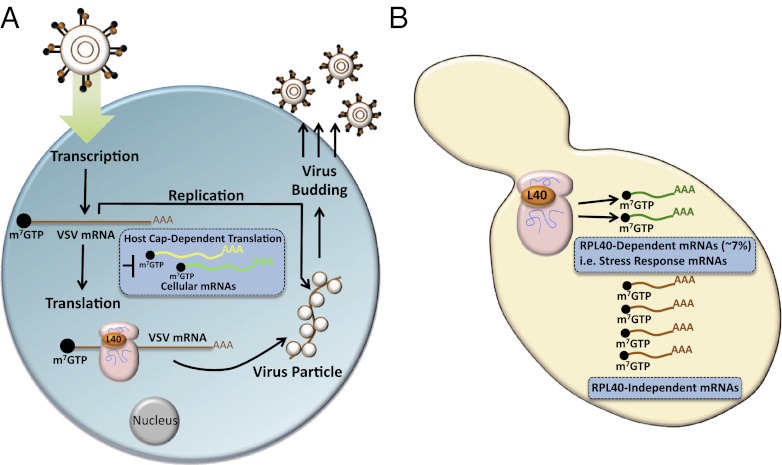
Banking on the knowledge that individual ribosome components may have greater specificity in viral mRNA translation, Lee et al. (4) set out to understand whether certain components of the ribosome may be required for translation of vesicular stomatitis virus (VSV) mRNAs. VSV mRNA translation is very intriguing because, despite the fact that this virus shuts off the host cap-dependent translation machinery, the VSV mRNA itself does not appear to possess an IRES element and requires cap-dependent translation. Moreover, VSV mRNAs are virtually indistinguishable from cellular mRNAs, as they are capped, methylated, and polyadenylated. Thereby, the mechanism that bestows the escape of VSV mRNA translation from shutoff of host translation is an outstanding question. In seeking an answer to this question, Lee et al. (4) carry out an unbiased siRNA screen of RPs in HeLa cells to determine whether specific RPs promote VSV mRNA translation. The results are striking and reveal that a single RP, RPL40, is necessary for VSV mRNA translation, but is largely dispensable for general cap-dependent protein synthesis, ribosome biogenesis, cell viability, and cell proliferation (Fig. 1). Furthermore, knockdown of RPL40 correlates with a significant decrease in VSV virus output. This effect is remarkably specific to cap-dependent translation of VSV viral mRNAs, as RPL40 is not required for viral entry, transcription, or IRES-dependent translation of other classes of viral mRNAs. Although certain RPs proteins exert secondary jobs in the cell, by existing in complexes outside of the ribosome, RPL40 does not. Consistent with its function as a constituent of the ribosome, RPL40 plays an early role in 80S complex formation on VSV mRNAs. Remarkably, these results are not only important for VSV mRNA translation, but Lee et al. (4) further show that the translation of a wide array of the Mononegavirales, of which VSV is a member, including rabies, measles, and Newcastle disease viruses, is dependent on RPL40.
Fig. 1.
RPL40 confers a ribosome-specialized function in translational control. (A) VSV polymerase first transcribes the individual mRNAs, for each viral gene, that are capped and virtually identical to cellular mRNAs. VSV infection shuts down host general cap-dependent translation (box). A specialized mechanism for translation control is required for synthesis of VSV proteins. A single RP (RPL40) is, at least in part, required for this translation event. Surprisingly, the effects of RPL40 are very specialized, as it does not appear to be required for general protein synthesis or cell viability. (B) Additional studies in an RPL40-deficient yeast strain suggest that this RP is required for transcript-specific translation control of eukaryotic mRNAs, including a subset of stress response mRNAs.
The studies revealing that RPL40-dependent translation is transcript-specific and is required for the replication of an entire array of viruses are important and make a significant contribution to our understanding of specialized translational control mechanisms. However, these findings also raise additional and important unanswered questions. As sequences or structures in the 5′ and 3′ UTRs of VSV mRNAs are sufficient for translational control, it remains unknown whether RPL40 is acting through a specific cis-acting element. This could be facilitated by direct binding of RPL40 itself or through an RNA binding intermediary. Alternatively, RPL40 may instead promote the translation of certain cellular mRNA(s), which in turn direct the translation of VSV mRNAs. Curiously, Lee et al. (4) show that knock-down of at least eight additional RPs of the large ribosome subunit are also required for VSV infection, but not cell viability. This raises the possibility that the coordinate actions of subgroups of RPs either directly or indirectly may promote viral mRNA transcript-specific translational control through a mechanism that is not yet understood. As VSV mRNAs are capped, the expectation is that the 40S subunit, along with initiation factors, are recruited to the mRNA cap structure, where this complex scans along the mRNA until it reaches the initiator codon. The specificity of RPs belonging to the 60S large subunit in this process is highly unusual because the 60S typically only joins at the AUG to form an elongation-competent 80S, and therefore the mechanisms by which these RPs play a role in translational “selectivity” remain puzzling.
Lee et al. extend beyond viral mRNA translation and suggest that similar RPL40 mechanism of translational control may apply to cellular mRNAs.
The studies of Lee et al. (4) extend beyond viral mRNA translation and suggest that a similar RPL40 mechanism of translational control may apply to cellular mRNAs (Fig. 1). This is indeed an exciting area of exploration, as some RPs appear to regulate the translation of specific mRNAs as constituents of the ribosome. For example, a single RP belonging to the large ribosome subunit, RPL38, is critically required for formation of the mammalian body plan by controlling the selective translation of a subset of Hox mRNAs (12). Furthermore, certain RPs appear to control IRES-dependent translation of mRNAs important for human erythroid differentiation (13). Lee et al. (4) address the role of RPL40 in control of cellular mRNA translation by employing a yeast strain deficient for RPL40 and by sequencing polysome or translationally active mRNAs. Interestingly, in yeast, RPL40 exists as two paralogues, RPL40A and RPL40B (14). Although RPL40 is an essential gene, Lee et al. (4) examine the consequences of short-term RPL40 depletion compared with a strain in which RPL40A is ectopically expressed from a galactose-inducible promoter. In this experimental setting, the authors find that the role of RPL40 appears to be specific for translational control of only ∼7% of total cellular mRNAs that stratify into many distinct cellular categories. This includes translation of stress-response mRNAs, which might provide exciting parallels between VSV mRNA translation and similar mechanisms of translation control during stress, regulated by RPL40. However, it remains unclear whether, in yeast, there may be a specific requirement for distinct RPL40 paralogues in this process. Moreover, it remains unknown whether mRNAs regulated by RPL40 share common features, for example within their 5′UTRs, such as length, folding free energies, uORFs, or GC content.
It is extremely fascinating that one single RP is required for translational control of so many viruses, whereas its loss does not appear to have major consequences on general protein synthesis or for cell viability. Therefore, any means to down-regulate RPL40 may be a novel therapeutic approach for viral infections. Moreover, these findings also support the notion of greater specialized regulatory control in how the genomic template is translated into functional proteins by individual constituents of the ribosome (3). The nature of specific cis-acting elements on mRNAs that confer ribosome-mediated translational specificity has to date remained very elusive. Future studies will be required to identify these specific sequences and/or structures, as well as the molecular mechanisms for their recognition. Moreover, deeper understanding is critically needed to determine whether the few RPs shown to exert ribosome-mediated specificity reflects a harbinger of a new mechanism for gene regulation. What the Lee et al. study (4) strongly suggests is that the ribosome is capable of much greater control in key cellular processes than previously anticipated.
Acknowledgments
I thank Kim Tong for help in preparation of the illustration. This work was supported by the Program for Breakthrough Biomedical Research (University of California, San Francisco) and by National Institutes of Health Director’s New Innovator Award DP2OD008509.
Footnotes
The author declares no conflict of interest.
See companion article on page 324.
References
- 1.Kressler D, Hurt E, Bassler J. Driving ribosome assembly. Biochim Biophys Acta. 2010;1803(6):673–683. doi: 10.1016/j.bbamcr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Schwanhäusser B, et al. Global quantification of mammalian gene expression control. Nature. 2011;473(7347):337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 3.Xue S, Barna M. Specialized ribosomes: A new frontier in gene regulation and organismal biology. Nat Rev Mol Cell Biol. 2012;13(6):355–369. doi: 10.1038/nrm3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AS-Y, Burdeinick-Kerr R, Whelan SPJ. A ribosome-specialized translation initiation pathway is required for cap-dependent translation of vesicular stomatitis virus mRNAs. Proc Natl Acad Sci USA. 2013;110:324–329. doi: 10.1073/pnas.1216454109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh D, Mohr I. Viral subversion of the host protein synthesis machinery. Nat Rev Microbiol. 2011;9(12):860–875. doi: 10.1038/nrmicro2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kieft JS. Viral IRES RNA structures and ribosome interactions. Trends Biochem Sci. 2008;33(6):274–283. doi: 10.1016/j.tibs.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell. 2009;136(4):731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon A, et al. Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science. 2006;312(5775):902–906. doi: 10.1126/science.1123835. [DOI] [PubMed] [Google Scholar]
- 9.Jack K, et al. rRNA pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol Cell. 2011;44(4):660–666. doi: 10.1016/j.molcel.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landry DM, Hertz MI, Thompson SR. RPS25 is essential for translation initiation by the Dicistroviridae and hepatitis C viral IRESs. Genes Dev. 2009;23(23):2753–2764. doi: 10.1101/gad.1832209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhs M, et al. Structural basis for the binding of IRES RNAs to the head of the ribosomal 40S subunit. Nucleic Acids Res. 2011;39(12):5264–5275. doi: 10.1093/nar/gkr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kondrashov N, et al. Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell. 2011;145(3):383–397. doi: 10.1016/j.cell.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horos R, et al. Ribosomal deficiencies in Diamond-Blackfan anemia impair translation of transcripts essential for differentiation of murine and human erythroblasts. Blood. 2012;119(1):262–272. doi: 10.1182/blood-2011-06-358200. [DOI] [PubMed] [Google Scholar]
- 14.Finley D, Bartel B, Varshavsky A. The tails of ubiquitin precursors are ribosomal proteins whose fusion to ubiquitin facilitates ribosome biogenesis. Nature. 1989;338(6214):394–401. doi: 10.1038/338394a0. [DOI] [PubMed] [Google Scholar]