Abstract
Administration of anti-inflammatory cytokines is a common therapeutic strategy in chronic inflammatory diseases. Gene therapy is an efficient method for delivering therapeutic molecules to target cells. Expression of the cell adhesion molecule E-selectin (ESEL), which is expressed in the early stages of inflammation, is controlled by proinflammatory cytokines, making its promoter a good candidate for the design of inflammation-regulated gene therapy vectors. This study describes an ESEL promoter (ESELp)-based lentiviral vector (LV) that drives localized transgene expression during inflammation. Mouse matrigel plug assays with ESELp-transduced endothelial cells showed that systemic lipopolysaccharide (LPS) administration selectively induces ESELp-controlled luciferase expression in vivo. Inflammation-specific induction was confirmed in a mouse model of arthritis, showing that this LV is repeatedly induced early in acute inflammation episodes and is downregulated during remission. Moreover, the local acute inflammatory response in this animal model was efficiently blocked by expression of the anti-inflammatory cytokine interleukin-10 (IL10) driven by our LV system. This inflammation-regulated expression system has potential application in the design of new strategies for the local treatment of chronic inflammatory diseases such as cardiovascular and autoimmune diseases.
Introduction
Chronic inflammatory diseases are characterized by episodes of relapse and remission that often involve superposition of acute inflammation on top of the inflammation already present. Altering the cytokine network is a common therapeutic strategy in inflammatory diseases. Therapies based on natural cytokines are very promising as they are more effective, better tolerated, and more specific than pharmacological treatments.1 One way to regulate inflammatory cytokine activity is to administer anti-inflammatory cytokines, such as interleukin-10 (IL10). Compared with other anti-inflammatory molecules such as IL4 or IL13, IL10 has a broader spectrum of anti-inflammatory activities, inhibiting the production of several proinflammatory cytokines [e.g., IL1, IL2, IL6, IL8, and tumor necrosis factor-α (TNF-α)] and inducing the production of anti-inflammatory agents (such as IL1 receptor antagonist). IL10 also suppresses nitric oxide release in lymphocytes.2 Local administration of recombinant IL10 effectively reduces proinflammatory cytokine activity in animal models of diseases such as sepsis, stroke, multiple sclerosis, and chronic inflammatory disorders.3,4,5,6
Studies of inflammatory flare-up reactions in animal models have shown the applicability and viability of local gene therapy in arthritis.7 Ideally, treatments for chronic inflammatory diseases should parallel the course of the disease itself, matching the varying pathological conditions and thereby avoiding undesirable secondary effects. An ideal vector system would therefore be disease-regulated, expressing high levels of anti-inflammatory agents during relapses and lower levels during remission phases of the disease.
Several viral expression systems that respond to inflammatory stimuli in vivo have been devised.8 The first was based on a two-component adenoviral expression system in which the promoter of complement factor 3 drives transcription of the HIV transactivator of transcription (Tat).9 However, the immunogenicity of Tat has been implicated in central nervous system disorders. Subsequently, van de Loo et al.10 developed a hybrid inflammation-inducible adenoviral expression system, consisting of the human IL6 promoter fused to the enhancer region of the human IL1 promoter. The therapeutic efficacy of adenoviral systems is, however, compromised by the induction of an adenovirus-mediated immune response and by the rapid loss of transgene expression due to the episomal localization of the viral genomes. More recently, an inflammation-inducible expression system based on serotype 5 adeno-associated virus (AAV) has been developed by employing a chimeric promoter based on NF-kB-binding sites.11 However, although the nonpathogenicity of AAVs makes them promising candidates for long-term gene therapy, under some circumstances they too can cause an inflammatory response in the host.12,13,14 A potentially more appropriate system for gene therapy in chronic inflammatory processes would be lentiviral vectors (LVs), since they can infect both dividing and quiescent cells, provide long-term expression, and display low immunogenicity.15
Currently, the most frequently used promoters used for transgene expression are viral, but these strong promoters are usually silenced, and result in only transient expression in vivo. Moreover, even if high expression of the transgene were successful, the constant high levels of anti-inflammatory molecules might increase the risk of infection, as already observed with anti-TNF-α and anti-IL1 treatment of patients with rheumatoid arthritis (RA).16,17,18,19 In addition, there might be an adaptive response to the constant high concentration of transgene protein, counteracting its therapeutic effect. Several vectors driven by drug-controlled promoters have been developed to achieve regulated transgene expression; however, this approach requires constant monitoring of the disease to achieve optimal efficacy, and this is further complicated by the unpredictable, relapsing course of the disease.
TNF-α and IL1 are early induced proinflammatory cytokines that act locally on vascular endothelium to induce the expression of adhesion molecules, including selectins and ligands for leukocyte integrins, which participate in the capture and recruitment of blood cells.20 The selectins are a family of three type-I cell surface glycoproteins (E-, L-, and P-selectin) involved in chronic and acute inflammation. E-selectin (ESEL) is rapidly and transiently expressed in response to inflammatory cytokines such as TNF-α and IL1, and is not expressed under basal conditions except in skin microvessels.21 The promoter of human ESEL has been used for in vitro gene delivery in recombinant retroviral and adenoviral vectors22,23 and for in vivo delivery in nonviral vectors.24,25,26 Here, we report the development of a lentiviral expression system based on the ESEL promoter (ESELp). We show that ESELp-driven transgene expression is induced in response to proinflammatory cytokines in cell culture, and is regulated in vivo during chronic paw inflammation. This long-term expression system shows low basal activity during remission and high expression during the acute inflammatory response. The LV system drives in vivo expression of the anti-inflammatory cytokine IL10 at levels sufficient to efficiently attenuate repetitive local acute inflammation episodes induced by zymosan injection. This attenuation is also observed when the LV system is administered after zymosan injection. Therefore, this new expression system fulfills the requirements for a disease-regulated on/off system, suggesting potential use for autoregulated treatment of chronic inflammatory diseases.
Results
ESELp-driven transgene expression is efficiently activated by proinflammatory cytokines in lentivirus-transduced endothelial cells
To assess the ability of lentivectors to efficiently transduce endothelial cells, we infected mouse or human primary endothelial cell cultures [mouse lung endothelial cells (MLEC) and human umbilical vein endothelial cells] with a LV encoding green florescent protein (GFP) under the control of the constitutive SFFV viral promoter (LV-SFFVp-GFP). In addition, we infected immortalized MLEC (iMLEC).27 GFP expression was analyzed after 48 hours, and the efficiency of transduction was close to 100% in all cases (Supplementary Figure S1).
Since ESEL is the earliest endothelium-specific adhesion molecule induced by proinflammatory cytokines, we tested whether the ESELp might be a useful tool for achieving targeted transgene expression at sites of inflammation. We generated a LV encoding GFP under the control of ESELp (LV-ESELp-GFP; Supplementary Figure S2) and infected iMLEC and human umbilical vein endothelial cells. Treatment of infected cells with TNF-α strongly increased GFP expression in both cell types, paralleling the expression of endogenous ESEL (Figure 1a,b). In contrast, GFP expression from the constitutively active LV-SFFVp-GFP vector was not modified by TNF-α treatment (Supplementary Figure S3). The potent induction by TNF-α of endogenous ESEL in vitro is greatly enhanced by preincubation with the proangiogenic factor vascular endothelial growth factor (VEGF).28 We therefore preincubated infected cells for 24 hours with VEGF and then with TNF-α for different periods. As in the case of endogenous ESEL, VEGF pretreatment potentiated TNF-α-induced ESELp-driven expression of GFP; induction of GFP expression peaked at 6 hours both in VEGF and in vehicle pretreated cells, and declined after 12 hours (Supplementary Figure 4a).
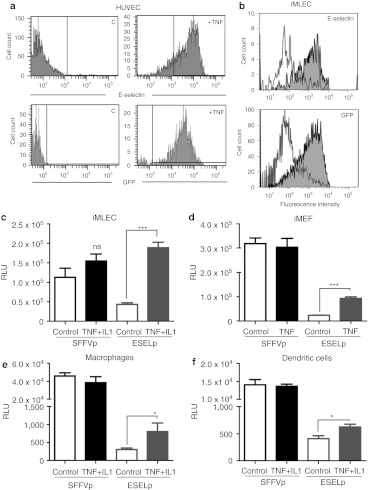
Figure 1.
Inducibility of the E-selectin promoter (ESELp)-based lentiviral system in vitro. (a) Human umbilical vein endothelial cells (HUVEC) were infected with the LV-ESELp-GFP and left untreated (left) or incubated with 30 ng/ml tumor necrosis factor-α (TNF-α) (right) for 6 hours. Flow cytometry histograms show the expression of endogenous E-selectin (top panels) and green florescent protein (GFP) (bottom panels). (b) LV-ESELp-GFP-infected iMLEC were treated as in (a). Histograms show the fluorescence intensity of untreated cells (gray line) and TNF-treated cells (filled). (c,d) Immortalized and (e,f) primary cells were transduced for 5 hours with either LV-SFFVp or LV-ESELp, serum-starved for 12 hours and stimulated with the indicated cytokines for 6 hours. The MOI employed was 5 for iMLEC and iMEF, 50 for peritoneal macrophages and 100 for bone-marrow derived dendritic cells. Three experiments were performed and one representative is shown (mean +SEM; n = 3). *P < 0.05 and ***P < 0.001 versus untreated cells. LV, lentiviral vector; RLU, relative light units.
As VSV-pseudotyped LVs have a broad host range, we tested the ESELp activity in non endothelial immortalized and primary cells susceptible to be infected after intraplantar administration by employing a lentivector containing the luciferase reporter gene under the control of the ESELp (LV-ESELp-Luc; Supplementary Figure S2). Treatment of infected cells with proinflammatory cytokines increased luciferase expression in all cell types assayed (Figure 1c–f). However, only in the case of stimulated LV-ESELp transduced endothelial cells the level of luciferase was similar to that observed in LV-SFFVp transduced cells (Figure 1c). In the rest of cell types we found a low basal level of luciferase expression and modest induction upon stimulation (Figure 4d–f).
We next investigated whether LV-ESELp could be activated by an inflammatory milieu. Injection of mice with lipopolysaccharide (LPS) induces the release of proinflammatory cytokines, resulting in activation of the endothelium and the expression of additional proinflammatory factors and cell-specific adhesion molecules that participate in the inflammatory response.27 To test the effect of these inflammatory mediators on LV-ESELp, we transduced endothelial cells with LV-ESELp-Luc and treated these cells with culture supernatant from LPS-activated macrophages.29 The cytokine-containing supernatant increased luciferase activity in iMLEC transduced with LV-ESELp-Luc but had no effect on cells transduced with LV-SFFVp-Luc (Supplementary Figure 4b). LV-ESELp-Luc driven luciferase activity was also increased by treatment with VEGF plus TNF-α, but direct treatment with LPS did not modify luciferase activity in endothelial cells driven by either SFFVp or ESELp (data not shown).
ESELp-driven transgene expression is induced by proinflammatory cytokines in a mouse subcutaneous matrigel model
The inducibility of LV-ESELp-Luc by inflammatory mediators was further investigated by subcutaneous matrigel experiments in mice. iMLEC were transduced with LV-SFFVp-Luc or LV-ESELp-Luc, and, after 24 hours, cells were embedded in VEGF-containing matrigel and injected subcutaneously into syngenic mice. Forty-eight hours later, mice were treated with LPS [intraperitoneal (i.p.)], and luciferase activity in matrigel plugs was monitored by in vivo optical bioluminescence imaging. LPS administration led to similar increases in serum IL6 levels in all mice, but luciferase activity was increased only in LV-ESELp-Luc matrigel implants, thus confirming the selectivity of ESELp induction by inflammatory cytokines in vivo (Figure 2 and Supplementary Figure S5).
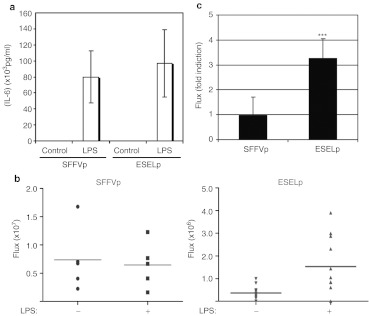
Figure 2.
Proinflammatory cytokines induce the E-selectin promoter (ESELp)-based lentiviral system in vivo. Mice were injected subcutaneously with matrigel containing mouse lung endothelial cells (MLEC) infected with either LV-SFFVp-GFP or LV-ESELp-GFP, and were subsequently injected [intraperitoneal (i.p.)] as indicated with 40 mg/kg lipopolysaccharide (LPS). (a) Graph showing serum IL6 levels (mean ± SD) from a representative experiment (n = 9). (b) Scatter plots showing in vivo luciferase activity (flux) of mice from a representative experiment (ESELp: P = 0.0090; n = 9). (c) Graph showing in vivo luciferase activity (flux) as fold induction of LPS-treated versus control mice (mean± SD of three independent experiments;***P < 0.001 versus untreated mice). GFP, green florescent protein; LV, lentiviral vector.
ESELp-driven transgene expression is modulated by proinflammatory cytokines in a mouse model of chronic inflammation
We next addressed the inducibility of the LV system in a mouse model of RA, a disease characterized by chronic inflammation of the joints which leads to destruction of cartilage and bone. We used a zymosan-induced arthritis (ZIA) model, in which intraplantar administration of zymosan, a glucan obtained from yeast cell wall, induces the secretion of inflammatory ILs.30 Intraplantar injection of zymosan causes pronounced, dose-dependent edema. This inflammatory response is time-dependent and can last up to 14 days following zymosan administration. LV-SFFVp-Luc or LV-ESELp-Luc was injected subcutaneously into both hind paws 1 week before zymosan administration to allow sufficient time for integration of the vector genome. After 1 week the right paw was injected with zymosan and the left paw with physiological saline solution. Luciferase expression was monitored by imaging of luciferase bioluminescence every 3–4 days over the first 2 weeks, when the signal intensity started to decrease, with additional measurements after 20 and 30 days. Compared with control paws, the inflamed paws showed a notable induction of ESELp-driven luciferase expression 4 days after zymosan injection, reaching a maximum after 7 days and decreasing from this point (Figure 3a–c). In contrast, paws injected with SFFVp-Luc showed no differences in luciferase expression upon injection with zymosan (Figure 3a–c). Comparable progression of ZIA in LV-SFFVp-Luc and LV-ESELp-Luc-injected paws was confirmed by measuring paw diameter (Figure 3d). In addition, inflammation was measured by bioluminescence after i.p. administration of luminol, which allows quantitative longitudinal monitoring of myeloperoxidase (MPO) system activity31 (Figure 3e). LV injection by itself produced no inflammatory reaction that could contribute to ZIA, since luminol reactions were only observed in zymosan-treated paws.
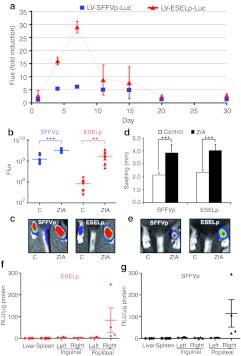
Figure 3.
The E-selectin promoter (ESELp)-based lentivector drives local transgene expression at inflammatory sites. Mice received an intraplantar injection of either LV-SFFVp-Luc or LV-ESELp-Luc in both hind paws. After 1 week, mice received intraplantar injections of 180 µg zymosan in the right paw and saline solution in the left paw (as a negative control for inflammation). In vivo luciferase activity was determined by bioluminescence at different times after zymosan injection. (a) Fold induction of in vivo luciferase activity (flux) in zymosan- and saline-injected paws at the indicated times. (b) Scatter plot showing in vivo luciferase activity (flux) of control and zymosan-induced arthritis (ZIA)-treated paws at day 7 (P < 0.0016; n ≥ 8). (c) Representative bioluminescence images of paws infected with the indicated vectors and treated with saline (c) or zymosan (ZIA) for 7 days. (d) Graph showing the diameters (mm) of mouse hind paws infected with the indicated vectors and treated with saline (control) or zymosan (ZIA) for 7 days (mean ± SEM; n ≥ 8). **P < 0.01 and ***P < 0.001. (f,g) Luciferase activity detected in tissues from mice locally transduced with the indicated lentiviral vector (LV). Total tissue homogenates from liver, spleen, and the indicated lymph nodes were employed to measure luciferase activity (n = 4), expressed as RLU (relative light units) per microgram of protein (µg). LV, lentiviral vector.
It has been demonstrated that the majority of transduced cells after lentivector foot pad administration are dendritic cells (DCs) (90% CD11c+ cells) which can migrate to the draining lymph nodes and spleen.32 To test whether locally infected cells were migrating and luciferase was expressed in these organs, lymph nodes, spleen and liver were extracted from LV-transduced animals 5 days after zymosan administration. Total tissue homogenates from these organs were employed to measure the luciferase activity. The results showed that only very low-luciferase activity was detected in the popliteal lymph node draining the paw infected with the luciferase-bearing LV (Figure 3f,g). Although we cannot rule out that LV particles may reach these and other organs, this result suggests that transgene expression remains restricted to the paw after zymosan injection.
To identify the uncharacterized 10% of transduced cells, we performed double immunostaining in cross-sections of paraffin-embedded transduced paws. Our preliminary data indicate that transgenes are expressed in transduced endothelial cells in vivo (data not shown); however further experiments would need to be performed to confirm these data and investigate whether other cell types are contributing to the overall transgene expression in vivo.
ESELp-driven transgene expression in vivo responds to inflammation flare-ups
An important aim in gene therapy is the development of expression systems which can be switched on and off on demand. Such vectors would allow cessation of transgene expression upon resolution of the pathological process, and its restoration should the disorder reactivate. We therefore wanted to determine whether our lentiviral ESELp-driven expression system is modulated by the inflammatory conditions induced by zymosan. We monitored the inflamed paws after the first injection of zymosan by weekly measurement of the bioluminescence produced in response to i.p. administration of luminol. After one month, no detectable bioluminescence signal was generated in the paws, and correspondingly control and zymosan-injected paws showed no differences in ESELp-driven luciferase activity (day 30, Figure 4a). At this point, we reactivated the inflammation by administering a second zymosan injection to the same paw, and monitored SFFVp- and ESELp-controlled luciferase expression by bioluminescence. The new inflammatory process again led to an increase in ESELp-driven transgene expression in the zymosan-injected paws, whereas no apparent changes were observed in paws infected with LV-SFFVp-Luc (Figure 4a,b and Supplementary Figure S6a). The acute inflammatory reaction induced by the second zymosan injection was similar in LV-SFFVp-Luc and LV-ESELp-Luc-infected mice, as estimated by paw diameter and luminol bioluminescence (Figure 4c and Supplementary Figure S6b). These data indicate that the ESELp-driven lentiviral expression system has the potential to selectively target inflammatory tissues and can be reinduced by acute inflammatory episodes.
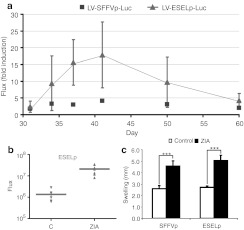
Figure 4.
Expression of the E-selectin promoter (ESELp)-based lentiviral system is regulated by the level of inflammation in vivo. (a) One month after the first zymosan injection, when the inflammation had receded, hind paws were reinjected with saline or 180 µg zymosan to reactivate inflammation. The graph shows fold induction of in vivo luciferase activity measured by bioluminescence in zymosan and saline reinjected paws of mice at the indicated times. (b) Scatter plot showing in vivo luciferase activity (flux) of control and zymosan-induced arthritis (ZIA)-treated paws 7 days after the second zymosan boost (P = 0.0027; n = 7). (c) The graph shows the diameters (mean ± SEM; n ≥ 8) of mouse hind paws infected with the indicated vectors, measured 7 days after the second treatment with saline (control) or zymosan (ZIA). **P < 0.01 and ***P < 0.001.
Comparative study of transgene expression under different inflammation-inducible expression systems
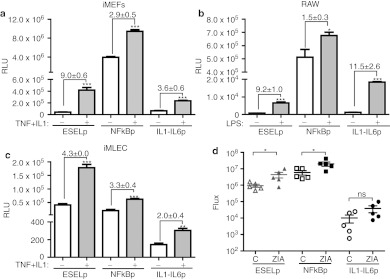
As other viral expression systems that respond to inflammatory stimuli have been described, we decided to compare the transcriptional inducibility of ESELp with two other inflammation-inducible promoters: a NF-kB responsive promoter (NFkBp) and a hybrid promoter based on the human IL6 promoter fused to the enhancer region of the human IL1 promoter (IL1-IL6p).10,11 After replacing ESELp with either NFkBp or IL1-IL6p and generating the corresponding luciferase-containing LVs, we employed them to transduce different cell lines. We found that LV-NFkBp displayed the highest luciferase activity in non endothelial cells (Figure 5a,b); however, in endothelial cells LV-ESELp and LV-NFkBp showed similar basal luciferase activity (Figure 5a,c). Upon stimulation, LV-ESELp was induced in all the cell types tested (Figure 6a–c), displaying the highest luciferase activity in endothelial cells (Figure 5c). In the case of LV-IL1-IL6p, it showed the highest induction in RAW cells after treatment with LPS, displaying luciferase units similar to those already published.10
Figure 5.
Comparative study of different inflammation-regulated systems. The E-selectin promoter (ESELp)-based system was compared with two other inflammation-inducible systems, the chimeric NFkB promoter (NFkBp) and the human IL1-IL6 hybrid promoter (IL1-IL6p), (a–c) in cell cultures and (d) in vivo. (a–c) The indicated lentivector vector (LV) was employed to transduce (a) iMEF (MOI = 5), (b) RAW (MOI = 10), and (c) iMLEC (MOI = 5) for 5 hours. After serum starvation, cells were incubated with (a,c) tumor necrosis factor (TNF) (100 ng/ml) plus IL1 (10 ng/ml) for 6 hours or (b) with lipopolysaccharide (LPS) (2 µg/ml) for 24 hours. Three experiments were performed and one representative is shown (mean + SEM; n = 3). Numbers indicate the fold induction (mean + SD; n = 3). (d) Mice received an intraplantar injection of either LV-ESELp, LV-NFkBp-Luc, or LV-IL1-IL6p-Luc in both hind paws. After 1 week, mice received intraplantar injections of zymosan (180 µg) in the right paw and saline solution in the left paw (C = control). In vivo luciferase activity was determined by bioluminescence after zymosan injection. Scatter plot shows in vivo luciferase activity (flux) of control and zymosan-induced arthritis (ZIA)-treated paws at day 5 (n = 5). *P < 0.05, **P < 0.01, and ***P < 0.001 versus untreated cells or control hind paw; P value. ns, not significant.
Figure 6.
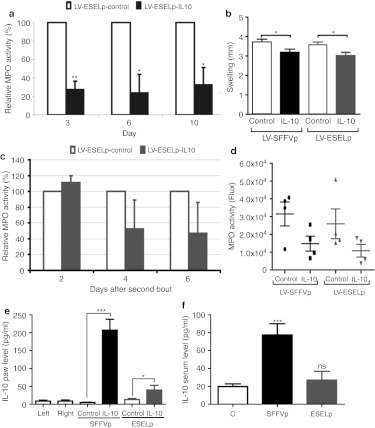
Interleukin-10 (IL10) release from the E-selectin promoter (ESELp) lentiviral system attenuates zymosan-induced inflammation. One group of mice received an intraplantar injection of LV-ESELp-Luc in the left hind paw and LV-ESELp-IL10 in the right one. A second group received similar intraplantar injections of LV-SFFVp-Luc and LV-SFFVp-IL10. After 1 week, mice received intraplantar injections of 180 µg zymosan in both hind paws. (a) Myeloperoxidase (MPO) activity was measured by bioluminescence at different times after zymosan injection, and the percentage of relative MPO activity was calculated; 100% = MPO activity in paws injected with LV-ESELp-Luc or LV-SFFVp-Luc (*P < 0.05 and **P < 0.01; n > 5). (b) Paw diameters measured 3 days after the first zymosan injection (*P < 0.05; n > 5). (c) After remission of the first acute inflammation, hind paws were reinjected with zymosan (180 µg per paw) to reactivate inflammation, and MPO activity was measured by bioluminescence at different times (n = 4). (d) The scatter plot shows in vivo MPO activity (flux) of zymosan-induced arthritis (ZIA)-treated paws 6 days after the second bout. (e,f) IL10 level present in (e) footpad supernatant and (f) serum from the indicated mice 5 days after the first zymosan bout was measured by ELISA (mean ± SEM; n ≥ 4). *P < 0.05 and ***P < 0.001 versus control; ns, not significant P value. LV, lentiviral vector.
Next, we compared these inflammation-inducible systems in vivo. For this, LV-ESELp-Luc, LV-NFkBp-Luc or LV-IL1-IL6p-Luc was injected subcutaneously into both hind paws and 1 week later the right paw was injected with zymosan and the left paw with physiological saline solution. Luciferase expression was monitored by imaging of luciferase bioluminescence 4 days after zymosan injection. As shown in Figure 5d, LV-NFkBp displayed very high-luciferase basal activity that was further increased after zymosan injection. LV-IL1IL6p displayed the lowest luciferase activity and the modest induction observed has no statistical significance. Once again, LV-ESELp showed a low basal activity which was significantly increased after zymosan administration.
ESELp-driven IL10 expression attenuates local acute inflammation in vivo
Since IL10 shows potential as an anti-arthritic agent which counteracts the actions of proinflammatory molecules, we used the inflammation-regulated LV-ESELp system to express this cytokine. Cells infected with LV-driving expression of IL10 (LV-ESELp-IL10) released this IL into the culture medium (Supplementary Table S1). To test the efficacy of lentiviral-driven IL10 expression in vivo, we injected LV-ESELp-IL10 subcutaneously into the right hind paw and LV-ESELp-Luc into the left hind paw of the same animal, so that each mouse served as its own control. Another group of animals was similarly administered with LV-SFFVp-IL10 and LV-SFFVp-Luc. One week later, ZIA was induced in both hind paws, and MPO (luminol) and luciferase activities were monitored by imaging of bioluminescence every other day until inflammation remission began (10 days). Compared with the luciferase-expressing inflamed paws, the IL10-expressing paws showed a notable reduction in MPO activity, by 90% and 70% 3 days after zymosan injection in the case of LV-SFFVp and LV-ESELp, respectively (Figure 6a and Supplementary Figure S7). This was accompanied by corresponding reductions in paw swelling (Figure 6b).
To test the reactivation of the system, we administered a second zymosan injection after remission of the first acute episode, and monitored luciferase and MPO activity by bioluminescence. The second acute inflammatory episode was again attenuated in the IL10-expressing paws (Figure 6c,d). The smaller attenuation observed correlated with the lower degree of inflammation (MPO activity) after a repeated zymosan administration (Figure 4). These results show that the anti-inflammatory effect obtained by low and transient level of IL10 expressed under LV-ESELp was similar to that observed after the high and constant level of IL10 released by the LV-SFFVp. Therefore, the expression of IL10 under the LV-ESELp would avoid the side effects associated with a prolonged release of IL10.
Locally released IL10 might reach the blood stream, raising its serum level and therefore increasing the risk of infection. To test this, we measured IL10 paw and serum levels in the two animal groups 5 days after the first zymosan bout. We found that both LV-SFFVp-IL10 and LV-ESELp-IL10 increased the local amount of IL10 (Figure 6e); however, only the injection of LV-SFFVp-IL10 significantly raised IL10 serum level which might cause immunosuppression and put the animals at risk of opportunistic infections (Figure 6f). Therefore, these results suggest that LV-ESELp-IL10 local administration does not increase susceptibility to infections.
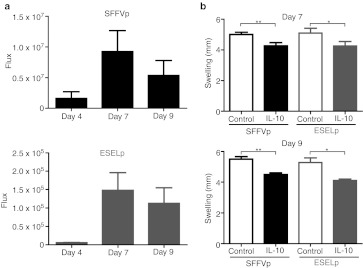
As the local environment in the animal paw is different after zymosan injection, we wondered whether our lentiviral system was also effective when it was administered after zymosan injection. We injected LV-ESELp-IL10 subcutaneously into the right hind paw and LV-ESELp-Luc into the left hind paw of the same animal 1 day after zymosan administration. Another group of animals was similarly administered with LV-SFFVp-IL10 and LV-SFFVp-Luc. We monitored luciferase activity and found the peak at day 7 after zymosan injection (Figure 7a). At that time there was a clear swelling reduction in the IL10-expressing paws in both groups which was maintained at least for two more days (Figure 7b). These results showed that LV-ESELp also works when it is administered after the initiation of inflammation; therefore, it might be useful as a therapeutic tool after detecting a new inflammatory episode.
Figure 7.
Therapeutic effect of E-selectin promoter (ESELp)-IL10 local administration. Mice received intraplantar injections of 180-µg zymosan in both hind paws. The next day, one group of mice received an intraplantar injection of LV-ESELp-Luc in the left hind paw and LV-ESELp-IL10 in the right one. A second group received similar intraplantar injections of LV-SFFVp-Luc and LV-SFFVp-IL10. (a) In vivo luciferase activity was determined by bioluminescence after zymosan injection in both animal groups. (b) Paw diameters measured (top) 7 and (bottom) 9 days after the zymosan injection. The graph shows the diameters (mean ± SEM; n = 5) of mouse hind paws infected with the indicated vectors. *P < 0.05 and **P < 0.01 versus control. LV, lentiviral vector.
Discussion
The inflammatory response is precisely controlled by the expression of cytokines whose local levels are directly related to the severity of the process. A major challenge in the treatment of chronic inflammatory diseases is the development of an expression system that is tightly regulated by the variable levels of these cytokines. This study describes a long-term lentiviral expression system based on the ESELp, and which is locally induced by inflammatory stimuli in direct correlation with the intensity and duration of the inflammatory response.
Studies in animal models have shown that gene therapy is an alternative for the local treatment of chronic inflammatory diseases. One of the critical factors in gene transfer is the type of vector employed. Nonviral vectors commonly yield low gene transfer efficiency.33,34 Among the viral vectors, adenoviruses are the most widely used, but they are poor candidates for the treatment of chronic inflammatory diseases because of the immune response associated with their application and the rapid loss of transgene expression due to lack of persistence of the viral genomes.35,36 AAV have emerged as a very promising alternative, since although AAV vectors have limited cargo capacity, they stably transduce host cells and show low immunogenicity. However, recent studies have reported an inflammatory response after AAV application.12,13,14 In addition, technical limitations limit the scalability of AAV vectors, making it difficult to produce adequate viral titers.37,38,39 As an alternative, lentivirus-derived expression systems have been employed in animal models of neuroinflammation.40 Lentivectors not only infect dividing and quiescent cells, but they also provide long-term expression and show low immunogenicity. In addition, the biosafety profile of LVs has been improved significantly by minimizing the regions of homology between vector and helper sequences (split configuration), and by using heterologous promoters.41 Furthermore, the use of vesicular stomatitis virus glycoprotein confers efficient transduction in a wide range of cell types from many species, and allows high titers of the lentiviral particles for clinical applications.42,43 Our study suggests that lentivectors may also be a valuable alternative in the treatment of chronic inflammatory diseases.
Several inflammation-inducible systems have been described recently, all of which are based on chimeric promoters. The precise in vivo regulation of these tailored promoters is still unknown. Our expression system is based on the proximal promoter region that controls the expression of the ESEL gene. This gene is particularly attractive as it is induced early and transiently upon inflammation and its promoter region contains the binding sites for transcription factors induced by the early induced proinflammatory cytokines TNF-α and IL1. Compared to other described expression systems, the ESELp-based system shows the highest transcriptional activity in endothelial cells. In addition, our expression system is highly induced in endothelial cells by the early induced proinflammatory cytokines TNF-α and IL1. These results are important as activated endothelium plays an important role in inflammation initiation. We tested our expression system in an experimental model of chronic inflammation by administering repeated local injections of zymosan. ESELp-driven transgene expression is rapidly induced after zymosan administration, coinciding with the peak of inflammation 7 days after the first treatment, and is maintained until inflammation recedes. Compared to other inflammation-inducible systems, the ESELp-based is characterized by a low basal activity that after zymosan administration increased to levels similar to those observed with a strong viral promoter. The other expression systems analyzed showed either very high basal activity (NFkB-based system) or very low inducibility after inflammation (IL1-IL6p-based system). Our results show that ESELp-dependent transgene expression increases several fold, correlating with the severity of inflammation in the animal system tested. Future experiments will enable more detailed comparison of how these expression systems perform in gene transfer models.
Since chronic inflammatory diseases are characterized by flare-ups and remission phases, it was important to test whether the promoter was silenced in vivo and whether transgene expression could be reinduced after a second zymosan boost. Transgene expression again correlated with inflammatory status after a second zymosan boost, showing no evidence of promoter silencing. We therefore consider the ESELp a valuable tool for the development of gene expression systems for the treatment of chronic inflammatory diseases. The use of ESELp-based gene delivery systems to selectively express anti-inflammatory agents in arthritis-affected joints might eliminate some of the problems of tolerability and compliance associated with systemic drug therapies.
One important issue in locally applied gene therapy is to study the putative migration of transduced cells in vivo. In this regard, it has been demonstrated that LV injection into the mouse footpad transduces DCs which are able to migrate to the draining lymph nodes and spleen.32 However, we only detected residual luciferase activity in the popliteal lymph nodes draining the infected paw suggesting that transduced DCs remain in the local inflammatory focus (Figure 3f,g). This is in agreement with previously published results showing that IL10 transgene expression modulates DC maturation.44,45 The authors observed that DCs transduced with adenoviral vectors expressing IL10 maintained an immature state characterized by low MHC class II, CD86, and IL12 expression. The immaturity might affect to their migratory ability which would support our in vivo results. Further experiments should be performed to further characterize the impact of IL10 expression on DC migration in vivo.
The occurrence of unpredictable relapses complicates the treatment of chronic inflammatory diseases. RA, the most frequent inflammatory rheumatic disorder, is a paradigm of chronic inflammatory diseases characterized by an imbalance of pro- and anti-inflammatory molecules. Although systemic administration of anti-inflammatory agents is beneficial to patients with chronic RA, these treatments are limited by loss of efficiency and relapse after treatment cessation. There are also significant side effects associated with a prolonged systemic imbalance of the natural inflammatory response.46,47 Viral vectors are promising candidates for gene therapy for local treatment of RA, and several clinical trials are underway.48 However, there is still a need to develop new therapeutic approaches that provide prolonged remission from disease with limited side-effects by targeting anti-inflammatory mediators to the diseased joints. The use of disease-regulated promoters to drive transgene expression might provide therapeutic levels of the anti-inflammatory agent exclusively during flare-ups. In addition, administration of virus directly into arthritic joints should avoid the side-effects associated with systemic administration and increase the site-specific effects of the therapeutic agent.
It has been shown that local administration of recombinant IL10 effectively reduces proinflammatory cytokine activity in several animal models of human diseases; however, constant high levels of anti-inflammatory molecules might increase the risk of infection, therefore prolonged administration of IL10 is limited by associated side-effects. Our study demonstrates that activation of the LV-ESELp lentiviral expression system is regulated by the local level of inflammation. Moreover, the LV-ESELp system drives inflammation-regulated IL10 expression at levels sufficient to reduce acute inflammation induced by zymosan with no effect in the IL10 serum levels. Although the LV-ESELp system releases considerably lower local concentrations of IL10 than the constitutive expression vector LV-SFFVp, it is noteworthy that regulated IL10 expression is as effective as constant expression in reducing inflammation in vivo. Furthermore, the inducible system is switched off during remission of the inflammation, thus avoiding the risks associated with a sustained release of IL10. Altogether, these results suggest that local administration of LV-ESELp-IL10 should not increase animal susceptibility to opportunistic infections. As expected, the levels of inflammation and transgene induction are lower after the second bout than observed at disease onset. This endogenously regulated system for the local expression of anti-inflammatory molecules provides a potential new approach for the local treatment of chronic inflammatory diseases.
Materials and Methods
Plasmid constructs. The human ESELp (–940;+40) (ESELp) was amplified from human genomic DNA by PCR. The PCR product was cloned into the pHRSIN HIV-derived transfer vector to replace the ubiquitously expressed spleen forming Focus virus promoter, SFFVp. Firefly luciferase cDNA was PCR amplified from a commercially available plasmid (pGL3 Basic).
The NFkB chimeric promoter (NFkBp) was generated by annealing sense and antisense oligonucleotides and direct cloning into pBlueScript in the following order: HindIII-EcoRI minimal CMV promoter (sense 5′ AGCTTGTAGGCGTGTACGGTGGAGGTCTATATAAGCAGAGCTCG 3′ antisense 5′ AATTCGAGCTCTGCTTATATAGACCTCCACCGTACA CGCCTACA 3′), XhoI-3xNFkB-HindIII (sense 5′ TCGAGGGACTTTCC ACAAGGGGACTTTCCACAAGGGGACTTTCC 3′ antisense 5′ AGCTGG AAAGTCCCCTTGTGGAAAGTCCCCTTGTGGAAAGTCCC 3′) and KpnI-MluI-3xNFkB-XhoI (sense 5′ CACGCGTGGGACTTT CCACAAGGGGACTTTCCAC AAGGGGACTTTCC 3′ antisense 5′ TCGAGGAA AGTCCCCTTGTGG AAAGTCCCCTTGTGGAAAGTCC CACGCGTGGTAC 3′). The IL1-IL6 hybrid promoter (IL1-IL6p) was generated by cloning the PCR products containing the human IL1 enhancer (–3,690; –2,720) and the human IL6 promoter (–172;+12) into pBlueScript. The PCR primers employed were MluI-IL1-fwd (5′ CCACGCGTGATCCAAGAGGGAGAAGAAGC 3′), XbaI-IL1-rv (5′ GGTCTAGACTGATGCTTTCGCTCGAGGG 3′), IL6-fwd (5′ GGCTTAGCGCTAGCCTCAATGAC 3′) and BamHI-IL6-rv (5′ GGGGATCCGAGACTCTAATATTGAGAC TCATGGG 3′). The ESELp sequence was removed from pHRSIN-ESELp-Luc-IRES-GFP by MluI and BamHI digestion and replaced by either NFkBp or IL1-IL6p.
The IL10 coding sequence was amplified by PCR using cDNA generated from total RNA extracted from LPS-treated RAW cells. The PCR products were directly cloned into the SIN-BX plasmid to generate the bicistronic cassettes. The luciferase-IRES-GFP and IL10-IRES-GFP inserts were cloned into the pHRSIN-SFFVp and pHRSIN-ESELp transfer plasmids. The sequences of all plasmids were confirmed and are available in our lab websites (http://www.rodriguezlab.com or http://www.lablife.org/labs/947).
Lentivirus production and titration. HEK-293 cells were transiently transfected by the calcium phosphate method. For viral particle production, the indicated pHRSIN transfer plasmid was cotransfected with two helper plasmids (8.9 and pMD2-G). Supernatants were collected 48 hours after transient transfection and cell debris was removed by centrifugation (10 minutes, 740 x g, 4 °C). Viral particles were concentrated by ultracentrifugation in a swing bucket rotor for 2 hours at 121,986 x g, 4 °C (Ultraclear Tubes, SW28 rotor and Optima L-100 XP Ultracentrifuge; Beckman Coulter, Fullerton, CA). After removal of the supernatant, viral particles were resuspended in phosphate-buffered saline (PBS) and stored at –80 °C until use.
Total viral content was determined by quantitative PCR.49 The concentration of transducing units was calculated by infecting 50,000 Jurkat cells (in a p96 plate), with 1, 0.1, and 0.01 µl (duplicates) of concentrated viral supernatant. After 12 hours, viruses were removed and cells were suspended in PBS and analyzed by flow cytometry (FACS Canto HTS; Beckton Dickinson, Franklin Lakes, NJ); the number of infective particles/µl was calculated from the percentage of GFP+ cells.
Cell culture, transient transfection, and transduction. HEK-293 (ATCC #CRL-1573), RAW 264.7 (ATCC #TIB-71) cells were grown in Dulbecco's modified Eagle's medium (Bio-Whittaker; Lonza, Walkersville, MD) supplemented with 10% fetal bovine serum (FBS; Sigma -Aldrich, St Louis, MO) and L-glutamine plus antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin). Jurkat cells were cultured in RPMI medium (RPMI; Bio-Whittaker, Lonza) containing 10% FBS and L-glutamine plus antibiotics. Mouse embryonic fibroblasts (MEFs) were derived from wild-type mice, immortalized with SV40 T large antigen using standard protocols (iMEFs), and grown in Dulbecco's modified Eagle's medium supplemented with 10% FBS plus antibiotics. Elicited peritoneal macrophages were obtained from male mice 4 days after intraperitoneal administration of 1 ml of 10% thioglycolate broth. Peritoneal macrophages were seeded at densities of 0.5–1.0 × 106 cells in 24-multiwell plates in RPMI supplemented with 10% FBS and antibiotics for 12 hours and transduced. Mouse granulocyte-macrophage colony-stimulating factor bone marrow-derived DCs were generated using granulocyte-macrophage colony-stimulating factor-containing media by standard protocols. DCs cultured in the presence of granulocyte-macrophage colony-stimulating factor were transduced at day 5 of culture. Primary cultures of human umbilical vein endothelial cells and MLEC were isolated and maintained as described.50 iMLEC were grown in Dulbecco's modified Eagle's medium F-12 (Bio-Whittaker, Lonza) supplemented with 10% FBS, L-glutamine plus antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin), and 50 µg/ml ECGF.27 Cells were transduced for 5 hours at the indicated multiplicity of infection, washed with PBS and serum-deprived for 12 hours before stimulation with the indicated reagents. After stimulation, cells were trypsinized and harvested for further analysis.
Flow cytometry, ELISA, and luciferase assay. GFP expression was analyzed by flow cytometry (FACScanto, BD Biosciences, Franklin Lakes, NJ) of harvested cells washed with PBS. For footpad cytokine extraction, each one was cut into small pieces in 0.2 ml RPMI with 10% FBS and were incubated for 45 minutes at 37 °C to allow cytokine release. Cytokine-containing media were collected and centrifuged at 2,000g for 20 minutes. Footpad or cell culture supernatants were collected for cytokine detection by employing commercial ELISA kits (Quantikine immune assays; R&D Systems, Minneapolis, MN) and measured in a Benchmark Plus microplate spectrophotometer (Bio-Rad, Hercules, CA). To determine luciferase activity, transduced cells were collected after stimulation, washed with PBS, lysed, and analyzed in an AutoLumat LB953 luminometer (Berthold Technologies, Bad Wildbad, Germany). Results are expressed in relative light units. For tissue luciferase assays, 1 ml of reporter lysis buffer was added to 100 mg of each sample and homogenized at 4 °C using a tissue grinder. Tissue homogenates were centrifuged for 10 minutes at 12,800xg at 4 °C. Supernatants were saved and employed for luciferase (20 µl) and protein quantification (Bradford; Bio-Rad). Reporter gene expression is shown as relative light units/µg of protein.
Animals. Four-week-old male and female C57/BL6 mice (Charles River, Burlington, MA) were fed lab chow and kept on a 12 hours light/dark cycle. The animals were cared for according to the CNIC Animal Facility guidelines for the care and use of laboratory animals.
In vivo optical bioluminescence imaging. Bioluminescent imaging analysis was conducted with the IVIS 200 in vivo imaging system (Caliper, Hopkinton, MA). Mice were i.p. injected with 150 mg/kg firefly luciferin (Promega, Madison, WI) 15 minutes before imaging and anesthetized with isoflurane during the procedure. Photons emitted from live mice were acquired as photons per s/cm2 per steradian, using Living Imaging 3.0 (Caliper). For photon quantification, a region of interest was manually selected and kept constant within each experiment.
In vivo matrigel plug assay. iMLEC transduced with lentiviral particles containing luciferase cDNA were embedded in matrigel (Sigma) which was implanted subcutaneously in the chest of mice. After 24 hours, lipopolyssaccharide (40 mg/ml LPS from Escherichia coli; Sigma) was intraperitoneally injected and blood samples were obtained 4-6 hours later to determine serum cytokine levels (IL6) by ELISA. Bioluminescence due to luciferase activity was monitored (see above) 24, 48, and 72 hours after LPS treatment.
ZIA animal model. Viral particles (2 × 107 transducing units) were injected into each hind paw of the mouse. After 1 week, the left paw was injected with saline solution (30 µl) and arthritis was induced in the right paw by intraplantar administration of 180 µg zymosan.30 Luciferase expression was monitored over one month using the IVIS 200 system (Caliper). Inflammation was determined by paw diameter and by monitoring the luminescence after i.p. administration of luminol (Sigma).31
Statistical analysis. Data were analyzed for statistical significance using GraphPad Prism (version 5.01). Data shown in Figure 2 were analyzed by one-way ANOVA followed by Newman–Keuls multiple comparison test. Data from ZIA experiments were analyzed by t-test. Statistical significance was assigned at P < 0.05. SEM, standard error of the mean; SD, standard deviation.
SUPPLEMENTARY MATERIAL Figure S1. Endothelial cells are efficiently infected by lentivectors in vitro. Figure S2. Lentiviral vectors containing the human E-selectin promoter sequence. Figure S3. Constitutive GFP expression driven by the SFFVp viral promoter is not affected by TNF-treatment. Figure S4. ESELp-based lentiviral system is induced by an inflammatory milieu in endothelial cells. Figure S5. In vivo optical bioluminescence images of luciferase activity from a representative experiment of the ESELp-driven transgene expression induced by proinflammatory cytokines in a mouse subcutaneous matrigel model. Figure S6. Inflammation induced by the second zymosan administration. Figure S7. IL10 release from the SFFVp lentiviral system in vivo. Table S1. IL10 release by LV-transduced cells.
Acknowledgments
We thank Dr Filip Lim for critical reading of the manuscript, and Dr S. Bartlett for English editing and helpful discussions. We also thank Drs David Sancho and M.A. del Pozo for providing us with DCs and immortalized MEFs, respectively. A.R. is supported by Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica (I+D+I) and Instituto de Salud Carlos III (FIS; PI060122), the Spanish Ministry of Science and Innovation (MICINN;SAF2009-10691) and the Comunidad Autónoma de Madrid (S2006/BIO-0236 and S2010/BMD-2312). J.M.R. is supported by MICINN (RECAVA RD06/0014/005) and by from Fundació La Marató de TV3 (Grant 080731).
Supplementary Material
Endothelial cells are efficiently infected by lentivectors in vitro.
Lentiviral vectors containing the human E-selectin promoter sequence.
Constitutive GFP expression driven by the SFFVp viral promoter is not affected by TNF-treatment.
ESELp-based lentiviral system is induced by an inflammatory milieu in endothelial cells.
In vivo optical bioluminescence images of luciferase activity from a representative experiment of the ESELp-driven transgene expression induced by proinflammatory cytokines in a mouse subcutaneous matrigel model.
Inflammation induced by the second zymosan administration.
IL10 release from the SFFVp lentiviral system in vivo.
IL10 release by LV-transduced cells.
REFERENCES
- Leung PS, Dhirapong A, Wu PY., and, Tao MH. Gene therapy in autoimmune diseases: challenges and opportunities. Autoimmun Rev. 2010;9:170–174. doi: 10.1016/j.autrev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Moore KW, O'Garra A, de Waal Malefyt R, Vieira P., and, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Oberholzer A, Oberholzer C., and, Moldawer LL. Interleukin-10: A complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med. 2002;30 1 Supp:S58–S63. [PubMed] [Google Scholar]
- Spera PA, Ellison JA, Feuerstein GZ., and, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- Rott O, Fleischer B., and, Cash E. Interleukin-10 prevents experimental allergic encephalomyelitis in rats. Eur J Immunol. 1994;24:1434–1440. doi: 10.1002/eji.1830240629. [DOI] [PubMed] [Google Scholar]
- Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W.et al. (2000Treatment of murine colitis by Lactococcus lactis secreting interleukin-10 Science 2891352–1355. [DOI] [PubMed] [Google Scholar]
- van de Loo FA., and, van den Berg WB. Gene therapy for rheumatoid arthritis. Lessons from animal models, including studies on interleukin-4, interleukin-10, and interleukin-1 receptor antagonist as potential disease modulators. Rheum Dis Clin North Am. 2002;28:127–149. doi: 10.1016/s0889-857x(03)00073-5. [DOI] [PubMed] [Google Scholar]
- van de Loo FA. Inflammation-responsive promoters for fine-tuned gene therapy in rheumatoid arthritis. Curr Opin Mol Ther. 2004;6:537–545. [PubMed] [Google Scholar]
- Varley AW, Geiszler SM, Gaynor RB., and, Munford RS. A two-component expression system that responds to inflammatory stimuli in vivo. Nat Biotechnol. 1997;15:1002–1006. doi: 10.1038/nbt1097-1002. [DOI] [PubMed] [Google Scholar]
- van de Loo FA, de Hooge AS, Smeets RL, Bakker AC, Bennink MB, Arntz OJ.et al. (2004An inflammation-inducible adenoviral expression system for local treatment of the arthritic joint Gene Ther 11581–590. [DOI] [PubMed] [Google Scholar]
- Khoury M, Adriaansen J, Vervoordeldonk MJ, Gould D, Chernajovsky Y, Bigey P.et al. (2007Inflammation-inducible anti-TNF gene expression mediated by intra-articular injection of serotype 5 adeno-associated virus reduces arthritis J Gene Med 9596–604. [DOI] [PubMed] [Google Scholar]
- Mingozzi F., and, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- Zaiss AK, Cotter MJ, White LR, Clark SA, Wong NC, Holers VM.et al. (2008Complement is an essential component of the immune response to adeno-associated virus vectors J Virol 822727–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden CS, Manfredsson FP, Reimsnider SK, Poirier AE, Burger C, Muzyczka N.et al. (2009Striatal readministration of rAAV vectors reveals an immune response against AAV2 capsids that can be circumvented Mol Ther 17524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchschacher GL., Jr, and, Wong-Staal F. Development of lentiviral vectors for gene therapy for human diseases. Blood. 2000;95:2499–2504. [PubMed] [Google Scholar]
- Roth S, Pulcini C, Vandenbos F, Bernard E, Dellamonica P, Kaphan R.et al. (2002[Pulmonary localization of hairy cell leukemia] Rev Med Interne 23870–872. [DOI] [PubMed] [Google Scholar]
- Mayordomo L, Marenco JL, Gomez-Mateos J., and, Rejon E. Pulmonary miliary tuberculosis in a patient with anti-TNF-alpha treatment. Scand J Rheumatol. 2002;31:44–45. doi: 10.1080/030097402317255372. [DOI] [PubMed] [Google Scholar]
- Núñez Martínez O, Ripoll Noiseux C, Carneros Martín JA, González Lara V., and, Gregorio Marañón HG. Reactivation tuberculosis in a patient with anti-TNF-alpha treatment. Am J Gastroenterol. 2001;96:1665–1666. doi: 10.1111/j.1572-0241.2001.03836.x. [DOI] [PubMed] [Google Scholar]
- Sicotte NL., and, Voskuhl RR. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology. 2001;57:1885–1888. doi: 10.1212/wnl.57.10.1885. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Keelan ET, Licence ST, Peters AM, Binns RM., and, Haskard DO. Characterization of E-selectin expression in vivo with use of a radiolabeled monoclonal antibody. Am J Physiol. 1994;266 1 Pt 2:H278–H290. doi: 10.1152/ajpheart.1994.266.1.H279. [DOI] [PubMed] [Google Scholar]
- Jaggar RT, Chan HY, Harris AL., and, Bicknell R. Endothelial cell-specific expression of tumor necrosis factor-alpha from the KDR or E-selectin promoters following retroviral delivery. Hum Gene Ther. 1997;8:2239–2247. doi: 10.1089/hum.1997.8.18-2239. [DOI] [PubMed] [Google Scholar]
- Walton T, Wang JL, Ribas A, Barsky SH, Economou J., and, Nguyen M. Endothelium-specific expression of an E-selectin promoter recombinant adenoviral vector. Anticancer Res. 1998;18 3A:1357–1360. [PubMed] [Google Scholar]
- Xu N, Rahman A, Minshall RD, Tiruppathi C., and, Malik AB. beta(2)-Integrin blockade driven by E-selectin promoter prevents neutrophil sequestration and lung injury in mice. Circ Res. 2000;87:254–260. doi: 10.1161/01.res.87.3.254. [DOI] [PubMed] [Google Scholar]
- Xu N, Gao XP, Minshall RD, Rahman A., and, Malik AB. Time-dependent reversal of sepsis-induced PMN uptake and lung vascular injury by expression of CD18 antagonist. Am J Physiol Lung Cell Mol Physiol. 2002;282:L796–L802. doi: 10.1152/ajplung.00298.2001. [DOI] [PubMed] [Google Scholar]
- Maxwell IH, Kaletta C, Naujoks K., and, Maxwell F. Targeting diphtheria toxin A-chain transcription to activated endothelial cells using an E-selectin promoter. Angiogenesis. 2003;6:31–38. doi: 10.1023/a:1025894616613. [DOI] [PubMed] [Google Scholar]
- Hortelano S, López-Fontal R, Través PG, Villa N, Grashoff C, Boscá L.et al. (2010ILK mediates LPS-induced vascular adhesion receptor expression and subsequent leucocyte trans-endothelial migration Cardiovasc Res 86283–292. [DOI] [PubMed] [Google Scholar]
- Stannard AK, Khurana R, Evans IM, Sofra V, Holmes DI., and, Zachary I. Vascular endothelial growth factor synergistically enhances induction of E-selectin by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 2007;27:494–502. doi: 10.1161/01.ATV.0000255309.38699.6c. [DOI] [PubMed] [Google Scholar]
- Girón N, Través PG, Rodríguez B, López-Fontal R, Boscá L, Hortelano S.et al. (2008Suppression of inflammatory responses by labdane-type diterpenoids Toxicol Appl Pharmacol 228179–189. [DOI] [PubMed] [Google Scholar]
- Jain NK, Ishikawa TO, Spigelman I., and, Herschman HR. COX-2 expression and function in the hyperalgesic response to paw inflammation in mice. Prostaglandins Leukot Essent Fatty Acids. 2008;79:183–190. doi: 10.1016/j.plefa.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S, Gammon ST, Moss BL, Rauch D, Harding J, Heinecke JW.et al. (2009Bioluminescence imaging of myeloperoxidase activity in vivo Nat Med 15455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslinger C, Chapatte L, Finke D, Miconnet I, Guillaume P, Lévy F.et al. (2003In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8(+) T cell responses J Clin Invest 1111673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., and, Huang L. Nonviral gene therapy: promises and challenges. Gene Ther. 2000;7:31–34. doi: 10.1038/sj.gt.3301110. [DOI] [PubMed] [Google Scholar]
- Niidome T., and, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647–1652. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- Newman KD, Dunn PF, Owens JW, Schulick AH, Virmani R, Sukhova G.et al. (1995Adenovirus-mediated gene transfer into normal rabbit arteries results in prolonged vascular cell activation, inflammation, and neointimal hyperplasia J Clin Invest 962955–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen MO, Turunen MP, Turunen AM, Rissanen TT, Laitinen M, Kosma VM.et al. (2000Biodistribution of adenoviral vector to nontarget tissues after local in vivo gene transfer to arterial wall using intravascular and periadventitial gene delivery methods FASEB J 142230–2236. [DOI] [PubMed] [Google Scholar]
- Pajusola K, Gruchala M, Joch H, Lüscher TF, Ylä-Herttuala S., and, Büeler H. Cell-type-specific characteristics modulate the transduction efficiency of adeno-associated virus type 2 and restrain infection of endothelial cells. J Virol. 2002;76:11530–11540. doi: 10.1128/JVI.76.22.11530-11540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslami MH, Gangadharan SP, Sui X, Rhynhart KK, Snyder RO., and, Conte MS. Gene delivery to in situ veins: differential effects of adenovirus and adeno-associated viral vectors. J Vasc Surg. 2000;31:1149–1159. [PubMed] [Google Scholar]
- Vassalli G, Büeler H, Dudler J, von Segesser LK., and, Kappenberger L. Adeno-associated virus (AAV) vectors achieve prolonged transgene expression in mouse myocardium and arteries in vivo: a comparative study with adenovirus vectors. Int J Cardiol. 2003;90:229–238. doi: 10.1016/s0167-5273(02)00554-5. [DOI] [PubMed] [Google Scholar]
- van Strien ME, Mercier D, Drukarch B, Brevé JJ, Poole S, Binnekade R.et al. (2010Anti-inflammatory effect by lentiviral-mediated overexpression of IL-10 or IL-1 receptor antagonist in rat glial cells and macrophages Gene Ther 17662–671. [DOI] [PubMed] [Google Scholar]
- VandenDriessche T, Naldini L, Collen D., and, Chuah MK. Oncoretroviral and lentiviral vector-mediated gene therapy. Meth Enzymol. 2002;346:573–589. doi: 10.1016/s0076-6879(02)46078-8. [DOI] [PubMed] [Google Scholar]
- Burns JC, Friedmann T, Driever W, Burrascano M., and, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz SR, Rogel ME., and, Emerman M. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberholzer A, Oberholzer C, Efron PA, Scumpia PO, Uchida T, Bahjat K.et al. (2005Functional modification of dendritic cells with recombinant adenovirus encoding interleukin 10 for the treatment of sepsis Shock 23507–515. [PubMed] [Google Scholar]
- Kushwah R, Oliver JR, Duan R, Zhang L, Keshavjee S., and, Hu J. Induction of immunological tolerance to adenoviral vectors by using a novel dendritic cell-based strategy. J Virol. 2012;86:3422–3435. doi: 10.1128/JVI.06172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL., and, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- Scott DL., and, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med. 2006;355:704–712. doi: 10.1056/NEJMct055183. [DOI] [PubMed] [Google Scholar]
- Chan JM, Villarreal G, Jin WW, Stepan T, Burstein H., and, Wahl SM. Intraarticular gene transfer of TNFR:Fc suppresses experimental arthritis with reduced systemic distribution of the gene product. Mol Ther. 2002;6:727–736. doi: 10.1006/mthe.2002.0808. [DOI] [PubMed] [Google Scholar]
- Scherr M, Battmer K, Blömer U, Ganser A., and, Grez M. Quantitative determination of lentiviral vector particle numbers by real-time PCR. BioTechniques. 2001;31:520, 522, 524, passim. doi: 10.2144/01313st05. [DOI] [PubMed] [Google Scholar]
- Hernández GL, Volpert OV, Iñiguez MA, Lorenzo E, Martínez-Martínez S, Grau R.et al. (2001Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2 J Exp Med 193607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Endothelial cells are efficiently infected by lentivectors in vitro.
Lentiviral vectors containing the human E-selectin promoter sequence.
Constitutive GFP expression driven by the SFFVp viral promoter is not affected by TNF-treatment.
ESELp-based lentiviral system is induced by an inflammatory milieu in endothelial cells.
In vivo optical bioluminescence images of luciferase activity from a representative experiment of the ESELp-driven transgene expression induced by proinflammatory cytokines in a mouse subcutaneous matrigel model.
Inflammation induced by the second zymosan administration.
IL10 release from the SFFVp lentiviral system in vivo.
IL10 release by LV-transduced cells.