Abstract
Microvesicles (MVs) play an important role in intercellular communication by carrying mRNAs, microRNAs (miRNAs), non-coding RNAs, proteins, and DNA from cell to cell. To our knowledge, this is the first report of delivery of a therapeutic mRNA/protein via MVs for treatment of cancer. We first generated genetically engineered MVs by expressing high levels of the suicide gene mRNA and protein–cytosine deaminase (CD) fused to uracil phosphoribosyltransferase (UPRT) in MV donor cells. MVs were isolated from these cells and used to treat pre-established nerve sheath tumors (schwannomas) in an orthotopic mouse model. We demonstrated that MV-mediated delivery of CD-UPRT mRNA/protein by direct injection into schwannomas led to regression of these tumors upon systemic treatment with the prodrug (5-fluorocytosine (5-FC)), which is converted within tumor cells to 5-fluorouracil (5-FU)–an anticancer agent. Taken together, these studies suggest that MVs can serve as novel cell-derived “liposomes” to effectively deliver therapeutic mRNA/proteins to treatment of diseases.
Introduction
Cancer therapeutic strategies include gene delivery to target cancer cells in order to replace dysfunctional tumor suppressor genes, elicit immune rejection or drive tumor cells into apoptotic pathways. To date, several biological delivery vehicles, including DNA, cationic liposomes, viral vectors, and small-interfering RNA (siRNA) nanoparticles have been used with advantages and limitations.1,2 Naked genetic materials are inefficient in targeting because of rapid clearance by extracellular nucleases.3 Liposomes can efficiently load genetic molecules, however, their clearance rate and immunogenicity put limitations on clinical applications.4 Many viral gene delivery vehicles, such as herpes simplex virus, adenovirus, adeno-associated virus, and retrovirus/lentivirus vectors can efficiently transfer genetic material inside tumor cells, however, with limitations in some cases, such as small packaging capacity, loss with cell division, immune response to viral particles, and insertional mutagenesis.5,6,7 Virus and liposome delivery tools are recognized by the host immune system as foreign particles resulting in generation of antibodies against them and thereby decreasing transgene delivery dramatically upon repeated administration, as well as causing immune rejection of transduced cells.8 Lipid nanoparticles are susceptible to opsonin and complement system-mediated clearance in the blood and following uptake into endosomes can trigger the activation of TLR7/8 resulting in transgene silencing.9 Polymeric siRNA nanoparticles such as polyethylenimine-siRNA can be subjected to rapid clearance upon binding to serum proteins. Although polymeric nanoparticles can escape from endosomes through the proton sponge effect of polyethylenimine and effectively deliver siRNA to the cytosol, leakage of endosomal and lysosomal membrane components can result in release of cathepsin B and inflammasome activation.10 In addition, polyethylenimine complexes, as well as virus vectors, tend to accumulate in lung, liver, and spleen, which make targeting to other tissues and tumors challenging.11
Microvesicles (MVs), on the contrary, are a natural mammalian delivery system used by many cell types under both normal physiological and pathological conditions.12 They include a variety of different vesicle types, variously termed exosomes, shed MVs and microparticles, ranging in size from 50 to 800 nm in diameter. They are released into the extracellular environment through fusion of endosome-derived multivesicular bodies with the plasma membrane and by budding from the plasma membrane.12,13,14,15,16,17 These small vesicles are released by cancer cells in abundance as a means to modify the tumor microenvironment.18,19,20,21,22,23 Recent studies have shown that MVs can function to carry a multitude of cargos, including mRNAs, proteins, microRNA (miRNA), non-coding RNAs, and DNA between cells.22,23,24,25,26,27
Based on the capacity of MVs to transfer cargo, in the present study, we evaluated whether MVs can serve as a novel cell-derived gene delivery vehicle carrying therapeutic mRNA/protein for cancer treatment. To test this hypothesis, we generated cells which stably expressed the suicide therapeutic mRNA/protein for cytosine deaminase (CD) fused in-frame with uracil phosphoribosyltransferase (UPRT), previously shown to be a potent prodrug-activating combination.28 MVs were harvested from these cells and used to treat schwannoma tumors. CD converts 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU), which is especially toxic to cells expressing UPRT due to its conversion to 5-fluoro-deoxyuridine monophosphate (5-FdUMP), an irreversible inhibitor of thymidine synthetase, thereby restricting the production of dTMP and downstream phosphorylated products. Depletion of dTTP results in inhibition of DNA synthesis and causes cells to go under apoptosis.29,30 We showed that with overexpression of the suicide gene in donor cells, high amounts of CD-UPRT message and protein were incorporated into MVs. These MVs were capable of transferring this therapeutic mRNA/protein to target tumor cells thereby achieving high level expression of functional protein in these recipient cells. Two different in vivo experiments resulted in significant inhibition of schwannoma tumor growth when CD-UPRT carrying MVs were injected into tumors in combination with systemic delivery of the prodrug, 5-FC.
Results
Use of genetically engineered MVs as a novel gene delivery tool
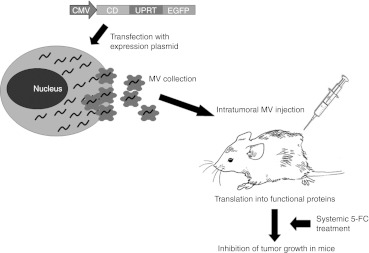
The general plan of action was to transduce donor cells with an expression cassette for a therapeutic gene, in this case CD-UPRT-EGFP (enhanced green fluorescent protein) under a strong promoter (the cytomegalovirus promoter) and to allow time for high level expression of the cassette (72 hours). Then MVs were isolated from the conditioned medium by differential centrifugation, ultracentrifugation, and filtration. These MVs were evaluated for enrichment of CD-UPRT-EGFP mRNA and protein (see below) and injected into tumors, followed, in this case, by systemic prodrug administration (5-FC) which should trigger apoptosis of tumor cells and regression of tumors. A schematic overview of the experiments performed in this study is illustrated in Figure 1.
Figure 1.
Engineering MVs as novel gene delivery tools. The therapeutic expression vector, in this case CD-UPRT-EGFP is delivered into donor cells via DNA transfection or infection with a viral vector. A few days later, MVs enriched with expressed mRNAs/protein are harvested from the conditioned medium and concentrated by ultracentrifugation. Recipient cancer cells/tumor are treated with those MVs followed a few days later by an administration of an activating agent, in this case the prodrug, 5-FC. CD and UPRT converts 5-FC to 5-FdUMP, an irreversible inhibitor of thymidine synthetase, thereby restricting the production of dTMP. Depletion of dTTP results in inhibition of DNA synthesis and leads to apoptosis of cancer cells. 5-FC, 5-fluorocytosine; 5-FdUMP, 5-fluoro-deoxyuridine monophosphate; CD, cytosine deaminase; CMV, cytomegalovirus; EGFP, enhanced green fluorescent protein; MV, microvesicle; UPRT, uracil phosphoribosyltransferase.
CD-UPRT-EGFP mRNA and protein are enriched in MVs
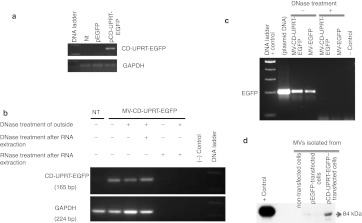
To test whether the CD-UPRT-EGFP mRNAs are enriched in MVs, we first transfected HEK-293T either with pCD-UPRT-EGFP or pEGFP, and 3 days after transfection MVs were collected from medium and treated with DNaseI to remove any residual plasmid DNA bound to the surface of the MVs. Then RNA was isolated from MVs and quantitative reverse transcription-PCRs (RT-PCRs) were performed for CD-UPRT-EGFP and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNAs and end PCR products were loaded onto agarose gel. As shown in Figure 2a, we detected the CD-UPRT-EGFP mRNA only in MVs isolated from pCD-UPRT-EGFP–transfected cells, compared with control pEGFP or non-transfected cells (Figure 2a). Moreover, to investigate the origin of the quantitative RT-PCR signal, we performed the following treatments: with and without DNaseI on intact MVs and after release of MV contents, and RNase treatment after RNA isolation from MVs. As shown in Figure 2b, DNaseI treatment on the outside of MVs did not prevent amplification of the RT-PCR product, whereas after RNase treatment of the contents of MVs, no signal was observed (Figure 2b). In order to determine whether DNaseI treatment of MVs was functional, we also performed quantitative PCR (qPCR) directly (without RT) on the contents of isolated MVs and found no PCR product, supporting the conclusion that the PCR amplicons observed in our experimental conditions came entirely from mRNAs within MVs and not from any plasmid DNA contamination during MVs isolation (Figure 2c). Since MVs can carry proteins as well as RNAs, we next determined whether CD-UPRT-EGFP protein was also incorporated into MVs. We performed western blots directly on isolated MVs lysates using anti-CD antibody and found that MVs from CD-UPRT-EGFP–transfected cells also contained CD-UPRT-EGFP protein (Figure 2d).
Figure 2.
The CD-UPRT-EGFP mRNA and protein are enriched in MVs. (a) Total RNA was isolated from MVs collected from HEK-293T cells transfected with pCD-UPRT-EGFP vector and qRT-PCR was performed for the CD-UPRT and GAPDH mRNAs. The end PCR products were loaded onto agarose gel. CD-UPRT mRNAs levels to GAPDH are shown. (b) After MV collection in a, DNase and RNase treatment were performed on MVs and/or their contents, as indicated. Similar qRT-PCR reactions were performed as in a and the end product DNA was loaded onto agarose gels. A representative agarose gel from three independent qRT-PCRs is shown. (c) Nucleic acid content of MVs were treated with DNase or left non-treated and then qPCRs were performed directly without RT and PCR products were loaded onto agarose gels. (d) Western blot analysis was carried out on MVs (40 µg protein) collected from HEK-293T transfected with pEGFP or pCD-UPRT-EGFP or non-transfected. HEK-293T cell lysates (10 µg) from pCD-UPRT-EGFP plasmid-transfected cells were used as a positive control for the CD-UPRT-EGFP fusion protein. CD, cytosine deaminase; EGFP, enhanced green fluorescent protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MV, microvesicle; Nt, nucleotide; qPCR, quantitative PCR; qRT-PCR, quantitative reverse transcription-PCR; UPRT, uracil phosphoribosyltransferase.
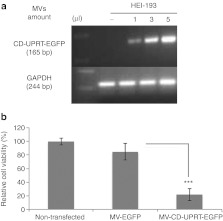
We next examined whether MVs could mediate mRNA/protein delivery into recipient cells in culture. We employed HEI-193 cells, human NF2 schwannoma cells immortalized with an oncogene31 as recipient tumor cells based on our recently developed orthotopic schwannoma mouse model.32,33 Donor HEK-293T cells (4 × 107) were transfected with pCD-UPRT-EGFP and 3 days later MVs were collected and concentrated as above in a 50 µl volume. HEI-193 cells (105) were treated for 2 days with increasing volumes of MV concentrate–1, 3, and 5 µl corresponding to MVs released from 8 × 105 (1 µl), 2.4 × 106 (3 µl), and 4 × 106 (5 µl) cells, respectively. Then total RNA was isolated from recipient cells and RT-PCR was performed for the CD-UPRT-EGFP and GAPDH mRNAs. As shown in Figure 3a, increasing amounts of the CD-UPRT-EGFP mRNA were observed in HEI-193 cells exposed to the higher numbers of MVs, supporting MV-mediated transfer of mRNA into the recipient cells. To test whether our delivery system was functional in culture, we treated HEI-193 cells (105) with CD-UPRT-EGFP mRNA/protein-enriched MVs (15 µl out of 50 µl MVs collected from 4 × 107 donor cells) and 2 days later added the prodrug, 5-FC to the medium followed by an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) viability assay 24 hours later. We found that HEI-193 cells treated with MVs carrying the CD-UPRT-EGFP mRNA/protein showed significant cell death (about 80%), compared with cells exposed to the control MVs carrying EGFP mRNA/protein after treatment with 5-FC (Figure 3b). In order to evaluate any nonspecific cell toxicity due to aggregated proteins which co-pelleted with MVs during MV isolation, we also purified MVs via sucrose gradient ultracentrifugation (fractions 3–7)34 and repeated the experiments performed in Figure 3b and observed a pronounced and significant cell death (about 40%) in cells treated with these sucrose density isolated CD-UPRT-EGFP MVs compared with cells exposed to control MVs (prepared in a similar manner) carrying EGFP mRNA/protein after treatment with 5-FC (Supplementary Figure S1). This supports the tumor toxicity of MVs, but does not exclude some portion being contributed by protein aggregates using centrifugal pelleting. The ultracentrifugation protocol used for MV isolation in Figure 3b yielded MVs which were mostly 100–150 nm in diameter (mean 159 nm), but included an additional larger fraction 200–350 nm in diameter, whereas the sucrose gradient protocol yielded mostly MVs of a smaller size 50–60 nm in diameter (mean 104 nm; Supplementary Figure S2). Moreover, ultracentrifugation gave ~3–4 times more MVs than that sucrose gradient method from the same number of HEK-293T cells. The somewhat decreased cell death using sucrose density prepared MVs as compared with pelleted MVs observed in Figure 3b may also be a consequence in the latter of larger MVs, known to be produced by tumorigenic cells27 like HEK-293T cells carrying a greater amount per vesicle of the EGFP-CD-UPRT mRNA/protein, as well as delivery of more MVs per cell. For treatment of tumor cells, the number of MVs per cell was the same for MVs isolated by the two methods. Treatment of HEI-193 cells with MVs isolated with sucrose gradient method is also show in Supplementary Figure S3, which also provided evidence that larger MVs are lost during sucrose gradient preparation and produces relatively less MVs.
Figure 3.
MVs carrying the CD-UPRT-EGFP mRNA/protein are functional in recipient cells. (a) Three days after treatment of HEI-193 with MVs carrying CD-UPRT-EGFP mRNA/protein in increasing concentrations (1, 3, and 5 µl), total RNA was isolated and RT-PCR was performed for the CD-UPRT-EGFP and GAPDH mRNAs and products resolved by ethidium bromide gel electrophoresis. (b) MTT assays were performed on HEI-193 cells 3 days after exposure to MVs containing CD-UPRT-EGFP mRNA/protein or control EGFP, in both cases with prodrug 5-FC treatment. The experiments were performed in triplicate, and the values are expressed as the mean ± SD (***P < 0.001, Student's t-test). 5-FC, 5-fluorocytosine; CD, cytosine deaminase; EGFP, enhanced green fluorescent protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; MV, microvesicle; RT-PCR, reverse transcription-PCR; UPRT, uracil phosphoribosyltransferase.
Treatment of other human cancer cell lines, glioblastoma U87 and meningioma SF443 with the same concentration of CD-UPRT-EGFP–loaded MVs also resulted in significant cell death after prodrug administration in culture (Supplementary Figure S4a,b). Transfer of the CD-UPRT-EGFP mRNAs in those cells via MVs was confirmed with RT-PCR (Supplementary Figure S4c), together suggesting that this MV-mediated suicide gene therapy approach is effective for a number of tumor cell types.
Under similar experimental conditions, HEI-193 cells were treated for 16 and 24 hours with MVs isolated from HEK-293T transfected with pCD-UPRT-EGFP or pEGFP and examined by fluorescence microscopy at indicated time points. In these experimental conditions, MVs were directly obtained from the medium of the transfected cells without any ultracentrifugation. We observed increased intracellular EGFP signal over time in MV-CD-UPRT-EGFP– and MV-EGFP–treated cells indicating uptake of MVs by recipient cells with expression of EGFP continuing over at least 24 hours, apparently due at least in part to translation of the CD-UPRT-EGFP mRNA rather than just MV transfer of this protein (Supplementary Figure S5a,b). EGFP signal was observed prominently in the cytoplasm of the cells treated with MV-CD-UPRT-EGFP, whereas MV-EGFP–treated cells exhibited a diffused EGFP signal both in nucleus and cytoplasm. Similar localization patterns of CD-UPRT-EGFP fusion protein and EGFP alone were also observed when they expressed from plasmid DNAs, pCD-UPRT-EGFP or pEGFP (Supplementary Figure S6).
Intratumoral delivery of MVs carrying CD-UPRT-EGFP mRNA/protein inhibits schwannoma tumor growth in vivo
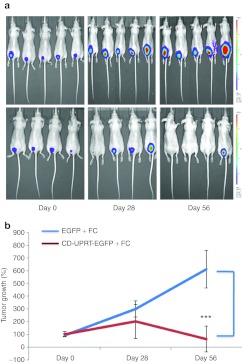
To test whether this therapeutic MV delivery system was functional in vivo, we used an orthotopic pre-established schwannoma tumor model which we recently developed.32,33 HEI-193 cells were stably transduced to express firefly luciferase (Fluc) and mCherry (mCh), yielding HEI-193FC cells.35 In order to establish tumors, 3 × 104 HEI-193FC cells in 1 µl of medium were implanted directly into the sciatic nerve of nude mice. Tumor development was monitored by in vivo bioluminescence imaging over 3 weeks and mice were regrouped so that both groups harbored a range of similarly sized tumors based on photon counts. MVs carrying CD-UPRT-EGFP mRNAs/protein or EGFP mRNA/protein were prepared under similar experimental conditions and resuspended in 20 µl. One µl of the MVs were injected into each tumor once a week for 2 months, with fresh, loaded MVs prepared for each injection. Prodrug, 5-FC was administered intraperitoneally at daily intervals (12 mg/day) after the first MV injection. Two independent in vivo studies were conducted using a total of 10 mice in the control group (EGFP + 5-FC treated) and 9 in the treatment group (CD-UPRT-EGFP + 5-FC treated). Tumor growth was monitored by in vivo bioluminescence imaging at 28-day intervals. Bioluminescence images of tumors in these mice in two independent experiments are shown in Figure 4a,b and Supplementary Figure S7a,b including quantification of the average photon counts. As shown in Figure 4 and Supplementary Figure S7 in the treatment groups (CD-UPRT-EGFP + 5-FC), tumor growth was completely inhibited in six of nine mice, whereas all tumors in EGFP + 5-FC control mice continued to grow. The lack of regression of the tumor in three of the treated mice is believed to be due to the difficulty in injecting directly into the tumor within the sciatic nerve. In control groups, in vivo imaging was terminated at day 56 because of excessive size of tumors. In the treatment group, in vivo imaging was carried out for an additional 2 weeks beyond that and none of the mice developed tumors.
Figure 4.
Intratumoral delivery of MVs carrying the CD-UPRT-EGFP mRNA/protein inhibited schwannoma tumor growth in vivo. (a) HEI-193FC cells were injected (3 × 104 cells in 1 µl of culture medium) into the sciatic nerve of nude mice starting 3 weeks after tumor implantation. MVs harvested from HEK-293T cells transfected with either pCD-UPRT-EGFP or pEGFP plasmids were injected weekly into tumors for 2 months (using 1 µl per tumor out of 20 µl MVs isolated from 4 × 107 cells) and prodrug 5-FC was given daily through intraperitoneal injections following the initial MV injection. Tumor growth was monitored by in vivo bioluminescence imaging using the Xenogen IVIS system to monitor photon emission. Bioluminescent images are shown with a pseudocolor bar to indicate degree of bioluminescence at 1, 28, and 56 days after tumor cell implantation. (b) The average photon counts of the groups is represented as an index of tumor growth, starting with 100% as the initial value. The values are expressed as the mean ± SD (***P < 0.001, Student's t-test). 5-FC, fluorocytosine; CD, cytosine deaminase; EGFP, enhanced green fluorescent protein; MV, microvesicle; UPRT, uracil phosphoribosyltransferase.
Discussion
This study provides the first evidence for the potential therapeutic use of cell-derived MVs as novel and natural gene delivery vehicles for cancer treatment. In this strategy the “donor” cells are genetically engineered to express high levels of a conditionally therapeutic message and protein which are then incorporated into MVs derived from these cells. We first transfected HEK-293T cells with pCD-UPRT-EGFP plasmid and then allowed the CD-UPRT mRNA/protein to be incorporated into the MVs for 3 days followed by isolation and concentration of the MVs by ultracentrifugation. We showed that both the CD-UPRT-EGFP message and protein were enriched in these MVs and were collectively functional. When recipient tumor cells were exposed to these loaded MVs and the prodrug, 5-FC, it was converted to its active form leading to cell death. Furthermore, in in vivo studies, when pre-established mouse sciatic nerve schwannomas were injected intratumorally with CD-UPRT-mRNA/protein-bearing MVs and treated with 5-FC, there was marked inhibition of tumor growth and regression of tumor size in two independent studies. It is likely that this therapeutic effect is mediated by both mRNA and protein delivery, but we are not able to evaluate the contribution of each in the current assays, but together make up a novel gene delivery tool: MVs carrying mRNAs/protein. Taken together, these data suggest that MVs can be used as a gene delivery tool to treat cancer.
Ideal therapeutic delivery vehicles should have a good packaging size and no immunogenic response in the host organism.1 Due to the fact that MVs are stable enough to have a relatively long halflife in tissues, small enough to diffuse throughout target tissues, and large enough to carry sufficient amounts of genetic and protein material for different purposes, they may prove to be among the most potent biological gene/protein delivery vehicles.1 Since therapeutic MVs can be derived from the host own cells placed in culture, they have the potential to evade the host's immune system, and with further modifications of membrane components, immunogenicity may be further decreased. For example, the host cells can be engineered to produce immunosuppressive ligands or cytokines which MVs derived from those engineered cells will also carry.36 In addition, introduction of specific peptides, such as targeting moieties onto the MV membrane should serve to target MVs to specific cell populations37 or tissues, such as the brain with access across the blood–brain barrier.38 Further, these cell-derived “physiologic liposomes” can carry multiple components including miRNA, mRNA, non-coding regulatory RNAs, proteins, and DNA (for review see ref. 26). Interestingly, we have recently described a zipcode-like 25 nt sequence in the 3′UTRs of many of the most enriched mRNAs in MVs derived from human primary glioblastoma cells, which enhances mRNA incorporation into MVs, in part through interaction with miR-1289.39
When compared with current viral gene delivery tools, MVs derived from an individual's cells should be recognized as “self” by the body, resulting in less immune response in the host organism to the delivery vehicle, as compared with virus vectors. Furthermore, some viral constructs also induce protein kinase R stress signaling in host cells.40 Herpes simplex virus also encodes proteins, e.g., UL41 (vhs) that block host cell protein translation,41 and these cellular and viral proteins can be toxic to cells in their own right and may interfere with action of therapeutic proteins.42
MVs can be taken up by endocytosis or fusion with the recipient cell plasma membrane as determined by membrane-bound protein interactions, after which genetic and protein material within the MVs are released into the cells. Although protein kinase R signaling and many other ribonucleases recognize foreign bacterial or viral RNA/DNA molecules, MVs mRNA molecules should have the common eukaryotic signals, such as untranslated regions at the 5′ and 3′ terminals, capping at the 5′ terminal, and coding sequences, which are distinct from microbial mRNAs. In addition, the immune system should recognize MVs produced by normal cells as self due to immunologic memory as these MVs are present in all body fluids, including blood and urine.43,44 Several studies also support the functional nature of transferred miRNAs and mRNAs which modify the translational profile and phenotype of the recipient cells.22,23,38,45,46,47,48 A recent study also showed that MVs derived from genetically engineered dendritic cells can successfully transfer siRNA molecules to mouse brain with consequent downregulated translation of targeted mRNAs.38
Taken together–less immunogenic response, abundant gene/protein transfer capacity, and ample packaging size, cell-derived MVs have immense potential as therapeutic delivery vehicles for disease applications in the future. Two applications can be envisioned. In one strategy, cells from the affected individual would be placed in culture, genetically modified as needed for targeting and delivery of the therapeutic protein/RNA and then MVs isolated from cultures administered to the patient, as modeled in Alvarez-Erviti et al.38 In the context of cancer therapy, it will be important that the therapeutic gene does not kill the donor cells, so, for example, it could be a prodrug-activating enzyme, as in this study, or a protein such as a membrane-bound form of TRAIL which is not toxic to most normal cells, but kills tumor cells upon transfer.49 In a second strategy, it should be possible to genetically modify cells in vivo so that they produce conditionally therapeutic MVs, such as the prodrug-activating scheme used in this study to empower tumor cells so that they “become their own worst enemies”. The results presented here provide a proof-of-concept that MVs can serve as therapeutic delivery vehicles for cancer and other diseases.
Materials and Methods
Plasmids. Yeast CD – yeast UPRT ORF (pORF-FcyFur; Invitrogen, Grand Island, NY) was fused to the N terminus of EGFP in pEGFP-N1 plasmid (Clontech, Mountain View, CA) under the cytomegalovirus promoter and the plasmid construct is referred to as pCD-UPRT-EGFP in these studies. Forward 5′-GCTTCGAATTCATGGTCACAGGAGGCATGGCTTC and reverse 5′-GACCGGTGGATCCACACAGTAGTATCTGTCCC primers were used to amplify CD:UPRT by conventional PCR with Pfu Polymerase (Stratagene, Santa Clara, CA) in cloning steps.
Cells. HEK-293T cells (from Dr Maria Calos, Stanford University, Stanford, CA) were cultured in Dulbecco's Modified Eagle Medium (DMEM; Cellgro, Mediatech, Manassas, VA) containing 10% fetal bovine serum (FBS). Human schwannoma cell line HEI-193 established from a schwannoma tumor from a NF2 patient and immortalized with retroviral-mediated HPV E6-E7 transduction (from Dr David Lim, House Ear Institute, Los Angeles, CA) was cultured, as described.31 These cells were transduced with a lentivirus vector expressing Fluc and mCh, termed HEI-193FC cells, as described.35 Meningioma cells, SF443 (from Dr Anita Lal, University of California, San Francisco, CA) were cultured, as described previously.50,51 U87 cells (ATCC, Manassas, VA) were cultured in DMEM containing 10% FBS. All cells were grown in the presence of 100 IU/ml penicillin and 100 µg/ml streptomycin and incubated at 37 °C in a 5% CO2 atmosphere. Cells were determined to be mycoplasma negative by testing with a mycoplasma detection kit (MycoAlert Mycoplasma Detection Assay; Lonza, Rockland, ME).
MVs isolation. HEK-293T cells (maintained within 10 passages) were transfected either with pEGFP-N1 or pCD-UPRT-EGFP using Lipofectamine 2000 (Invitrogen), according to manufacturer's protocol. Five hours later, transfection media was replaced with media containing 5% MV-free FBS.22 Three days after transfection, MVs were harvested, as described.22 Briefly, culture medium from 2 × 150 mm plates containing 2 × 107 cells per plate was first centrifuged at 300g for 15 minutes to separate cells from medium, then at 16,000g for 30 minutes to precipitate cellular debris. The supernatants were filtered through 0.22 µm filters (Millex, Billerica, MA) and then a final ultracentrifugation was performed at 110,000g for 80 minutes using Beckman Quick seal tubes and a 70Ti rotor (Beckman Coulter, Fullerton, CA). Pelleted MVs were eluted either in 50 µl (for in vitro experiments) or 20 µl (for in vivo studies) in a mixture containing 1× phosphate-buffered saline, RNase inhibitor (2 µl, 10U-RNAse-OUT—Invitrogen) and rDNase I (1 µl, 2U-DNA-free—Ambion, Grand Island, NY). One µl MVs were delivered into each tumor once a week for 2 months, with fresh MVs prepared for each injection. Every 2 weeks, we generated new, early passage cultures of the cells to isolate MVs to make sure that there were no extended passage differences between the cells from which the MVs were isolated. MV yields were determined by measuring total RNA content using a NanoDrop 1000 (NanoDrop, Wilmington, DE) which correlated directly with MV number as assessed using a Nanosight, NS500 (Supplementary Figure S8).
For in vivo studies, MVs were prepared in the same manner and similar amounts of MVs were used in both groups as assessed by total RNA content: 189 ± 21 ngin 20 µl for CD-UPRT-EGFP MVs and 201 ± 38 ng in 20 µl for the control EGFP MVs. In some cases MVs were treated with 1 µl DNaseI (2U; Ambion) in 50 µl of total reaction for 30 minutes at 37 °C to remove DNA bound to the surface. In order to make sure that our DNase treatment protocol was functional, we performed qPCR reactions in the absence of RT directly from the MVs content and found no PCR amplification suggesting that the treatment protocol completely removed any plasmid DNA purified during MVs isolation.
Sucrose gradient ultracentrifugation. Sucrose gradient ultracentrifugation was performed, as described previously.34 Briefly, MVs were layered onto a sucrose density gradient (8, 30, 45, 60% layers) and centrifuged for 38 minutes at 50,000 rpm in SW40Ti swinging bucket rotor (Beckman Coulter) in a Beckman Optima ultracentrifuge with deceleration set to slow. Fractions 3–7 (density: fraction 3 contains: half 8%, half 30%, fractions 4 and 5 contains 30% only, fractions 6 and 7 contains 45% only) were collected, diluted in phosphate-buffered saline and MVs pelleted at 100,000g for 75 minutes in the S50A rotor using a Sorvall MX-120 microcentrifuge (Thermo Fisher Scientific, Agawam, MA) and used for western blot analysis for MV-associated proteins.
Total RNA isolation, reverse transcription, and qPCR. RNA was isolated from MVs using the miRvana kit (Ambion), according to manufacturer's protocol. Reverse transcription reaction was performed with 150 ng of MV RNA using Omniscript (Qiagen, Valencia, CA). Relative mRNA amounts were quantified with Applied Biosystems 7000 series qPCR using SYBR Green (Applied Biosystems, Grand Island, NY). Forward 5′-CACAACATGAGGTTCCAGAA and reverse 5′-GAAGTTGA CATTCTCTCCCA primers were used to detect CD-UPRT-GFP message and forward 5′-GAAGGTGAAGGTCGGAGT and reverse 5′-GAA GATGGTGATGGGATTTC primers for GAPDH mRNA. Threshold cycles (CT) were analyzed using the Δ-CT formula and normalized to GAPDH mRNA levels. The quantitive RT-PCR analysis was based on threshold cycles of the non-transfected cells-derived MVs and the CD-UPRT-GFP transfected cell-derived MVs. The fold enrichment was calculated by comparing CT values. The highest CT value of qPCR (CT = 30), indicating non-detectable levels of mRNA, was taken as our reference point in non-transfected and control plasmid, pEGFP, transfected cells and normalized to GAPDH CT values. Although the non-transfected cells-derived MVs did not have the CD-UPRT-GFP message, the Δ-CT value of the non-transfected cells-derived MV samples were considered as 1 (CT = 30) in order to determine the minimal level of enrichment of CD message in CD-UPRT-MVs.
Western blot analysis. MVs were collected as described above and total protein (40 µg/lane) was resolved by electrophoresis in SDS–8% polyacrylamide gels and blotted onto nitrocellulose membranes, as described previously.51 The primary antibodies used were CD (#4012; Cell Signaling Technology, Danvers, MA) at 1:1,000 dilution, and β-actin (#A5441; Sigma-Aldrich, St Louis, MO) at 1:1,000 dilution. Goat-anti-mouse IgG HRP conjugated (DAKO, Glostrup, Denmark; 1:5,000, cat. no. P0447) was used as a secondary antibody.
Cell viability assay. HEI-193 cells were seeded (5,000 per well) into 96-well plates 1 day before MV introduction. MVs were harvested from HEK-293 cells and eluted in 50 µl of cocktail mix (see above) and 15 µl was added into the medium in each well. Cells were incubated with MVs for 2 days before prodrug treatment and then treated with 250 µg/ml 5-FC (cat. no. sud-5fc; Invitrogen). Three days later, MTT (Invitrogen) assays were performed, according to manufacturer's protocol to quantify cell viability.
Schwannoma tumor development and bioluminescence imaging. Schwannoma tumors were developed as described previously.32,33 Briefly, HEI-193FC cells were trypsinized and rinsed, and then 3 × 104 cells in 1 µl culture medium were injected directly into the sciatic nerve of athymic mice (nu/nu, 5-week-old females; Cox 7 breeding facility, Massachusetts General Hospital, Boston, MA). In vivo bioluminescence imaging was performed, as described previously.32,33
SUPPLEMENTARY MATERIAL Figure S1. Effect of MV-CD-UPRT-EGFP and MV-EGFP on cell viability after purification by sucrose gradient ultracentrifugation. Figure S2. MVs size comparison by NanoSight. Figure S3. MVs uptake of HEI-193 cells after purification by sucrose gradient ultracentrifugation. Figure S4. Genetically engineered MVs kill other cancer cell types. Figure S5. CD-UPRT-EGFP expression increases in recipient cells over time. Figure S6. pCD-UPRT-EGFP and pEGFP expression patterns in plasmid DNA transfected HEK-293T cells. Figure S7. Inhibition of schwannoma tumor growth after intratumoral delivery of MVs carrying the CD-UPRT-EGFP mRNA/protein. Figure S8. Correlation between MVs numbers and RNA content.
Acknowledgments
We thank Suzanne McDavitt for skilled editorial assistance and Shilpa Prabhakar for technical help. Support for this work was provided by NIH NCI grant CA141150 (X.O.B.), NIH NINDS grant NS037409 (X.O.B., O.S.), Forschungsgesellschaft for Brain Tumors (O.S.), EU-FP7-PEOPLE-2011-CIG (O.S.), and Association for conduct of scientific research in the field of neonatology and pediatric intensive care: “Unser Kind” (O.S.). The authors declared no conflict of interest.
Supplementary Material
Effect of MV-CD-UPRT-EGFP and MV-EGFP on cell viability after purification by sucrose gradient ultracentrifugation.
MVs size comparison by NanoSight.
MVs uptake of HEI-193 cells after purification by sucrose gradient ultracentrifugation.
Genetically engineered MVs kill other cancer cell types.
CD-UPRT-EGFP expression increases in recipient cells over time.
pCD-UPRT-EGFP and pEGFP expression patterns in plasmid DNA transfected HEK-293T cells.
Inhibition of schwannoma tumor growth after intratumoral delivery of MVs carrying the CD-UPRT-EGFP mRNA/protein.
Correlation between MVs numbers and RNA content.
REFERENCES
- Seow Y., and, Wood MJ. Biological gene delivery vehicles: beyond viral vectors. Mol Ther. 2009;17:767–777. doi: 10.1038/mt.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae KH, Chung HJ., and, Park TG. Nanomaterials for cancer therapy and imaging. Mol Cells. 2011;31:295–302. doi: 10.1007/s10059-011-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura Y, Nishikawa M, Yamashita F., and, Hashida M. Development of gene drug delivery systems based on pharmacokinetic studies. Eur J Pharm Sci. 2001;13:71–76. doi: 10.1016/s0928-0987(00)00209-8. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Liu F., and, Huang L. Implications of pharmacokinetic behavior of lipoplex for its inflammatory toxicity. Adv Drug Deliv Rev. 2005;57:689–698. doi: 10.1016/j.addr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Coura Rdos S., and, Nardi NB. The state of the art of adeno-associated virus-based vectors in gene therapy. Virol J. 2007;4:99. doi: 10.1186/1743-422X-4-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E.et al. (2008Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1 J Clin Invest 1183132–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman ZC, Appledorn DM., and, Amalfitano A. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132:1–14. doi: 10.1016/j.virusres.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss AK., and, Muruve DA. Immune responses to adeno-associated virus vectors. Curr Gene Ther. 2005;5:323–331. doi: 10.2174/1566523054065039. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Aggarwal P, Hall JB., and, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5:487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL.et al. (2008Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization Nat Immunol 9847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boorn JG, Schlee M, Coch C., and, Hartmann G. SiRNA delivery with exosome nanoparticles. Nat Biotechnol. 2011;29:325–326. doi: 10.1038/nbt.1830. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Racchetti G., and, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- van Niel G, Porto-Carreiro I, Simoes S., and, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W, Kleijmeer MJ, Geuze HJ., and, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- Johnstone RM. Exosomes biological significance: A concise review. Blood Cells Mol Dis. 2006;36:315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Keller S, Sanderson MP, Stoeck A., and, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Shen B, Wu N, Yang JM., and, Gould SJ. Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem. 2011;286:14383–14395. doi: 10.1074/jbc.M110.208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ.et al. (1996B lymphocytes secrete antigen-presenting vesicles J Exp Med 1831161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G.et al. (2002TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex J Immunol 1683235–3241. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D.et al. (1998Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes Nat Med 4594–600. [DOI] [PubMed] [Google Scholar]
- Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C.et al. (2001Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming Nat Med 7297–303. [DOI] [PubMed] [Google Scholar]
- Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M.et al. (2008Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers Nat Cell Biol 101470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ., and, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P.et al. (2006Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery Leukemia 20847–856. [DOI] [PubMed] [Google Scholar]
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A.et al. (2008Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells Nat Cell Biol 10619–624. [DOI] [PubMed] [Google Scholar]
- van der Vos KE, Balaj L, Skog J., and, Breakefield XO. Brain tumor microvesicles: insights into intercellular communication in the nervous system. Cell Mol Neurobiol. 2011;31:949–959. doi: 10.1007/s10571-011-9697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO.et al. (2011Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences Nat Commun 2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbs P, Regulier E, Kintz J, Leroy P, Poitevin Y, Exinger F.et al. (2000In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene Cancer Res 603813–3822. [PubMed] [Google Scholar]
- Huber BE, Austin EA, Richards CA, Davis ST., and, Good SS. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci USA. 1994;91:8302–8306. doi: 10.1073/pnas.91.17.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen CA, Coale MM, Lowe R., and, Blaese RM. Tumors expressing the cytosine deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild type tumor. Cancer Res. 1994;54:1503–1506. [PubMed] [Google Scholar]
- Hung G, Li X, Faudoa R, Xeu Z, Kluwe L, Rhim JS.et al. (2002Establishment and characterization of a schwannoma cell line from a patient with neurofibromatosis 2 Int J Oncol 20475–482. [PubMed] [Google Scholar]
- Saydam O, Ozdener GB, Senol O, Mizrak A, Prabhakar S, Stemmer-Rachamimov AO.et al. (2011A novel imaging-compatible sciatic nerve schwannoma model J Neurosci Methods 19575–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saydam O, Senol O, Würdinger T, Mizrak A, Ozdener GB, Stemmer-Rachamimov AO.et al. (2011miRNA-7 attenuation in Schwannoma tumors stimulates growth by upregulating three oncogenic signaling pathways Cancer Res 71852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire CA, Balaj L, Sivaraman S, Crommentuijn MH, Ericsson M, Mincheva-Nilsson L.et al. (2012Microvesicle-associated AAV vector as a novel gene delivery system Mol Ther 20960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar S, Brenner GJ, Sung B, Messerli SM, Mao J, Sena-Esteves M.et al. (2010Imaging and therapy of experimental schwannomas using HSV amplicon vector-encoding apoptotic protein under Schwann cell promoter Cancer Gene Ther 17266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Bianco N, Menon R, Lechman ER, Shufesky WJ, Morelli AE.et al. (2006Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive Mol Ther 13289–300. [DOI] [PubMed] [Google Scholar]
- Maguire CA, Balaj L, Sivaraman S, Crommentuijn M, Ericsson M, Mincheva-Nilsson L.et al. (2012Microvesicle-associated AAV vector as a novel gene delivery system Mol Ther 20960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S., and, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Bolukbasi MF, Mizrak A, Ozdener BG, Madlener S, Ströbel T, Skog J.et al. (2012). miR-1289 and “zipcode”-like sequence enrich mRNAs in microvesicles Mol Ther-Nucleic Acids1: e10. [DOI] [PMC free article] [PubMed]
- Patel CV, Handy I, Goldsmith T., and, Patel RC. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J Biol Chem. 2000;275:37993–37998. doi: 10.1074/jbc.M004762200. [DOI] [PubMed] [Google Scholar]
- Matis J., and, Kúdelová M. Early shutoff of host protein synthesis in cells infected with herpes simplex viruses. Acta Virol. 2001;45:269–277. [PubMed] [Google Scholar]
- García MA, Meurs EF., and, Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89:799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G., and, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- Gonzales P, Pisitkun T., and, Knepper MA. Urinary exosomes: is there a future. Nephrol Dial Transplant. 2008;23:1799–1801. doi: 10.1093/ndt/gfn058. [DOI] [PubMed] [Google Scholar]
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL.et al. (2010Functional delivery of viral miRNAs via exosomes Proc Natl Acad Sci USA 1076328–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB.et al. (2009Transfer of microRNAs by embryonic stem cell microvesicles PLoS ONE 4e4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González Má.et al. (2011Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells Nat Commun 2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM.et al. (2012Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes Blood 119756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Bureau E, Kim DE, Yang K, Tang Y, Weissleder R.et al. (2005Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression Ann Neurol 5734–41. [DOI] [PubMed] [Google Scholar]
- Cuevas IC, Slocum AL, Jun P, Costello JF, Bollen AW, Riggins GJ.et al. (2005Meningioma transcript profiles reveal deregulated Notch signaling pathway Cancer Res 655070–5075. [DOI] [PubMed] [Google Scholar]
- Saydam O, Shen Y, Würdinger T, Senol O, Boke E, James MF.et al. (2009Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/beta-catenin signaling pathway Mol Cell Biol 295923–5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of MV-CD-UPRT-EGFP and MV-EGFP on cell viability after purification by sucrose gradient ultracentrifugation.
MVs size comparison by NanoSight.
MVs uptake of HEI-193 cells after purification by sucrose gradient ultracentrifugation.
Genetically engineered MVs kill other cancer cell types.
CD-UPRT-EGFP expression increases in recipient cells over time.
pCD-UPRT-EGFP and pEGFP expression patterns in plasmid DNA transfected HEK-293T cells.
Inhibition of schwannoma tumor growth after intratumoral delivery of MVs carrying the CD-UPRT-EGFP mRNA/protein.
Correlation between MVs numbers and RNA content.