Abstract
Only a subset of cancer patients inoculated with oncolytic herpes simplex virus (oHSV) type-1 has shown objective response in phase 1 and 2 clinical trials. This has raised speculations whether resistance of tumor cells to oHSV therapy may be a limiting factor. In this study, we have identified established and patient derived primary glioblastoma multiforme (GBM) stem cell lines (GSC) resistant to oHSV and also to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) that has recently shown promise in preclinical and initial clinical studies. We created a recombinant oHSV bearing a secretable TRAIL (oHSV-TRAIL) and hypothesized that oHSV-TRAIL could be used as a cancer therapeutic to target a broad spectrum of resistant tumors in a mechanism-based manner. Using the identified resistant GBM lines, we show that oHSV-TRAIL downregulates extracellular signal-regulated protein kinase (ERK)-mitogen-activated protein kinase (MAPK) and upregulates c-Jun N-terminal kinase (JNK) and p38-MAPK signaling, which primes resistant GBM cells to apoptosis via activation of caspase-8, -9, and -3. We further show that oHSV-TRAIL inhibits tumor growth and invasiveness and increases survival of mice bearing resistant intracerebral tumors without affecting the normal tissues. This study sheds new light on the mechanism by which oHSV and TRAIL function in concert to overcome therapeutic-resistance, and provides an oncolytic virus based platform to target a broad spectrum of different cancer types.
Introduction
Glioblastoma multiforme (GBM) is a high-grade glioma and the most common primary malignant brain tumor.1 GBMs are diffuse and infiltrating with no clear border between normal brain and tumor. Current treatment regimens that include temozolomide have significantly improved the median, 2- and 5-year survival compared to radiotherapy alone in patients with newly diagnosed GBM.2,3 Nevertheless, GBM patients have a poor prognosis with a median survival of 14.6 months.2 The inherent or acquired resistance of tumor cells to antitumor agents and the highly invasive nature of tumor cells are the major impediments to the currently employed anti-GBM therapies and pose an urgent need for novel therapeutics with substantial efficacy. Oncolytic herpes simplex virus (oHSV) and TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) have recently shown promise in both preclinical and clinical trials.4,5,6,7,8,9,10,11,12,13 Oncolytic viruses are genetically modified viruses that, upon infection, selectively replicate in and kill neoplastic cells while sparing normal cells.4,8,14 Among them, oHSV type 1-derived virus is one of the most extensively studied and considered a promising agent for treating brain tumors as well as other types of cancer.4,15 Recombinant oHSV vectors such as G207 and G47Δ have been previously investigated in both preclinical and clinical studies.9,16,17,18 Unlike replication-incompetent vectors, replication-competent or conditional vectors can amplify to produce virus progeny that then infects surrounding tumor cells resulting in multiple waves of infection in situ, virus spread and extensive cell death. In a direct comparison between oncolytic adenovirus and oHSV in GBM cell lines, oHSV was shown to be more efficacious.19 Mutations of specific HSV genes, namely γ34.5 and UL39, have been shown to confer selectivity to cancer cells, which has enabled translational studies to humans.4,15 Although phase 1 and 1b clinical trials for oHSV proved its safety, the efficacy for human GBMs seems marginal as only a subset of patients showed decrease in tumor volume9 which could in part be due to the insensitivity of a subset of GBM cells to HSV mediated oncolysis. TRAIL has emerged as a promising antitumor agent due to its tumor-specific induction of apoptosis in a death receptor-dependent manner.20 Both recombinant human TRAIL ligand and TRAIL receptor agonist monoclonal antibodies are currently being evaluated in clinical trials,21 however, short half-life and off-target toxicity of systemically delivered TRAIL pose challenges in the clinic.22 We have previously established that a secreted form of TRAIL (S-TRAIL) exerts more potent apoptotic effects compared to TRAIL itself and when delivered by viruses or different stem cell types has significant antitumor effects as compared to systemically administrated TRAIL in different mouse models of GBMs.5,7,10,11,12,23 However, malignant GBMs show heterogeneity in their response to TRAIL; with ~50% showing sensitivity to TRAIL-mediated apoptosis and others showing varying resistance to TRAIL-mediated apoptosis.7,24
In this study, we screened a panel of established and patient derived primary GBM stem cell lines for their sensitivity to a recombinant version of G47Δ (referred to oHSV in this study) and TRAIL. In an effort to develop anti-GBM therapies that target a broad spectrum of GBMs that are either resistant to TRAIL-mediated apoptosis or resistant to both oHSV-mediated oncolysis and TRAIL, we have engineered oHSV-bearing secretable-TRAIL (oHSV-TRAIL) and extensively studied a mechanism-based therapeutic approach to target resistant GBMs in vitro and in malignant and invasive GBM models in mice.
Results
Screening of different GBM lines reveals differential sensitivities to oHSV-mediated oncolysis and S-TRAIL-mediated apoptosis
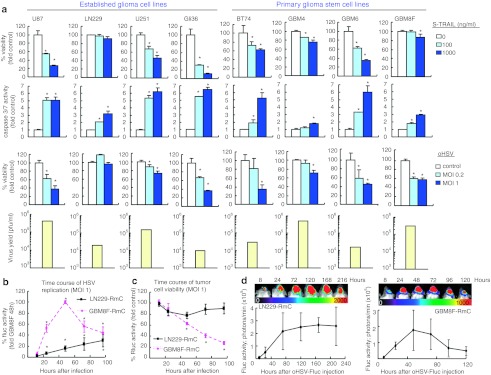
We screened a cohort of both established GBM cell lines (Gli36, U87, U251, and LN229) and primary glioma stem cell (GSC) lines obtained from surgical specimens (GBM4, GBM6, BT74, and GBM8F) for their sensitivity to purified S-TRAIL- or oHSV-mediated cell death. While three established lines had varying sensitivity to TRAIL-induced apoptosis-mediated by caspase-3 and -7, one established line (LN229) was fully resistant to TRAIL-mediated apoptosis. Among the primary GSC, GBM8F was fully resistant to TRAIL whereas other GSC lines had varying sensitivity to TRAIL-induced apoptosis. Next, we evaluated the sensitivity of established GBM lines and GSC lines to oHSV (G47Δ empty) mediated oncolysis. Among the established lines, TRAIL resistant LN229 line was also resistant to oHSV-mediated oncolysis whereas all the GSC lines were sensitive to oHSV-mediated oncolysis. The amounts of virus released by the oHSV-infected cells greatly varied among the GBM cell lines tested, and did not necessarily correspond to the sensitivity to oHSV-mediated oncolysis. These results reveal the identification of GBM lines that are either resistant to TRAIL-mediated apoptosis (LN229, GBM8F) or resistant to both oHSV-mediated oncolysis and TRAIL (LN229). Based on these results, we used LN229 and GBM8F GSC for further therapeutic evaluation (Figure 1a).
Figure 1.
Differential sensitivities of glioblastoma multiforme (GBM) cells to S-TRAIL-mediated apoptosis and oncolytic herpes simplex virus (oHSV)-mediated oncolysis. (a) Screening of different GBM lines reveals differential sensitivities to S-TRAIL-mediated apoptosis and oHSV-mediated oncolysis. Established GBM lines (U87, LN229, U251, and Gli36) and primary glioma stem cell (GSC) lines (BT74, GBM4, GBM6, and GBM8F) were treated with different concentrations of purified S-TRAIL and assayed for viability at 48 hours post-treatment (top row), and for caspase-3/7 activity at 18 hours post-treatment (second row). Established GBM lines and primary GSC lines were infected with oHSV at multiplicities of infection (MOIs) 0.2 and 1 and assayed for viability at 72 hours postinfection (third row), and virus titration on Vero cells using the supernatant of oHSV-infected GBM cell lines (MOI1, bottom row). (b,c) Pharmacodynamics of oHSV in vitro. LN229-RmC and GBM8F-RmC were infected with oHSV-Fluc at MOI = 1. Viral replication indicated by (b) firefly luciferase (Fluc) activity and tumor cell viability indicated by (c) Renilla luciferase (Rluc) activity were monitored by dual Fluc and Rluc bioluminescence imaging, respectively at different time points. (d) Pharmacodynamics of oHSV in vivo. Mice- (n = 3 per line) bearing intracranial LN229-RmC (left) or GBM8F-RmC (right) GBMs were injected with oHSV-Fluc intratumorally and viral distribution was followed by Fluc bioluminescence imaging at different indicated times. One representative image of mice and the average Fluc bioluminescence intensities are shown. *P < 0.05. Error bars indicate SD.
To evaluate oHSV replication and spread in oHSV and TRAIL resistant GBM cells and corresponding changes in cell viability, LN229 and GBM8F cells engineered to express Renilla luciferase (Rluc)-mCherry (LN229-RmC, GBM8F-RmC) were infected with oHSV-bearing firefly luciferase (oHSV-Fluc, Supplementary Figure S1), and monitored by in vitro and in vivo dual bioluminescence imaging. In vitro, oHSV-Fluc replicates in both LN229 and GBM8F cells as indicated by firefly luciferase (Fluc) expression, but oHSV replication was more robust and peaked earlier in GBM8F cells than LN229 cells (Figure 1b and Supplementary Figure S2). Accordingly, while oHSV killed GBM8F cells efficiently (indicated by Rluc expression), LN229 cells exhibited apparent resistance to the killing effect by oHSV (Figure 1c). In vivo monitoring of virus infection demonstrated the ability of intratumorally injected oHSV-Fluc to replicate in intracerebral tumors generated with these GBM cells, and revealed patterns of virus replication similar to the ones observed in vitro (Figure 1b and d). These data reveal that although oHSV replicates in both oHSV resistant and sensitive lines but results in killing only the sensitive GBM8F line and suggest that GBMs might be heterogeneous in the responses to oHSV.
Combination of oHSV and S-TRAIL leads to apoptosis in TRAIL resistant GBM cells in vitro
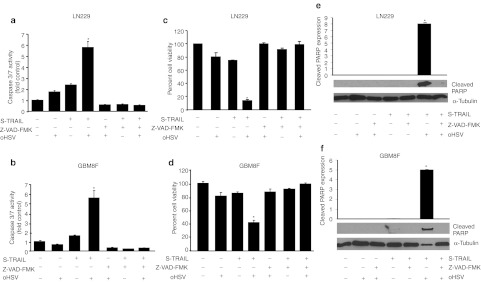
In order to evaluate the therapeutic potential of oHSV and S-TRAIL in resistant GBMs, established LN229 GBM cells and primary GBM8F (TRAIL resistant) GBM cells were infected with oHSV and treated with purified S-TRAIL 6 hours post-infection. oHSV infection and subsequent S-TRAIL treatment resulted in significant decrease in viability of LN229 and GBM8F cells in culture, which was associated with increased caspase-3/7 activation (Figure 2a–d). A significantly impaired activation of caspase-3/7 activity was observed when LN229 and GBM8F cells were infected with oHSV and treated with S-TRAIL in the presence of pan-caspase inhibitor, Z-VAD-FMK (Figure 2a, b). This resulted in complete reversal of both LN229 and GBM8F cell death in oHSV-infected and S-TRAIL-treated cells (Figure 2c–d). Western blotting revealed that cleavage of poly-ADP ribose polymerase (PARP), one of the main downstream targets of caspase-3, was undetectable by either S-TRAIL or oHSV treatment in both LN229 and GBM8F cells, indicating that oHSV-mediated cell death is primarily not dependent on caspase/PARP activation, However, cleaved PARP was increased in cells treated with combined oHSV and S-TRAIL (Figure 2e, f). A significant reduction in PARP cleavage was observed when LN229 and GBM8F cells were infected with oHSV and treated with S-TRAIL in the presence of pan-caspase inhibitor, Z-VAD-FMK (Figure 2e, f). These results revealed that oHSV and TRAIL combination leads to caspase-mediated apoptosis in TRAIL resistant and both TRAIL and oHSV resistant GBM cells.
Figure 2.
Combination of oncolytic herpes simplex virus (oHSV) and S-TRAIL leads to caspase-3/7-mediated apoptosis in resistant glioblastoma multiforme (GBM) cells. (a–d) Caspase-3/7 activity and cell viability of (a,c) LN229 and (b,d) GBM8F treated with purified S-TRAIL (100 ng/ml), oHSV (multiplicity of infection (MOI) = 1) or both oHSV and S-TRAIL in the presence or absence of pan-caspase inhibitor, Z-VAD-FMK (20 µmol/l). *P < 0.05 in the comparison of oHSV plus S-TRAIL treatment group with other groups. (e,f) Immunoblot analysis using cleaved poly-ADP ribose polymerase (PARP) antibody on whole cell lysates prepared from LN229 and GBM8F cells treated with purified S-TRAIL (100 ng/ml), oHSV (MOI = 1) or both oHSV and S-TRAIL in the presence or absence of pan-caspase inhibitor, Z-VAD-FMK (20 µmol/l). Cleaved PARP expression was normalized to α-tubulin expression. *P <0.05 in the comparison of oHSV plus tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to TRAIL. Error bars indicate SD.
oHSV-TRAIL targets both cell proliferation and death pathways in resistant GBM cells
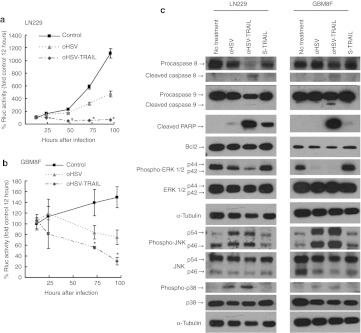
In order to target both TRAIL resistant and oHSV resistant cell lines, we engineered an oHSV-bearing S-TRAIL (oHSV-TRAIL) using a BAC containing the backbone structure of G47Δ, G47Δ-BAC25,26,27 (Supplementary Figure S1). The replicating ability of oHSV-TRAIL was similar to that of oHSV as revealed by the virus yield quantified using plaque assay on Vero cells (Supplementary Figure S3a). The secretion of TRAIL in oHSV-TRAIL infected LN229 GBM cells was confirmed by ELISA (Supplementary Figure S3b). To evaluate the effect of oHSV-TRAIL, LN229-RmC cells and GBM8F-RmC cells were infected with oHSV or oHSV-TRAIL at multiplicity of infection (MOI) = 1 for 24, 48, 72, and 96 hours. The changes in cell viability (Rluc activity) revealed that oHSV-TRAIL infection resulted in significantly more potent cell killing in both LN229 and GBM8F cells as compared to oHSV infection (Figure 3a and b and Supplementary Figure S3c, d). Annexin V staining analysis revealed that the number of apoptotic cells (Annexin V positive, propidium iodide negative) was considerably increased in both LN229 and GBM8F cell populations post-oHSV-TRAIL infection (Supplementary Figure S3e). oHSV-TRAIL also resulted in significantly greater killing in oHSV and TRAIL sensitive human Gli36 GBM line as compared to oHSV infection (Supplementary Figure S4). Western blot analysis of oHSV-TRAIL-infected LN229 and GBM8F cell lysates showed cleavage of caspases-8, -9 and PARP and no significant difference was observed in Bcl2 expression (Figure 3c) and death receptor (DR)4/5 expression (Supplementary Figure S5). These results reveal that oHSV-TRAIL infection induces caspase-mediated apoptosis in both TRAIL resistant LN229 and GBM8F glioma lines.
Figure 3.
Oncolytic herpes simplex virus (oHSV)-tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediates potent cytotoxicity in resistant glioblastoma multiforme (GBM) cells by altering both cell proliferation and death pathways in vitro. (a,b) Cell viability of GBM cells assessed by Renilla luciferase (Rluc) bioluminescence at different time points. (a) LN229-RmC and (b) GBM8F-RmC were infected with oHSV or oHSV-TRAIL at multiplicity of infection (MOI) = 1. *P <0.05 in the comparison of oHSV-TRAIL to control and to oHSV. Error bars indicate SD. (c) Immunoblot analysis using antibodies against caspase-8, -9, cleaved poly-ADP ribose polymerase (PARP), Bcl2, phospho-ERK, ERK, phospho-c-Jun N-terminal kinase (JNK), JNK, phospho-p38, and p38 on whole cell lysates prepared from LN229 and GBM8F cells untreated, or treated with oHSV, oHSV-TRAIL (MOI = 1) or S-TRAIL for 18 hours. α-Tubulin was used as a loading control.
We next examined the mechanism of oHSV-TRAIL induced apoptosis in resistant GBM cells. As such, we assessed the activation of molecules involved in the cell proliferation pathways, mitogen-activated protein kinases (MAPKs) including extracellular signal-regulated protein kinase 1 and 2 (ERK 1/2), c-Jun N-terminal kinase (JNK) and p38 in both LN229 and GBM8F. A significantly impaired activation of ERK 1/2 was observed in LN229 cells infected with oHSV, which was further impaired by oHSV-TRAIL (Figure 3c). There was a complete shutdown of ERK 1/2 phosphorylation in both oHSV and oHSV-TRAIL infected GBM8F cells. JNK and p38 were activated in LN229 and GBM8F cells after oHSV and oHSV-TRAIL infection.
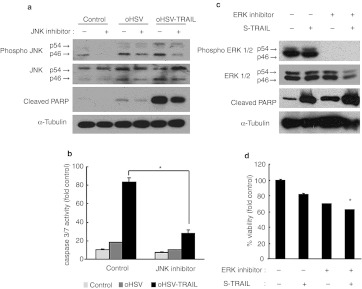
We next sought to determine the significance of the JNK/ERK MAP kinase signaling alterations in oHSV-TRAIL-mediated cytotoxicity of both TRAIL and oHSV resistant GBM cells. To examine the role of JNK upregulation, we treated resistant GBM cells with JNK inhibitor, SP600125. LN229 were infected with oHSV or oHSV-TRAIL in the absence and presence of SP600125. Western blotting analysis showed SP600125 treatment resulted in the inhibition of JNK phosphorylation in oHSV and oHSV-TRAIL-infected cells and also a significant reduction in the cleavage of PARP in oHSV-TRAIL-infected cells (Figure 4a). Caspase-3/7 activity assays showed a significant decrease in the caspase-3/7 activation in oHSV-TRAIL-infected cells in the presence of SP600125 as compared to the infected cells in the absence of SP600125 (Figure 4b). We also investigated whether inhibition of ERK1/2 with MEK-ERK inhibitor, U0126, could mimic oHSV-mediated inhibition of ERK1/2 and sensitize TRAIL resistant GBM cells to TRAIL-induced apoptosis. Western blotting analysis showed that U0126 treatment of LN229 cells inhibited ERK phosphorylation and subsequent addition of purified S-TRAIL markedly increased PARP cleavage (Figure 4c). Combined treatment with U0126 and S-TRAIL decreased cell viability in resistant LN229 GBM cells compared with single treatment (Figure 4d). These results thus suggest that oHSV-mediated downregulation of the ERK-MAPK and upregulation of JNK signaling may contribute to apoptotic cell death in oHSV-TRAIL-infected resistant GBM cells.
Figure 4.
Oncolytic herpes simplex virus (oHSV)-tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in resistant glioblastoma multiformes (GBMs) depends on c-Jun N-terminal kinase (JNK) activation and ERK inhibition. (a) Immunoblot analysis using antibodies against JNK, phosho-JNK, and cleaved poly-ADP ribose polymerase (PARP), on whole cell lysate prepared from LN229 cells treated with oHSV, oHSV-TRAIL (multiplicity of infection (MOI) = 1) or control in the absence (–) and presence (+) of JNK inhibitor (SP600125, 20 µmol/l) for 18 hours. (b) Caspase 3/7 activity of LN229 cells treated with oHSV, oHSV-TRAIL (MOI = 1), or control for 20 hours in the absence (–) and presence (+) of JNK inhibitor. *P < 0.05 in the comparison of oHSV-TRAIL treated cells in the absence and presence of JNK. (c) Immunoblot analysis using antibodies against ERK, phosho ERK, and cleaved PARP on whole cell lysate prepared from LN229 cells treated with S-TRAIL (100 ng/ml) in the absence (–) and presence (+) of ERK inhibitor (U0126, 20 µmol/l) for 18 hours. α-Tubulin was used as a loading control. (d) Cell viability assay showing the % viable LN229 cells after treatment with different combinations of U0126 (20 µmol/l) and TRAIL (100 ng/ml) for 48 hours. *P <0.05 JNK inhibitor group in the comparison with control (in b) and ERK inhibitor and S-TRAIL treatment group in the comparison with other treatment groups (in d). Error bars indicate SD.
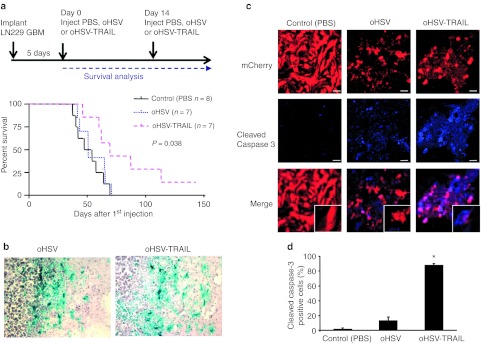
oHSV-TRAIL inhibits GBM growth and invasion in vivo and prolongs survival of mice bearing both TRAIL and oHSV resistant GBM
We assessed the therapeutic efficacy of oHSV-TRAIL in intracranial GBMs, using LN229 and GBM8F glioma lines which were engineered to express Fluc-mCherry (LN229-FmC, GBM8F-FmC). Mice bearing established intracranial LN229-FmC GBMs were administered intratumorally with oHSV, oHSV-TRAIL, or phosphate-buffered saline (PBS) and followed for changes in tumor volumes by Fluc imaging. As expected, oHSV injection had no impact on both tumor burden (Supplementary Figure S6a) and survival (Figure 5a, PBS: 50.5 days, oHSV: 49 days) in this resistant GBM model. In contrast, oHSV-TRAIL injection resulted in significant decrease in tumor volumes compared to both control (PBS) and oHSV treated mice (Supplementary Figure S6a) and prolonged survival of mice-bearing intracranial GBMs (Figure 5a). The median survival of oHSV-TRAIL-treated mice was 69 days, which was significantly longer than PBS- and oHSV-treated mice (Figure 5a; P = 0.038 oHSV and oHSV-TRAIL comparison, log-rank test). X-gal staining of the brain sections collected 48 hours post oHSV or oHSV-TRAIL injection showed an extensive distribution of reporter β-galactosidase positivity, which overlapped with the tumor area, suggesting that the spread of virus infection was comparable between oHSV and oHSV-TRAIL (Figure 5b). Immunohistochemical analysis and confocal microscopy showed significantly increased cleaved caspase-3 staining in sections from oHSV-TRAIL-treated tumors as compared to oHSV and PBS treated tumors. Furthermore, no cleaved caspase-3 staining was seen in normal brain cells in all treated groups revealing the tumor specificity of oHSV-TRAIL treatment (Figure 5c, d).
Figure 5.
Oncolytic herpes simplex virus (oHSV)-tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) prolongs survival of mice-bearing both TRAIL and oHSV resistant glioblastoma multiforme (GBM). (a) Timeline and survival curves of LN229-FmC GBM-bearing mice treated with oHSV, oHSV-TRAIL, or control (phosphate-buffered saline (PBS)). P = 0.038 in oHSV and oHSV-TRAIL comparison, log-rank test. (b) X-gal staining revealing virus-infected areas. oHSV-injected (left) and oHSV-TRAIL-injected (right) tumor sections. Original magnification ×20. (c) Immunofluorescence of cleaved caspase-3 staining on brain sections from LN229-FmC GBM-bearing mice injected with oHSV, oHSV-TRAIL, or PBS (control). Original magnification ×20. (d) Plot showing the percentage of cleaved caspase-3 positive LN229-FmC GBM cells on brain sections. *P < 0.05 in the comparison of oHSV-TRAIL to control and to oHSV. n = 3 in each group. Error bars indicate SD.
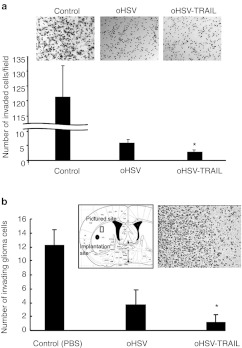
To examine the effect on GBM cell invasion, we used matrigel-coated assays in vitro and intracranial mouse GBM model using invasive GBM8F as opposed to LN229 GBM line which is a noninvasive line (Supplementary Figure S7). oHSV and oHSV-TRAIL treatment reduced the number of GBM8F cells that had invaded in matrigel-coated invasion assay, with oHSV-TRAIL having a stronger inhibitory effect than oHSV (Figure 6a). In vivo, both oHSV and oHSV-TRAIL injection into intracranial GBM8F tumors reduced the number of invading GBM8F cells as compared to the control, with oHSV-TRAIL having a stronger inhibitory effect than oHSV (Figure 6b). These results demonstrate that oHSV-TRAIL induces apoptosis in TRAIL and oHSV resistant intracranial GBM, inhibits GBM growth and invasiveness and significantly increases survival in mice.
Figure 6.
Oncolytic herpes simplex virus (oHSV)-tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) inhibits glioblastoma multiforme (GBM) invasion in vitro and in vivo in TRAIL resistant GBM. (a) In vitro invasion assay. Photomicrographs and graph showing the change in cell invasion after treatment with oHSV or oHSV-TRAIL in GBM8F glioma line. Multiplicity of infection (MOI) = 1. *P <0.05 in the comparison of oHSV-TRAIL to control and to oHSV. (b) In vivo invasion assay. Mice-bearing intracranial GBM8-FmC gliomas were injected with oHSV, oHSV-TRAIL, or phosphate-buffered saline (PBS) (control) and mice were sacrificed on day 14 and invasion of the GBM cells on brain sections was evaluated. Photomicrograph of hematoxylin and eosin (H&E) staining of GBM8F tumor cell invasion towards adjacent normal brain tissue and illustration of brain revealing GBM8F implantation-site and pictured-site. n = 3 per group. *P < 0.05 in the comparison of oHSV-TRAIL to control and to oHSV. Error bars indicate SD.
Discussion
In this study, we identified GBM lines that are either resistant to TRAIL-mediated apoptosis or resistant to both oHSV-mediated oncolysis and TRAIL, and created a novel oHSV-bearing secretable-TRAIL (oHSV-TRAIL) to study the mechanism-based targeted therapy in resistant GBM lines in vitro and in vivo. We show that oHSV-TRAIL induces apoptosis in TRAIL and oHSV resistant GBMs by targeting both cell proliferation and death pathways. Furthermore, oHSV-TRAIL inhibits GBM growth and invasion and prolongs survival of mice-bearing intracranial brain tumors.
GBMs are molecularly heterogeneous tumors usually containing a subset of tumor cells that are resistant to a number of currently used anti-GBM therapies.1 These cells rapidly take over and ultimately result in tumor progression. oHSV as a single agent has been tested in preclinical studies and shown to result in oncolysis in most GBM cells including GBM stem cells.13,28,29 However, while phase I clinical trials using intracranial oHSV inoculation demonstrated safety in GBM patients, they showed only partial radiologic responses.9,30,31 These results suggest that GBMs might be heterogeneous in the responses to oHSV and contain a subset of cells resistant to oHSV-mediated oncolysis. In this study, we screened a panel of eight GBM cell lines that covers established GBM cell lines and patient derived primary GSCs. We found that GBM cells have differential sensitivities to oHSV-mediated oncolysis as well as TRAIL-mediated apoptosis. To our knowledge, this is the first study which identifies GBM lines resistant to oHSV. Interestingly, our screening indicated that the yields of oHSV do not always parallel the efficiency of oHSV-mediated cell killing, implicating a presence of cell death mode other than oncolysis that affects oHSV efficacy.
A number of previous studies by others and our laboratory have shown that dual bioluminescence imaging of tumor cells and therapeutics enables rapid and noninvasive measurement of both tumor load and fate of therapeutics in vitro and in mice-bearing intracranial tumors.10,12,32,33,34 Utilizing oHSV-Fluc, GBM cells expressing Rluc and dual bioluminescence imaging, our studies reveal that two distinct biological events, oHSV replication and its effects on cell viability can be monitored. Our results indicate that oHSV can replicate in a GBM line which is resistant to oHSV, even though the viral replication and spread is greatly decreased and slow when compared to the GBM lines which are sensitive to oHSV. The ability of oHSV to replicate in GBM lines resistant to oHSV provides a great rationale of using oHSV to deliver cytotoxic agents like TRAIL and studying both the molecular mechanism and therapeutic effect of oHSV and TRAIL treatments in GBMs that are resistant to oHSV-mediated oncolysis and TRAIL-mediated apoptosis.
Various strategies have been employed to overcome resistance of tumor cells to different drug regimens. The ability of TRAIL to selectively target tumor cells while remaining harmless to most normal cells makes it an attractive candidate for an apoptotic therapy for highly malignant brain tumors. However, a large percentage of primary GBM lines are resistant to TRAIL-induced apoptosis.35 Our results reveal that oHSV infection and subsequent S-TRAIL treatment results in a caspase-3/7-mediated cell killing in a line that is resistant to both oHSV and TRAIL. Although TRAIL is a potent tumor-specific agent, its short biological half-life and limited delivery across the blood–brain barrier limit its applicability in treating brain tumors when delivered systemically. oHSV can replicate in situ, spread and exhibit oncolytic activity via a direct cytocidal effect, and at the same time can deliver substantial quantities of therapeutic molecules in situ. As compared to the other oncolytic viruses, the capacity to incorporate large transgenes into its genome is one of the advantages of HSV-1.36 The intratumoral delivery of oHSV-TRAIL can overcome the limitations posed by systemic administration of TRAIL as it remedies the short half-life of drugs, the use of multiple drug regimens to target resistant GBMs, in addition to circumventing the restrictions posed by the blood–brain barrier to deliver drugs to brain.
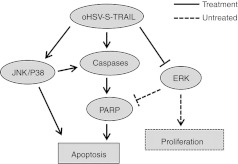
HSV like other viruses have genes such as Us3 and Rs1 (ICP4) whose products have an anti-apoptotic function.37,38 These genes appear to facilitate HSV replication by preventing premature cell death. Therefore, it is possible that combining proapoptotic molecule with oHSV dampens viral replication and reduces oHSV potency. Our data, however, showed that replication of oHSV and oHSV-TRAIL in glioma cells is comparable (Supplementary Figure S3a), indicating that expression of TRAIL does not suppress viral replication. We show that oHSV-TRAIL downregulates ERK phosphorylation, upregulates p38 and JNK phosphorylation and induces cleavage of caspases in two GBM cell lines either TRAIL or TRAIL and oHSV resistant. Downregulation of ERK phosphorylation and upregulation of P38 and JNK phosphorylation have been previously reported following infection with HSV-1.39,40 ERK1/2, members of the MAPK super family, can mediate cell proliferation and apoptosis41 while activation of ERK is primarily associated with promoting cellular proliferation. Activation of cell proliferation signals that block apoptosis is associated with tumorigenesis and resistance to chemotherapeutic drugs.42 Activation of ERK is reported to prevent Fas-induced apoptosis in activated T cells43 and inhibit activation of caspases despite the release of cytochrome c from mitochondria.44,45,46 In contrast to ERK, the JNK and p38-MAPK signaling is activated by proinflammatory cytokines and a variety of cellular stresses, and is typically linked to differentiation and apoptosis. Xia et al. reported that activation of JNK and p38 and concurrent inhibition of ERK are critical for induction of apoptosis.47 We show that: (i) the inhibition of JNK activation suppresses oHSV-TRAIL-mediated activation of caspase pathway, and (ii) a small-molecule inhibitor of MEK/ERK sensitizes resistant cells to TRAIL-mediated apoptosis. Therefore, our results suggest that oHSV-mediated downregulation of the ERK signaling and upregulation of the JNK and p38 signaling play a vital role in priming resistant cells so that S-TRAIL expressed in oHSV-TRAIL infected GBM cells promotes activation of caspase-3, -8, and -9, leading to apoptotic cell death (Figure 7). Our studies thus underscore targeting both cell proliferation and death pathways as a crucial mechanism underlying oHSV-TRAIL-mediated robust induction of apoptosis in resistant GBMs.
Figure 7.
Schematic presentation showing the mechanism underlying the efficacy of oncolytic herpes simplex virus (oHSV)-tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on resistant glioblastoma multiforme (GBM) cells.
Our in vivo studies reveal that oHSV-TRAIL results in marked attenuation of intracranial tumor growth and survival prolongation in mice-bearing TRAIL and oHSV resistant GBM. This makes a clear contrast to the lack of long-lasting antitumor efficacy mediated by an oncolytic adenovirus-expressing TRAIL.48 Although established GBM lines are the most commonly used models in vitro and in vivo, they fail to recapitulate the clinical properties of tumors. Given this limitation of GBM lines as a representative GBM model, recent studies have focused on primary GBM lines and indicated a role for tumor-initiating cells in these lines. In an effort to test the effect of oHSV-TRAIL in such models, we used GBM8F primary GBM line that contains a subpopulation of CD133+ cells13 and exhibits highly invasive behavior in vivo. Our in vitro studies showed that despite resistance to TRAIL, oHSV-TRAIL is significantly more effective in killing and inhibiting invasiveness of GBM8F cells than oHSV. Furthermore, the number of invading cells in vivo was strongly reduced by a single inoculation of oHSV-TRAIL into GBM8F-generated tumors. This suggests that the oHSV spread in migrating tumor cells combined with in situ release of S-TRAIL can cooperate to block invasiveness of these cells. Future studies will need to address whether this is solely due to oHSV-TRAIL-mediated increased cell death or its additional but unknown functions that inhibit cellular migratory or invasive machineries.
In conclusion, our findings shed a new light on targeting oHSV and TRAIL resistant GBMs and pave the way for how oHSV and TRAIL can function in concert to target both cell proliferation and death pathways in heterogeneous GBM cells. A recent promising report on oncolytic virus targeting of metastatic cancer cells of multiple cancer types in humans highlighted the feasibility of achieving high concentrations of anticancer molecules in situ in the context of oncolytic virus therapy.49 Therefore, this study may provide the key to ultimately develop novel oHSV-based therapies for patients with different tumors presenting different molecular profiles.
Materials and Methods
Parental and engineered cell lines. Established human GBM lines (Gli36, U87, U251, and LN229) and GBM8F were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and penicillin/streptomycin. GBM stem cells (GBM4, GBM6, and BT74) were cultured in Neurobasal medium (Invitrogen, Carlsbad, CA) supplemented with 3 mmol/l L-glutamine (Mediatech, Manassas, VA), B27 (Invitrogen, Carlsbad, CA), 2 µg/ml heparin (Sigma-Aldrich, St Louis, MN), 20 ng/ml human EGF (R&D Systems, Minneapolis, MN), and 20 ng/ml human FGF-2 (Peprotech, Rocky Hills, NJ) as described previously.13 Two lentiviral vectors were used: (i) Pico2-Fluc-mCherry, a kind gift from Dr Andrew Kung (Dana Farber Cancer Institute; Boston, MA), (ii) Pico2-Rluc-mCherry, which is created by ligating Rluc fragment (the cDNA sequences encoding Rluc were amplified by PCR) into BamH1/BstB1-digested Pico2-Fluc-mcherry. Lentiviral packaging was performed by transfection of 293T cells as previously described.33 LN229 and GBM8F were transduced with LV-Pico2-Fluc-mCherry and LV-Pico2-Rluc-mCherry at a MOI of 2 in medium-containing protamine sulfate (2 µg/ml) and LN229-Fluc-mCherry (LN229-FmC); GBM8-Fluc-mCherry (GBM8-FmC) LN229-Rluc-mCherry (LN229-RmC); GBM8-Rluc-mCherry (GBM8-FmC) lines were obtained after puromycin (1 ug/ml) selection in culture.
Recombinant oHSVs and viral growth assay. G47ΔBAC contains the genome of G47Δ (γ34.5–, ICP6–, ICP47–) and a cytomegalovirus promoter driven enhanced green fluorescent protein (EGFP) in place of lacZ in G47Δ.17 Recombinant oHSV vectors, G47Δ-empty (oHSV; referred to oHSV in this study), G47Δ-TRAIL (oHSV-TRAIL), and G47Δ-Fluc (oHSV-Fluc), were generated using the methods described previously.25,26,27,50 Briefly, the respective shuttle plasmids were integrated into G47ΔBAC using Cre-mediated recombination in DH10B Escherichia coli, and proper recombination confirmed by restriction analysis of BAC clones. Next, the resulting BAC and an Flpe-expressing plasmid were cotransfected to Vero cells, to remove the BAC-derived sequences and the EGFP gene, and allow virus to be produced. Each recombinant virus was plaque purified and expanded. All the recombinant oHSVs, oHSV, oHSV-TRAIL, and oHSV-Fluc, express E. coli lacZ driven by endogenous ICP6 promoter (Supplementary Figure S1a). oHSV bears no additional transgene sequences, and oHSV-TRAIL carries S-TRAIL driven by herpes simplex virus immediate early 4/5 promoter. Cytomegalovirus immediate early promoter was used to express Fluc-by oHSV-Fluc. For the viral growth assay, cells plated on 96-well plates were infected with oHSV at MOI = 1. After virus adsorption, media was replaced and culture continued. Forty eight hours after infection, culture supernatant was harvested. Titers of infectious virus were determined by plaque assay on Vero cells (American Type Culture Collection, Manassas, VA). The concentrations of TRAIL in conditioned media of GBM cells infected with oHSV-TRAIL at various MOIs were determined by ELISA using a TRAIL Immunoassay Kit (Biosource International, Camarillo, CA) using recombinant hTRAIL expressed in E. coli as a standard.
In vitro bioluminescence assays. To determine the effects of S-TRAIL, oHSV, and oHSV-TRAIL on GBM viability and caspase activation, GBM cells were seeded on 96-well plates (0.5 × 104/well) and treated with different doses S-TRAIL (0–1,000 ng/ml) or different MOIs of oHSV or oHSV-TRAIL 24 hours after plating. Cell viability was measured by determining the aggregate cell metabolic activity using an ATP-dependent luminescent reagent (CellTiter-Glo; Promega, Madison, WI) and caspase activity was determined using a DEVD-aminoluciferin (Caspase-Glo-3/7, Promega) according to manufacturer's instructions. For dual-luciferase imaging of GBM cell viability and oHSV-Fluc distribution in the cells, Fluc and Rluc activity were measured in LN229-RmC and GBM8F-RmC cells by Dual-Glo luciferase assay system (Promega) according to manufacturer's instructions. All experiments were performed in triplicates.
Detection of apoptosis by flow cytometry using annexin V staining. After a 24 hours treatment with oHSV, oHSV-TRAIL, or PBS (control), cells were stained with FITC-conjugated annexin V (Invitrogen) and propidium iodide (2 µg/ml) in accordance with the manufacturer's instructions. Cells were subjected to FACS analysis with a FACSCaliber (Becton Dickinson, Franklin Lakes, NJ). Data acquisition and analysis were performed by CellQuest program (Becton Dickinson).
Western blot analysis. Following treatment, GBM cells were lysed with NP40 buffer supplemented with protease (Roche, Indianapolis, IN) and phosphatase inhibitors (Sigma-Aldrich). Twenty micrograms of harvested proteins from each lysate were resolved on 10% SDS-PAGE, and immunoblotted with antibodies against caspase-8 (Cell Signaling), cleaved PARP (Cell Signaling, Danvers, MA), p-44/42MAPK (ERK 1/2) (Cell Signaling), phospho-p44/42MAPK (ERK 1/2) (Thr202,Thr204) (Cell Signaling), SAPK/JNK (Cell Signaling), phospho-SAPK/JNK (Thr183/Thr185) (Cell Signaling), p38-MAPK (Cell Signaling), phospho-p38 MAPK (Cell Signaling), caspase-9 (Stressgen, Pharmingdale, NY), Bcl2 (Abcam, Cambridge, MA) or α-tubulin (Sigma-Aldrich); and blots were developed by chemiluminescence after incubation with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz, Santa Cruz, CA). Blots were then exposed to film (3 seconds to 5 minutes) and quantification of western blot signals was performed using Image J. The data was normalized to α-tubulin expression. For inhibition studies, pan-caspase inhibitor, Z-VAD-FMK (Promega) JNK inhibitor SP600125 (Sigma-Aldrich), and the MEK inhibitor, U0126, (Promega Corporation) were used.
Intracranial GBM cell implantation and in vivo bioluminescence imaging. To follow viral distribution in intracranial GBMs, LN229-RmC, and GBM8F-RmC GBM (5 × 105 cells per mouse; n = 3 each GBM line) were stereotactically implanted into the brains (right striatum, 2.5-mm lateral from bregma and 2.5-mm deep) of SCID mice (6 weeks of age; Charles River Laboratories, Wilmington, MA). Five days later, mice-bearing intracranial GBMs were injected with oHSV-Fluc (6 µl of 2.0 × 108 plaque-forming unit/ml) intratumorally at the same coordinate as the tumor implantation, and viral distribution was followed by Fluc bioluminescence imaging over time as described previously.33 To follow changes in tumor volume and mice survival after treatment, LN229-FmC GBM cells (5 × 105 per mouse; n = 31) were stereotactically implanted into the brains (right striatum, 2.5-mm lateral from bregma and 2.5-mm deep) of SCID mice (6 weeks of age). Mice were imaged for the presence of tumors by Fluc bioluminescence imaging and mice-bearing tumors were injected with 6 µl of 2.0 × 108 plaque-forming unit/ml of oHSV (n = 10), oHSV-TRAIL (n = 10), or PBS (n = 11) intratumorally. Two days post-treatment, mice (n = 3 in each group) were sacrificed for immunohistochemical analysis as described below. Fourteen days post-treatment, mice were again injected intratumorally with oHSV, oHSV-TRAIL, or PBS as described above. Mice were imaged for Fluc bioluminescence imaging and followed for survival and sacrificed when neurological symptoms became apparent. All in vivo procedures were approved by the Subcommittee on Research Animal Care at MGH.
Tissue processing and immunohistochemistry. Mice were perfused by pumping ice-cold 4% paraformaldehyde directly into the heart and the brains were fixed in 4% paraformaldehyde and frozen sections were obtained for hematoxylin and eosin staining and immunohistochemistry. 5-Bromo-4-choloro-3-indolyl-β-D-galactopyranoside (X-gal) staining was performed to identify lacZ-expressing infected cells. For cleaved-caspase-3 staining, sections were incubated for 1 hour in a blocking solution (0.3% bovine serum albumin, 8% goat serum, and 0.3% Triton-X100) at room temperature, followed by incubation at 4 °C overnight with anti-cleaved-caspase-3 (Cell Signaling) diluted in blocking solution. Sections were incubated in Alexa Fluor 649 goat anti-rabbit secondary antibody (Invitrogen), and visualized using confocal microscope (LSM Pascal; Zeiss, Oberkochen, Germany). The percentage of cleaved caspase-3 positive cells was calculated by counting the positive cells in randomly selected field of views under a microscope.
In vitro invasion assay. The invasive capacity of GBM8F cells was tested using two chamber in vitro invasion assays (BD BioCoat Matrigel Invasion Chambers). GBM8F cells were seeded in the matrigel-coated upper chamber, infected with oHSV and oHSV-TRAIL at MOI = 1 and 24 hours later the noninvading cells were removed from the upper surface of the invasion membrane and the cells on the lower surface were stained with Diff-Quick staining kit (IMEB Inc, San Marco, CA). The average number of cells/field was determined by counting the cells in 8 random fields/well in ×10 images of each well captured.
In vivo invasion study. GBM8F-FmC GBM cells (3 × 105 per mouse; n = 9) were stereotactically implanted into the brain (right striatum, 2.5-mm lateral from bregma and 2.5-mm deep) of SCID mice (6 weeks of age; Charles River Laboratories). Tumor-bearing mice were intratumorally injected with oHSV, oHSV-TRAIL, or PBS (n = 3, each group) and 14 days post injection, mice were perfused and brains were removed and sectioned for hematoxylin and eosin staining and mCherry visualization. Brain sections on slides were visualized for mCherry expression and the number of GBM8F tumor invading toward adjacent normal brain tissue was counted and compared in different mice groups.
Statistical analysis. Data were analyzed by Student t-test when comparing two groups. Data were expressed as mean ± SD and differences were considered significant at P < 0.05. Kaplan–Meier analysis was used for mouse survival studies, and the groups were compared using the log-rank test.
SUPPLEMENTARY MATERIAL Figure S1. Schema of oHSV-bearing Fluc or S-TRAIL transgene. Figure S2. Pharmacodynamics of oHSV in vitro at different MOIs. Figure S3. oHSV-TRAIL virus yield is similar to oHSV yield and mediates potent cytotoxicity in resistant GBM cells. Figure S4. oHSV-TRAIL mediates potent cytotoxicity in sensitive GBM cells. Figure S5. oHSV-TRAIL-mediated potent cytotoxicity is independent of DR4/DR5 expression. Figure S6. oHSV-TRAIL inhibits growth of both TRAIL and oHSV resistant LN229-Fluc-mCherry GBMs in vivo. Figure S7. H&E stained mouse brain sections harboring intracerebral tumors.
Acknowledgments
We thank Dr Howard Chen for his help with Annexin-V staining and FACS analysis; Dr Andrew Kung for providing pico2-Fluc-mCherry lentiviral vector. This work was supported in part by Alliance for Cancer Gene Therapy (K.S.) National Institutes of Health (NS03677; R.L.M. and CA138922-01; NS076873; K.S.) American Cancer Society (K.S.) and James McDonnell Foundation (K.S.). The authors declared no conflict of interest.
Supplementary Material
Schema of oHSV-bearing Fluc or S-TRAIL transgene.
Pharmacodynamics of oHSV in vitro at different MOIs.
oHSV-TRAIL virus yield is similar to oHSV yield and mediates potent cytotoxicity in resistant GBM cells.
oHSV-TRAIL mediates potent cytotoxicity in sensitive GBM cells.
oHSV-TRAIL-mediated potent cytotoxicity is independent of DR4/DR5 expression.
oHSV-TRAIL inhibits growth of both TRAIL and oHSV resistant LN229-Fluc-mCherry GBMs in vivo.
H&E stained mouse brain sections harboring intracerebral tumors.
REFERENCES
- Wen PY., and, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- Aghi M., and, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24:7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- Corsten MF., and, Shah K. Therapeutic stem-cells for cancer treatment: hopes and hurdles in tactical warfare. Lancet Oncol. 2008;9:376–384. doi: 10.1016/S1470-2045(08)70099-8. [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Frew AJ., and, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- Kock N, Kasmieh R, Weissleder R., and, Shah K. Tumor therapy mediated by lentiviral expression of shBcl-2 and S-TRAIL. Neoplasia. 2007;9:435–442. doi: 10.1593/neo.07223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TC, Galanis E., and, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD.et al. (2000Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial Gene Ther 7867–874. [DOI] [PubMed] [Google Scholar]
- Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G.et al. (2009Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy Proc Natl Acad Sci USA 1064822–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Tung CH, Breakefield XO., and, Weissleder R. In vivo imaging of S-TRAIL-mediated tumor regression and apoptosis. Mol Ther. 2005;11:926–931. doi: 10.1016/j.ymthe.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Shah K, Tung CH, Yang K, Weissleder R., and, Breakefield XO. Inducible release of TRAIL fusion proteins from a proapoptotic form for tumor therapy. Cancer Res. 2004;64:3236–3242. doi: 10.1158/0008-5472.can-03-3516. [DOI] [PubMed] [Google Scholar]
- Wakimoto H, Kesari S, Farrell CJ, Curry WT, Jr, Zaupa C, Aghi M.et al. (2009Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors Cancer Res 693472–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parato KA, Senger D, Forsyth PA., and, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- Varghese S., and, Rabkin SD. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002;9:967–978. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- Mineta T, Rabkin SD, Yazaki T, Hunter WD., and, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- Todo T, Martuza RL, Rabkin SD., and, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M.et al. (2009Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM Mol Ther 17199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann D., and, Wildner O. Comparison of herpes simplex virus- and conditionally replicative adenovirus-based vectors for glioblastoma treatment. Cancer Gene Ther. 2007;14:627–639. doi: 10.1038/sj.cgt.7701055. [DOI] [PubMed] [Google Scholar]
- LeBlanc, HN., and, Ashkenazi, A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Diff. 2003;10:66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- Wiezorek J, Holland P., and, Graves J. Death receptor agonists as a targeted therapy for cancer. Clin Cancer Res. 2010;16:1701–1708. doi: 10.1158/1078-0432.CCR-09-1692. [DOI] [PubMed] [Google Scholar]
- Rozanov DV, Savinov AY, Golubkov VS, Rozanova OL, Postnova TI, Sergienko EA.et al. (2009Engineering a leucine zipper-TRAIL homotrimer with improved cytotoxicity in tumor cells Mol Cancer Ther 81515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S., and, Shah K. Stem-cell based therapies for brain tumors. Curr Opin Mol Ther. 2008;10:334–342. [PubMed] [Google Scholar]
- Bagci-Onder T, Wakimoto H, Anderegg M, Cameron C., and, Shah K. A dual PI3K/mTOR inhibitor, PI-103, cooperates with stem cell-delivered TRAIL in experimental glioma models. Cancer Res. 2011;71:154–163. doi: 10.1158/0008-5472.CAN-10-1601. [DOI] [PubMed] [Google Scholar]
- Fukuhara H, Ino Y, Kuroda T, Martuza RL., and, Todo T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system. Cancer Res. 2005;65:10663–10668. doi: 10.1158/0008-5472.CAN-05-2534. [DOI] [PubMed] [Google Scholar]
- Kuroda T, Martuza RL, Todo T., and, Rabkin SD. Flip-Flop HSV-BAC: bacterial artificial chromosome based system for rapid generation of recombinant herpes simplex virus vectors using two independent site-specific recombinases. BMC Biotechnol. 2006;6:40. doi: 10.1186/1472-6750-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Deckter LA, Kasai K, Chiocca EA., and, Saeki Y. Imaging immediate-early and strict-late promoter activity during oncolytic herpes simplex virus type 1 infection and replication in tumors. Gene Ther. 2006;13:1731–1736. doi: 10.1038/sj.gt.3302831. [DOI] [PubMed] [Google Scholar]
- Han ZQ, Assenberg M, Liu BL, Wang YB, Simpson G, Thomas S.et al. (2007Development of a second-generation oncolytic Herpes simplex virus expressing TNFalpha for cancer therapy J Gene Med 999–106. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Hardcastle J, Thakur R, Yang M, Christoforidis G, Fulci G.et al. (2007Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy J Natl Cancer Inst 991768–1781. [DOI] [PubMed] [Google Scholar]
- Kirn D, Martuza RL., and, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat Med. 2001;7:781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- Markert E, Siebolts U, Odenthal M, Kreuzer KA., and, Wickenhauser C. High diagnostic value of morphologic examination and molecular analysis of bone marrow biopsies in a case of BCR-ABL+ CML with clusters of blasts. Int J Hematol. 2009;89:294–297. doi: 10.1007/s12185-009-0257-x. [DOI] [PubMed] [Google Scholar]
- Corsten MF, Miranda R, Kasmieh R, Krichevsky AM, Weissleder R., and, Shah K. MicroRNA-21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cell delivered S-TRAIL in human gliomas. Cancer Res. 2007;67:8994–9000. doi: 10.1158/0008-5472.CAN-07-1045. [DOI] [PubMed] [Google Scholar]
- Shah K, Hingtgen S, Kasmieh R, Figueiredo JL, Garcia-Garcia E, Martinez-Serrano A.et al. (2008Bimodal viral vectors and in vivo imaging reveal the fate of human neural stem cells in experimental glioma model J Neurosci 284406–4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Tang Y, Breakefield X., and, Weissleder R. Real-time imaging of TRAIL-induced apoptosis of glioma tumors in vivo. Oncogene. 2003;22:6865–6872. doi: 10.1038/sj.onc.1206748. [DOI] [PubMed] [Google Scholar]
- Rieger J, Frank B, Weller M., and, Wick W. Mechanisms of resistance of human glioma cells to Apo2 ligand/TNF-related apoptosis-inducing ligand. Cell Physiol Biochem. 2007;20:23–34. doi: 10.1159/000104150. [DOI] [PubMed] [Google Scholar]
- Todo T. “Armed ” oncolytic herpes simplex viruses for brain tumor therapy. Cell Adh Migr. 2008;2:208–213. doi: 10.4161/cam.2.3.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopardi R, Van Sant C., and, Roizman B. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc Natl Acad Sci USA. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopardi R., and, Roizman B. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc Natl Acad Sci USA. 1996;93:9583–9587. doi: 10.1073/pnas.93.18.9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D, Gyure KA, Pereira EF., and, Aurelian L. Herpes simplex virus type 1-induced encephalitis has an apoptotic component associated with activation of c-Jun N-terminal kinase. J Neurovirol. 2003;9:101–111. doi: 10.1080/13550280390173427. [DOI] [PubMed] [Google Scholar]
- Walsh D., and, Mohr I. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 2004;18:660–672. doi: 10.1101/gad.1185304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebratu Y., and, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer. Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan LA, Morrice N, Brady S, Magee G, Pathak S., and, Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol. 2003;5:647–654. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- Holmström TH, Schmitz I, Söderström TS, Poukkula M, Johnson VL, Chow SC.et al. (2000MAPK/ERK signaling in activated T cells inhibits CD95/Fas-mediated apoptosis downstream of DISC assembly EMBO J 195418–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt P, Schremser EJ., and, Cooper GM. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol Cell Biol. 1999;19:5308–5315. doi: 10.1128/mcb.19.8.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MD, Weil M., and, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Tashker JS, Olson M., and, Kornbluth S. Post-cytochrome C protection from apoptosis conferred by a MAPK pathway in Xenopus egg extracts. Mol Biol Cell. 2002;13:393–401. doi: 10.1091/mbc.01-06-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ., and, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Wohlfahrt ME, Beard BC, Lieber A., and, Kiem HP. A capsid-modified, conditionally replicating oncolytic adenovirus vector expressing TRAIL Leads to enhanced cancer cell killing in human glioblastoma models. Cancer Res. 2007;67:8783–8790. doi: 10.1158/0008-5472.CAN-07-0357. [DOI] [PubMed] [Google Scholar]
- Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ.et al. (2011Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans Nature 47799–102. [DOI] [PubMed] [Google Scholar]
- Saeki Y, Ichikawa T, Saeki A, Chiocca EA, Tobler K, Ackermann M.et al. (1998Herpes simplex virus type 1 DNA amplified as bacterial artificial chromosome in Escherichia coli: rescue of replication-competent virus progeny and packaging of amplicon vectors Hum Gene Ther 92787–2794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Schema of oHSV-bearing Fluc or S-TRAIL transgene.
Pharmacodynamics of oHSV in vitro at different MOIs.
oHSV-TRAIL virus yield is similar to oHSV yield and mediates potent cytotoxicity in resistant GBM cells.
oHSV-TRAIL mediates potent cytotoxicity in sensitive GBM cells.
oHSV-TRAIL-mediated potent cytotoxicity is independent of DR4/DR5 expression.
oHSV-TRAIL inhibits growth of both TRAIL and oHSV resistant LN229-Fluc-mCherry GBMs in vivo.
H&E stained mouse brain sections harboring intracerebral tumors.