Abstract
Epidermal growth factor receptor (EGFR) over-signaling leads to more aggressive tumor growth. The antitumor effect of Cetuximab, an anti-EGFR antibody, depends on oncogenic-signal blockade leading to tumor cell apoptosis and antibody dependent cell-mediated cytotoxicity (ADCC). However, whether adaptive immunity plays a role in Cetuximab-mediated tumor inhibition is unclear, as current xenograft models lack adaptive immunity and human-EGFR–dependent mouse tumor cell lines are unavailable. Using a newly developed xenograft model with reconstituted immune cells, we demonstrate that the Cetuximab effect becomes more pronounced and reduces the EGFR+ human tumor burden when adaptive immunity is present. To further study this in a mouse tumor model, we created a novel EGFR+ mouse tumor cell line and demonstrated that Cetuximab-induced tumor regression depends on both innate and adaptive immunity components, including CD8+ T cells, MyD88, and FcγR. To test whether strong innate signals inside tumor tissues amplifies the Cetuximab-mediated therapeutic effect, Cetuximab was conjugated to CpG. This conjugate is more potent than Cetuximab alone for complete tumor regression and resistance to tumor rechallenge. Furthermore, Cetuximab-CpG conjugates can activate tumor-reactive T cells for tumor regression by increasing dendritic cell (DC) cross-presentation. Therefore, this study establishes new models to evaluate immune responses induced by antibody-based treatment, defines molecular mechanisms, and provides new tumor-regression strategies.
Introduction
The epidermal growth factor receptor (EGFR) belongs to the ErbB family consisting of four closely related cell membrane receptors: EGFR (HER1 or ErbB1), ErbB2 (HER2), ErbB3 (HER3), and ErbB4 (HER4).1,2 These receptors are all transmembrane glycoproteins that consist of an extracellular ligand-binding domain, a transmembrane domain, and an intracellular domain with tyrosine kinase activity for signal transduction.3 After ligand binding, receptor dimerization activates intercellular tyrosine kinases via autophosphorylation. The signals produced by this process induce transcription of essential growth and survival factors in both normal and tumor tissues.2,4 EGFR is highly expressed in a variety of tumor types, such as lung, testis, breast, gastric, colorectal, and ovarian tumors. High expression of EGFR usually correlates with disease progression, poor prognosis, poor survival, and poor response to therapy.5
The US Food and Drug Administration has approved multiple drugs targeting EGFR, including monoclonal antibodies (Cetuximab and Panitumumab) and tyrosine kinase inhibitors (Erlotinib and Gefitinib).6,7 Cetuximab is a human-mouse chimeric immunoglobulin G1 (IgG1) antibody against EGFR that is approved for squamous cell carcinoma of the head and neck as well as for colorectal cancer.8 Cetuximab binds specifically to EGFR and competitively inhibits the binding of epidermal growth factor.9 In vitro assays and in vivo animal studies have shown that binding of Cetuximab to EGFR blocks phosphorylation and activation of receptor-associated kinase, resulting in inhibition of cell growth10 and induction of apoptosis10 as well as decreased production of matrix metalloproteinase and vascular endothelial growth factor.11 In vitro, Cetuximab can mediate antibody dependent cell-mediated cytotoxicity (ADCC) against certain human tumor types.12 Data from mouse xenograft models have revealed that Cetuximab can inhibit tumor growth in vivo but cannot regress tumors;13 supporting a role for ADCC, this therapeutic effect requires the Fc portion of the antibody.14 The addition of Cetuximab to radiation therapy or chemotherapy in human xenograft models in mice increases antitumor effects compared to radiation therapy or chemotherapy alone.13,15 Collectively, these observations lead to the idea that the antitumor effect of this antibody therapy is mediated via two mechanisms: direct oncogenic-signal stress through competing with natural ligands of EGFR and inducing blockade of EGFR signal pathway, and ADCC effect mediated through the Fc portion of Cetuximab. Consistent with this, the ability of antitumor antibodies to induce apoptosis was recently reported to be dependent on host FcR-positive cells.16
Due to the lack of appropriate experimental tumor cell lines that can respond to Cetuximab in immune-competent hosts, it has been difficult not only to study whether the adaptive immune response is also involved in Cetuximab-induced tumor inhibition in vivo, but also to determine how to improve the limited therapeutic effect of Cetuximab in broader types of cancers in order to treat more patients. Current evaluation of the antitumor effect for human antibody therapy is biased toward direct inhibition of EGFR-dependent cell lines without consideration of adaptive immune responses. To overcome these obstacles, we created both human and mouse EGFR+ tumor model systems in the presence of various immune cells to test the essential role of the innate and adaptive immune system for Cetuximab-mediated tumor control in both human and mouse tumors, and demonstrate an essential role for both. Furthermore, our data suggests that a modified Cetuximab that includes an innate-immune modulator can be developed to greatly improve tumor control.
Results
The therapeutic effect of Cetuximab requires adaptive immunity in immune-reconstituted human tumor xenograft model
Xenograft models have been used as the standard to evaluate human antibody-based treatment. But potential immune-mediated regulation of Cetuximab effects cannot be evaluated, as human EGFR-dependent tumors cannot grow in immune-competent wild type (Wt) mice and there is no mouse tumor cell line that responds to Cetuximab. To overcome those obstacles, we first developed a new xenograft model using immune-reconstituted mice.17 In this modified mouse model, Rag1 knockout (KO) mice were inoculated subcutaneously with the human lung epidermoid carcinoma cell line A431. This cell line has been shown to respond to Cetuximab treatment in a xenograft model when very high dose of Cetuximab is repeatedly used14 and it also responds to the same treatment schedule quite well in our lab (Supplementary Figure S1). After the tumor was established, 2 × 106 lymph node (LN) cells (~50% T cells) from OTI TCR transgenic mice were adoptively transferred via the intravenous route. Among the transferred OTI TCR transgenic CD8 T cells, ~95% are OTI-peptide–reactive CD8+ T cells. Therefore, most of the adoptively transferred cells do not recognize A431 tumor antigens, and only 200–1,000 cells of the remaining non-OTI T cells can potentially mount antihuman tumor response. This number and frequency is comparable to the actual number of human T cells against various cancer conditions.17,18,19 In this immune-reconstituted A431 xenograft model, low dose of Cetuximab treatment could induce tumor regression only in the presence of LN cells from OTI-transgenic donors. Neither adoptive transfer of OTI LN cells alone (without Cetuximab) nor Cetuximab treatment alone could significantly inhibit tumor growth (Figure 1a). Because tumor-antigen–reactive T cells must be non-OTI T cells, we expected that an increased percentage of the non-OTI T cell population would indicate the potential increase of tumor-specific T cells after treatment. Indeed, we found that the percentage of potential tumor-antigen reactive non-OTI T cells dramatically increased from 5 to 25% after Cetuximab treatment as compared to human IgG (hIgG) control (Figure 1b). To further determine the tumor-specific T cell effector response, we collected host LN cells after Cetuximab treatment and cocultured them with A431 tumor cells and measured interferon-γ (IFNγ) production with an enzyme-linked immunosorbent spot (ELISPOT) assay. As shown in Figure 1c, Cetuximab treatment increased the number of tumor-antigen specific IFNγ-producing T cells in this immune-reconstituted xenograft model. These data demonstrate that adaptive immune cells are necessary for effective Cetuximab therapy, and suggest that the tumor-reactive T cell population is activated by Cetuximab and mediates this antitumor effect. In addition, these results suggest that this new model allows for the efficacy evaluation of clinically relevant reagents (such as antibodies against human molecules) on human tumors in the presence of an adaptive immune system.
Figure 1.
The antitumor effect of Cetuximab depends on adaptive immunity in a human A431 tumor xenograft model. (a) Rag1 knockout (KO) mice (n = 5/group) were injected subcutaneously with 6 × 106 A431 cells, and 2 × 106 OTI lymph node (LN) cells were adoptively transferred on day 13. Two hundred micrograms of Cetuximab or control human immunoglobulin G (IgG) was administered on days 14, 21, and 28. The growth of tumor was measured and compared twice a week. (b) Draining LN cells were collected for fluorescence-activated cell sorting (FACS) analysis on day 35 after treatment. *P < 0.05 compared with control treated group. One of three experiments is shown. (c) Rag1 KO mice (n = 5/group) were injected subcutaneously with 6 × 106 A431 cells, and 2 × 106 OTI LN cells were adoptively transferred on day 13. Two hundred micrograms of Cetuximab or control human IgG was administered on days 14, 21, and 28. Draining LN cells were collected 9 days after the last treatment and were stimulated with 1 × 104 A431 cells in an interferon-γ (IFNγ) enzyme-linked immunosorbent spot (ELISPOT) assay. *P < 0.05 compared with control group. One representative experiment of three is depicted.
Adaptive immunity is essential for the therapeutic effect of Cetuximab treatment in a mouse tumor model
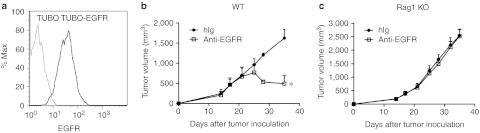
To further create an experimental model to study antibody-mediated immune responses against a murine tumor in an immune-competent host, we developed a new EGFR-responding murine tumor cell line amenable for transfer into immune-competent syngeneic hosts. To this end, we transfected the HER2/neu-dependent TUBO cell line, derived from BALB/c mice transgenic for the neu oncogene, with human EGFR (Figure 2a). This cell line is able to grow in immune-competent Wt BALB/c mice similar to the parental TUBO cell line. Moreover, immunoprecipitation experiments confirmed association between human EGFR and HER2 (Supplementary Figure S2), suggesting that this receptor can bind to ligand and promote signals through dimerization with HER2. As predicted, Cetuximab treatment inhibited tumor growth of TUBO-EGFR in vivo (Figure 2b). To evaluate the role of adaptive immunity during Cetuximab treatment, TUBO-EGFR–bearing BALB/c Rag1 KO mice were treated with Cetuximab. The therapeutic effect of Cetuximab was abolished in these immune-compromised Rag1 KO mice (Figure 2c). Thus, these data further support that the therapeutic effect of Cetuximab requires adaptive immunity. Collectively, these models demonstrate that Cetuximab can induce antitumor adaptive immune responses in both human and mouse tumors.
Figure 2.
Adaptive immunity is essential for the therapeutic effect of Cetuximab treatment in mouse epidermal growth factor receptor (EGFR) tumor model. (a) TUBO and TUBO-EGFR cells were stained with Cetuximab and antihuman–IgG-PE. (b) Wild type (Wt) BALB/c mice (n = 5/group) were injected subcutaneously with 5 × 105 TUBO-EGFR cells and treated with 200 µg of Cetuximab or control human immunoglobulin G (IgG) on days 14, 21, and 28. The growth of tumor was measured and compared twice a week. *P < 0.05 compared with control group. (c) TUBO-bearing Rag1 knockout (KO) mice (n = 5/group) were treated with 200 µg of Cetuximab or control human IgG on days 14, 21, and 28. One of three representative experiments is shown.
Anti-EGFR Ab treatment induced tumor-specific CD8+ T cell responses
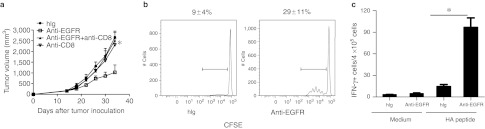
CD8+ T lymphocytes are the major cell population involved in controlling the growth of many tumors.20 To address whether CD8+ cells are essential for the therapeutic effect of Cetuximab, we administered a CD8-depleting antibody during Cetuximab treatment in TUBO-EGFR–bearing Wt BALB/c mice and measured tumor growth. Depletion of CD8+ cells eliminated the therapeutic effect of Cetuximab (Figure 3a). We therefore hypothesized that Cetuximab may induce more effective priming of tumor-specific CTL. To trace the antitumor T cell response during the priming phase, we established a TUBO-EGFR-HA tumor cell line, in which human EGFR was ectopically expressed with an hemagglutinin (HA) peptide (IYSTVASSL) fused to the intracellular C-terminal domain of EGFR; this HA peptide serves as a surrogate marker that can be specifically recognized and detected by CD8+ T cells from HA-specific clone 4 (CL4) TCR transgenic mice.21 Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled CL4 CD8+ T cells were adoptively transferred into TUBO-EGFR-HA–bearing Wt BALB/c mice that were then treated with Cetuximab. Cetuximab treatment increased the proliferation of tumor-antigen specific CL4 CD8+ T cells in the draining LN (dLN) (Figure 3b). To determine whether Cetuximab affected the T cell effector response, we measured IFNγ production as an effector function of activated T cells. After treatment with Cetuximab, dLN lymphocytes from tumor-bearing mice that were adoptively transferred with CL4 splenocytes were isolated and stimulated with HA peptide (hereafter referred to as a tumor-specific antigen) to evaluate IFNγ production by an ELISPOT assay. As shown in Figure 3c, Cetuximab treatment increased IFNγ production from tumor-antigen specific T cells. Taken together, these data suggest that Cetuximab treatment can increase the priming and effector function of tumor-specific T cells.
Figure 3.
Anti-EGFR Ab induced a tumor-specific CTL response, which is required for the therapeutic effect. (a) Wild type (Wt) BALB/c mice (n = 5/group) were injected subcutaneously with 5 × 105 TUBO-EGFR and treated with 200 µg of Cetuximab on days 14, 21, and 28. A CD8-depleting antibody (200 µg/mouse) was administered on the same day as Cetuximab. The tumor growth was measured and compared twice a week. *P < 0.05, compared to Cetuximab-treated Wt mice. One of three representative experiments is shown. (b) TUBO-EGFR-HA–bearing Wt mice received an adoptive transfer of 6 × 106 carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled clone 4 (CL4) T cells on day 10 after tumor inoculation. Six hours later, mice were treated with 500 µg of Cetuximab or control human immunoglobulin G (IgG). Draining lymph node (LN) cells were collected 3 days later and analyzed for CFSE dilution. Data is gated on the Thy1.1+CD8+Vβ8+ population. *P < 0.05 compared to control group. One of two similar experiments is represented. (c) TUBO-EGFR-HA–bearing Wt BALB/c mice received an adoptive transfer of 2 × 103 CL4 splenocytes on day 10 after tumor inoculation. Six hours later, mice were treated with 500 µg of Cetuximab or control human IgG. Draining LN cells were collected 9 days later and stimulated with hemagglutinin (HA) peptide in an interferon-γ (IFNγ) enzyme-linked immunosorbent spot (ELISPOT) assay. *P < 0.05 compared with control group. One representative experiment of three is depicted.
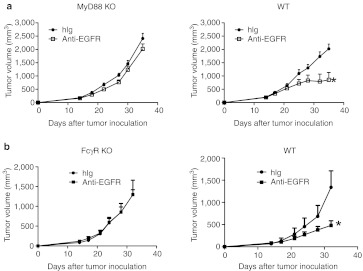
The MyD88 signaling pathway and FcγR contribute to the therapeutic effect of Cetuximab
Danger signaling molecules released by tumor cells are necessary for dendritic cell (DC) activation and cross-presentation in a TLR4- and MyD88-dependent manner after chemotherapy and radiotherapy.22 Therefore, we sought to address whether Cetuximab treatment induced a stress response from TUBO- EGFR that triggered a MyD88-dependent inhibition of tumor growth. To evaluate this, TUBO-EGFR–bearing MyD88 KO mice were treated with Cetuximab. Indeed, the therapeutic effect of Cetuximab was impaired in MyD88 KO mice as compared to Wt mice (Figure 4a). To test whether ADCC is responsible for the reduction of tumor mass in vivo, Wt and FcγR KO mice were inoculated with TUBO-EGFR. Indeed, the therapeutic effect of Cetuximab was FcγR dependent, as FcγR KO mice failed to show Cetuximab-induced inhibition (Figure 4b). These data indicate that both ADCC and the MyD88 signaling pathway contribute to the therapeutic effect of Cetuximab.
Figure 4.
The MyD88 signaling pathway and FcγR contribute to the therapeutic effect of Cetuximab. (a) Wild type (Wt) and MyD88 knockout (KO) BALB/c mice (n = 5/group) were injected subcutaneously with 5 × 105 TUBO-EGFR cells and treated with 200 µg of Cetuximab or control human immunoglobulin G (IgG) on days 14, 21, and 28. *P < 0.05 compared to control treated mice. One of three experiments is shown. (b) Wt and FcγR KO BALB/c mice (n = 5/group) were injected subcutaneously with 5 × 105 TUBO-EGFR cells and were treated with 200 µg of Cetuximab or control human IgG on days 14, 21, and 28. *P < 0.05 compared to control treated mice. One of two experiments is shown. EGFR, epidermal growth factor receptor.
CpG conjugation to Cetuximab improves the antitumor effect of Cetuximab alone
Our previous study showed that anti-neu treatment could cure the parental neu-expressing TUBO tumor in a CD8+ T cell-dependent fashion in immune-competent mice.23 However, low dose anti-EGFR Cetuximab treatment is less effective, as it can only delay tumor growth. Therefore, we speculate that TUBO-EGFR growth is more dependent on the Neu pathway than on EGFR pathway. Indeed, we found that anti-neu has more inhibitory effect on TUBO-EGFR proliferation than Cetuximab by in vitro cell proliferation assay (Figure 5a). We have previously shown that anti-neu–mediated tumor regression depends on high-mobility group protein B1 (HMGB-1), a danger signal released from stressed cells.23 However, when we tested the effect of anti-HMGB-1 in our model, HMGB-1 blockade has no significant effect on Cetuximab-mediated tumor inhibition (data not shown), suggesting that Cetuximab might either use different pathway or does not induce a strong stress response from TUBO-EGFR cells.
Figure 5.
CpG conjugation improves the antitumor effect of Cetuximab. (a) Approximately 1 × 104 TUBO-EGFR cells were plated in 96-well plate and incubated with 10, 3, or 0.3 µg/ml Cetuximab or anti-neu antibodies. Cell viability was measured by an MTT assay at 24, 36, and 72 hours after incubation. Cell growth rate was normalized to control cell growth. (b) Wild type (Wt) BALB/c mice (n = 5/group) were injected subcutaneously with 5 × 105 TUBO-EGFR and treated with 40 µg of human Ig (hIg), Cetuximab, Cetuximab-CpG, or 10 µg of CpG on days 14, 18, and 22. Tumor growth was measured and compared twice a week. *P < 0.05 compared to Cetuximab-treated Wt mice. One of three representative experiments is shown. (c) Wt BALB/c mice (n = 5/group) were injected subcutaneously with 5 × 105 TUBO-EGFR and treated with 40 µg of hIg or Cetuximab-CpG on days 14, 18, and 22. A CD8-depleting antibody (200 µg/mouse) was administered on days 14, 21, and 28. Tumor growth was measured and compared twice a week. *P < 0.05, compared to Cetuximab-CpG-treated Wt mice. One of three representative experiments is shown. (d) Wt BALB/c mice or cured Cetuximab-CpG treated mice (n = 5/group) were injected subcutaneously with 2 × 106 TUBO-EGFR, and the growth of tumor was measured and compared twice a week. *P < 0.05, compared to naive Wt mice. One of three representative experiments is shown. EGFR, epidermal growth factor receptor.
Since Cetuximab cannot induce strong innate signaling on its own, we wondered whether employing the use of an innate stimulator in combination with Cetuximab could enhance the antitumor effect of Cetuximab; we therefore asked if increasing innate responses and DC cross-presentation could achieve this goal. Many TLR ligands have been reported to activate DCs, especially the TLR9 agonist CpG.24 Tumor-targeted delivery of CpG has a much greater effect on tumor regression when compared to systemic delivery of CpG, which has almost no antitumor effect.24 We therefore hypothesized that a conjugate of CpG and Cetuximab will increase innate signals inside tumor tissues through both the CpG and antibody components. In our conjugation protocol, 4–6 CpGs are linked to one Cetuximab antibody. To examine the synergistic effects with CpG, we reduced the dose of Cetuximab in these experiments. Treating mice with 40 µg of Cetuximab-CpG conjugate delivers ~6–10 µg of CpG, which is a dose of CpG that has been reported to be ineffective in curing TUBO tumors when used alone.24 Impressively, treatment with this low dose of Cetuximab-CpG conjugate resulted in regression of TUBO-EGFR tumors (Figure 5b), indicating that the combined effect is more powerful and effective than either treatment alone. After 50 days of observation, no relapse was observed in any of the conjugate-treated cured mice, while a similar dose of Cetuximab treatment alone was limited in its ability to prevent relapse. Therefore, the CpG-mediated increase of innate signaling might synergize with antibody treatment to induce tumor regression.
The CpG component of the conjugate has multiple effects on various cell types, mostly on innate cells. To address whether the overall antitumor effect of Cetuximab-CpG conjugate depends on CTLs similar to Cetuximab treatment alone, a CD8+-depleting antibody was administered during the treatment. Loss of CD8+ cells significantly impaired the therapeutic effect of Cetuximab-CpG (Figure 5c), suggesting that the antitumor effect of Cetuximab-CpG conjugate is indeed dependent on adaptive immune CD8+ T cells. Since the generation of an adaptive immune memory response is essential for preventing tumor relapse and metastasis, we sought to address whether Cetuximab-CpG therapy could also induce effective antitumor immune memory. To do so, we used tumor-rechallenge assays, as they are a robust readout not only for established memory responses but also for the ability of the host immune system to control systemic cancer relapse. Cured mice with no detectable tumor for over 40 days were therefore rechallenged with a lethal dose of TUBO-EGFR. All cured mice were protected from a lethal-dose rechallenge of TUBO-EGFR (Figure 5d). Taken together, these data suggest that Cetuximab-CpG treatment can generate both strong effector and memory immune responses.
Both Cetuximab antibody and CpG contribute to the antitumor effect of Cetuximab-CpG conjugates
When used together, the Cetuximab-CpG conjugate treatment showed an impressive antitumor effect; however, we wondered how Cetuximab and CpG individually contributed to this effect. Therefore, we addressed whether the Cetuximab-CpG conjugate maintained the individual function of both Cetuximab and CpG. As shown in Figure 6a, the conjugate specifically bound to the surface of EGFR-positive A431 cells through the Cetuximab antibody. Moreover, an anti-Fc antibody detected and bound to the exposed Fc portion of Cetuximab on the cells. Testing the CpG-mediated effects, the Cetuximab-CpG conjugate-activated DCs to produce tumor necrosis factor and interleukin-6 in similar quantities to CpG treatment alone, according to previously published reports25 (Figure 6b). Since MyD88 is downstream of CpG-TLR9 signaling, we predicted that MyD88 deficiency could impair the therapeutic effect of Cetuximab-CpG conjugates. To evaluate this, Cetuximab-CpG conjugate treatment was performed in TUBO-EGFR–bearing MyD88 KO mice. Indeed, the therapeutic effect of Cetuximab-CpG was diminished in MyD88 KO mice (Figure 6c). To test whether ADCC, via the antibody component of the conjugate, is required for the conjugate-mediated reduction of tumor mass in vivo, Wt, and FcγR KO mice were inoculated with TUBO-EGFR and then treated with Cetuximab-CpG conjugates. Indeed, the therapeutic effect of Cetuximab-CpG was FcγR-dependent, as FcγR KO mice failed to show Cetuximab-CpG conjugate-induced inhibition of tumor growth (Figure 6d). Recently, CpG was reported to be able to reprogram the regulatory T cell (Treg).26 To test whether Cetuximab-CpG has the similar effect in our tumor model, we detect the percentage of Treg after Cetuximab-CpG treatment. As shown in Supplementary Figure S3, Cetuximab-CpG does decrease the percentage of Treg in dLN and tumor tissue. Considering the importance modulation role of Treg in antitumor immunity, this reprogramming Treg ability may contribute to the antitumor effect of Cetuximab-CpG. Collectively, these data indicate that both the antibody component and the CpG component contribute to the therapeutic effect of Cetuximab-CpG conjugate treatment: whereas the Cetuximab component increased the targeted delivery of CpG to EGFR+ tumor cells and FcγR+ immune cells, the CpG component increased the immune-activating ability on DCs by engaging TLR9 and decrease the inhibitory effect from Treg.
Figure 6.
The TLR-MyD88 signal pathway and FcγR contribute to the therapeutic effect of Cetuximab-CpG conjugates. (a) A431 cells were stained with human Ig (hIg), Cetuximab, or Cetuximab-CpG, and antihuman immunoglobulin G (IgG) Fcγ-PE. (b). Approximately 1 × 105 purified dendritic cells (DCs) from splenocytes were incubated with 3 µg/ml of hIg or Cetuximab-CpG. Supernatants were collected and CBA assay was performed to detect the tumor necrosis factor (TNF) and interleukin-6 (IL-6) levels 48 hours later. (c) Wild type (Wt) and MyD88 knockout (KO) BALB/c mice (n = 5/group) were injected subcutaneously with 5 × 105 TUBO-EGFR cells and treated with 40 µg of hIg or Cetuximab-CpG on days 14, 18, and 22. The growth of tumor was measured and compared twice a week. *P < 0.05 compared to control-treated mice. One of three experiments is shown. (d) Wt and FcγR KO BALB/c mice (n = 5/group) were injected subcutaneously with 5 × 105 TUBO-EGFR cells and treated with 40 µg of hIg or Cetuximab-CpG on days 14, 18, and 22. The growth of tumor was measured and compared twice a week. *P < 0.05 compared to control treated mice. One of two experiments is shown. EGFR, epidermal growth factor receptor.
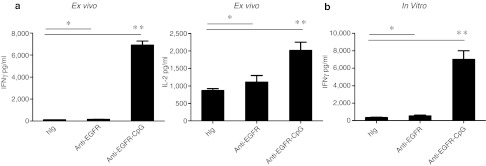
Cetuximab-CpG conjugates activate DCs to enhance the antitumor CTL response through cross-presentation
Given the essential role of CD8+ cells in Cetuximab-CpG treatment, we further explored how this therapy enhanced the antitumor CTL response. We predicted that Cetuximab-CpG conjugates activate DCs that then function to enhance the antitumor CTL response through cross-presentation. To test this, TUBO-EGFR-HA–bearing mice were first treated with Cetuximab-CpG to activate DCs, followed by isolation of activated DCs from the dLN 1 day after the final conjugate treatment. We assessed whether these activated DCs could enhance the specific antitumor CTL response by incubating the activated DCs with HA-reactive CL4 T cells and exogenous HA peptide for re-stimulation in an ex vivo assay. With such dose, the DCs from the Cetuximab-CpG-treated mice induced more IFNγ and interleukin-2 production from the CL4 T cells than compared to control Ig or Cetuximab treatment alone (Figure 7a). This suggests that Cetuximab-CpG conjugate-activated DCs enhanced CD8+ T cell priming and activation; further, these results indicate that the CpG component of the conjugate is responsible for activating the DCs, as Cetuximab alone did not induce strong DC activation. To further dissect whether CpG can directly activate DCs to promote T cell priming by cross-presentation, TUBO-EGFR-HA tumor cells, purified DCs, and HA-reactive CL4 T cells were cocultured and treated with hIg, Cetuximab, or Cetuximab-CpG in vitro. Importantly, the Cetuximab-CpG treatment significantly increased the production of IFNγ from the CL4 T cells only when all three cell types–tumor, DC, and antigen-specific CTL–were present (Figure 7b), as Cetuximab-CpG had no effect on IFNγ production from T cells when DC or tumor cells were missing from the coculture (data not shown). These data collectively suggest that Cetuximab-CpG conjugate increased antitumor CTL response by directly activating DCs and increasing cross-presentation.
Figure 7.
Cetuximab-CpG conjugate treatment activates dendritic cells (DCs) to enhance the antitumor CTL response through cross-presentation. (a) TUBO-EGFR-HA bearing mice were treated with 40 µg of human Ig (hIg), Cetuximab, or Cetuximab-CpG on days 14 and 17. Approximately 1 × 105 draining lymph node (LN) DC were purified and incubated with 2 × 105 purified clone 4 (CL4) T cells on day 18. Hemagglutinin (HA) peptides were supplied at concentration of 10 µg/ml. Supernatants were collected 48 hours later and interferon-γ (IFNγ) and interleukin-2 (IL-2) concentration was determined by CBA assay. (b) Approximately 1 × 105 purified DCs from splenocytes were incubated with 2 × 105 purified CL4 T cells and 1 × 104 TUBO-EGFR-HA. The mixture was stimulated with 3 µg/ml hIg, Cetuximab, or Cetuximab-CpG. Supernatants were collected 48 hours later and IFNγ concentration was determined by CBA assay. EGFR, epidermal growth factor receptor.
Discussion
Cetuximab has been used to treat patients with EGFR-dependent cancer, and some tumors regressed rapidly after treatment.20 Current dogma holds that antitumor antibody treatments like Cetuximab block growth signal induction, arrest cell cycle, and/or induce apoptosis20 or ADCC.20,27 However, most studies that address the mechanisms of antitumor antibodies are based on human tumor experiments from in vitro culture studies or in vivo xenograft models. Both of these experimental systems fail to take the adaptive immune system into account; therefore, the role of T cells in antibody therapy has not been well defined. Although human tumor cell lines, such as A431, express high levels of EGFR and respond to Cetuximab, these cell lines cannot grow in immune-competent mice. Using an entirely mouse-based experimental system is also not a viable option, as no EGFR-dependent mouse cell lines have been developed. Thus, a lack of appropriate experimental models has prevented the proper evaluation of the immune response influence on the antitumor effect of to Cetuximab. Here, we use both an immune-reconstituted human tumor xenograft model and a novel EGFR+ mouse tumor-cell line in immune-competent BALB/c mice to explore the role of T cells in Cetuximab-based antitumor therapy. We revealed that the therapeutic effect of Cetuximab depends on the adaptive immune system, particularly CD8+ T cells, based on the following evidence: (i) Rag1 KO mice bearing human EGFR-dependent A431 tumors failed to clear the tumor mass after Cetuximab treatment, while partially reconstituted Rag1 KO mice completely cleared the tumors; (ii) CD8+ cell depletion diminished the Cetuximab-mediated tumor regression; (iii) elevated tumor-specific CTL responses were observed in the dLN of tumor-bearing mice after Cetuximab treatment, as measured by both CFSE-based T cell proliferation assay and IFNγ ELISPOT assay; and (iv) enhanced innate response by conjugation of Cetuximab with CpG facilitated immune-mediated rejection. These results are consistent with recently published data demonstrating that long-lasting antitumor protection by anti-CD20 or anti-neu antibody therapy also depends on the cellular immune response.23,28,29 In addition, our study has revealed a requirement for innate signaling, including MyD88 and FcγR, for Cetuximab-mediated tumor inhibition.
This study has several important implications: first, it establishes an effective tumor model that improves screening for the adaptive immune response influence in various antihuman cancer treatments; second, it provides strong evidence that adaptive immune responses are required for effective and optimal Cetuximab-mediated tumor regression. After standard antitumor treatments, including surgery, chemotherapy, radiotherapy, and immune therapy, most patients undergo relapse or develop metastasis.30 This study argues the importance of generating effective antitumor effector and memory T cell responses to control these detrimental outcomes. We show that antibody therapies can induce tumor-specific CTL responses that are required for their antitumor effect. Thus, further improving antibody-initiated adaptive immunity should be considered for antibody-based antitumor drug design. Many reagents can be helpful for this process, such as cytokine-antibody fusion proteins to increase DC and T cell activation30 and anti-CD3-based bispecific antibodies to directly activate T cells.31
While the local tumor environment provides a rich source of antigens, the lack of danger signals to activate DCs could prevent effective T cell priming. Therefore, increasing danger signals after antibody treatment might trigger better DC activation and T cell priming inside the tumor. Here, we demonstrated that while Cetuximab treatment alone had limited antitumor effect, Cetuximab-CpG conjugates were able to effectively generate both antitumor effector and memory T cell immunity. This not only cured the primary tumor but also controlled tumor growth upon distal tumor rechallenge in our model. Therefore, conjugation with CpG might increase the therapeutic effect of Cetuximab in patients already approved to receive Cetuximab as well as in a broader scope of tumor types in order to effectively treat more patients.
Finally, this study expands on understanding the mechanism of an antitumor treatment procedure currently used in the clinic. As standard of care, antitumor antibody treatment is often combined with chemotherapy; however, the standard dose of these therapies is high, killing not only dividing tumor cells but also activated immune cells. Importantly, this includes effector and memory tumor-specific CD8+ T cells that are essential for controlling relapse and metastasis. Without these important antitumor immune defenses in place, undivided but still-alive tumor cells left behind after treatment might become key sources of relapse, since the antitumor CTL response would not be present. Thus, suitable treatment combinations and schedules that avoid killing CD8+ T cells should be considered during treatment plan development.
We propose in this study that Cetuximab can inhibit tumor growth by blocking oncogenic signals and initiating ADCC, which not only suppresses tumor growth but also triggers innate immunity to improve CTL cross-priming by DC. This enhanced CTL response, in turn, can then kill more tumor cells to create a positive loop that initiates danger/innate signaling that further generates both innate and adaptive immunity against the tumor, and ultimately leads to tumor regression. Collectively, this study provides insight into a novel antitumor mechanism of anti-EGFR Ab therapy that promotes cooperation between innate and adaptive immunity and warrants reconsideration of the adaptive immune system in current therapy regimens and antitumor therapy design. Furthermore, the strategy used in this study can be also used to evaluate other human antibody treatments against human tumors, or murine tumor models expressing human-targeted molecules.
Materials and Methods
Mice. BALB/c, BALB/c recombination activating gene-1 (Rag1) KO, C57BL/6 OTI, and C57BL/6 Rag1 KO mice were purchased from Harlan (Indianapolis, IN) at 6–7 weeks of age. BALB/c FcγR KO mice were purchased from Taconic (Cambridge City, IN). BALB/c MyD88 KO mice were kindly provided by Dr Anita Chong, University of Chicago. All mice were maintained under specific pathogen free conditions and used between 6 and 16 weeks of age in accordance to the animal experimental guidelines set by the Institutional Animal Care and Use Committee.
Cell lines and reagents. A431 was purchased from ATCC (Manassas, VA). TUBO was cloned from a spontaneous mammary tumor in a BALB/c Neu-transgenic mouse.32 TUBO-EGFR and TUBO-EGFR-HA were selected after transfection of pSEB-EGFR or pSEB-EGFR-HA plasmid with 2 µg/ml of Blasticidin (InvivoGen, San Diego, CA), respectively. A431, TUBO, and its derivatives were cultured in 5% CO2 and maintained in vitro in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St Louis, MO), 2 mmol/l L-glutamine, 0.1 mmol/l MEM nonessential amino acids, 100 U/ml penicillin, and 100 µg/ml streptomycin. Anti-EGFR mAb Cetuximab was purchased from Imclone (Bridge Water, NJ). Anti-CD8 depleting antibody YTS 169.4.2 and anti-HMGB-1 neutralizing mAb 3B1 were produced in-house. Anti-HMGB-1 mAb is capable of neutralizing HMGB-1 in vivo.33 All antibodies for fluorescence-activated cell sorting were purchased from BD Biosciences (San Jose, CA).
Detection of endotoxin in mAb preparation. Endotoxin was measured by the limulus amebocyte lysate assay (Cambrex, Walkersville MD). For all mAb preparations, the amount of endotoxin was determined to be <0.2 EU/mg mAb (limit of detection).
In vivo treatments. Approximately 5 × 105 TUBO-EGFR or TUBO-EGFR-HA cells were injected subcutaneously on the right flank into 6–8-week-old mice. Tumor volumes were measured along three orthogonal axes (a, b, and c) and calculated as tumor volume = abc/2. Mice were treated with three intraperitoneal injections of 200 µg of anti-EGFR antibody. For CD8 depletion experiments, 200 µg of anti-CD8 antibody (YTS 169.4.2, ATCC) was injected intraperitoneally at the same time as the anti-EGFR antibody treatment. For the HMGB-1 neutralizing experiment, 150 µg of mouse anti-HMGB-1 antibody (3B1) was injected intraperitoneally at the same time as anti-EGFR antibody treatment. For the Cetuximab-CpG treatment, tumor-bearing mice were treated by 40 µg of hIg, Cetuximab, Cetuximab-CpG, or 10 µg of CpG intratumorally on day 14, 18, and 22 after tumor inoculation.
Approximately 6 × 106 A431 cells were injected subcutaneously on the right flank into 6–8-week-old Rag1 KO mice. After the tumor was established (~14 days), 2 × 106 LN cells from OTI TCR transgenic mice were adoptively transferred to mice by intravenous injection. One day later, mice were treated with 3 intraperitoneal injections of 200 µg of Cetuximab weekly.
Measurement of IFNγ-secreting T cells by ELISPOT assay. Influenza HA peptide (IYSTVASSL)-reactive T cells were measured by ELISPOT assay.29 Spleen or LN cells were resuspended in RPMI 1640 supplemented with 10% fetal calf serum, 2 mmol/l L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. A total of 1–4 × 105 spleen or LN cells were added to each well of a 96-well HTS IP plate (Millipore, Billerica, MA), which was precoated with 2.5 µg/ml rat anti-mouse IFNγ (clone R4-6A2; BD-PharMingen). HA peptide was added at concentration of 10 µg/ml, or 1 × 104 A431 was added as stimulation antigen in the A431 tumor model. After 48 hours of incubation, cells were removed and 2 µg/ml biotinylated rat anti-mouse-IFNγ (clone XMG 1.2; BD-PharMingen) was added. Plates were incubated for another 12 hours at 4 °C, then washed to remove unbound antibody. Bound antibody was detected by incubating the plates with 0.9 µg/ml avidin-horseradish peroxidase (BD-PharMingen, San Jose, CA) for 2 hours at room temperature. The substrate 3-amino-9-ethylcarbazole (AEC; PharMingen) was diluted in 0.1 mol/l acetic acid, 0.003% hydrogen peroxide was added, and the plate was incubated for 3–5 minutes. The AEC solution was discarded, and the plates were washed six times with water. The visualized cytokine spots were enumerated with the ImmunoSpot analyzer (CTL).
CFSE dilution assay in vivo. Influenza HA-reactive CD8 T cells from CL4 TCR transgenic mice were purified with the mouse T cell enrichment kit (Stemcell, Vancouver, BC). Then, purified CL4 cells were labeled with CFSE according to a previous publication.34 About 5 × 106 CL4 T cells were adoptively transferred to TUBO-EGFR-HA–bearing mice by intravenous injection. Six hours later, mice were treated with hIg or Cetuximab. Two days later, LN cells were collected and analyzed with FACS Canto (BD Biosciences) for CFSE dilution.
CpG conjugation. CpG DNA (ODN1826: tccatgacgttcctgacgtt) was synthesized by IDT with 5′ amino modification. Cetuximab-CpG conjugates were produced with cleavable linker by All Purpose Crosslinking Kit according to the manufacturer's instruction (Solulink, San Diego, CA).
Ex vivo CL4 T cell activation assay. TUBO-EGFR-HA–bearing mice were treated with 40 µg of hIg, Cetuximab, or Cetuximab-CpG intratumoral injection on days 14 and 17. One day later, dLN DCs were purified by CD11c positive selection (Stemcell). Approximately 1 × 105 DCs were mixed together with 2 × 105 CL4 T cells and 10 µg/ml of HA peptide was added in to restimulate the T cells. Two days later, the supernatants were collected, and IFNγ was measured by CBA assay (BD Biosciences).
In vitro CL4 T cell activation assay. TUBO-EGFR-HA (2 × 104), DC (1 × 105), and CL4 T cells (2 × 105) were mixed together and stimulated with 3 µg/ml of hIg, Cetuximab, or Cetuximab-CpG. Three days later, the supernatants were collected and IFNγ was measured by CBA assay (BD Biosciences).
Statistical analysis. Differences between groups were analyzed using the two-tailed Student's t-test. Error bars represent SEM. *P < 0.05 and **P < 0.01.
SUPPLEMENTARY MATERIAL Figure S1. High dose of Cetuximab could control the A431 tumor growth. Figure S2. Transfected human EGFR could interact with rat Neu in TUBO-EGFR cells. Figure S3. Anti-EGFR-CpG could reduce the Treg population in both dLN and tumor microenvironment.
Acknowledgments
We thank William Pao (Vanderbilt-Ingram Cancer Center) for plasmid DNA encoding human EGFR. This research was in part supported by US National Institutes of Health grants CA134563 and CA97296 to Y.X.F. The authors declared no conflict of interest.
Supplementary Material
High dose of Cetuximab could control the A431 tumor growth.
Transfected human EGFR could interact with rat Neu in TUBO-EGFR cells.
Anti-EGFR-CpG could reduce the Treg population in both dLN and tumor microenvironment.
REFERENCES
- Citri A., and, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- Arteaga CL. ErbB-targeted therapeutic approaches in human cancer. Exp Cell Res. 2003;284:122–130. doi: 10.1016/s0014-4827(02)00104-0. [DOI] [PubMed] [Google Scholar]
- Chen WS, Lazar CS, Poenie M, Tsien RY, Gill GN., and, Rosenfeld MG. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. Nature. 1987;328:820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Hynes NE., and, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- Sharma SV, Bell DW, Settleman J., and, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- Giaccone G. Epidermal growth factor receptor inhibitors in the treatment of non-small-cell lung cancer. J Clin Oncol. 2005;23:3235–3242. doi: 10.1200/JCO.2005.08.409. [DOI] [PubMed] [Google Scholar]
- Moyer JD, Barbacci EG, Iwata KK, Arnold L, Boman B, Cunningham A.et al. (1997Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase Cancer Res 574838–4848. [PubMed] [Google Scholar]
- Merlano M., and, Occelli M. Review of cetuximab in the treatment of squamous cell carcinoma of the head and neck. Ther Clin Risk Manag. 2007;3:871–876. [PMC free article] [PubMed] [Google Scholar]
- Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P., and, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Li X, Lu Y, Liang K, Pan T, Mendelsohn J., and, Fan Z. Requirement of hypoxia-inducible factor-1alpha down-regulation in mediating the antitumor activity of the anti-epidermal growth factor receptor monoclonal antibody cetuximab. Mol Cancer Ther. 2008;7:1207–1217. doi: 10.1158/1535-7163.MCT-07-2187. [DOI] [PubMed] [Google Scholar]
- Huang SM, Li J., and, Harari PM. Molecular inhibition of angiogenesis and metastatic potential in human squamous cell carcinomas after epidermal growth factor receptor blockade. Mol Cancer Ther. 2002;1:507–514. [PubMed] [Google Scholar]
- Kurai J, Chikumi H, Hashimoto K, Yamaguchi K, Yamasaki A, Sako T.et al. (2007Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines Clin Cancer Res 131552–1561. [DOI] [PubMed] [Google Scholar]
- Steiner P, Joynes C, Bassi R, Wang S, Tonra JR, Hadari YR.et al. (2007Tumor growth inhibition with cetuximab and chemotherapy in non-small cell lung cancer xenografts expressing wild-type and mutated epidermal growth factor receptor Clin Cancer Res 131540–1551. [DOI] [PubMed] [Google Scholar]
- Fan Z, Masui H, Altas I., and, Mendelsohn J. Blockade of epidermal growth factor receptor function by bivalent and monovalent fragments of 225 anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1993;53:4322–4328. [PubMed] [Google Scholar]
- Raben D, Helfrich B, Chan DC, Ciardiello F, Zhao L, Franklin W.et al. (2005The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer Clin Cancer Res 112 Pt 1795–805. [PubMed] [Google Scholar]
- Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D.et al. (2011An Fc? receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells Cancer Cell 19101–113. [DOI] [PubMed] [Google Scholar]
- Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y.et al. (2009Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment Blood 114589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM.et al. (2007Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude Immunity 27203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ.et al. (2007The genomic landscapes of human breast and colorectal cancers Science 3181108–1113. [DOI] [PubMed] [Google Scholar]
- Dougan M., and, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- Martinez X, Kreuwel HT, Redmond WL, Trenney R, Hunter K, Rosen H.et al. (2005CD8+ T cell tolerance in nonobese diabetic mice is restored by insulin-dependent diabetes resistance alleles J Immunol 1751677–1685. [DOI] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A.et al. (2007Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy Nat Med 131050–1059. [DOI] [PubMed] [Google Scholar]
- Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X.et al. (2010The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity Cancer Cell 18160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Dominguez AL, Hoelzinger DB., and, Lustgarten J. CpG-ODN but not other TLR-ligands restore the antitumor responses in old mice: the implications for vaccinations in the aged. Cancer Immunol Immunother. 2008;57:549–561. doi: 10.1007/s00262-007-0393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Redecke V, Ellwart JW, Scherer B, Kremer JP, Wagner H.et al. (2001Bacterial CpG-DNA triggers activation and maturation of human CD11c-, CD123+ dendritic cells J Immunol 1665000–5007. [DOI] [PubMed] [Google Scholar]
- Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR.et al. (2010Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice Immunity 33942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes RA, Towers TL, Presta LG., and, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- Abès R, Gélizé E, Fridman WH., and, Teillaud JL. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood. 2010;116:926–934. doi: 10.1182/blood-2009-10-248609. [DOI] [PubMed] [Google Scholar]
- Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H.et al. (2011Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy Proc Natl Acad Sci USA 1087142–7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M, Mak TW, Ohashi PS. Fighting cancers from within: augmenting tumor immunity with cytokine therapy. Trends Pharmacol Sci. [DOI] [PubMed]
- Lutterbuese R, Raum T, Kischel R, Hoffmann P, Mangold S, Rattel B.et al. (2010T cell-engaging BiTE antibodies specific for EGFR potently eliminate KRAS- and BRAF-mutated colorectal cancer cells Proc Natl Acad Sci USA 10712605–12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovero S, Amici A, Di Carlo E, Bei R, Nanni P, Quaglino E.et al. (2000DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice J Immunol 1655133–5142. [DOI] [PubMed] [Google Scholar]
- Chen GY, Tang J, Zheng P., and, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quah BJ, Warren HS., and, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–2056. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High dose of Cetuximab could control the A431 tumor growth.
Transfected human EGFR could interact with rat Neu in TUBO-EGFR cells.
Anti-EGFR-CpG could reduce the Treg population in both dLN and tumor microenvironment.