Abstract
Gene transfer vectors derived from the adeno-associated virus (AAV) have recently received increasing attention due to substantial therapeutic benefit in several clinical trials. Nevertheless, their great potential for in vivo gene therapy can only be partially exploited owing to their broad tropism. Current cell surface targeting strategies expanded vector tropism towards transduction of cell types that are inefficiently infected naturally, but failed to restrict or fully re-direct AAV's tropism. Hypothesizing that this limitation can be overcome by equipping natural receptor-blinded AAV vectors with high-affinity ligands, we displayed designed ankyrin repeat proteins (DARPin) as VP2 fusion proteins on AAV capsids ablated for natural primary receptor binding. These second generation targeting vectors demonstrated an as of yet unachieved efficiency to discriminate between target and non-target cells in mono- and mixed cultures. Moreover, DARPin-AAV vectors delivered a suicide gene precisely to tumor tissue and substantially reduced tumor growth without causing fatal liver toxicity. The latter caused death in animals treated with conventional AAV vectors with unmodified capsids, which accumulated in liver tissue and failed to affect tumor growth. This novel targeting platform will be key to translational approaches requiring restricted and cell type-specific in vivo gene delivery.
Introduction
After a period of setbacks, gene therapy is gaining renewed interest because substantial clinical benefit was achieved for a number of severely debilitating disorders.1 From the portfolio of gene delivery vehicles, adeno-associated virus (AAV) vectors are thought to be best suited for in vivo delivery as they mediate stable long-term expression, can be produced at very high titers, and are of low immunogenicity.2,3 However, following systemic application in mice or nonhuman primates AAV particles tend to accumulate in the liver, limiting efficient transduction of other target tissues.4,5 Furthermore, owing to the promiscuous expression of AAV's natural receptors, target as well as non-target cells are transduced even following local administration.6 Although gene expression may be controlled by tissue-specific promoters, vector particles can still enter non-target cells and eventually induce cytotoxicity.3,7
Consequently, cell entry targeting technologies aiming to modify the interaction of vector particles with cell surface receptors were developed.7 These approaches either use adaptors that bridge between vector and target receptor (indirect or non-genetic approaches) or permanently modify viral capsid or envelope proteins by incorporation of receptor-binding moieties (direct or genetic approaches).2,7 Due to these newly established ligand–receptor interactions transduction efficiencies were significantly improved.2,7 However, restricting vector entry and gene delivery to the cell type of choice, i.e., a true re-direction of vector biodistribution upon systemic application remains an unsolved task in particular for nonenveloped viruses. The latter requires permanent ablation of natural receptor binding, stable incorporation of the adaptor/ligand, and availability of adaptors/ligands with high-target selectivity.
The nonenveloped AAV capsid is a tightly packaged 60-mer composed of three capsid proteins (VP1, VP2, and VP3) that share most of their amino acid sequences.8 The common region of all three capsid proteins (“common VP3 region”) form the capsid surface, whereas the N-termini of VP1 and most likely also of VP2 are buried within the capsid interior.8 Natural viral infectivity of the most commonly used serotype, AAV-2, can be abolished by site-directed mutagenesis without influencing capsid assembly, capsid stability or vector genome packaging fulfilling one of the requirements for vector re-targeting.9,10,11 However, so far only peptide ligands with limiting receptor affinity, frequently identified by AAV peptide display screening procedures, could be incorporated into the rigid structure of the AAV capsid and as a consequence AAV-targeting vectors demonstrated low cell type selectivity.2,12,13,14,15,16,17 In order to overcome this limitation, we used the N-terminus of VP2, which was previously used to label AAV particles,18,19,20 as platform for the incorporation of designed ankyrin repeat proteins (DARPins). DARPins are high-affinity binding molecules derived from ankyrin repeat proteins that have been developed as alternative to antibody-based scaffolds and which are selected by high-throughput screens from DARPin libraries.21,22 With 14–17 kDa DARPins largely exceed the size tolerated as insertion within the tight structure of the AAV capsid,15,23 but did not affect vector packaging when fused to VP2. As VP2 fusion proteins, DARPins were readily displayed on the viral capsid and conferred the AAV vectors with ablated HSPG-binding ability with an as of yet unprecedented level of cell type specificity of vector genome delivery and cell transduction resulting in a restricted biodistribution and the safe delivery of suicide genes following systemic application. Hence, by using DARPins as targeting device the current limitations imposed to gene therapy by off-target transduction can be overcome at least for AAV-based vectors.
Results
Display of functionally active DARPins on the AAV particle surface
Recently, we developed an enhanced green fluorescent protein (EGFP)-VP2 fusion construct that incorporated despite its size into the AAV-2 capsid without interfering with viral assembly or genome packaging and which retained infectivity.19 Key for the latter was the unaffected incorporation of VP1 into the modified capsid, which was achieved by placing the fusion construct under control of the human cytomegalovirus promoter, while retaining the natural AAV p40 promoter for expression of VP1 and VP3.19,20
Following these findings, we here hypothesize the N-terminus of VP2 as suitable insertion position for DARPins, multidomain proteins slightly smaller than EGFP. We therefore substituted the EGFP-coding sequence in pGFP-VP2 for that of DARPin 9.2924 which is specific for HER2/neu, a receptor tyrosine kinase overexpressed on human cancer cells.25
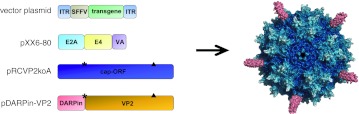
In order to re-direct the tropism, insertion of a new ligand has to be accompanied by destruction of the natural tropism. Five positively charged residues located in the common VP3 region of each capsid protein constitute AAV-2's primary receptor-binding motif.10,11 Since insertions at the N-terminus of VP2 are unlikely to interfere with HSPG binding, we ablated natural receptor binding by substituting R585 and R588, the two main residues involved in HSPG binding, with alanine.9 The same amino acids substitutions were introduced into the AAV helper plasmid pRCVP2koA, which encoded the AAV-2 nonstructural (Rep78, Rep68, Rep52, Rep40, AAP) proteins as well as the capsid proteins VP1 and VP3. In addition, expression of native VP2 was avoided by VP2-start codon deletion in pDARPin-VP2 and pRCVP2koA (Figure 1).
Figure 1.
Production of Her2-AAV particles. Plasmids used for generation of AAV-targeting vectors showing the open reading frames for AAV capsid proteins (cap ORF) only. In pRCVP2koA and pDARPin-VP2, the VP2-start codon was mutated (labeled by asterisk) to prevent expression of unmodified VP2. To abolish HSPG binding, R585 and R588 were substituted by alanine in pDARPin-VP2 and pRCVP2koA (labeled by triangle). Reporter and suicide genes are under control of the SSFV promoter. Right: Schematic illustration of the up to five DARPin molecules (red) extruding from pores at the fivefold symmetry axis of the AAV capsid. Illustration was created using Pymol and PDB files 1LP346 and 2XEE.47 AAV, adeno-associated virus; DARPin, designed ankyrin repeat protein; ITR, inverted terminal repeat; SSFV, spleen focus-forming virus.
The DARPin 9.29-displaying AAV particles (Her2-AAV particles) and—as control–AAV-2 with unmodified capsid (AAV-2) were packaged as vectors encoding EGFP and firefly luciferase (luc-2), respectively. Following density gradient purification, genomic and capsid titers were determined. Although the genomic-to-capsid ratios of Her2-AAV preparations were higher than those determined for AAV-2, they were below or equal to 50, a packaging efficiency previously determined to be wild-type phenotype,10 revealing that the capsid modifications did not interfere with vector packaging (Supplementary Figure S1).
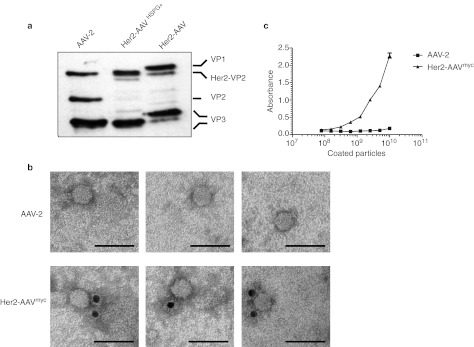
Next, we assessed whether DARPin-VP2 fusion proteins had been incorporated into the AAV capsid by western blot using the anti-capsid antibody B1,26 which recognizes an epitope located at the C′-terminus of all three capsid proteins (Figure 2a). Since substitution of the HSPG-binding residues R585 and R588 by alanine results in a retarded movement of all capsid proteins,9 we included Her2-AAV particles with an unmodified HSPG-binding motif (Her2-AAVHSPG+) as further control. For AAV-2, bands of ~90, 72, and 60 kDa corresponding to VP1, VP2, and VP3 were detectable. As consequence of the VP2-start codon deletion, VP2 expression was impaired in Her2-AAV and Her2-AAVHSPG+. Instead a new band of the size of the DARPin-VP2 fusion protein (86 kDa) was detected.
Figure 2.
Incorporation and surface display of DARPins on AAV-2 particles depleted for natural receptor binding. (a) Western blot analysis of iodixanol step gradient-purified AAV particles. In accordance with our previous observation, the R585A and R588A mutations lead to a reduced mobility of AAV capsid proteins in SDS-PAGE.9 (b) Electron microscopy analysis of AAV particles. Iodixanol step gradient-purified Her2-AAV particles containing a myc-tag fused to the DARPin-VP2 (Her2-AAVmyc) or AAV-2 particles were coated on formvar-carbon–coated copper grids and DARPin surface exposure was detected using an anti-myc antibody. Representative gold-labeled particles at a magnification of ×140,000 are shown. Scale bar corresponds to 50 nm. (c) Surface display of DARPins was assayed by ELISA. Her2-AAVmyc particles were bound to ELISA-plates coated with a myc-tag–specific antibody. AAV-2 particles were used as control. Bound vector particles were quantified using the AAV-2 capsid-specific antibody A20. N = 3 experiments; mean ± SD. AAV, adeno-associated virus; DARPin, designed ankyrin repeat protein; ELISA, enzyme-linked immunosorbent assay; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis.
To further confirm the incorporation of DARPin-VP2 into AAV-2 capsids, we produced Her2-AAV particles, which contained a myc-tag fused at the N-terminal end of the DARPin-VP2 fusion protein (Her2-AAVmyc). Following incubation with a gold-labeled anti-myc antibody, electron microscopy images of density gradient-purified Her2-AAVmyc and AAV-2 preparations were taken (Figure 2b). In both preparations, viral particles were easily detectable. Detection of DARPin, however, was exclusively found in the Her2-AAVmyc preparation associated with the viral capsids.
Key for their function as targeting device, DARPins have to be displayed on the viral surface. We therefore measured the accessibility of the DARPins by enzyme-linked immunosorbent assay (ELISA). Specifically, ELISA plates were coated with a myc-tag–specific antibody, incubated with serial dilutions of Her2-AAVmyc and AAV-2, respectively, followed by detection of bound viral particles by the anti-AAV-2 capsid antibody A20.26 As indicated in Figure 2c, signals for AAV-2 remained at background level, whereas signals for Her2-AAVmyc increased with increasing particle numbers.
In summary, the results show that DARPins can be incorporated into AAV-2 particles as fusion to VP2 without interfering with capsid assembly or packaging efficacy and are accessible for receptor binding likely by extruding from pores at the fivefold symmetry axis (Figure 1).
DARPin-displaying AAV particles discriminate between HER2/neu+ and HER2/neu− cells
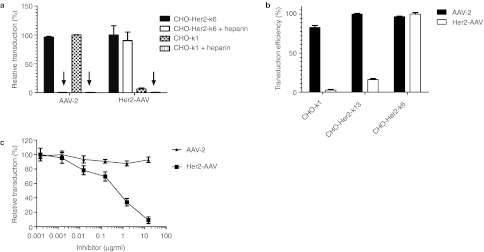
To evaluate whether DARPin 9.29 conferred Her2-AAV with a novel tropism, we assessed transduction efficacy of Her2-AAV and AAV-2 on the CHO cell line CHO-Her2-k6,27 stably expressing HER2/neu, and the HER2/neu-negative parental cell line CHO-K1.
Both cell lines were efficiently transduced by AAV-2 in an HSPG-dependent manner as indicated by the blockage of cell transduction in the presence of heparin, a soluble analog of HSPG. In line with previous reports on the loss of viral infectivity by site-directed mutagenesis of the HSPG-binding motif,9 transduction efficacy of the Her2-AAV preparation on HER2/neu-negative cell line CHO-K1 remained at background level even at a particle-per-cell ratio of 60,000. In contrast, Her2-AAV efficiently transduced CHO-Her2-k6 cells and was not affected by the addition of heparin (Figure 3a, Supplementary Figure S2). Its transduction efficiency correlated directly with increasing HER2/neu receptor density (Figure 3b). Also SK-OV-3 cells that naturally express HER2/neu were efficiently transduced by Her2-AAV while AAV-2ΔHSPG, carrying the mutated HSPG-binding motif but no DARPin, was unable to do so (Supplementary Figure S3).
Figure 3.
Her2-AAV transduction is heparin-independent and HER2/neu-dependent. (a) CHO-K1 (HER2/neu−) or CHO-Her2-k6 (HER2/neu+) cells were incubated with 60,000 genomic particles per cell (GOI) of AAV-2 and Her2-AAV encoding for EGFP, respectively, in the presence or absence of heparin (425 IU/ml). The percentage of EGFP-positive cells was quantified by flow cytometry 60 hours post-transduction. Values are expressed relative to the highest transduction efficiency for each vector. N = 3 experiments; mean ± SD. (b) CHO-Her2-k6 cells (2.1 × 105 HER2/neu molecules per cell27), CHO-Her2-k13 cells (3.7 × 104 HER2/neu molecules per cell27), and CHO-K1 cells (negative for HER2/neu27) were transduced with Her2-AAV (GOI = 300,000) or AAV-2 (GOI = 10,000). Sixty hours after transduction, cells were analyzed by flow cytometry. Values are expressed relative to the highest transduction efficiency observed for each vector type. Mean values from three independent experiments with SD are shown. (c) AAV-2 (GOI = 3,000) or Her2-AAV (GOI = 90,000) were incubated for 1 hour at 4 °C with increasing amounts of the entire HER2/neu receptor extracellular domain. Following incubation, SK-OV-3 cells were transduced and analyzed for EGFP expression. Data are normalized to the transduction efficiency measured without preincubation with HER2/neu domain. N = 3 experiments; mean ± SD. AAV, adeno-associated virus; EGFP, enhanced green fluorescent protein; GOI, genomic particles per cell.
Receptor competition experiments further confirmed that Her2-AAV transductions were strictly dependent on the DARPin ligand. Briefly, SK-OV-3 cells were incubated with Her2-AAV or AAV-2 in the presence of increasing concentrations of a soluble form of the extracellular domain of the HER2/neu receptor. As indicated in Figure 3c, transduction of SK-OV-3 cells by Her2-AAV was inhibited in a dose-dependent manner, whereas AAV-2 was not affected. Hence, cell entry by Her2-AAV is HER2/neu-dependent, but HSPG-independent.
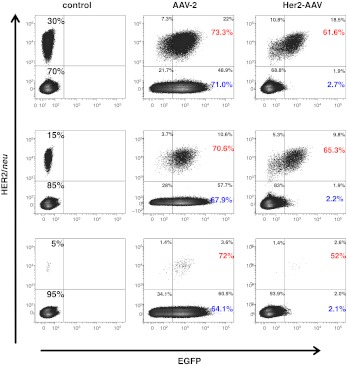
To evaluate whether the targeting capacity of Her2-AAV vectors is sufficiently potent to discriminate between target and non-target cells not only in monocultures, but in mixed cultures of receptor-positive and -negative cells, we transduced a series of cell mixtures comprising defined ratios of CHO-Her2-k6 and CHO-K1 cells (Figure 4). Of note, Her2-AAV transduced more than 50% of the target cell population even when representing only 5% of the total cell number further confirming the high level of specificity.
Figure 4.
Her2-AAV selectively transduces HER2/neu-positive cells. CHO-Her2-k6 and CHO-K1 cells were mixed in different ratios and transduced with Her2-AAV (GOI = 300,000) or AAV-2 (GOI = 10,000) or left untreated (control). After 60 hours, the cells were analyzed as described previously.27 Percentage of all measured cells for each gate is indicated in black. Values in red and blue express the percentage of transduced, HER2/neu-positive or -negative cells. AAV, adeno-associated virus; EGFP, enhanced green fluorescent protein; GOI, genomic particles per cell.
In vivo tumor targeting of Her2-AAV
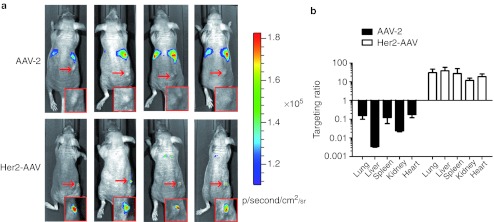
We next aimed to evaluate the targeting capacity of Her2-AAV in vivo. We therefore changed from EGFP to luciferase-expressing vectors to track cell transduction by charged-coupled device imaging in the living animal. In vitro specificity of Her2-AAV vectors carrying the luc-2 expression cassette was confirmed by selective transduction of HER2/neu-positive cells (Supplementary Figure S4). For in vivo analysis, mice carrying subcutaneous SK-OV-3 tumors were injected intravenously with equal amounts of Her2-AAV and AAV-2 particles, respectively (Figure 5). No signal was detectable in the tumors of AAV-2–injected mice, which instead showed prominent signals in the liver (Figure 5a). In sharp contrast, for Her2-AAV a strong luciferase activity was confined to the site of tumor injection (Figure 5a), while with exception of a very weak signal in the chest region in two of the four animals no off-target signals were detected. A further increase in the Her2-AAV dose injected, resulted in a significant increase in luciferase activity in the tumor tissue with no increase in off-target signals (Supplementary Figure S5a).
Figure 5.
Her2-AAV targets HER2/neu-positive tumors in vivo. (a) In vivo imaging of nude mice carrying subcutaneously growing SK-OV-3–derived tumors (arrow). Data were monitored 1 week after intravenous injection of 2 × 1010 gc of AAV-2 or Her2-AAV, transferring the luciferase expression cassette, respectively. Luciferase signal intensity is expressed as photons/second/square centimeter/steradian (p/sec/cm2/sr). Red boxes show a magnification of the tumor cell injection site. (b) Biodistribution of AAV particles. Immediately after imaging, mice were sacrificed, indicated organs were isolated and the vector genome copies (luc-2 gene) were quantified by qPCR. The targeting coefficient was calculated by normalizing the copy number in tumor tissue to that of the organ indicated. N = 4; mean ± SEM. AAV, adeno-associated virus; gc, genome copies; qPCR, quantitative PCR.
As VP2 is not essential for capsid assembly, we hypothesized that HER2/neu vector preparations consist of DARPin-proficient and -deficient particles. The latter are composed of VP1 and VP3, are deficient in HSPG binding and lack the targeting ligand. This hypothesis is supported by the weaker band of DARPin-VP2 detected for Her2-AAV (and Her2-AAVHSPG+) compared with VP2 for AAV-2 detected by western blot (Figure 2). Although non-infectious in vitro, AAV vectors deficient in HSPG binding have been reported to transduce heart10,28 and to a lower extend lung28 tissue following intravenous injection. In order to evaluate whether the weak signals observed in the chest region are caused by DARPin-deficient particle in Her2-AAV stocks, we injected AAV-2ΔHSPG in comparison to Her2-AAV preparations into SK-OV-3–bearing mice. In AAV-2ΔHSPG–treated animals, luciferase signals were confined to the chest region whereas Her2-AAV mediated signals again were clearly located at the tumor site (Supplementary Figure S5b). These findings suggest that the weak off-targeting signals in the chest region observed in mice injected with Her2-AAV are indeed caused by DARPin-deficient particles. Moreover, since tumor localization of luciferase activity is solely observed in Her2-AAV–injected mice, passive tumor targeting caused by natural receptor deletion can be excluded.
Quantification of the luciferase activities in organ lysates confirmed the in vivo imaging data. Luciferase expression in the tumor tissue of Her2-AAV–injected animals exceeded the activities elsewhere in the body by at least 20-fold (Supplementary Figure S6). In contrast, AAV-2–injected mice produced the most prominent signal in the liver, whereas the tumor tissue emitted signals just above background. Luciferase activity, however, depends on transgene expression. In order to assess, if our targeting strategy also re-directed particle distribution to the target tissue, we quantified AAV genome copy numbers in tumor and non-target organs by quantitative PCR. AAV-2 accumulated mainly in the liver, which exceeded the number of genome copies found in the tumor tissue more than 100-fold. All other tested organs contained at least tenfold more vector particles than the tumor tissue. The distribution of Her2-AAV genome copies was completely different. Here, tumor tissue contained between ten (kidney) to 100-fold (liver) more genome copies than the other organs (Figure 5b, Supplementary Figure S7). Thus, relative to liver, Her2-AAV was by more than four orders of magnitude more efficient in targeting the tumor tissue than AAV-2. To roughly estimate the extent of transduction in tumor tissue reached by Her2-AAV, we normalized the Her2-AAV genome copy number in tumor to the toll-like-receptor 2 gene. Based on this measurement, we calculated that 17.63 ± 3.75% (n = 4; mean ± SEM) of all SK-OV-3 tumor cells were positive for the luciferase transgene. This is a considerable fraction of vector-positive cells reached by single intravenous injection which should enable an effective suicide gene therapy.
HER2/neu targeting prevents liver toxicity
The most stringent assay to assess target cell selectivity is in vivo vector-mediated delivery of a suicide gene through the intravenous application route. We therefore equipped Her2-AAV with the herpes simplex virus-thymidine kinase (HSV-TK) under control of the strong and ubiquitously active spleen focus-forming virus promoter.29 HSV-TK converts the prodrug ganciclovir (GCV) into cytotoxic compounds30 inducing cell killing in HSV-TK expressing and neighboring cells.
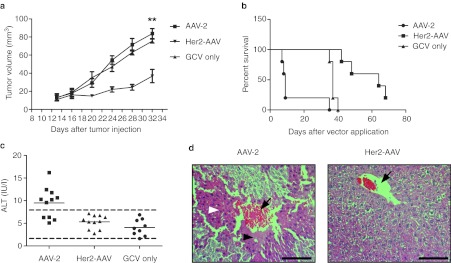
A single systemic injection of this vector into tumor-bearing mice was sufficient to significantly delay tumor growth, while the tumor volume of AAV-2–treated animals increased at the same rate as tumors in control animals (Figure 6a). Of note, although the tumors of AAV-2–injected mice were still far below the critical size, more than half of these animals developed liver failure, substantial weight loss, and had to be sacrificed within the first days of GCV treatment (N = 11, P = 0.0009; logrank test) (Figure 6b). Further investigations of these mice revealed elevated serum alanine transaminase levels (Figure 6c) and massive acute multifocal central lobular necrosis as typically induced by hepatotoxins (Figure 6d). Her2-AAV–treated animals in contrast showed a substantially prolonged survival time, also when compared to the GCV-only treated group (Figure 6b). Neither mock-treated nor Her2-AAV–treated animals showed apparent liver abnormalities, thus confirming the absence of cytotoxic side effects in Her2-AAV–treated animals (Supplementary Figure S8).
Figure 6.
Her2-AAV reduces tumor growth without inducing liver toxicity. (a) SK-OV-3 tumor-bearing mice received a single intravenous injection of HSV-TK transferring AAV-2 or Her2-AAV (8 × 1011 gc, respectively) or PBS, followed by GCV treatment for seven consecutive days and tumor volume was monitored. AAV-2: N = 6, Her2-AAV: N = 5, PBS: N = 4; mean ± SEM, asterisks indicate P < 0.005 (unpaired t-test). (b) SK-OV-3 cell-derived tumor-bearing mice were intravenously administered with 8 × 1011 gc per animal of AAV-2 (N = 11), Her2-AAV (N = 11) or an equal volume of PBS (N = 9) on day 1. GCV treatment (100 mg/kg body weight) was carried out daily from days 3–9. More than half of the AAV-2–treated mice had to be sacrificed due to dramatic weight loss during the GCV treatment phase. Mock-treated animals had to be sacrificed around day 40 due to high tumor burden. (c) Serum alanine transaminase (ALT) levels of mice described in b. The upper and lower limits of normal are indicated by dotted lines. (d) Representative liver histology slices of AAV-2– and Her2-AAV–treated mice. Vessels are indicated by black arrows, areas of central lobular necrosis are indicated by black arrow heads, and the hypertrophic state with karyorrhexis by white arrow heads. Scale bar represents 100 µm. AAV, adeno-associated virus; gc, genome copies; GCV, ganciclovir; PBS, phosphate-buffered saline.
Discussion
The remarkable clinical benefit recently reported for the treatment of rare diseases impressively highlights the potency of gene therapy.1 Nevertheless, gene therapy stays far below its potential due to the inability to control vector tropism. Almost two decades ago, cell entry targeting has been proposed as an approach that might allow systemic in vivo gene therapy by preventing gene transfer into non-target cells in an effort to reduce therapeutic vector dose, enhance bioavailability at the target site, and improve safety.7 Here we describe an engineered AAV vector that finally fulfills these requirements demonstrating that this obstacle can be overcome by incorporation of high-affine targeting devices into vectors stripped for their natural tropism.
As targeting devices we chose DARPins–artificial proteins that consist like their natural ancestors, the ankyrin repeat proteins, of helical repeats with a concave protein interaction surface.22 Upon randomizing the contact residues of this surface, DARPin libraries are generated which are used in high-throughput selection screens against target proteins of choice.21 By this way, highly specific binders with affinities in the pmol/l range have been obtained, which can be used as alternative to antibodies. Clear advantages of DARPins compared with antibodies are their smaller size, the lack of effector domains, a low tendency to aggregate, and the lack of cysteine residues. In particular, the latter feature impairs the use of antibodies as targeting ligand.
We incorporated a DARPin specific for HER2/neu. This receptor tyrosine kinase belongs to the epidermal growth factor family and is overexpressed in ~20–30% of breast cancers.25 Upon approval of trastuzumab, a HER2/neu-specific monoclonal antibody, this tumor-associated antigen has entered clinical phase as drug target.31 In addition, HER2/neu has become a promising model antigen in preclinical and basic research.32,33 We here exploited DARPin 9.29 that was selected by in vitro high-throughput selection of a DARPin library against the extracellular domains of ErbB2.24 Incorporation of this DARPin as N-terminal fusion to VP2, the second largest AAV capsid protein, did not interfere with capsid assembly or vector genome packaging (Supplementary Figure S1), but made it available for target receptor binding (Figure 2). The latter observation is of particular importance, since it is the first direct evidence demonstrating that fusions to the N-terminus of VP2 are exposed on the capsid exterior. Previous studies solely reported on the successful particle incorporation of fusion proteins. Investigations on the locations of the VP2 fusion proteins were either technically not possible since VP1 and VP2 had been modified34,35,36 or have not been conducted.18,19,20
In order to develop true re-targeting vectors, we not only incorporated a highly specific targeting ligand, but ablated simultaneously natural receptor binding by site-directed mutagenesis of two residues, R585 and R588, of the AAV-2 HSPG-binding motif. When assaying this new generation of targeting vectors for specificity, we observed a yet unprecedented level of selectivity which was not only demonstrated in monocultures, but also in mixed cultures of target receptor-positive and -negative cells (Figure 4). The clinically most important finding of our study, however, is that our approach resulted in a true tumor targeting (Figure 5, Supplementary Figure S5). As a consequence of this high level of tumor specificity, vector distribution to other organs in particular to liver was substantially reduced and off-target expression-associated toxicity was prevented. The latter caused death in animals treated by AAV-2 vectors with unmodified capsids (Figure 6). Our data, thus, impressively illustrate that effective cell entry targeting is essential to avoid the detrimental consequences of non-targeted systemic delivery of a toxic gene leading to massive organ damage, as observed here for the delivery of TK combined with GCV treatment. Furthermore, our biodistribution analysis of AAVΔHSPG suggests that residual off-targeting observed for Her2-AAV is caused by DARPin-deficient particles in the vector stock (Supplementary Figure S5). Such DARPin-deficient particles can principally be depleted from DARPin-AAV vector stocks by chromatography.
In contrast to our study that describes the genetic incorporation of DARPin into the capsid of a nonenveloped virus, Dreier and colleagues exploited a non-genetic or indirect-targeting approach for adenovirus.37 Specifically, an adaptor consisting of a DARPin and an adenovirus-binding protein was produced, which non-covalently bridged the vector with its target receptor. By choosing this approach the challenging task of modifying the tightly packaged capsid of nonenveloped viruses, which in particular for adenoviruses frequently results in vector destruction, is circumvented. However, this comes to the expenses of stability of the complex, as the adaptor binds non-covalently. Obviously well suited for ex vivo transductions on target receptor overexpressing cells,37 the in vivo applicability of this approach remains questionable since noncovalent interactions are prone to competition, e.g., by serum components.38
The only further use of DARPins as targeting ligands was reported for lentiviral vectors.27 Lentiviral vectors are enveloped viruses that enter cells by membrane fusion following binding of envelope-anchored glycoproteins to cell surface receptors. Hence, the tropism of enveloped viruses can be altered by exchanging or modifying the envelope-anchored glycoproteins. Compared to non-targeted lentiviral vectors that showed a broad tropism, marker gene expression of the HER2/neu-targeted lentiviral vector was restricted to HER2/neu-positive tumors with a significant lower activity to spleen. Particle redistribution to tumor tissue, as demonstrated here for Her2-AAV, was not assessed in this study. Moreover, it is impossible to transfer targeting strategies successfully applied to enveloped vectors to nonenveloped vector particles such as adenovirus or AAV, where the outer shell is composed of tightly connected proteins.
In summary, we demonstrate the first example for a nonenveloped vector that has been genetically engineered with a multimodal, high-affinity ligand. The here-described approach resulted in major improvements in vector and gene delivery in a therapeutically relevant setting. Besides avoiding transgene product-mediated toxicity, our cell entry targeting strategy that not only restricts transgene expression, but also gene delivery to target cells, lowers the risk of liver carcinogenicity39 and the loss of AAV-transduced cells by anti-AAV immune responses.40 Hence, using the N-terminus of VP2 as position for DARPin insertion into an AAV-2 vector with ablated HSPG binding, opens up the avenue for a plethora of new applications in gene therapy.
Materials and Methods
Plasmids. DARPin 9.29-coding sequences 24 were amplified by PCR using the primers A1-forward and A1-reverse or A2-forward and inserted into pGFP-VP219 by sticky end ligation using AgeI and BsrGI restriction sites. In order to ablate natural HSPG-binding of AAV-2 capsids, R585 and R588 coding residues were mutated to alanine.9 Three different AAV vector plasmids were generated (scLUC, scGFP, and scHSV-TK). Briefly, the coding sequences for firefly luciferase GL4 (primers L1-forward and L1-reverse) and of the spleen focus-forming virus promoter29 (primers S1-forward and S1-reverse) were amplified by PCR. Fragments were inserted into scAAV/EGFP41 by sticky end ligation at SpeI/NotI or SpeI/KpnI restriction sites, respectively, resulting in scLUC and scGFP. HSV-TK coding sequence42 was inserted into scLUC—replacing the luciferase-coding sequences–using the primers H1-forward and H1-reverse and restriction sites SacII/NotI.
Vector particle production. AAV-targeting vectors and AAV-2 were generated using the adenovirus-helper free AAV-packaging strategy.43 For production of the targeting vectors, HEK-293 cells were transfected with plasmids pXX6-80,43 pRCVP2koA, pDARPin-VP2, and the vector plasmid in an equimolar ratio mixed with polyethyleneimine as previously described for lentiviral vectors.44 For production of AAV-2, HEK-293 cells were transfected with plasmids pXX6-80, pRC,45 and the vector plasmid, while for production of AAV-2ΔHSPG (previously termed rWTKO) pRC was replaced by pRC-A2.9 Forty-eight hours after transfection cells were harvested, pelleted by centrifugation, and lysed. Cell lysate was treated by Benzonase (Sigma-Aldrich, Taufkirchen, Germany), pre-cleared by low speed centrifugation and subjected to iodixonal density gradient centrifugation as described previously.41 All vectors contained a self-complementary vector genome conformation.
Determination of genomic and capsid titers. Genomic particle titers were determined as described previously9 using primers specific for each transgene (Supplementary Table S1). Capsid titers were determined using an ELISA-based assay. AAV vector preparations were coated on Maxisorp immunoplates (Nunc, Wiesbaden, Germany) and detected with the anti-AAV-2-capsid antibody A20 (1:4 diluted in phosphate-buffered saline (PBS) containing 3% bovine serum albumin, 5% sucrose, 0.05% Tween20; Progen, Heidelberg, Germany). Subsequently, plates were incubated with a donkey anti-mouse biotin-conjugated antibody (1:25,000 in PBS containing 3% bovine serum albumin, 5% sucrose, 0.05% Tween20; Jackson ImmunoResearch, Suffolk, UK) and HRP-conjugated streptavidin (1:500 in PBS containing 3% bovine serum albumin, 5% sucrose, 0.05% Tween20; Dianova, Hamburg, Germany). Upon addition of TMB-liquid substrate (Sigma-Aldrich, Hamburg, Germany) according to the manufacturers' instruction, the reaction product was quantified at 450 nm wavelength.
Western blot. 2 × 1010 genomic particles of density-purified AAV vectors were separated on 8% SDS PAGE and then transferred to nitrocellulose membrane (Amersham Biosciences, Freiburg, Germany). The AAV capsid proteins were detected using the AAV capsid protein-specific antibody B1 and secondary anti-mouse peroxidase-conjugated antibodies (Dianova). Signals were visualized by enhanced chemiluminescence using ECL Plus Western Blotting Detection System (GE Healthcare, Munich, Germany).
DARPin surface display ELISA. Serial dilutions of Her2-AAVmyc and AAV-2 were bound to ELISA-plates coated with a myc-tag–specific antibody (Abcam, Cambridge, UK). Bound vector particles were quantified using the AAV-2 capsid-specific antibody A20 (Progen). Subsequent detection was carried out as described under the capsid ELISA section.
Electron microscopy. Formvar-carbon–coated 300-mesh copper grids were placed on a suspension drop containing iodixanol density gradient-purified Her2-AAVmyc or AAV-2 particles and anti-myc monoclonal antibody (diluted 1:100 in PBS) for 1 hour at room temperature. After washing twice with PBS, the grid was incubated for 30 minutes with 10 nm gold-labeled secondary anti-mouse antibody diluted 1:100 in PBS. Subsequently, grids were washed five times with ddH2O and stained for 15 seconds in 2% aqueous uranyl acetate. Samples were examined in an EM109 transmission electron microscope (Zeiss, Jena, Germany). Representative gold-labeled particles at a magnification of ×140,000 are shown. Scale bar corresponds to 50 nm.
Competition assay. AAV-EGFP vector preparations were incubated for 1 hour at 4 °C with increasing amounts of the entire HER2/neu receptor extracellular domain (Sino Biological, Beijing, China). Following incubation, SK-OV-3 cells were transduced. After 48 hours, the percentage of EGFP-positive cells was determined by flow cytometry.
In vivo analysis of Her2-AAV. For biodistribution analysis, 6- to 8-week-old Beige nude mice (Harlan Laboratories, Eystrup, Germany) were engrafted subcutaneously with SK-OV-3 tumor cells as described previously.27 AAV-2 and Her2-AAV were injected systemically through the tail vein and luciferase signals were detected by in vivo imaging 1 week post-injection. For tumor killing experiments, SK-OV-3 cell-derived tumor-bearing mice received a single intravenous injection of 8 × 1011 genome copies (gc) of HSV-TK encoding AAV-2- or Her2-AAV, respectively. From 2 days after vector application on, mice received a daily intraperitoneal injection of GCV (100 mg/kg body weight) for seven consecutive days. Tumor size was determined every 3 days using calipers.
The level of alanine transaminase in blood was determined using the VETTEST-8008-system (IDEXX, Ludwigsburg, Germany) in accordance with the manufacturer's instructions.
For histology, organ or tumor tissue was fixed in 4% formalin for 6 days, paraffin embedded and 3 µm slices were stained with hematoxylin and eosin.
For in vivo imaging, mice were intraperitoneally injected with 150 mg D-Luciferin/kg body weight (Caliper Life Sciences, Mainz, Germany) and anesthetized. Imaging data were obtained 10 minutes after substrate injection using a noninvasive cooled charged-coupled device (IVIS Spectrum; Caliper Life Sciences). Data were analyzed using the Living Image Software (Caliper Life Sciences).
All experimental mouse work was carried out in compliance with the German animal protection law.
Quantification of vector copy number in tumor tissue. Tumor tissue was explanted from Her2-AAV–injected mice. After isolation, samples were homogenized and genomic DNA was isolated. Vector copy number was subsequently determined by quantitative PCR-analysis using transgene- and housekeeping gene-specific primers (Supplementary Table S1).
Statistics. Data were analyzed using the unpaired two-tailed t-test. Survival curves were analyzed by the Kaplan–Meier logrank test. P ≤ 0.05 was taken to be significant. Graph Pad Prism 5 provided the software for statistical analysis.
SUPPLEMENTARY MATERIAL Figure S1. Incorporation of DARPins does not interfere with packaging efficiency. Figure S2. Absolute values for transduction efficiencies shown in Figure 3a. Figure S3. AAV-2ΔHSPG does not transduce HER2/neu-positive cells. Figure S4. Selectivity of luciferase gene delivery by Her2-AAV. Figure S5. Tumor targeting by Her2-AAV is DARPin-mediated and enhanced at increased vector dose. Figure S6. Luciferase activities in Her2-AAV- and AAV-2–injected animals. Figure S7. Absolute values for particle biodistribution shown in Figure 5b. Figure S8. Her2-AAV–mediated suicide gene transfer prevents severe liver toxicity. Table S1. Primer sequences.
Acknowledgments
This work was supported by grants from the priority program “Mechanisms of gene vector entry and persistence” of the Deutsche Forschungsgemeinschaft to H.B. (BU1310/1-2) and C.J.B. (BU1301-2/2) as well as by the Center for Molecular Medicine (CMMC) to H.B. (B1) and the LOEWE Center for Cell and Gene Therapy Frankfurt funded by Hessisches Ministerium für Wissenschaft und Kunst (III L 4- 518/17.004 (2010)) to C.J.B. The authors wish to thank Andreas Plückthun (Zurich University, Switzerland) for critical reading of the manuscript and providing the coding sequence of DARPin 9.29, Jude Samulski (University of North Carolina at Chapel Hill, USA) for providing plasmid pXX6-80, Jürgen Kleinschmidt (DKFZ, Germany) for providing antibody B1, Klaus Boller (Paul-Ehrlich-Institut, Germany) for help with electron microscopy, Laura Escalona Espinosa (University of Cologne, Germany) and Anke Huber (University of Cologne, Germany) for technical assistance, and Ulrike Müller (Heidelberg University, Germany) and Oliver Coutelle (University Hospital Cologne, Germany) for critically reading the manuscript. The authors declared no conflict of interest.
Supplementary Material
Incorporation of DARPins does not interfere with packaging efficiency.
Absolute values for transduction efficiencies shown in Figure 3a.
AAV-2ΔHSPG does not transduce HER2/neu-positive cells.
Selectivity of luciferase gene delivery by Her2-AAV.
Tumor targeting by Her2-AAV is DARPin-mediated and enhanced at increased vector dose.
Luciferase activities in Her2-AAV- and AAV-2–injected animals.
Absolute values for particle biodistribution shown in Figure 5b.
Her2-AAV–mediated suicide gene transfer prevents severe liver toxicity.
Primer sequences.
REFERENCES
- Kay MA. State-of-the-art gene-based therapies: the road ahead. Nat Rev Genet. 2011;12:316–328. doi: 10.1038/nrg2971. [DOI] [PubMed] [Google Scholar]
- Büning H, Perabo L, Coutelle O, Quadt-Humme S., and, Hallek M. Recent developments in adeno-associated virus vector technology. J Gene Med. 2008;10:717–733. doi: 10.1002/jgm.1205. [DOI] [PubMed] [Google Scholar]
- Mingozzi F., and, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X.et al. (2004Clades of Adeno-associated viruses are widely disseminated in human tissues J Virol 786381–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Rosales C, McIntosh J, Rastegarlari G, Nathwani D, Raj D.et al. (2011Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins Mol Ther 19876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toromanoff A, Chérel Y, Guilbaud M, Penaud-Budloo M, Snyder RO, Haskins ME.et al. (2008Safety and efficacy of regional intravenous (r.i.) versus intramuscular (i.m.) delivery of rAAV1 and rAAV8 to nonhuman primate skeletal muscle Mol Ther 161291–1299. [DOI] [PubMed] [Google Scholar]
- Waehler R, Russell SJ., and, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag F, Bleker S, Leuchs B, Fischer R., and, Kleinschmidt JA. Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J Virol. 2006;80:11040–11054. doi: 10.1128/JVI.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucas J, Lux K, Huber A, Schievenbusch S, von Freyend MJ, Perabo L.et al. (2009Engineering adeno-associated virus serotype 2-based targeting vectors using a new insertion site-position 453-and single point mutations J Gene Med 111103–1113. [DOI] [PubMed] [Google Scholar]
- Kern A, Schmidt K, Leder C, Müller OJ, Wobus CE, Bettinger K.et al. (2003Identification of a heparin-binding motif on adeno-associated virus type 2 capsids J Virol 7711072–11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie SR, Warrington KH, Jr, Agbandje-McKenna M, Zolotukhin S., and, Muzyczka N. Identification of amino acid residues in the capsid proteins of adeno-associated virus type 2 that contribute to heparan sulfate proteoglycan binding. J Virol. 2003;77:6995–7006. doi: 10.1128/JVI.77.12.6995-7006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Chang M., and, Davidson BL. Molecular signatures of disease brain endothelia provide new sites for CNS-directed enzyme therapy. Nat Med. 2009;15:1215–1218. doi: 10.1038/nm.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelfelder S, Kohlschütter J, Skorupa A, Pfennings S, Müller O, Kleinschmidt JA.et al. (2009Successful expansion but not complete restriction of tropism of adeno-associated virus by in vivo biopanning of random virus display peptide libraries PLoS ONE 4e5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelfelder S, Varadi K, Raupp C, Hunger A, Körbelin J, Pahrmann C.et al. (2011Peptide ligands incorporated into the threefold spike capsid domain to re-direct gene transduction of AAV8 and AAV9 in vivo PLoS ONE 6e23101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumer M, Ying Y, Michelfelder S, Reuter A, Trepel M, Müller OJ.et al. (2012Development and validation of novel AAV2 random libraries displaying peptides of diverse lengths and at diverse capsid positions Hum Gene Ther 23492–507. [DOI] [PubMed] [Google Scholar]
- Varadi K, Michelfelder S, Korff T, Hecker M, Trepel M, Katus HA.et al. (2012Novel random peptide libraries displayed on AAV serotype 9 for selection of endothelial cell-directed gene transfer vectors Gene Ther 19800–809. [DOI] [PubMed] [Google Scholar]
- Ying Y, Müller OJ, Goehringer C, Leuchs B, Trepel M, Katus HA.et al. (2010Heart-targeted adeno-associated viral vectors selected by in vivo biopanning of a random viral display peptide library Gene Ther 17980–990. [DOI] [PubMed] [Google Scholar]
- Asokan A, Johnson JS, Li C., and, Samulski RJ. Bioluminescent virion shells: new tools for quantitation of AAV vector dynamics in cells and live animals. Gene Ther. 2008;15:1618–1622. doi: 10.1038/gt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux K, Goerlitz N, Schlemminger S, Perabo L, Goldnau D, Endell J.et al. (2005Green fluorescent protein-tagged adeno-associated virus particles allow the study of cytosolic and nuclear trafficking J Virol 7911776–11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington KH, Jr, Gorbatyuk OS, Harrison JK, Opie SR, Zolotukhin S., and, Muzyczka N. Adeno-associated virus type 2 VP2 capsid protein is nonessential and can tolerate large peptide insertions at its N terminus. J Virol. 2004;78:6595–6609. doi: 10.1128/JVI.78.12.6595-6609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binz HK, Amstutz P, Kohl A, Stumpp MT, Briand C, Forrer P.et al. (2004High-affinity binders selected from designed ankyrin repeat protein libraries Nat Biotechnol 22575–582. [DOI] [PubMed] [Google Scholar]
- Stumpp MT, Binz HK., and, Amstutz P. DARPins: a new generation of protein therapeutics. Drug Discov Today. 2008;13:695–701. doi: 10.1016/j.drudis.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Ried MU, Girod A, Leike K, Büning H., and, Hallek M. Adeno-associated virus capsids displaying immunoglobulin-binding domains permit antibody-mediated vector retargeting to specific cell surface receptors. J Virol. 2002;76:4559–4566. doi: 10.1128/JVI.76.9.4559-4566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D, Forrer P., and, Plückthun A. Efficient selection of DARPins with sub-nanomolar affinities using SRP phage display. J Mol Biol. 2008;382:1211–1227. doi: 10.1016/j.jmb.2008.07.085. [DOI] [PubMed] [Google Scholar]
- Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61 suppl. 2:1–13. doi: 10.1159/000055396. [DOI] [PubMed] [Google Scholar]
- Wobus CE, Hügle-Dörr B, Girod A, Petersen G, Hallek M., and, Kleinschmidt JA. Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. J Virol. 2000;74:9281–9293. doi: 10.1128/jvi.74.19.9281-9293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch RC, Mühlebach MD, Schaser T, Kneissl S, Jost C, Plückthun A.et al. (2011DARPins: an efficient targeting domain for lentiviral vectors Mol Ther 19686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller OJ, Leuchs B, Pleger ST, Grimm D, Franz WM, Katus HA.et al. (2006Improved cardiac gene transfer by transcriptional and transductional targeting of adeno-associated viral vectors Cardiovasc Res 7070–78. [DOI] [PubMed] [Google Scholar]
- Baum C, Hegewisch-Becker S, Eckert HG, Stocking C., and, Ostertag W. Novel retroviral vectors for efficient expression of the multidrug resistance (mdr-1) gene in early hematopoietic cells. J Virol. 1995;69:7541–7547. doi: 10.1128/jvi.69.12.7541-7547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räty JK, Pikkarainen JT, Wirth T., and, Ylä-Herttuala S. Gene therapy: the first approved gene-based medicines, molecular mechanisms and clinical indications. Curr Mol Pharmacol. 2008;1:13–23. doi: 10.2174/1874467210801010013. [DOI] [PubMed] [Google Scholar]
- Nahta R. Pharmacological strategies to overcome HER2 cross-talk and Trastuzumab resistance. Curr Med Chem. 2012;19:1065–1075. doi: 10.2174/092986712799320691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro L, Lucchesi M, Caparello C, Vasile E, Caponi S, Ginocchi L.et al. (2011Anti-HER agents in gastric cancer: from bench to bedside Nat Rev Gastroenterol Hepatol 8369–383. [DOI] [PubMed] [Google Scholar]
- Higgins MJ., and, Baselga J. Targeted therapies for breast cancer. J Clin Invest. 2011;121:3797–3803. doi: 10.1172/JCI57152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiler SA, Conlon TJ, Song S, Tang Q, Warrington KH, Agarwal A.et al. (2003Targeting recombinant adeno-associated virus vectors to enhance gene transfer to pancreatic islets and liver Gene Ther 101551–1558. [DOI] [PubMed] [Google Scholar]
- Shi W, Arnold GS., and, Bartlett JS. Insertional mutagenesis of the adeno-associated virus type 2 (AAV2) capsid gene and generation of AAV2 vectors targeted to alternative cell-surface receptors. Hum Gene Ther. 2001;12:1697–1711. doi: 10.1089/104303401750476212. [DOI] [PubMed] [Google Scholar]
- Wu P, Xiao W, Conlon T, Hughes J, Agbandje-McKenna M, Ferkol T.et al. (2000Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism J Virol 748635–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier B, Mikheeva G, Belousova N, Parizek P, Boczek E, Jelesarov I.et al. (2011Her2-specific multivalent adapters confer designed tropism to adenovirus for gene targeting J Mol Biol 405410–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare R, Chen CY, Weaver EA., and, Barry MA. Advances and future challenges in adenoviral vector pharmacology and targeting. Curr Gene Ther. 2011;11:241–258. doi: 10.2174/156652311796150363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW.et al. (2007AAV vector integration sites in mouse hepatocellular carcinoma Science 317477. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ.et al. (2006Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response Nat Med 12342–347. [DOI] [PubMed] [Google Scholar]
- Hacker UT, Wingenfeld L, Kofler DM, Schuhmann NK, Lutz S, Herold T.et al. (2005Adeno-associated virus serotypes 1 to 5 mediated tumor cell directed gene transfer and improvement of transduction efficiency J Gene Med 71429–1438. [DOI] [PubMed] [Google Scholar]
- Funke S, Maisner A, Mühlebach MD, Koehl U, Grez M, Cattaneo R.et al. (2008Targeted cell entry of lentiviral vectors Mol Ther 161427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Li J., and, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anliker B, Abel T, Kneissl S, Hlavaty J, Caputi A, Brynza J.et al. (2010Specific gene transfer to neurons, endothelial cells and hematopoietic progenitors with lentiviral vectors Nat Methods 7929–935. [DOI] [PubMed] [Google Scholar]
- Girod A, Ried M, Wobus C, Lahm H, Leike K, Kleinschmidt J.et al. (1999Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2 Nat Med 51052–1056. [DOI] [PubMed] [Google Scholar]
- Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A.et al. (2002The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy Proc Natl Acad Sci USA 9910405–10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MA, Wetzel SK, Plückthun A, Mittl PR., and, Grütter MG. Structural determinants for improved stability of designed ankyrin repeat proteins with a redesigned C-capping module. J Mol Biol. 2010;404:381–391. doi: 10.1016/j.jmb.2010.09.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Incorporation of DARPins does not interfere with packaging efficiency.
Absolute values for transduction efficiencies shown in Figure 3a.
AAV-2ΔHSPG does not transduce HER2/neu-positive cells.
Selectivity of luciferase gene delivery by Her2-AAV.
Tumor targeting by Her2-AAV is DARPin-mediated and enhanced at increased vector dose.
Luciferase activities in Her2-AAV- and AAV-2–injected animals.
Absolute values for particle biodistribution shown in Figure 5b.
Her2-AAV–mediated suicide gene transfer prevents severe liver toxicity.
Primer sequences.