Abstract
The disease ataxia-telangiectasia (A-T) has no cure and few treatment options. It is caused by mutations in the ATM kinase, which functions in the DNA-damage response and redox sensing. In addition to severe cerebellar degeneration, A-T pathology includes cancer predisposition, sterility, immune system dysfunction, and bone marrow abnormalities. These latter phenotypes are recapitulated in the ATM null (ATM−/−) mouse model of the disease. Since oxidative stress and mitochondrial dysfunction are implicated in A-T, we determined whether reducing mitochondrial reactive oxygen species (ROS) via overexpression of catalase targeted to mitochondria (mCAT) alleviates A-T–related pathology in ATM−/− mice. We found that mCAT has many beneficial effects in this context, including reduced propensity to develop thymic lymphoma, improved bone marrow hematopoiesis and macrophage differentiation in vitro, and partial rescue of memory T-cell developmental defects. Our results suggest that positive effects observed on cancer development may be linked to mCAT reducing mitochondrial ROS, lactate production, and TORC1 signaling in transforming double-positive cells, whereas beneficial effects in memory T cells appear to be TORC1-independent. Altogether, this study provides proof-of-principle that reducing mitochondrial ROS production per se may be therapeutic for the disease, which may have advantages compared with more general antioxidant strategies.
Introduction
Ataxia telangiectasia (A-T) is a recessive genetic disease resulting from mutations in the ATM DNA-damage response kinase.1 Human A-T patients have severe ataxia due to cerebellar degeneration, a heightened risk T- and B-cell lymphomas and leukemias, as well as a variety of other pathological features.1 Although ATM null (ATM−/−) mouse models of A-T display only very mild neurodegenerative phenotypes, they often succumb to thymic lymphomas as early as 3 months of age.2 In addition, they have fewer T- and B-cells, defective memory T-cell development in response to a viral infection,3 and deficiencies in hematopoietic stem cells (HSCs).2,4,5 Mouse and cell-culture studies have implicated increased oxidative stress and dysregulated Akt/TORC1 signaling as potentially important for A-T pathology.3,5,6,7,8,9 Entirely consistent with this, antioxidants (NAC and tempol) or the TORC1 inhibitor rapamycin enhance lifespan by suppressing thymic lymphoma, HSC instability or both.10,11,12 A-T patient cells also have intrinsic mitochondrial dysfunction, suggesting a probable cause of oxidative stress,13,14,15 as mitochondria are a major source of reactive oxygen species (ROS).16 Finally, ATM has been shown to be redox sensitive17 and localized in the cytoplasm and perhaps also mitochondria,15 where it may carry out a ROS-signaling function that is distinct from its DNA-damage signaling role.18
Rabinovitch and colleagues19 generated transgenic mice that globally overexpress the human antioxidant enzyme catalase targeted to mitochondria (mCAT). These mice have an enhanced capacity to detoxify hydrogen peroxide and are reported to impart many beneficial effects, including extended lifespan,19 reduced cancer,20,21,22 age-related hearing loss,23 insulin resistance,24 heart disease,25 and other age-related pathologies.22 In this study, we examined the ability of mCAT to rescue thymic lymphoma, defective bone marrow hematopoiesis, and memory CD8+ T-cell function which are robust, disease-related phenotypes that are recapitulated in the ATM−/− mouse model of A-T, hypothesizing that mitochondrial ROS contribute to the oxidative stress associated with this disease.
Results
Mitochondrial catalase suppresses development of thymic lymphoma in ATM−/− mice
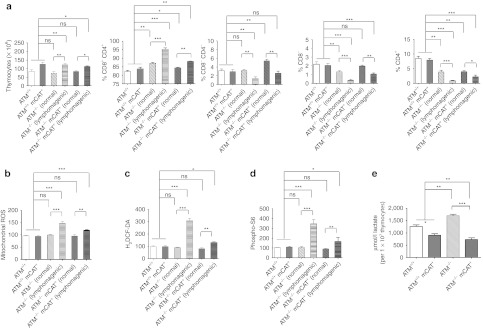
Most ATM−/− mice die from thymic lymphoma within ~20 weeks, but some can live substantially longer due to significantly delayed cancer development.2,4,26 Thymic lymphoma involves a block in T-cell development in ATM−/− mice at the double-positive (CD4+ CD8+) stage and subsequent proliferation and malignant transformation of these cells.27,28 We attempted to identify molecular changes involved in this tumorigenesis process, hypothesizing that oxidative stress and pro-growth TORC1 signaling, previously implicated in ATM−/− mice,12 are involved. We also crossed ATM−/− mice to those that overexpress human mCAT19 to determine the effect of reducing mitochondrial ROS on cancer development. First, we analyzed thymuses of 12-week-old ATM+/+, ATM +/+ mCAT+, ATM−/−, and ATM−/− mCAT+ animals for changes in the distribution of T-cell subsets. ATM−/− animals fell into two groups. Approximately half had a significantly greater total number of thymocytes, with a higher than normal percentage of double-positive cells, and far fewer double-negative (CD4− CD8−) and individual single-positive (CD4+ or CD8+) cells (Figure 1a, “lymphomagenic”) than ATM+/+ controls. The aberrantly proliferating double-positive cells in these “lymphomagenic” animals eventually resulted in death due to thymic lymphoma and metastasis to the spleen and other tissues. The other ~50% of 12-week-old ATM−/− animals had the typical distribution of the four thymic T-cell types seen in 6-week-old ATM−/− animals (Supplementary Figure S1a,b) that had not progressed toward lymphoma (Figure 1a, “normal”). That is, a moderate increase in double-positive cells compared with ATM+/+ controls and correspondingly fewer single-positive cells, due to the documented partial block in differentiation of the single-positive cell types from the double-positive population.2 Similar results were observed in ATM−/− mCAT+ animals (Figure 1a, ATM−/− mCAT+ “normal” and “lymphomagenic”), but the percentage of animals that showed T-cell subtype distributions indicative of progression toward lymphoma was lower (~33%, 4 out of 12 vs. ~50%, 5 out of 10). The mCAT transgene had little effect on the distribution of these T-cell subtypes in ATM+/+ mice, but did appear to result in a greater total number of thymocytes (Figure 1a).
Figure 1.
Mitochondrial catalase suppresses thymic lymphomagenesis in ATM−/− mice. (a) Total thymocytes from 12-week-old ATM+/+ (white-filled bar, n = 10), ATM+/+ mCAT+ (gray-filled bar, n = 10), normal ATM−/− (white filled with black diagonal lines, n = 6), lymphomagenic ATM−/− (white filled with black vertical lines, n = 6), normal ATM−/− mCAT+ (gray filled with black diagonal lines, n = 8), and lymphomagenic ATM−/− mCAT+ (gray filled with black vertical lines, n = 4) mice. Analyses of percent CD8+ CD4+ (double-positive), CD8− CD4− (double-negative), CD8+ (single-positive), and CD4+ (single-positive) cells from the mice in a. FACS analyses of (b) mitochondrial ROS (MitoSox), (c) cellular ROS/redox (H2DCFDA), and (d) TORC1 activity (phospho-S6) of CD8+ CD4+ (double-positive) cells from the mice in a. (e) L-lactate measured from 107 thymocytes from 12-week-old ATM+/+ (white-filled bar, n = 3), ATM+/+ mCAT+ (gray-filled bar, n = 3), ATM−/− (white filled with black diagonal lines, n = 3), and ATM−/− mCAT+ (gray filled with black diagonal lines, n = 3) mice. Statistical significance is denoted by *(<0.05), **(<0.005), ***(<0.0005) or ns (not significant). FACS, fluorescence-activated cell sorting; ROS, reactive oxygen species.
We next analyzed mitochondrial ROS (MitoSox staining) and TORC1 activity (S6 phosphorylation) in 12-week-old mice. Regardless of mCAT status, double-positive cells in ATM−/− animals that had begun to progress to cancer had enhanced mitochondrial ROS and S6 phosphorylation compared with double-positive cells from the asymptomatic ATM−/− or ATM+/+ mice (Figure 1b–d, compare “lymphomagenic” to “normal”). They also exhibited an increase in H2DCFDA staining consistent with an increase in cellular hydrogen peroxide and/or a change in cellular redox status (Figure 1c).29 However, the mCAT transgene reduced the magnitude of these increases (Figure 1b–d, compare “lymphomagenic” ATM−/− to “lymphomagenic” ATM−/− mCAT+). Significant differences in these parameters in double-positive thymocytes (Supplementary Figure S1c–e) were not observed in 6-week-old ATM−/− mice and only minor differences in mitochondrial ROS and H2DCFDA staining were observed between the four genotypes (Supplementary Figure S1c,d). Lastly, we found that thymocytes from 12-week-old mice produce elevated lactate, consistent with increased glycolysis that might be permissive to tumorigenesis, and that this is suppressed by mCAT (Figure 1e). Altogether, these results suggest that enhanced ROS, TORC1 activity, and a shift in metabolism toward glycolysis represent signatures of proliferating and/or transforming thymic double-positive cells that can be suppressed by mCAT expression.
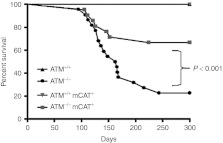
As a final readout of the ATM−/− cancer phenotype, we ascertained the survival of these mice over 300 days (Figure 2). While no ATM+/+ and ATM+/+ mCAT+ animals died during this time frame, ~80% of the ATM−/− animals died (or showed signs of impending lethality) from thymic lymphoma by the end of the experiment. In contrast, only ~40% of the ATM−/− mCAT+ animals died by 300 days. However, the majority of both the ATM−/− and ATM−/− mCAT+ mice died from lymphoma by ~120 days. Analysis of all of the longer-lived ATM−/− and ATM−/− mCAT+ animals revealed that they had similar ratios of the four major cell types (CD4− CD8−, CD4+ CD8+, CD4+, and CD8+) in their thymus as ATM+/+ and ATM+/+ mCAT+ animals, but had fewer single-positive (CD4+ or CD8+) cells (Supplementary Figure S2a,b). These results are consistent with our cellular data and indicate that reducing mitochondrial ROS via mCAT decreases the likelihood of developing thymic lymphoma or significantly delays onset of the cancer.
Figure 2.
Survival Analysis. Kaplan–Meier survival curves for ATM+/+ (n = 9), ATM+/+ mCAT+ (n = 14), ATM−/− (n = 22), and ATM−/− mCAT+ (n = 21) mice. P value was <0.0001 on comparing ATM−/− and ATM−/− mCAT+ curves. The experiment was terminated at 300 days.
Mitochondrial catalase partially rescues defective bone marrow hematopoiesis in ATM−/− mice
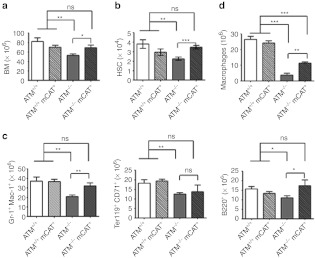
Given the positive effects of mCAT expression on cancer phenotypes and survivability we identified, we became interested if other defects in ATM−/− mice likewise benefit from reduced mitochondrial ROS production. ATM−/− mice have documented defects in the bone marrow hematopoiesis and in maintenance of HSCs,4,30,31 which we corroborated in our 12-week-old animals. That is, compared with ATM+/+ controls, ATM−/− mice had fewer total cells in the bone marrow, as well as deficiencies in the HSC (Lin− ckit+ sca-1+), erythroid (Ter119+ CD71+), granulocyte-monocyte (Gr-1+ Mac-1+), and lymphoid (B220+) cell populations (Figure 3a–c). Age-matched ATM−/− mCAT+ animals exhibited significant improvements in all of these lineages except the erythroid lineage (Figure 3b,c) that was reflected in also having total bone marrow cellularity approaching that of ATM+/+ animals (Figure 3a). However, these improvements were not sustained in older animals, as bone marrow cellularity and the HSC, erythroid, granulocyte-monocyte, and lymphoid compartments in the 300-day-old ATM−/− mCAT+ animals were similar to those in age-matched ATM−/− animals and, in both cases, significantly lower than in age-matched ATM+/+ animals (Supplementary Figure S2c,d). Finally, we also found that mCAT partially rescued the reduced ability of ATM−/− bone marrow HSCs to differentiate into macrophages in vitro (Figure 3d). These results point to a significant contribution of mitochondrial ROS to defects in bone marrow hematopoiesis in ATM−/− mice.
Figure 3.
Bone marrow analyses of 12-week-old ATM−/− and ATM−/− mCAT+ mice. (a) FACS analyses of total bone marrow cells from femurs of the same mice analyzed in a, followed by (b) total number of hematopoietic stem cells (HSC, Lin− cKit+ Sca-1+), (c) granulocytes-monocytes (Gr-1+ Mac-1+), erythroid cells (Ter119+ CD71+), and lymphoid cells (B220+). (d) Total macrophages obtained upon differentiation in vitro of bone marrow cells from ATM+/+ (n = 4), ATM+/+ mCAT+ (n = 4), ATM−/− (n = 4), and ATM−/− mCAT+ (n = 4) mice. Statistical significance is denoted by *(<0.05), **(<0.005), ***(<0.0005) or ns (not significant). FACS, fluorescence-activated cell sorting.
Defective memory CD8+ T-cell responses in ATM−/− mice and in vitro are partially rescued by mitochondrial catalase
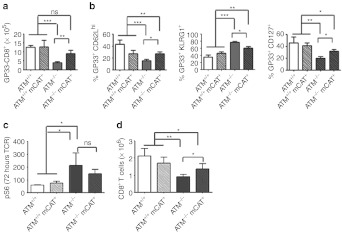
We showed previously that, although ATM−/− mice successfully clear a lymphochoriomeningitus virus (LCMV) infection, they exhibit defective memory CD8+ T-cell development, associated with hyperactive AKT/TORC1 activation.3 Therefore, we determined whether mCAT expression rescues these immunological phenotypes. We subjected a cohort of ATM+/+, ATM+/+ mCAT+, ATM−/−, and ATM−/− mCAT+ animals to LCMV infection and analyzed the splenic CD8+ T lymphocytes at day 30 post-infection (an early-memory time point). Although similar deficiencies in splenic CD8+ T cells were observed in ATM−/− and ATM−/− mCAT+ spleens relative to ATM+/+ and the ATM+/+ mCAT+ controls (Supplementary Figure S3a), ATM−/− mCAT+ CD8+ T mice had significantly more virus-specific (GP33+) CD8 T cells than ATM−/− mice (Figure 4a). In addition, infected ATM−/− mCAT+ mice had a higher ratio of long-lived (CD62Lhi or CD127+) to short-lived (KLRG1+) CD8+ T cells compared with their ATM−/− counterparts, although still deficient compared with ATM+/+ animals (Figure 4b). This was true for both total CD8+ (Supplementary Figure S3b) as well as viral-specific (GP33+) CD8+ T cells (Figure 4b), regardless of more CD44hi CD8+ T cells present (Supplementary Figure S3b). Thus, mCAT partially rescues the defective memory response to LCMV infection observed in ATM−/− mice.
Figure 4.
Analysis of CD8+ T cell memory-differentiation phenotypes in vivo and in vitro. (a) Analyses of LCMV-specific Gp33+ splenic CD8+ T cells on day 30 post-LCMV infection of ATM+/+ (white-filled bar, n = 10), ATM+/+ mCAT+ (white filled with black stripes, n = 7), ATM−/− (gray-filled bar, n = 10), and ATM−/− mCAT+ (gray filled with black stripes, n = 9) mice. (b) Percent CD62Lhi, KLRG1+ (effector-memory TEM) and CD127+ (central memory, TCM) GP33+ CD8+ T cells from the mice analyzed in a. (c) Phospho-S6 MFI of splenic CD8+ T cells 72 hours post-TCR activation in vitro and (d) number of TCR-activated CD8+ T cells after additional 72 hours of IL-15 treatment from ATM+/+ (white-filled bar, n = 5), ATM+/+ mCAT+ (white filled with black stripes, n = 4), ATM−/− (gray-filled bar, n = 5), and ATM−/− mCAT+ (gray filled with black stripes, n = 5) mice. Statistical significance is denoted by *(<0.05), **(<0.005), ***(<0.0005) or ns (not significant). IL, interleukin; LCMV, lymphochoriomeningitis virus; MFI, median fluorescence intensity; TCR, T cell receptor.
We showed previously that hyperactivation of TORC1 during T cell receptor (TCR) activation contributes to a CD8+ T-cell proliferation defect in response to interleukin (IL)-15,3 a cytokine important for memory CD8 T-cell development. Therefore, we analyzed TORC1 activity in ATM−/− mCAT+ T lymphocytes after TCR activation and upon subsequent addition of IL-15 in vitro. Similar to ATM−/− CD8+ T cells, ATM−/− mCAT+ CD8+ T cells had elevated phospho-S6 relative to ATM+/+ or ATM+/+ mCAT+ controls (Figure 4c). Thus, mCAT overexpression did not suppress the hyperactivation of TORC1 in ATM−/− CD8+ T cells in response to TCR activation. However, when we cultured TCR-activated CD8+ T cells with IL-15, we found that mCAT rescued the proliferation defect we documented previously3 in ATM−/− CD8+ T cells (Figure 4d).
Discussion
We contend that the results of this study in mice implicate mitochondrial ROS as a component of A-T disease pathology and might be a novel therapeutic angle to be pursued. Below we discuss our results showing that mCAT partially alleviates multiple pathogenic features in ATM−/− mice that are shared in human patients. We also interpret our results in terms of the recently documented role for ATM as a redox-sensing protein, and the promise and potential limitations of therapeutically targeting mitochondrial ROS for A-T in the context of other antioxidant studies.
The first significant finding of this study was the ability of mCAT to decrease the probability of ATM−/− mice developing cancer (Figure 2). Based on the analysis of T-cell populations in ATM−/− and ATM−/− mCAT+ mice (Figure 1a), we conclude that reducing mitochondrial ROS (via the mCAT transgene) delays or reduces the likelihood of double-positive cells progressing to lymphoma in ATM−/− mice. However, once these cells do progress, which still occurs in a substantial number of ATM−/− mCAT+ animals, mCAT has no obvious effect on cancer progression. These results may help explain the shape of the lifespan curve (Figure 2). That is, as is the case for ATM−/− animals, many ATM−/− mCAT+ mice die of cancer in the first 18–20 weeks, yet significantly more ATM−/− mCAT+ mice escape this fate than do ATM−/− without mCAT. Thus, mCAT is increasing the chance of an individual escaping cancer or delaying its onset beyond 300 days, but not affecting cancer progression once it starts. Finally, our results showing that double-positive cells from mice that have begun to progress toward cancer have increased mitochondrial ROS, TORC1 activity, and lactate production (Figure 1b,d,e) suggest that these features may contribute directly to the tumorigenesis process. And, that these parameters are reduced in ATM−/− mCAT+ cells, suggests this may be one mechanism that mCAT reduces the likelihood of transformation. These results are entirely consistent with those showing that the TORC1 inhibitor rapamycin delays thymic lymphoma in ATM−/− mice.12 In addition, our results likely relate to how other antioxidant treatments, including NAC, tempol, and EUK18 are beneficial in this regard.10,11,32,33 That is, mitochondrial ROS and, in particular, hydrogen peroxide (which mCAT detoxifies), not only damage mitochondria, but also can diffuse out of the organelle to cause more global cellular oxidative stress or altered redox balance that can be rectified by these more general antioxidant compounds. However, there are differences in how mCAT rescues compared with these other antioxidant treatments. For example, we observed a bi-phasic survival curve for ATM−/− mCAT+ mice (Figure 2), in which a major portion of the population dies at a rate similar to ATM−/−, whereas a subset appear to escape and live longer. This was not evident in the survival curves of ATM−/− mice treated with antioxidants NAC, EUK189 or tempol, which delay lymphoma in most or all of the animals.11,32 In addition, while NAC almost completely restored the thymic differentiation block between double-positive and single-positive cells,5 this was not significantly affected in the ATM−/− mCAT+ mice. Thus, the precise timing and mode of action of mitochondrial ROS in A-T pathology are likely different than ROS generated from other cellular sources or that accumulate and act in other compartments.
In addition to the beneficial effects on cancer development, our results clearly show that mCAT also partially rescues other salient A-T–related pathology in ATM−/− mice, including HSC and bone marrow hematopoiesis defects (Figure 3 and Supplementary Figure S2), and CD8 T-cell memory responses (Figure 4). The ability of mCAT to improve HSC function in ATM−/− is consistent with the known involvement of oxidative stress in this defect. For example, the antioxidant NAC also alleviates HSC proliferation defects in ATM−/− mice4 and Foxo3, a regulator of antioxidant responses, is defective in ATM−/− HSCs.31 Furthermore, quiescence of HSCs is regulated, in part, by TORC1 repressing mitochondrial ROS production,34 either or both of which might be disrupted in ATM−/− mice and remedied by mCAT expression. How mCAT improves memory T-cell development defects is less clear, but it does not involve restoration of deficits in peripheral CD4+ and CD8+ T cells in the ATM−/− mice (Figure 1a and Supplementary Figure S3a). And, even though we showed previously that hyperactive TORC1 signaling is involved in the ATM−/− T-cell memory phenotypes,3 mCAT does not appear to act via reducing TORC1 activity in this circumstance (Figure 4c). Thus, increased mitochondrial ROS probably affect other properties of ATM−/− CD8+ T cells during TCR and cytokine activation and/or effector to memory differentiation that involves their signaling or damaging roles that mCAT is helping to restore. One possibility worthy of speculation based on our results is via effects on metabolism. That is, mCAT reduced lactate production in ATM−/− thymocytes (Figure 1e), suggesting that mitochondrial ROS may normally signal to ATM to dampen glycolysis. This concept is supported by studies showing that ATM is activated downstream of insulin signaling and regulates TORC1 via AMPK or Akt under specific circumstances to alter cell metabolism.6,35,36
Clearly, oxidative stress is a key component of A-T pathology,4,7,10,17 yet the source and exact effects of this stress remain largely unknown. Furthermore, multiple lines of evidence have shown that ATM not only is involved in the DNA-damage response in the nucleus, but also senses ROS and cellular redox status.6,17,37 Our results implicate mitochondrial ROS in particular as a contributor to A-T–related pathology. This could be via damage caused by these reactive molecules, their roles in signaling, or both. We speculate that ATM senses mitochondrial ROS, perhaps even through its direct association with mitochondria15 and that this ROS-sensing function is needed for normal cellular antioxidant and stress defenses and perhaps also mitochondrial homeostasis, perhaps via the regulation of AMP kinase36,38 and TORC1.6,12 Disruption of this putative circuit might explain both the oxidative stress-related pathology and intrinsic mitochondrial defects observed in A-T cells.13,14,15 It is noteworthy that overexpression of superoxide dismutase, SOD1, enhances the pathology in ATM−/− mice.39 This may also be explained by our results, as overexpression of SOD1 would likely increase hydrogen peroxide, which, based on our results, is a major offending ROS in ATM−/− mice. Likewise the inability of SOD1 or SOD2 deletion to exacerbate thymic lymphoma in ATM−/− mice40 may also be explained by hydrogen peroxide being a primary pathogenic ROS, as opposed to superoxide.
Finally, our study opens the possibility that antioxidant therapies directed at mitochondrial ROS will be of therapeutic value for A-T patients. To do this, deciphering whether ROS damage or signaling malfunctions are at the heart of specific A-T pathology is a key future goal. Furthermore, the need to target specific ROS and sites of production is likewise critical, given that the signaling roles for these molecules will likely be context and tissue-specific. Finally, while our study clearly shows benefits of reducing mitochondrial ROS on A-T pathology in mice, none of the defects were rescued fully and some not at all. For example, many of the ATM−/− mice die of cancer at early ages, the restoration of bone marrow function declines with age, memory CD8+ T-cell differentiation is only partially rescued, and the differentiation defect in the thymus from double-positive to single-positive T cell is unaffected. Thus, clearly other molecular and cellular defects in A-T patients work alone or conspire with mitochondrial dysfunction and ROS to generate the full pathogenic picture, suggesting combination therapies will most likely be needed. Nonetheless, linking mitochondrial ROS to A-T pathogenesis is an important step forward and targeting mitochondrial ROS per se may be a strategy with fewer unintended consequences and side effects (e.g., dampening of normal ROS/redox signaling pathways) than more generalized antioxidant therapies.
Materials and Methods
Mouse breeding and genotyping. Mice were generated and maintained according to approved Institutional Animal Care and Use Committee protocols. To generate ATM−/− mCAT+, ATM−/−, ATM+/+, and ATM+/+ mCAT+ mice for all experiments, mCAT mice in the C57BL/6 background (obtained from Dr Peter Rabinovitch, University of Washington) were first crossed with ATM+/− mice in the 129SvEV background (obtained from Dr Howard Mount, University of Toronto) to obtain mixed background ATM+/− mCAT+ F1 mice. In all experiments, mice that were then backcrossed to the original 129SvEV ATM+/− mice at least five times were used for all experiments. Genotyping for ATM was performed according to the PCR conditions outlined by the Jackson laboratory (Bar Harbor, ME), whereas genotyping for mCAT was performed according to published protocols.19
Thymus and bone marrow analyses. Thymuses were harvested from mice and placed in a tissue culture dish on ice and single-cell suspension was prepared by pressing with a plunger of a 3-ml syringe. Cells were collected in ice-cold RPMI 1640 medium with 10% fetal bovine serum and the cell suspension was passed through a 40-micron strainer to eliminate clumps and debris. Cells were counted and 1 × 106 cells were stained for fluorescence-activated cell sorting (FACS) analyses.
For bone marrow analysis, femurs were harvested and ground with a mortar and pestle in Dulbecco's modified Eagle's medium medium with 10% fetal bovine serum, followed by filtering the single-cell suspension through a 100-µm nylon strainer to remove bone and cell debris and clumps; 1 × 106 cells were stained for FACS analyses with appropriate antibody cocktail. For HSC analyses, FITC anti-mouse Sca-1, PE-anti-mouse cKIT, and APC-anti-mouse lineage marker cocktail with antibodies against CD4, B220, TER-119, Mac-1, and Gr-1 were used. For analyzing granulocytes and monocytes, cells were doubly stained with PE-anti-mouse Gr-1 and APC-anti-mouse Mac-1. For erythrocyte populations, cells were doubly stained with PE-anti-mouse Ter119 and APC-anti-mouse CD71. For B-lymphocytes, PE-anti mouse B220 was used. All antibodies were from ebiosciences (San Diego, CA).
Macrophage differentiation in vitro. Single-cell suspensions were prepared from femurs of mice and 1 × 107 cells were cultured for a period of 7 days in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum plus 30% L929-conditioned media to promote macrophage differentiation as described.41 Media was replenished on day 4 of culture. On day 7, cells were detached from plates by incubating in cold TEN buffer (40 mmol/l Tris-HCl, pH 7.4, 150 mmol/l NaCl, 1 mmol/l EDTA) and counted under microscope after trypan blue staining.
LCMV infection. For LCMV-infection studies, 4-week-old mice (ATM−/− mCAT+, ATM−/−, ATM+/+, and ATM+/+ mCAT+) were infected with 2 × 105 pfu LCMV-Armstrong (intraperitoneally) as described.42 Spleens were harvested at day 30 post-infection after euthanizing the mice according to approved mouse protocols. Splenocytes were prepared for FACS analyses after lysing the red blood cells using 0.83% NH4Cl buffer for 2 minutes and suspended in RPMI 1640 medium containing 10% FBS, 200 mmol/l Glutamine, and 1× Penicillin/Streptomycin.
TCR activation and cytokine culturing of T lymphocytes in vitro. For in vitro experiments, fresh spleens from 12-week-old mice were harvested and total lymphocytes were prepared after lysing the red blood cells osmotically with 0.83% NH4Cl buffer for 2 minutes. Cells (1 × 105) were seeded in individual wells of 96-well plates coated with 10 µg/ml anti-CD3 and anti-CD28 antibodies in 1× phosphate-buffered saline (PBS) overnight at 4 °C. Samples were analyzed after 72 hours. When indicated, after 72-hour TCR activation, 1 × 105 CD8+ T lymphocytes were cultured in 12-well tissue culture plates with 10 pg/ml IL-15 (ebiosciences). The cells were supplemented with 10 ng/ml IL-15 at 36 hours and counted at 72 hours.
FACS analyses. For intracellular staining with phospho-S6-alexafluor 433, 1 × 106 CD8+ T cells or total splenocytes were fixed and permeabilized using the cytofix/cytoperm kit from BD (San Jose, CA). Surface staining with different markers was performed using appropriate dilutions of antibodies either as a cocktail or individually in 1× PBS buffer containing 0.5% fetal calf serum. Cells were incubated at 4 °C or on ice for 20 minutes, washed three times in 1× PBS, and resuspended in appropriate amounts of 1× PBS containing 0.1% fetal calf serum. Staining for mitochondrial and cellular ROS was performed using MitoSox (Molecular Probes, Grand Island, NY) and H2DCF-DA (Molecular Probes), respectively, on 2 × 105 cells in RPMI medium without FBS for 20 minutes followed by centrifugation and resuspending the cells in 0.1% FBS in 1× PBS. FACS analysis was performed using FACS Calibur (Yale FACS facility) and data were analyzed using FlowJo software (Ashland, OR). Median fluorescence intensities were used for comparative analyses.
Lactate measurements. L-lactate measurements were performed using the L-Lactate assay kit from Eton Biosciences (San Diego, CA). Briefly, 1 × 107 thymocytes or macrophages were extracted with 80% ethanol for 1 hour on ice. The cells were then removed by centrifugation at 10,000g for 10 minutes and 5 µl of supernatants were used for measurements. Quantification was achieved by comparison to a standard curve that was generated using known amounts of L-lactate provided in the kit.
Statistical analyses. Prism software was used to plot graphs and calculate statistics. The mean±SD was determined from at least three biological replicate samples and “P” values determined using unpaired two-tailed Student's t-test and indicated when appropriate in the figures. Values <=0.05 were interpreted as statistically significant. The Kaplan–Meier survival plot was generated using Prism software (Prism Software, Irvine, CA) and analyzed statistically using the log-rank test.
SUPPLEMENTARY MATERIAL Figure S1. Thymocyte analyses of 6-week-old ATM−/− and ATM−/− mCAT+ mice. Figure S2. Thymocyte and bone marrow analyses of long-living ATM−/− and ATM−/− mCAT+ mice. Figure S3. Analyses of CD8+ T lymphocytes from ATM−/− and mCAT+ ATM−/− mice post-LCMV infection.
Acknowledgments
The authors thank Dr Peter Rabinovitch for the mCAT mice, Dr Howard Mount for the original ATM knockout mice, Dr Sharen McKay for helpful advice and discussions, Zimei Zhang for mouse colony maintenance and genotyping, and Sharon Lin for bone marrow FACS analysis reagents. This work was supported by National Institutes of Health grant NS056206 awarded to G.S.S and The A-T Children's Project. The authors declared no conflict of interest.
Supplementary Material
Thymocyte analyses of 6-week-old ATM−/− and ATM−/− mCAT+ mice.
Thymocyte and bone marrow analyses of long-living ATM−/− and ATM−/− mCAT+ mice.
Analyses of CD8+ T lymphocytes from ATM−/− and mCAT+ ATM−/− mice post-LCMV infection.
REFERENCES
- Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L.et al. (1995A single ataxia telangiectasia gene with a product similar to PI-3 kinase Science 2681749–1753. [DOI] [PubMed] [Google Scholar]
- Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F.et al. (1996Atm-deficient mice: a paradigm of ataxia telangiectasia Cell 86159–171. [DOI] [PubMed] [Google Scholar]
- D'Souza AD, Parish IA, McKay SE, Kaech SM., and, Shadel GS. Aberrant CD8+ T-cell responses and memory differentiation upon viral infection of an ataxia-telangiectasia mouse model driven by hyper-activated Akt and mTORC1 signaling. Am J Pathol. 2011;178:2740–2751. doi: 10.1016/j.ajpath.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I.et al. (2004Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells Nature 431997–1002. [DOI] [PubMed] [Google Scholar]
- Ito K, Takubo K, Arai F, Satoh H, Matsuoka S, Ohmura M.et al. (2007Regulation of reactive oxygen species by Atm is essential for proper response to DNA double-strand breaks in lymphocytes J Immunol 178103–110. [DOI] [PubMed] [Google Scholar]
- Alexander A, Cai SL, Kim J, Nanez A, Sahin M, MacLean KH.et al. (2012ATM signals to TSC2 in the cytoplasm to regulate TORC1 in response to ROS Proc Natl Acad Sci USA 1074153–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamsler A, Daily D, Hochman A, Stern N, Shiloh Y, Rotman G.et al. (2001Increased oxidative stress in ataxia telangiectasia evidenced by alterations in redox state of brains from Atm-deficient mice Cancer Res 611849–1854. [PubMed] [Google Scholar]
- Barlow C, Dennery PA, Shigenaga MK, Smith MA, Morrow JD, Roberts LJ 2nd.et al. (1999Loss of the ataxia-telangiectasia gene product causes oxidative damage in target organs Proc Natl Acad Sci USA 969915–9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach J, Schubert R, Schindler D, Müller K, Böhles H., and, Zielen S. Elevated oxidative stress in patients with ataxia telangiectasia. Antioxid Redox Signal. 2002;4:465–469. doi: 10.1089/15230860260196254. [DOI] [PubMed] [Google Scholar]
- Reliene R., and, Schiestl RH. Antioxidant N-acetyl cysteine reduces incidence and multiplicity of lymphoma in Atm deficient mice. DNA Repair (Amst) 2006;5:852–859. doi: 10.1016/j.dnarep.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Schubert R, Erker L, Barlow C, Yakushiji H, Larson D, Russo A.et al. (2004Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice Hum Mol Genet 131793–1802. [DOI] [PubMed] [Google Scholar]
- Kuang X, Shen J, Wong PK., and, Yan M. Deregulation of mTOR signaling is involved in thymic lymphoma development in Atm-/- mice. Biochem Biophys Res Commun. 2009;383:368–372. doi: 10.1016/j.bbrc.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Eaton JS, Lin ZP, Sartorelli AC, Bonawitz ND., and, Shadel GS. Ataxia-telangiectasia mutated kinase regulates ribonucleotide reductase and mitochondrial homeostasis. J Clin Invest. 2007;117:2723–2734. doi: 10.1172/JCI31604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose M, Goldstine JV., and, Gatti RA. Intrinsic mitochondrial dysfunction in ATM-deficient lymphoblastoid cells. Hum Mol Genet. 2007;16:2154–2164. doi: 10.1093/hmg/ddm166. [DOI] [PubMed] [Google Scholar]
- Valentin-Vega YA, Maclean KH, Tait-Mulder J, Milasta S, Steeves M, Dorsey FC.et al. (2012Mitochondrial dysfunction in ataxia-telangiectasia Blood 1191490–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel GS. Expression and maintenance of mitochondrial DNA: new insights into human disease pathology. Am J Pathol. 2008;172:1445–1456. doi: 10.2353/ajpath.2008.071163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Kozlov S, Lavin MF, Person MD., and, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- Ditch S., and, Paull TT. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends Biochem Sci. 2012;37:15–22. doi: 10.1016/j.tibs.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M.et al. (2005Extension of murine life span by overexpression of catalase targeted to mitochondria Science 3081909–1911. [DOI] [PubMed] [Google Scholar]
- Woo DK, Green PD, Santos JH, D'Souza AD, Walther Z, Martin WD.et al. (2012Mitochondrial genome instability and ROS enhance intestinal tumorigenesis in APC(Min/+) mice Am J Pathol 18024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh J, Enns L, Fatemie S, Hopkins H, Morton J, Pettan-Brewer C.et al. (2011Mitochondrial targeted catalase suppresses invasive breast cancer in mice BMC Cancer 11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treuting PM, Linford NJ, Knoblaugh SE, Emond MJ, Morton JF, Martin GM.et al. (2008Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria J Gerontol A Biol Sci Med Sci 63813–822. [DOI] [PubMed] [Google Scholar]
- Someya S, Xu J, Kondo K, Ding D, Salvi RJ, Yamasoba T.et al. (2009Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis Proc Natl Acad Sci USA 10619432–19437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ.et al. (2010Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance Cell Metab 12668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintrón M, Chen T.et al. (2011Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure Circ Res 108837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS., and, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- Liyanage M, Weaver Z, Barlow C, Coleman A, Pankratz DG, Anderson S.et al. (2000Abnormal rearrangement within the alpha/delta T-cell receptor locus in lymphomas from Atm-deficient mice Blood 961940–1946. [PubMed] [Google Scholar]
- Kuang X, Yan M, Liu N, Scofield VL, Qiang W, Cahill J.et al. (2005Control of Atm-/- thymic lymphoma cell proliferation in vitro and in vivo by dexamethasone Cancer Chemother Pharmacol 55203–212. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB.et al. (2012Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations Free Radic Biol Med 521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishiya A, Ito M, Aburatani H, Motoyama N, Ikeda K., and, Watanabe K. Ataxia telangiectasia mutated (Atm) knockout mice as a model of osteopenia due to impaired bone formation. Bone. 2005;37:497–503. doi: 10.1016/j.bone.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Yalcin S, Zhang X, Luciano JP, Mungamuri SK, Marinkovic D, Vercherat C.et al. (2008Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells J Biol Chem 28325692–25705. [DOI] [PubMed] [Google Scholar]
- Reliene R., and, Schiestl RH. Antioxidants suppress lymphoma and increase longevity in Atm-deficient mice. J Nutr. 2007;137 suppl. 1:229S–232S. doi: 10.1093/jn/137.1.229S. [DOI] [PubMed] [Google Scholar]
- Browne SE, Roberts LJ 2nd, Dennery PA, Doctrow SR, Beal MF, Barlow C.et al. (2004Treatment with a catalytic antioxidant corrects the neurobehavioral defect in ataxia-telangiectasia mice Free Radic Biol Med 36938–942. [DOI] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y.et al. (2008TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species J Exp Med 2052397–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armata HL, Golebiowski D, Jung DY, Ko HJ, Kim JK., and, Sluss HK. Requirement of the ATM/p53 tumor suppressor pathway for glucose homeostasis. Mol Cell Biol. 2010;30:5787–5794. doi: 10.1128/MCB.00347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Connors KE., and, Yang DQ. AICAR induces phosphorylation of AMPK in an ATM-dependent, LKB1-independent manner. Mol Cell Biochem. 2007;306:239–245. doi: 10.1007/s11010-007-9575-6. [DOI] [PubMed] [Google Scholar]
- Yan M, Qiang W, Liu N, Shen J, Lynn WS., and, Wong PK. The ataxia-telangiectasia gene product may modulate DNA turnover and control cell fate by regulating cellular redox in lymphocytes. FASEB J. 2001;15:1132–1138. doi: 10.1096/fj.00-0601com. [DOI] [PubMed] [Google Scholar]
- Kuang X, Yan M, Ajmo JM, Scofield VL, Stoica G., and, Wong PK. Activation of AMP-activated protein kinase in cerebella of Atm-/- mice is attributable to accumulation of reactive oxygen species. Biochem Biophys Res Commun. 2012;418:267–272. doi: 10.1016/j.bbrc.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter Y, Rotman G, Lotem J, Elson A, Shiloh Y., and, Groner Y. Elevated Cu/Zn-SOD exacerbates radiation sensitivity and hematopoietic abnormalities of Atm-deficient mice. EMBO J. 2001;20:1538–1546. doi: 10.1093/emboj/20.7.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erker L, Schubert R, Elchuri S, Huang TT, Tarin D, Mueller K.et al. (2006Effect of the reduction of superoxide dismutase 1 and 2 or treatment with alpha-tocopherol on tumorigenesis in Atm-deficient mice Free Radic Biol Med 41590–600. [DOI] [PubMed] [Google Scholar]
- West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P.et al. (2011TLR signalling augments macrophage bactericidal activity through mitochondrial ROS Nature 472476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD., and, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Thymocyte analyses of 6-week-old ATM−/− and ATM−/− mCAT+ mice.
Thymocyte and bone marrow analyses of long-living ATM−/− and ATM−/− mCAT+ mice.
Analyses of CD8+ T lymphocytes from ATM−/− and mCAT+ ATM−/− mice post-LCMV infection.