Abstract
Genetic therapies, including transfected immune cells and viral vectors, continue to show clinical responses as systemically deliverable and targeted therapeutics, with the first such approaches having been approved for cancer treatment. The majority of these employ cytokine transgenes. However, expression of cytokines early after systemic delivery can result in increased toxicity and nonspecific induction of the immune response. In addition, premature immune-mediated clearance of the therapy may result, especially for viral-based approaches. Here, it was initially verified that cytokine (interleukin (IL)2) or chemokine (CCL5) expression from a systemically delivered oncolytic virus resulted in reduced oncolytic activity and suboptimal immune activation, while IL2 also resulted in increased toxicity. However, all these limitations could be overcome through incorporation of exogenous regulation of cytokine or chemokine transgene function through fusion of a small and externally controllable destabilizing domain to the protein of interest. Regulation allowed an initial phase without cytokine function, permitting enhanced delivery and oncolytic activity before activation of cytokine function and a subsequent phase of enhanced and tumor-targeted immunotherapeutic activity. As a result of this exogenous regulation of cytokine function, both oncolytic and immune-mediated mechanisms of action were optimized, greatly enhancing therapeutic activity, while toxicity was significantly reduced.
Introduction
Although the expression of cytokine and chemokine transgenes has been incorporated into many biological cancer therapies, their use has gathered significant attention recently as exciting clinical responses have been reported both with immune cell1 and oncolytic virus2 therapies expressing a variety of different cytokines. The first engineered cytokine-expressing dendritic cell (DC) vaccine has recently been approved in the United States,1 while the field of oncolytic virology has made significant strides in recent years with the publication of exciting phase II clinical results from several different cytokine-expressing vectors,2,3 including confirmation of the capacity for systemic viral delivery to the tumor in the clinic.4 Multiple vectors are currently undergoing phase IIb or phase III testing.5,6,7 The expression of selected cytokines from these biological therapies has demonstrated clear therapeutic benefits, however to date, little effort has been made to externally regulate the level, biodistribution, or kinetics of cytokine production.
Because early cytokine expression from either immune cell or viral therapies will likely be systemic or nonspecific, some toxicity may be expected. This potential for additional toxicity has not been explored in detail previously. Furthermore, expression of immunostimulatory cytokine transgenes from oncolytic viruses typically leads to a reduced capacity for viral replication within the tumor and earlier clearance of the therapy.8,9,10,11,12,13 This is despite the fact that cytokine expression still usually results in overall increases in therapeutic activity. Additional advances in therapeutic activity would therefore be expected through approaches that increase the immunotherapeutic potential while maintaining oncolytic activity.
It was hypothesized that oncolytic vectors typically function in two phases, with an initial directly oncolytic phase, characterized by rapid and selective viral replication and direct tumor cell destruction, followed by the induction of a potent immune response within the tumor, with a reduction in localized immunosuppression and an in situ vaccination effect. Because any approach that selectively enhances one of the phases (such as immune suppression to allow increased viral replication or expression of immunostimulatory factors to enhance the immune response) will likely lead to reduced effectiveness in the other phase, we restricted the activity to one phase only.
A protein-destabilizing domain14 was fused to different immunostimulatory molecules expressed as transgenes from an oncolytic vaccinia virus. This domain leads to rapid proteasomal degradation of the protein, so effectively blocking its functional capabilities.10,14,15 The addition of a small molecule (Shield-1; S-1) that specifically binds this protein region leads to stabilization of the protein, thus restoring its function. In this way, it is possible to exogenously activate the function of the protein produced by any genes or transgenes expressed from a virus or immune cell in both a targeted and a rapid and reversible fashion. Here, we demonstrate that this approach can result in multiple safety and therapeutic benefits.
Results
Viral transgene expression early after systemic delivery can lead to toxicity
Recombinant interleukin (IL)2 is approved for the treatment of several different cancers, including renal cancer and melanoma,16 however, the therapeutic benefits of its use are often countered by severe toxicities. This makes it an ideal candidate for tumor-selective expression from an oncolytic virus, such as the oncolytic vaccinia strain vaccinia virus double deleted (vvDD). This is a Western Reserve strain of vaccinia with deletions in the viral thymidine kinase and vaccinia growth factor genes, providing it with highly tumor-selective replication and gene expression.17,18 As such a version of vvDD was constructed expressing luciferase (for preclinical imaging of viral gene expression)19 and mIL2 expressed from the exclusively late p11 viral promoter,20 such that IL2 expression is linked to viral replication.
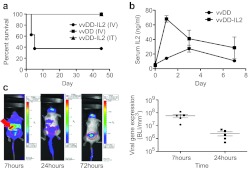
However, it was discovered (Figure 1a) that vvDD-IL2 delivered via tail vein injection to treat syngeneic tumor bearing immunocompetent mice resulted in significant toxicity, with mice becoming hunched and lethargic within 24 hours and more than 60% of the mice requiring sacrifice within 5 days (due to loss of >20% of body weight). The rapid onset of this toxicity, and the fact that it was not observed in mice treated with equivalent virus without IL2 expression, suggested that it was mediated by IL2 cytokine expression rather than viral replication. It was also noted that toxicity only occurred when virus was delivered intravenously, with intratumoral injection producing no significant toxicity, indicating that systemic rather than localized (tumor-specific) IL2 expression was mediating toxicity. It was further observed that an early peak in serum IL2 levels was seen only in mice treated systemically with vvDD-IL2 (Figure 1b). Of interest, this peak in serum IL2 levels was present only at 24 hours after viral delivery suggesting transient and early viral transgene expression was responsible for the acute toxicity. Of note, several mice treated with vvDD-IL2 for the enzyme-linked immunosorbent assay study needed to be killed before the 7-day timepoint was reached indicating that the acute toxicity seen with IL2 expression was not related to the presence of tumor.
Figure 1.
Expression of interleukin (IL)2 from oncolytic vaccinia produces unexpected acute toxicity. (a) Survival of mice (BALB/c) bearing RENCA subcutaneous tumors and injected intravenously (tail vein) or intratumoral with 3 × 108 PFU of the indicated viruses (n = 5 per group). Animals were killed if weight dropped below 20% of starting weight. Vaccinia virus double deleted (vvDD) and vvDD-IL2(IT) groups displayed 100% survival. Intravenous delivery of IL2 expressing virus resulted in significantly greater toxicity (P = 0.036). (b) Serum levels of IL2 were determined after intravenous delivery of 3 × 108 PFU of the indicated viruses into nontumor bearing BALB/c mice (n = 4 per group). vvDD-IL2 produced significantly greater levels of systemic IL2 (P < 0.0001 at 24 hours). (c) Bioluminescence imaging of viral luciferase gene expression demonstrates early peak in primarily splenic signal. Mice (BALB/c bearing subcutaneous RENCA tumors (circled)) were treated intravenously with vvDD-IL2 (that also expresses luciferase) and bioluminescence images taken at the indicated times. At 7 hours, a large signal is seen from the spleen (arrowed). The spleen signal is quantified for five mice (right panel) and is significantly greater at 7 hours (P = 0.014).
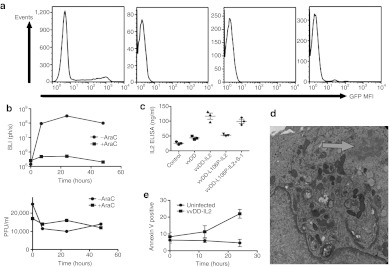
Because the p11 promoter is tied to late gene expression, IL2 production should be restricted to cells that actively replicate vvDD. Bioluminescence imaging was therefore used to help identify the cells producing IL2. It was noted that an early peak of viral gene expression primarily from within the spleen was witnessed at around 7 hours after viral delivery, and that this was significantly reduced even by 24 hours after delivery (Figure 1c). This early peak of viral gene expression was unexpected and has not been previously reported. It was determined that this gene expression was produced primarily by infection of DCs and macrophages (Figure 2a and Supplementary Figure S1 online), and that it occurred despite a lack of viral replication (Figure 2b).
Figure 2.
Viral acute toxicity is mediated by cytokine transgene production from dendritic cells (DCs). (a) Vaccinia preferentially infects monocyte lineage cells after exposure to a mixed population of lymphocytes. Human peripheral blood was exposed to vaccinia expressing green fluorescent protein (GFP) and the percentage of GFP+ cells in different subsets revealed that CD14+ cells (DC and macrophages) were preferentially infected; (b) in vitro viral replication and gene expression in DCs. vaccinia virus double deleted (vvDD) expressing luciferase was used to infect DCs (MOI 1.0) in culture with (left panel) bioluminescence imaging performed at the indicated times to determine gene expression and (right panel) plaque assay to titer level of viral replication. High level gene expression was seen despite a lack of viral replication. The level of viral gene expression from infected DCs was greatly reduced as a result of addition of AraC (cytosine arabinoside; 40 ug/ml) indicating that late gene expression is possible despite a lack of production of progeny virus. All experiments repeated in triplicate. (c) Interleukin (IL)2 expression in human DCs. DCs were expanded from human peripheral blood and infected with the indicated viruses with and without S-1 (MOI of 5.0). Media was collected after 24 hours for assay of IL2 levels by enzyme-linked immunosorbent assay. (d) Transmission electron microscopy of DC infected with vvDD and collected 24 hours after infection. Immature viral particles are formed (arrowed). (e) Apoptosis of DCs after vvDD infection. High levels of apoptosis occurred within 24 hours of infection. Vaccinia strain vvDD-GFP was used to infect DC and cells were collected amnd stained with Annexin V antibody at the indicated times. GFP, green fluorescent protein; S-1, Shield-1.
This toxicity may however represent a major concern for the future development of oncolytic viruses, and potentially other gene therapy approaches, as more biological therapies expressing similar transgenes are applied systemically in a clinical setting.
In vitro regulation of cytokine and chemokine expression from oncolytic vaccinia virus
Several novel viral vectors were constructed to test the hypothesis that regulating the function of the cytokine or immune modulating gene expressed from an oncolytic vaccinia virus would enhance the safety and therapeutic benefits. In one strain (vvDD-L106P-IL2), the IL2 gene was fused to the previously described L106P peptide degradation domain,14 whereas in the second strain (vvDD-L106P-CCL5), the chemokine CCL5 (RANTES) was similarly fused to the L106P domain (Supplementary Figure S3 online). Addition of the small molecule Shield-1 (S-1) that binds to and stabilizes the L106P domain was confirmed to restore stability to the protein of interest (either IL2 or CCL5) (Supplementary Figure S2a,b online). In vitro one-step viral replication assays confirmed that cytokine or chemokine expression did not directly effect viral replication (Supplementary Figure S2c,d online).
Further studies looked to define the mechanisms mediating the production of IL2 in the absence of viral replication. Human DCs were expanded from buffy coats and infected with different viral strains and the production of IL2 was measured by enzyme-linked immunosorbent assay (Figure 2c). It was found that vvDD-IL2 and vvDD-L106P-IL2 in the presence of S-1 produced high levels of IL2, providing further in vitro support that DC can act to express the IL2 transgene, despite the lack of viral replication. Also, of note, whereas vvDD-IL2 uses the p11 promoter to drive IL2 expression, vvDD-L106P-IL2 incorporates the p7.5 promoter and luciferase was driven by the pSE/L promoter, indicating that multiple promoters are all functionally active in DC despite the lack of viral replication.
Further transmission electron microscopy studies determined that immature viral particles were indeed formed in the DC (Figure 2d), however, these did not appear to process to the mature form, indicating that DC might block viral replication at a step in the viral replication cycle distinct from the switch from early to late promoter expression that is normally associated with susceptibility or resistance to viral replication. Finally, an apoptosis assay (Figure 2e) indicated that infected DC underwent high levels of apoptosis subsequent to the formation of immature viral particles.
Regulation of IL2 expression enhances safety of oncolytic vaccinia
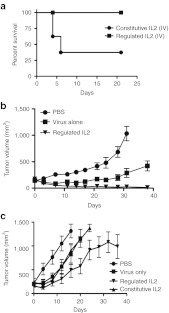
The expression of the L106P fusion to IL2 was therefore tested in vivo in the context of regulated transgene expression from an oncolytic vaccinia strain. It was found that vvDD-L106P-IL2 displayed no observable toxicity when delivered systemically without the addition of S-1, as expected as IL2 was destabilized (Figure 3a), while constitutive addition of S-1 resulted in toxicity equivalent to that seen with vvDD-IL2 (Figures 1a and 3a). Alternatively, however, if S-1 was not added until 72 hours after the viral therapy (thus stabilizing IL2 function at times after initial systemic viral infection and gene expression has been cleared, and when viral gene expression is primarily restricted to the tumor (Figure 1c)) no signs of toxicity were observed (Figure 3a). This capacity to regulate and confine IL2 expression to the tumor through destabilizing of protein activity during the first 72 hours after viral systemic delivery was therefore sufficient to prevent the associated toxicity from IL2 production within lymphoid tissues at early time points.
Figure 3.
Regulated interleukin (IL)2 activity results in a loss of acute toxicity and enhanced therapeutic effect. (a) Delayed stabilization of function resulted in a loss of acute toxicity. Mice (BALB/c) were treated with 3 × 108 PFU of vaccinia virus double deleted (vvDD)-L106P-IL2 via tail vein injection (n = 5 per group). IL2 protein is unstable in the absence of exogenously added Shield-1 (S-1). S-1 was delivered (intraperitoneal delivery) every 48 hour starting either before viral injection (constitutive IL2 function) or starting 72 hours after viral treatment (regulated IL2 function). Regulated IL2 activity resulted in a loss of the acute early toxicity seen with constitutive activity (P = 0.002). (b) Enhanced therapeutic effect of regulated IL2 function. Mice (C57/BL6 bearing subcutaneous MC38 tumors) were treated via tail vein injection of 3 × 108 PFU of vvDD or vvDD-L106P-IL2 with S-1 added before virus delivery (constitutive IL2) or 72 hours after viral delivery (regulated IL2) (n = 10 per group). Tumor growth was followed by caliper measurement. Constitutive IL2 treatment resulted in significant toxicity and 8 of 10 mice were euthanized within 7 days of treatment, meaning insufficient numbers were available to follow therapeutic effect. The regulated IL2 group produced significant greater therapeutic effect than any other group (P < 0.05 from day 21 onwards and 8 of 10 complete responses at the end of the experiment). (c) A second more aggressive tumor model (BALB/c mice bearing RENCA tumors, n = 8 per group) also displayed the therapeutic benefit of regulated IL2 activity. Mice were treated as before. In this model four of eight mice in the constitutive IL2 treatment group were killed due to acute treatment related toxicity, and were removed from the study, tumor growth in the remaining mice is plotted. The regulated IL2 treatment group displayed significantly greater therapeutic effect (P < 0.05) from day 20 onwards, and three of eight mice displayed complete responses.
Anti-tumor effects of regulated IL2 expression
To examine the effects of regulation of IL2 on the therapeutic activity of the oncolytic vector, several tumor models were examined. In both cases, virus was applied systemically (via tail vein) and S-1 was applied via intraperitoneal injection either (i) every 48 hours (for a total of 8 days) starting before viral delivery (resulting in constitutive IL2 activity); (ii) every 48 hours (for a total of 8 days) starting 72 hours after viral delivery (regulated IL2 activity); or (iii) not at all (virus alone; no IL2 activity). In the first model tested (MC38 tumors implanted subcutaneously into C57/BL6 mice) (Figure 3b), virus alone provided a significant therapeutic benefit, but all animals relapsed and eventually had to be killed. However, mice treated with vvvDD-L106P-IL2 with S-1 added after 72 hours (regulated IL2) displayed not only a significantly enhanced therapeutic benefit relative to virus alone, but 80% of the mice in this group (8 of 10) displayed complete responses, and remained tumor free up to 90 days after the treatment. In this experiment, mice treated with vvDD-L106P-IL2 and S-1 starting before the viral treatment (constitutive IL2 activity) displayed significant toxicity, with all but two mice requiring killing within 7 days of treatment, meaning that therapeutic effects could not be determined.
In a second experiment, RENCA tumors were implanted subcutaneously into BALB/c mice (Figure 3c). Again all treatments resulted in some therapeutic advantage over the phosphate-buffered saline (PBS) controls. As before, the group receiving vvDD with constitutive IL2 activity suffered from treatment related toxicities, and 50% of the animals had to be killed (the tumor growth of the remaining animals was plotted). However, the group that received virus with regulated IL2 (i.e., with IL2 activity stabilized from 72 hours after the viral delivery) displayed significantly greater therapeutic benefit than any other treatment, including 38% (three of eight) showing durable complete responses. Our results therefore demonstrate that temporally regulated expression of the inflammatory cytokine IL2 in conjunction with oncolytic vaccinia activity provided significantly improved therapeutic activity, in addition to the greatly improved safety profile.
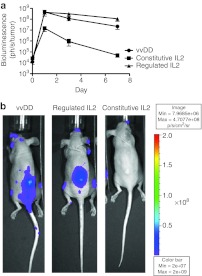
Regulating IL2 results in increased tumor-specific replication of vvDD
One possible factor mediating the enhanced therapeutic benefit seen after regulating the expression of IL2 could be the capability of the virus to establish a more robust initial infection within the tumor. In addition to acute early toxicity, constitutive IL2 activity may result in premature clearance of the virus from the tumor, thus directly reduced the oncolytic capacity of the vectors. This was examined through imaging of viral luciferase gene expression from different viral constructs (Figure 4a), with constitutive IL2 activity resulting in over 100-fold lower viral gene expression from within the tumor at 72 hours after delivery of the virus (in those animals that were not killed because of the increased toxicity). As expected, destabilizing IL2 function for the first 72 hours after viral delivery allowed an initial phase of extensive viral replication and spread within the tumor equivalent to vvDD virus alone (Figure 4a). Of note, the addition of S-1 at this time (72 hours after viral delivery) did not lead to a rapid immune-mediated clearance of the virus from the tumor, with the L106P-IL2 expressing vector retaining luciferase gene expression in the tumor at levels at least equivalent to vvDD used alone over subsequent days. However, restricting IL2 function to times from 72 hours after initial viral delivery appeared to result in more tumor-specific viral gene expression, with viral infection patterns limited to the tumor, whereas vvDD alone displayed increased secondary spread to organs and tissues outside of the tumor at later times (when an athymic nu−/nu− mouse strain was used) (Figure 4b).
Figure 4.
Regulated interleukin (IL)2 function resulted in improved tumor-specific viral replication. (a) Mice (BALB/c with RENCA tumors) were treated with 3 × 108 PFU of vaccinia virus double deleted (vvDD)-L106P-IL2 (expressing luciferase) and Shield-1 added as before (n = 5 per group). Viral gene expression from within the tumor was quantified by bioluminescence imaging of viral luciferase expression (not regulated). It was seen that constitutive IL2 function resulted in significantly (P < 0.001) reduced viral gene expression from within the tumor by 1 day after viral treatment. Regulated IL2 resulted in equivalent viral gene expression from within the tumor to virus with no IL2 activity. (b) In a second experiment athymic nu−/nu− mice with RENCA tumors were treated as before (n = 3 per group). It was noted that at later time points (7 days after viral treatment), regulated IL2 function led to less secondary spread of virus from the tumor (only seen in athymic nu−/nu− mice), indicating more restricted viral replication. Constitutive IL2 expression resulted in very low viral gene expression at this time.
Regulating IL2 activity leads to favorable changes in immune activation profiles
Because regulated IL2 results in significantly greater anti-tumor effects than any other approach tested, it is presumed that IL2 is acting to enhance the immunotherapeutic action of the therapies. This was examined through determination of several immune parameters.
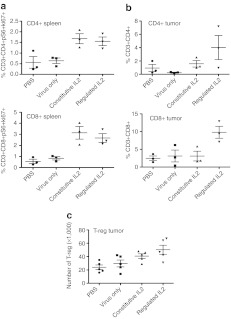
It was observed that the level of activated (Ki67+pS6+) CD4+ or CD8+ T-cells in the spleen at 7 days after the treatment with the different therapies was significantly higher for all therapies with IL2 activity, either constitutive or regulated (Figure 5a), highlighting the additional immune activation potential of the IL2 function. However, when the levels of CD4+ and CD8+ cells in the tumor itself were examined, it was noted that only the regulated IL2 treatment group resulted in additional increases in tumor infiltration of T-cell populations (Figure 5b). This may be due to the IL2 expression being exclusively from within the tumor after external regulation of the IL2 activity, meaning that the immune response is better targeted against the tumor.
Figure 5.
Regulated interleukin (IL)2 activity enhanced the tumor-targeted immune response. Tumor and spleen were collected from female BALB/c mice bearing subcutaneous RENCA tumors 7 days after animals were injected systemically with PBS or 3 × 108 PFU of vaccinia virus double deleted (vvDD) or vvDD-L106P-IL2. Shield-1 was used to stabilize IL2 function immediately after the administration of virus or 72 hours after the administration of virus as before. (a) Splenic lymphocytes were stained using antibodies against CD3, CD4, CD8, Ki-67, and pS6. Levels of activated CD4+ and CD8+ T-cells increased for treatments with both constitutive (P = 0.019 and 0.017) and regulated IL2 (P = 0.018 and 0.013) relative to vvDD alone. (b) Tumor-infiltrating T-cell lymphocytes were stained using antibodies against CD3, CD4, and CD8. Constitutive IL2 expression did not significantly increase tumor levels of either CD8+ or CD4+ relative to vvDD (P = 0.99 and 1.02), whereas regulated IL2 significantly increased the levels of CD8+ T-cells (P = 0.049), while CD4+ increases trended higher (P = 0.054) n = 3. (c) Tumor-infiltrating regulatory T-cells after treatment with different viral strains. Tumors as before were also stained for T-reg after permeabilization of dissociated cells (CD4+CD25+FoxP3+), levels of T-reg in the tumors are shown. PBS, phosphate-buffered saline.
It is also known that IL2 mediates the expansion of regulatory T-cells. Further experiments examined the effects of different viral treatments on the levels of T-regs within the tumor (Figure 5c). Although there was a trend toward increased T-reg levels in the tumor when IL2 was expressed from the viral therapy, this was not significant.
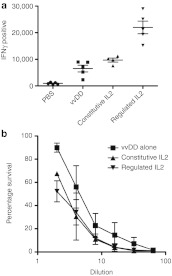
Furthermore, when the level of cytotoxic T-lynphocytes targeting tumor antigens were measured, it was confirmed that regulated IL2 activity resulted in significantly higher induction of anti-tumor cytotoxic T-lynphocyte than any other approach (Figure 6a). It is possible that constitutive expression of IL2 results in more systemic immune activation at times before tumor-selective replication of the virus, and that this led to a greater antiviral immune response without necessarily increasing targeting of tumor antigens.
Figure 6.
Regulated interleukin (IL)2 activity results in enhanced anti- tumor CTL response and reduced antiviral neutralizing antibody response. (a) Mice (BALB/c bearing RENCA tumors) were treated as before (n = 5 per group) and killed at 10 days after initial treatments. Anti-tumor CTL activity was determined via exposure of splenocytes to irradiated RENCA cells in an interferon (IFN)-γ ELISPOT assay. Regulated IL2 produced significantly greater lymphocytes induced to produce IFN upon exposure to tumor antigens than constitutive IL2 (P = 0.031). (b) Serum collected from the same animals was used to quantify levels of circulating antiviral neutralizing antibodies. Both groups expressing functional IL2 (regulated or constitutive activity) were found to have reduced levels of circulating antibody at this time. CTL, cytotoxic T-lynphocyte; ELISPOT, enzyme-linked immunosorbent spot.
The antiviral neutralizing antibody response was reduced in both constitutive and regulated IL2 expressing virus groups relative to virus alone, possible due to IL2 expression inducing a more Th1 weighted immune response (Figure 6b).
Regulation of chemokine CCL5 function also enhances therapeutic benefits
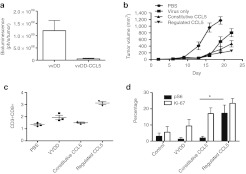
To demonstrate that external regulation of the function of immune modulating factors other than IL2 also produce therapeutic benefits the vvDD-L106P-CCL5 viral construct was tested in vivo in mouse tumor models (Figure 7). Unlike IL2, Constitutive CCL5 activity did not produce significant acute toxicity. However, it was seen that constitutive CCL5 expression did result greatly in reducing early viral gene expression from the tumor (Figure 7a) indicating that an inability to successfully colonize the tumor may be a common trait for oncolytic viruses expressing different immune activating transgenes. In addition, regulating CCL5 activity such that protein function is stabilized at times from 72 hours post-therapeutic delivery resulted in significantly greater overall therapeutic benefit than for either virus alone or for constitutive CCL5 expression (Figure 7b).
Figure 7.
Regulated CCL5 (RANTES) activity also results in therapeutic benefits. (a) Mice (BALB/c bearing RENCA tumors) were treated with vaccinia virus double deleted (vvDD) or vvDD-L106P-CCL5 with Shield-1 added from times before viral therapy (constitutive CCL5 activity) (n = 5 per group). These viruses also express luciferase and bioluminescence imaging was used to quantify viral gene expression at 72 hours after initial treatment. It was seen that (as for constitutive IL2 activity), CCL5 expression significantly reduced the level of initial viral infection, replication and gene expression from within the tumor (P = 0.024). (b) Regulated CCL5 results in greater therapeutic activity. Mice (BALB/c bearing RENCA tumors) were treated intravenously with PBS, vvDD or vvDD-L106P-CCL5 with Shield-1 added from before viral therapy (constitutive CCL5) or starting at 72 hours after viral therapy (regulated CCL5) (n = 8 per group). Subsequent tumor growth was followed by caliper measurement. Regulated CCL5 function resulted in significantly greater therapeutic activity than any other group) (P < 0.05 from day 22 onwards). (c) Enhanced immune activity in the tumor with regulated CCL5. Mice treated as before were sacrificed at 7 days after viral treatment and tumors recovered and dissociated for flow cytometry analysis of tumor infiltrating lymphocytes. (d) Regulated CCL5 activity resulted in greater overall numbers of tumor infiltrating CD3+CD8+ T-cells (left panel; P = 0.0003 for regulated vs. constitutive CCL5 expression) and a greater number of CD3+CD8+ tumor cells displaying both Ki-67 and phospho-S6 markers, a phenotype associated with greater effector and memory activity (P = 0.02 for regulated vs. constitutive CCL5 expression). N = 3. PBS, phosphate-buffered saline.
Regulated CCL5 expression also produced significant advantages in the level, type, and targeting of the immune response induced. It was determined, as for IL2, that significantly greater numbers of both total and activated T-cells were recovered from the tumor after the regulation of CCL5 activity (Figure 7c). Of note, CD8+ T-cells demonstrated greater levels of both Ki-67 and phosphorylated S6, markers associated with effective cytotoxic T-lynphocyte and memory immune response.21,22
Discussion
An improved understanding of the functioning of the immune system has determined differential roles for many cytokines during distinct phases of an immune response and in different tissues or lymphoid compartments, meaning that greater control over cytokine transgene expression would likely enhance therapeutic benefits for many biological therapies incorporating cytokine expression. Furthermore, expression of cytokines or other immune modulators from viral vectors, although frequently enhancing therapeutic benefit also often result in their premature clearance.
Inflammatory cytokines known to have potent anti-tumor effects (such as IL2) can severely compromise the activity of oncolytic viral therapies by decreasing the persistence of the oncolytic agent. It is therefore highly desirable to have a system where the oncolytic virus can produce the anti-tumor cytokine only after the peak of its lytic replication cycles, so that the in situ amplification of the therapeutic agent within the tumor mass is not compromised by increased immune-mediated clearance of the input virus. For these reasons, the ability to temporally regulate transgene expression from a viral vector may be essential for optimal activity.
Furthermore, here we demonstrate that some cytokines expressed from oncolytic viruses can be produced systemically at times early after infection leading to significant toxicity. Oncolytic viral vectors based on strains of vaccinia and herpes simplex virus are currently in advanced clinical testing, yet both DCs and macrophages can take up these viruses after systemic delivery,23,24 a prerequisite for the acute toxicity demonstrated here. Indeed, both vaccinia and herpes simplex virus expressing granulocyte macrophage colony stimulating hormone have been shown to induce early fevers and related adverse events in clinical testing and the high level of granulocyte macrophage colony stimulating hormone production was eventually dose limiting.3,25 It is possible that vectors expressing other cytokines may also result in even more severe toxicities, while the capability to block early cytokine function might limit this early toxicity.
The observation that even exclusively late promoters can result in viral gene expression from DC, despite no measurable viral replication is unexpected, and indicates that the use of late promoters may not be sufficient to limit transgene expression to cells that actively replicate the virus. Although not the primary focus of this work, some effort was made to define the mechanisms mediating this gene expression. It appeared that viral replication in DC was not blocked at the expected switch from early to late viral promoter expression, but instead early progeny viral particles began to form, but were unable to process successfully to mature virions. Instead the infected DC underwent apoptosis. It is still unknown exactly how the block is controlled, whether all DC subsets respond in the same way and how this affects viral pathogenicity or the immune response. These questions are being actively examined.
A simple, tunable, reversible, and externally controlled system for regulating protein function was therefore incorporated into different oncolytic vaccinia vectors. The system itself has been described previously10,14 and involves the fusion of a small destabilizing or degradation domain to a protein of interest, such that the protein is actively transcribed, but rapidly targeted for proteasomal degradation, meaning that protein function is blocked at the level of protein stability. The addition of a small molecule (S-1) can bind to the degradation domain, shielding it from the proteasomal pathway, and so restoring protein functional activity. Although the clinical use of S-1 will undoubtedly add complexity to the therapy, the S-1 small molecule has no natural cellular targets26 and has previously demonstrated safety in phase I testing27 and so it is believed this approach is directly and rapidly translatable in next generation vectors and that clinical use of S-1 is feasible. Alternatively the commonly used antibiotic rapamycin can substitute for S-1, simplifying clinical translation, but possibly confusing any immunotherapeutic approach.
We previously demonstrated that this system can be used to externally regulate protein function in mouse models.10 Here we demonstrate the capacity for external regulation of transgene function to enhance the safety and activity of oncolytic vaccinia strains on multiple levels, and for different immune modulators. Advantages include a reduction in acute toxicity, increased early viral infection and replication within the tumor, and induction of an enhanced and more targeted immune response.
In these experiments, the cytokine activity was destabilized over the first 72 hours after systemic viral delivery. This was designed to reduce toxicity related to transgene expression in lymphoid tissues over the first 24 hour after delivery (for IL2 expression), and because the peak of viral gene expression from the tumor (when viral strains without a therapeutic transgene were used) occurs at around 72 hours. This allows an optimal “oncolytic ” phase of therapeutic activity to complete before enhancing immune activation. However, it is likely that this approach would be broadly applicable to multiple biological therapies beyond oncolytic viral vectors expressing therapeutic cytokine transgenes. Immune cells transduced to express immune modulating proteins or any gene therapy system would be expected to benefit from external regulation of protein function, with different regimens of protein stabilization expected to enhance safety and/ or activity of different therapeutics. The fact the system employed is simple and protein stabilization is both rapid and reversible, and even tunable, makes this a potentially exciting approach for multiple different therapies.
Materials and Methods
Virus strains. Several novel vaccinia strains were constructed for this work, based on the Western Reserve vaccinia strain (BEI Resources; Manassas, VA). All strains contained mutations in both the viral growth factor and thymidine kinase genes and the firefly luciferase gene expressed from the synthetic vaccinia promoter pSE/L (vvDD). The pSE/L promoter contains both early and late promoter elements,28 however, late expression from this promoter is over 30-fold stronger than early gene expression, meaning that robust luciferase expression is likely to indicate viral replication. In strain vvDD-IL2, murine IL2 is expressed from the late vaccinia promoter p11; in strain vvDD-L106P-IL2, murine IL2 with an N-terminal fusion to the L106P degradation domain was expressed from the vaccinia p7.5 promoter; in strain vvDD-L106P-CCL5, murine CCL5 (RANTES) with an N-terminal fusion to the L106P degradation domain was expressed from the vaccinia p7.5 promoter. All transgenes were cloned into the locus of the viral thymidine kinase gene (Supplementary Figure S3 online). Virus was titered by plaque assay on BSC-1 cell line and growth kinetics examined on MC38 cells (ATCC, Manassas, VA). AraC (cytosine arabinoside, Sigma-Aldrich, St Louis, MO) was added at 40 ug/ml at the time of infection in some experiments.
DC culture and transmission electron microscopy. DCs were obtained from buffy coats obtained from the Central Pittsburgh bloodbank under IRB approved protocol. CD14+ cells were isolated from the lymphocyte fraction using microbeads (Miltenyi Biotec, Cambridge, MA) before being cultured in granulocyte macrophage colony stimulating hormone and IL4 for 6 days.
DCs were fixed at different times after infection with vaccinia strains with 2.5% glutaraldehyde in PBS, for 1 hour at room temperature before washing and treatment with 1% OsO4. Cells were then dehydrated, treated with epon and polymerized in beam capsules before sectioning and staining for transmission electron microscopy.
In vivo tumor models. All animal studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Female BALB/c, C57/BL6, and athymic nu−/nu− mice (4–8 weeks old) were purchased from Jackson Laboratories (Bar Harbor, Maine). Tumor cell lines RENCA, or MC38 (ATCC) were implanted subcutaneously at 5 × 105 cells per animal into BALB/c or C57/BL6 mice respectively. Virotherapy treatments began when tumors reached ~50–100 mm3. Where appropriate, S-1 (manufactured under contract at CheminPharma, Farmington, CT) was administered i.p. at 10 mg/kg per treatment at 48-hour intervals at indicated times (starting before viral treatment or 72 hours after viral treatment, and for a total of 8 days). For efficacy studies, tumor volumes were determined by caliper measurements and mice were killed once the tumor volume reached 1,400 mm3. Mouse weight was determined every 48 hours and mice were killed if weight dropped below 20% initial value.
Bioluminescence imaging. In some mouse studies, viral gene expression was determined through bioluminescence imaging of luciferase expression after injection of 150 ul of 30 mg/ml D-luciferin (GoldBio, St Louis, MO). Imaging was performed on an IVIS200 (Perkin Elmer, Waltham, MA) and images were analyzed with LivingImage software (Perkin Elmer).
Immune assays. The concentrations of IL2 and CCL5 in media samples or mouse serum were determined by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) run according to manufacturer's instructions.
In addition, whole splenocytes were exposed to irradiated tumor cells and activation (IFN-γ release) was determined by enzyme-linked immunosorbent spot assay (R&D Systems) run according to manufacturer's instructions.
Levels of antiviral neutralizing antibody in mouse serum samples was also determined according to our previously described assay. Briefly, 1,000 PFU of vvDD expressing luciferase was mixed with serum serially diluted in PBS for 30 minutes before being layered onto BSC-1 cells in a black-walled 96-well plate. Luciferase expression was determined after overnight incubation. Neutralizing antibody level was determined as the dilution needed to neutralize 50% of the vvDD (as determined by bioluminescence signal).
Flow cytometry. Freshly excised tumors were digested using a triple enzyme cocktail containing collagenase (1 mg/ml), hyaluronidase (0.1 mg/ml), and DNAse (20 mg/ml) at room temperature for 45 minutes. Cells were then washed in cold media and PBS containing 1% bovine serum albumin, then incubated with an anti-mouse CD16/32 Fc-blocking reagent (eBioscience, San Diego, CA) before staining with antibodies. Splenocytes were collected by grinding the spleen through a cell strainer into 1.6% paraformaldehyde (2.5 ml); after 15 minutes ice-cold methanol (22 ml) was added to the fixed cells, which were stored at −80 °C until the time of staining, at which point they were washed twice with PBS containing 1% bovine serum albumin before proceeding with the staining procedures. Fluorochrome-conjugated antibodies CD4-FITC, CD25-PE, FoxP3-APC (eBioscience), CD3-PE-Cy7, CD8-PE-Cy5, and Ki-67-PE-Cy7 (BD Pharmingen, Sparks, MD) and the anti-phospho-S6 Ribosomal Protein antibody (Cell Signaling Technology, Boston, MA) coupled with the Alexa Fluor 594 Goat Anti-Rabbit immunoglobulin G secondary antibody (Invitrogen, Grand Island, NY) were used to stain lymphocytes according to the manufacturers' instructions, where fixation/permeabilization was necessary for staining Foxp3 (for tumor-extracted lymphocytes not already previously permeabilized), the procedure was performed using the mouse regulatory T-cell staining kit (eBioscience). Flow cytometry was run on a Cyan (DAKO, Carpinteria, CA) and data were analyzed using the Flowjo software (TreeStar, Ashland, OR).
Statistical analysis. Standard Student's t-tests (two-tailed) were used throughout this work, except for comparison of survival curves, where Wilcoxon-Rank test was used. In all cases significance was achieved if P < 0.05.
SUPPLEMENTARY MATERIAL Figure S1. Vaccinia preferentially infects monocyte lineage cells after exposure to a mixed population of lymphocytes. Figure S2. Expression of L106P-IL2 or L106P-CCL5 from vaccinia strain vvDD produced viruses. Figure S3. Schematic diagrams of TK inserts of the novel viral constructs.
Acknowledgments
The authors thank Dr Tom Wandless (Stanford University) for supplying Shield-1, Dr Laura Banaszynski (Rockefeller University) for assistance in construction of vvDD-L106P-IL2, Dr Pawel Kalinski (University of Pittsburgh) for advise on immune assays, and Dr Ravikumar Muthuswamy (University of Pittsburgh) for provision of DCs. This work was supported directly by National Institute of Health (grant numbers R01 CA140215 and P01 CA132714, in addition, core facilities used in this work were supported by P30 CA047904-21S3). S.H.T. has a financial interest in Jennerex Biotherapeutics.
Supplementary Material
Vaccinia preferentially infects monocyte lineage cells after exposure to a mixed population of lymphocytes.
Expression of L106P-IL2 or L106P-CCL5 from vaccinia strain vvDD produced viruses.
Schematic diagrams of TK inserts of the novel viral constructs.
REFERENCES
- DeFrancesco L. Landmark approval for Dendreon's cancer vaccine. Nat Biotechnol. 2010;28:531–532. doi: 10.1038/nbt0610-531. [DOI] [PubMed] [Google Scholar]
- Schmidt C. Amgen spikes interest in live virus vaccines for hard-to-treat cancers. Nat Biotechnol. 2011;29:295–296. doi: 10.1038/nbt0411-295. [DOI] [PubMed] [Google Scholar]
- Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC.et al. (2008Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial Lancet Oncol 9533–542. [DOI] [PubMed] [Google Scholar]
- Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ.et al. (2011Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans Nature 47799–102. [DOI] [PubMed] [Google Scholar]
- Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS., and, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- Kirn DH. Redemption for the field of oncolytic virotherapy. Mol Ther. 2011;19:627–628. doi: 10.1038/mt.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington KJ, Vile RG, Melcher A, Chester J., and, Pandha HS. Clinical trials with oncolytic reovirus: moving beyond phase I into combinations with standard therapeutics. Cytokine Growth Factor Rev. 2010;21:91–98. doi: 10.1016/j.cytogfr.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmon CL, Saloura V, Fridlender ZG, Wongthida P, Diaz RM, Thompson J.et al. (2009Expression of IFN-beta enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma Cancer Res 697713–7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn DH, Wang Y, Le Boeuf F, Bell J., and, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszynski LA, Sellmyer MA, Contag CH, Wandless TJ., and, Thorne SH. Chemical control of protein stability and function in living mice. Nat Med. 2008;14:1123–1127. doi: 10.1038/nm.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloura V, Wang LC, Fridlender ZG, Sun J, Cheng G, Kapoor V.et al. (2010Evaluation of an attenuated vesicular stomatitis virus vector expressing interferon-beta for use in malignant pleural mesothelioma: heterogeneity in interferon responsiveness defines potential efficacy Hum Gene Ther 2151–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei N, Shen FB, Chang JH, Wang L, Li H, Yang C.et al. (2009An oncolytic adenovirus expressing granulocyte macrophage colony-stimulating factor shows improved specificity and efficacy for treating human solid tumors Cancer Gene Ther 1633–43. [DOI] [PubMed] [Google Scholar]
- Allen C, Paraskevakou G, Iankov I, Giannini C, Schroeder M, Sarkaria J.et al. (2008Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity Mol Ther 161556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AG., and, Wandless TJ. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell. 2006;126:995–1004. doi: 10.1016/j.cell.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszynski LA., and, Wandless TJ. Conditional control of protein function. Chem Biol. 2006;13:11–21. doi: 10.1016/j.chembiol.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Dutcher J. Current status of interleukin-2 therapy for metastatic renal cell carcinoma and metastatic melanoma. Oncology (Williston Park, NY) 2002;16 11 Suppl 13:4–10. [PubMed] [Google Scholar]
- McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK.et al. (2001Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes Cancer Res 618751–8757. [PubMed] [Google Scholar]
- Thorne SH, Hwang TH, O'Gorman WE, Bartlett DL, Sei S, Kanji F.et al. (2007Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963 J Clin Invest 1173350–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JJ., and, Thorne SH. Theranostic potential of oncolytic vaccinia virus. Theranostics. 2012;2:363–373. doi: 10.7150/thno.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AJ., and, Moss B. Structure of vaccinia virus late promoters. J Mol Biol. 1989;210:771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF.et al. (2009mTOR regulates memory CD8 T-cell differentiation Nature 460108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D.et al. (2008Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines Immunity 28710–722. [DOI] [PubMed] [Google Scholar]
- Drillien R, Spehner D, Bohbot A., and, Hanau D. Vaccinia virus-related events and phenotypic changes after infection of dendritic cells derived from human monocytes. Virology. 2000;268:471–481. doi: 10.1006/viro.2000.0203. [DOI] [PubMed] [Google Scholar]
- Mikloska Z, Bosnjak L., and, Cunningham AL. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J Virol. 2001;75:5958–5964. doi: 10.1128/JVI.75.13.5958-5964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ.et al. (2006A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor Clin Cancer Res 126737–6747. [DOI] [PubMed] [Google Scholar]
- Maynard-Smith LA, Chen LC, Banaszynski LA, Ooi AG., and, Wandless TJ. A directed approach for engineering conditional protein stability using biologically silent small molecules. J Biol Chem. 2007;282:24866–24872. doi: 10.1074/jbc.M703902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuliucci JD, Oliver SD, Morley S, Ward C, Ward J, Dalgarno D.et al. (2001Intravenous safety and pharmacokinetics of a novel dimerizer drug, AP1903, in healthy volunteers J Clin Pharmacol 41870–879. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Sisler JR., and, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques. 1997;23:1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vaccinia preferentially infects monocyte lineage cells after exposure to a mixed population of lymphocytes.
Expression of L106P-IL2 or L106P-CCL5 from vaccinia strain vvDD produced viruses.
Schematic diagrams of TK inserts of the novel viral constructs.