Last year the Nobel Prize in Physiology or Medicine was awarded to John B. Gurdon and Shinya Yamanaka for their groundbreaking research on reprogramming of somatic cells.1,2 Using a set of just four transcription factors, Yamanaka demonstrated that somatic cells could be reprogrammed into induced pluripotent stem (iPS) cells that exhibited most, if not all, of the hallmarks of bona fide pluripotent stem cells. This observation immediately raised the prospect of patient-specific pluripotent stem cells both for therapeutic applications using stem cell–based transplants and for disease modeling. However, six years after the appearance of this landmark study, the suitability of patient-specific iPS cells for disease modeling or drug screening remains challenged by the existence of clone-to-clone variability that can complicate such studies.
In this issue of Molecular Therapy, Thatava et al. describe the generation of three iPS cell lines from each of three individual patients suffering from type 1 diabetes (T1D) and the subsequent differentiation of these T1D-iPS cells into pancreatic cells.3 Interestingly, they observed a high degree of intrapatient variability in the capacity of the T1D-iPS cells to develop into glucose-responsive insulin-producing cells. Indeed, the iPS cell lines derived from a single donor seemed to be as different from each other as individual iPS cell lines derived from unrelated donors.
These observations highlight a number of caveats to iPS cell–based disease modeling (Figure 1). These include sequence variations such as point mutations in single cells of the original nonclonal somatic cell population, the fidelity of nuclear reprogramming and the impact of residual epigenetic signatures derived from the original cells, potential genomic alterations during the initial expansion of iPS cell colonies into established iPS cell lines, potential subclone-related immunogenic properties, and, finally, the variability between differentiation protocols per se and the variability of the phenotype of the differentiated cells derived from disease-affected and control iPS cell lines.
Figure 1.
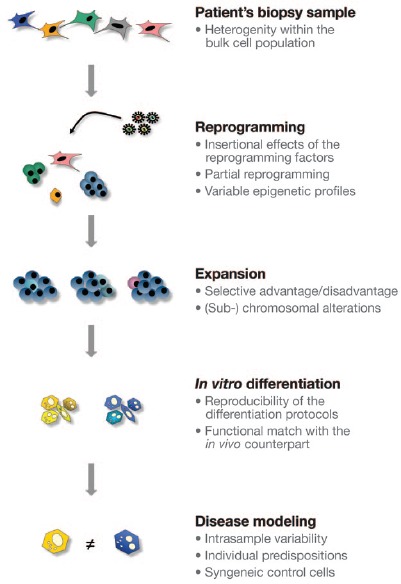
Disease modeling of patient-specific iPS cells. Within the heterogeneic bulk cell population of a patient's biopsy sample, cells with point mutations or copy-number variations providing a selective advantage may preexist and account for a significant number of intrasample variations of induced pluripotent stem (iPS) cells derived from the same donor. During reprogramming, insertional effects and off-target effects of the reprogramming factors can affect the pluripotency-associated transcriptome or epigenome. During expansion and establishing of late-passage iPS cells, disadvantageous mutations could be counterselected, but cell cycle–accelerating or other chromosomal alterations could also emerge. Upon differentiation of these iPS cell lines into the functional cells of interest, genetic alterations or misregulated epigenetic modifications may result in heterogeneous populations of differentiated cells. If these cells were applied for disease modeling, the intrasample variability and other individual predispositions by themselves could cause significant phenotype differences and thus could challenge the fidelity of the specific disease phenotype-related assay. Hence, syngeneic control cells generated with tailored genetic engineering tools should be considered for translational research studies.
Deep-sequencing strategies had previously demonstrated that at least half the point mutations identified in established human iPS cells were present in a very small subset of the starting cell population.4,5 Very recently, Abyzov et al. described “line-manifested” copy-number variations (CNVs) among dermal fibroblasts and iPS cells derived from them, which reflected a somatic mosaicism normally present in human skin.6 These observations underline the need for extensive genetic screening of established iPS cell lines intended for potential therapeutic applications. Moreover, such mutations might affect differentiation propensities or the cells' responses to pharmacological or genetic treatments—suggesting ramifications for disease modeling as well.
Recent data have shown that a preferential stoichiometry of reprogramming factors greatly enhances the generation of iPS cells,7,8 and there is increasing evidence that the extent of reprogramming of the epigenetic status of the original cells toward a fully pluripotent state is also very sensitive to factor stoichiometry.9 However, the latter can be ensured using elaborate polycistronic reprogramming constructs.10 Bock and colleagues have developed a valuable epigenetic scorecard11 that assesses the transcriptional and epigenetic similarity of iPS cell lines and that might prove useful in identifying individual lines with a full capacity to differentiate in vitro.
Notably, the degree of CNVs, as well as epigenetic and transcriptional differences, seems to be greater in early iPS-cell passages.12,13 In these studies it was postulated that replicative stress during the initial phase of reprogramming resulted in mosaic early-passage colonies that contain cells with a high number of CNVs. Quite a few of these CNVs might give rise to a growth disadvantage that gradually removes certain clones, which could explain why later passages of iPS cell lines exhibited fewer CNVs. However, given that the number of chromosomal abnormalities increases at higher passages,14 high-passage iPS cell lines could acquire other genetic aberrations that interfere with their capacity to differentiate or with the resulting cell type–specific phenotype.
The ideal differentiation protocol should lead to an iPS cell–derived, but tissue-specific, cell that closely mimics the phenotype of its in vivo counterpart, with high efficiency. So far, even the most sophisticated differentiation protocols result in a quite heterogeneous cell population and lack reproducible efficiency if different pluripotent starting cells are used. With respect to endodermal and, in particular, pancreatic cell differentiation, protocols are stepwise in nature, comprising induction of definitive endoderm through a meso-endodermal progenitor cell state followed by the specification of a more tissue-specific state (pancreatic precursor cell). These precursor cells can further mature in vivo following transplantation and can thus give rise to results that are superior to those obtained when using more mature cells, which might have failed to acquire the full metabolic capabilities of the intended target cell owing to missing or inefficient cues during in vitro differentiation.15 This observation casts doubt on the current concepts for in vitro disease modeling because an assumed maturation state of in vitro differentiated cells may not represent a state that provides metabolic function after engraftment in vivo.
Thatava and colleagues report the reproducible generation of just such a pancreatic progenitor cell from different patient-specific iPS cells. However, the further differentiation of these cells into functional (i.e., glucose-responsive) insulin-producing pancreatic β-cells was successful in only a subset of iPS cell lines.3 This observation of high intrapatient as well as interpatient variability between functionally differentiated cell phenotypes may support a paradigm shift for differentiation strategies applied in disease modeling—rather than aim for mature cell phenotypes in a direct and rapid differentiation protocol, one might first aim to generate well-defined and expandable precursor cells as an intermediate step.16,17 After such self-renewing progenitor cells were established, one could extensively characterize their epigenetic and transcriptional profile and identify progenitor cell lines that might most efficiently respond to subsequent differentiation cues during further in vitro differentiation. Then, in a second step, these cells could be differentiated toward mature cells in a more reproducible manner and presumably with greater functionality.
If the disease-related phenotype of patient-specific iPS cell derivatives was subtle compared with that of derivatives of iPS cells from healthy individuals, such intra- and intersample variation could cloud the capacity to detect disease-specific effects. Therefore, it has been postulated that syngeneic control iPS cell lines might be useful to minimize intersample differences between disease-specific and control cell lines. Such syngeneic control iPS cells could be generated by applying tailored strategies for genetic correction of disease-associated mutations, which might be achieved by homologous recombination mediated by zinc-finger nucleases or transcription activator–like effector nucleases (TALENs).18,19 However, off-target effects such as DNA double-strand breaks could occur using both techniques,20 which might also affect the cells' capacity to differentiate. Considering the advantages of a syngeneic source of mutated and normal stem cell derivatives, one could argue that such tailored gene-editing approaches could be utilized to introduce disease-specific mutations in well-established human embryonic stem cell lines, which would not exhibit epigenetic or transcriptional aberrations related to iPS cell generation per se. But this approach fails to take into account that a particular genetic mutation would not necessarily cause the same severe phenotype in individual patients with different genotypic backgrounds and that the onset of the particular disorder could further depend on other individual factors.
In conclusion, Thatava et al. provide good evidence that human disease–specific iPS cell lines exhibit considerable intra- and intersample variability that must be addressed if tissue-specific functional iPS cell derivatives are to be used for basic or translational research on T1D. Future research may identify additional factors that could provide improved tools that generate iPS cells with less intra- and intersample variation. Advanced profiling of the iPS cells' transcriptome and epigenome may not only assay the establishment of fully reprogrammed pluripotent stem cells but also more adequately predict the differentiation ability of a given iPS cell line such that cell lines with aberrant differentiation potential could be excluded. If more subtle disease-related phenotypes are investigated, syngeneic cell sources for the disease-specific and the control cells may provide an advantageous system, provided that relevant intersample genetic alterations could be excluded by applying array–comparative genomic hybridization or deep-sequencing techniques.
References
- Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962;10:622–640. [PubMed] [Google Scholar]
- Takahashi K., and, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Thatava T, Kudva YC, Edukulla R, Squillace K, De Lamo JG, Khan YK. Intrapatient variations in type 1 diabetes–specific iPS cell differentiation into insulin producing cells. Mol Ther. 2013;21:228–239. doi: 10.1038/mt.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J.et al. (2011Somatic coding mutations in human induced pluripotent stem cells Nature 47163–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MA, Larson DE, Sun CW, George DR, Ding L, Miller CA.et al. (2012Background mutations in parental cells account for most of the genetic heterogeneity of induced pluripotent stem cells Cell Stem Cell 10570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abyzov A, Mariani J, Palejev D, Zhang Y, Haney MS, Tomasini L.et al. (2012Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells Nature e-pub ahead of print 18 November 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetrou EP, Tomishima MJ, Chambers SM, Mica Y, Reed E, Menon J.et al. (2009Stoichiometric and temporal requirements of Oct4, Sox2, Klf4, and c-Myc expression for efficient human iPSC induction and differentiation Proc Natl Acad Sci USA 10612759–12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemann U, Sgodda M, Warlich E, Ballmaier M, Schöler HR, Schambach A.et al. (2011Optimal reprogramming factor stoichiometry increases colony numbers and affects molecular characteristics of murine induced pluripotent stem cells Cytometry A 79426–435. [DOI] [PubMed] [Google Scholar]
- Carey BW, Markoulaki S, Hanna JH, Faddah DA, Buganim Y, Kim J.et al. (2011Reprogramming factors stoichiometry influences the epigenetic state and biological properties of induced pluripotent stem cells Cell Stem Cell 9588–598. [DOI] [PubMed] [Google Scholar]
- Warlich E, Kuehle J, Cantz T, Brugman MH, Maetzig T, Galla M.et al. (2011Lentiviral vector design and imaging approaches to visualize the early stages of cellular reprogramming Mol Ther 19782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD.et al. (2011Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines Cell 144439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Närvä E.et al. (2011Copy number variation and selection during reprogramming to pluripotency Nature 47158–62. [DOI] [PubMed] [Google Scholar]
- Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY.et al. (2010Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells Nat Biotechnol 28848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayshar Y, Ben-David U, Lavon N, Biancotti JC, Yakir B, Clark AT.et al. (2010Identification and classification of chromosomal aberrations in human induced pluripotent stem cells Cell Stem Cell 7521–531. [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S.et al. (2008Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo Nat Biotechnol 26443–452. [DOI] [PubMed] [Google Scholar]
- Cheng X, Ying L, Lu L, Galvão AM, Mills JA, Lin HC.et al. (2012Self-renewing endodermal progenitor lines generated from human pluripotent stem cells Cell Stem Cell 10371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Sakaguchi Y, Shoji E, Nishino T, Maki I, Sakai H.et al. (2012In vitro modeling of paraxial mesodermal progenitors derived from induced pluripotent stem cells PLoS One 7e47078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Soldner F, Beard C, Gao Q, Mitalipova M, DeKelver RC.et al. (2009Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases Nat Biotechnol 27851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG.2ndet al. (2012In vivo genome editing using a high-efficiency TALEN system Nature 491114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson DF, Fahrenkrug SC., and, Hackett PB. Targeting DNA with fingers and TALENs. Mol Ther Nucleic Acids. 2012;1:e3. doi: 10.1038/mtna.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]


