Abstract
Wnt signaling is involved in numerous aspects of vertebrate development and homeostasis, including the formation and function of blood cells. Here, we show that canonical and noncanonical Wnt signaling pathways are present and functional in megakaryocytes (MKs), with several Wnt effectors displaying MK-restricted expression. Using the CHRF288-11 cell line as a model for human MKs, the canonical Wnt3a signal was found to induce a time and dose-dependent increase in β-catenin expression. β-catenin accumulation was inhibited by the canonical antagonist dickkopf-1 (DKK1) and by the noncanonical agonist Wnt5a. Whole genome expression analysis demonstrated that Wnt3a and Wnt5a regulated distinct patterns of gene expression in MKs, and revealed a further interplay between canonical and noncanonical Wnt pathways. Fetal liver cells derived from low-density-lipoprotein receptor-related protein 6-deficient mice (LRP6−/−), generated dramatically reduced numbers of MKs in culture of lower ploidy (2N and 4N) than wild-type controls, implicating LRP6-dependent Wnt signaling in MK proliferation and maturation. Finally, in wild-type mature murine fetal liver-derived MKs, Wnt3a potently induced proplatelet formation, an effect that could be completely abrogated by DKK1. These data identify novel extrinsic regulators of proplatelet formation, and reveal a profound role for Wnt signaling in platelet production.
Key Points
Wnt signaling is essential for MK proliferation and maturation in addition to profoundly stimulating proplatelet formation.
These observations suggest that mature megakaryocytes may be able to respond to known Wnt gradients in the osteoblastic and vascular niches.
Introduction
The Wingless (Wnt) family of secreted glycoproteins regulates diverse biologic processes, from embryonic development through to aspects of adult homeostasis and disease. The Wnt signaling pathways comprise 19 distinct Wnt ligands, a host of extracellular Wnt-modulating proteins and at least 10 members of the 7 transmembrane domain-containing Frizzled (FZD) receptor family.1 After ligand-receptor binding, multiple pathways and downstream events are triggered in a context specific manner. The most studied Wnt signaling pathway, the canonical pathway, is mediated by the stabilization and nuclear translocation of β-catenin (see Figure 1A). Under unstimulated conditions, β-catenin is phosphorylated by casein kinase 1 (CK1) and glycogen synthase kinase-3β (GSK3β) in the axin/APC destruction complex, which tags β-catenin for ubiquitination and degradation. Stimulation with a canonical Wnt ligand (such as Wnt3a) inhibits this process, stabilizing cytoplasmic β-catenin which then migrates to the nucleus. There, it acts as a transcriptional coactivator in complex with transcription factors, such as T-cell factor/lymphoid enhancer factor (TCF/LEF) family members to regulate gene expression. The canonical Wnt pathway inhibitor dickkopf-1 (DKK1) binds to low-density lipoprotein receptor-related protein 5/6 (LRP5/6) and prevents signal transduction from occurring. Multiple “noncanonical” Wnt signaling pathways also exist.2 Although these pathways all function in a β-catenin independent manner, there is an interplay between canonical and noncanonical signaling in some contexts.3,4
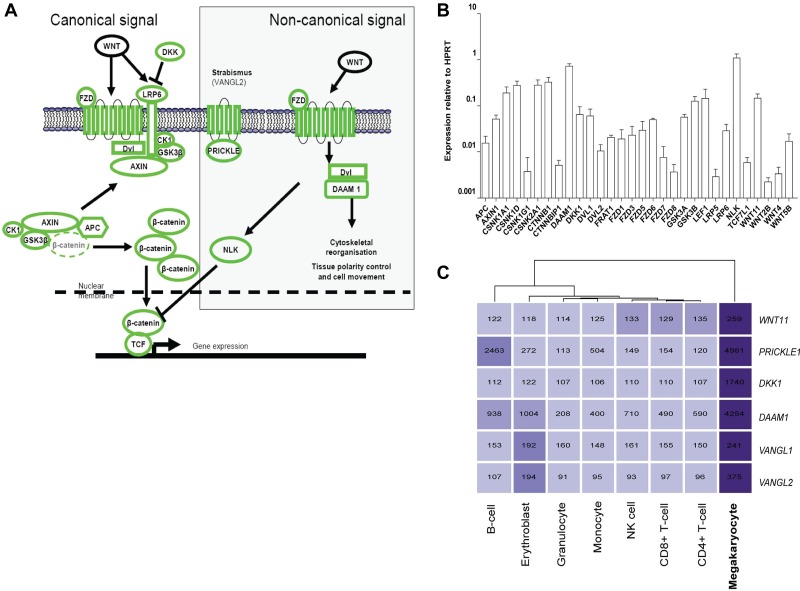
Figure 1.
Expression of Wnt signaling pathway components in the megakaryocyte. (A) Overview of the canonical and noncanonical Wnt signaling pathways. All components shown in green were detected at the transcript level in our previous microarray studies of in vitro differentiated MKs.21 (B) Expression of Wnt signaling pathway components in in vitro differentiated MKs. Data are presented as the mean and range of the expression relative to the HPRT gene from 2 biologic replicates. (C) Heatmap displaying MK-specific components of the Wnt signaling pathway. The intensity of the shading reflects the level of expression of each gene in each cell type. Numbers shown indicate the normalized expression value of each gene as determined in Watkins et al.21
Recently, Wnt signaling has been implicated in the development5–7 and function of mature blood cells.8,9 These have focused predominantly on the regulation of hematopoietic stem cell (HSC) function and T-cell development. Other effects of Wnt signaling on myeloid differentiation have been observed, yet its contribution to megakaryopoiesis and platelet formation remains unexplored.
We previously identified a role for the canonical Wnt signaling pathway in the regulation of platelet function,10 with Wnt3a modulating platelet adhesion and suppressing platelet activation to multiple platelet agonists (thrombin, collagen, ADP). More recently, noncanonical Wnt signaling via Wnt5a has also been described in platelets.11 Whether the elements of Wnt signaling are expressed and functional in MKs is unknown, although roles for GSK3β have been proposed in MK development.12,13 MKs differentiate from HSCs through a regulated hierarchy of changes in gene and receptor molecule expression, polyploidization, and cytoplasmic maturation. During differentiation, maturing MKs migrate from the osteoblastic niche to the vascular niche14,15 where they extend cytoplasmic protrusions known as proplatelets into sinusoidal blood vessels, from which platelets are ultimately released in the circulation.16–19 As several Wnts are expressed in hematopoietic niches,7 MKs probably encounter an array of Wnt ligands during this maturation process.
Here, we demonstrate that canonical and noncanonical Wnt signaling pathways are present and functional in MKs, and that Wnt signaling is essential for MK proliferation and maturation, and profoundly stimulate proplatelet formation from the mature MK.
Methods
Cell culture
CHRF288-11 cells were cultured in RPMI 1640, 10% fetal calf serum, 2mM l-glutamine in a humidified incubator with 5% CO2. For Wnt treatments, cells (106/mL) were cultured in serum free medium for 16 hours before treatment with recombinant mouse Wnt3a, Wnt5a or DKK1 (R&D Systems) at the stated doses. For protein analysis, the cells (which become weakly adherent after serum starvation) were gently scraped from the wells and washed twice in ice cold phosphate buffered saline (PBS). The cell pellet was then resuspended in PBS and lysed by addition of 2× reducing buffer (2% sodium dodecyl sulfate, 10% glycerol, 50mM dithiothreitol, 45mM Tris-HCl (pH 6.8), 0.1 g/L bromophenol blue). Subcellular fractionation was performed using the QProteome subcellular fractionation kit (Qiagen) following the manufacturer's instructions.
For RNA preparation, cells (106/mL) were cultured in serum free medium for 16 hours before treatment with recombinant Wnt3a (150 ng/mL), Wnt5a (1200 ng/mL) or both Wnt3a and 5a in combination. Cells were collected as described above, lysed in 200 μL Trizol reagent (Invitrogen) and prepared according to the manufacturer's instructions.
Wnt signaling PCR array
MK cDNA was prepared as previously described20 and analyzed using the Wnt signaling pathway PCR array (SABiosicences). Transcripts with a cycle threshold (Ct) value less than 35 were determined to be “present” in MKs, as per the manufacturer's instructions. The microarray data for in vitro differentiated MKs is available as supplemental material to Watkins et al.21
Western blotting
Western blotting was carried out as previously described.22 Briefly, protein lysates were separated on an 8% to 20% gradient gel (Thermo), transferred to a nitrocellulose membrane and β-catenin was detected using mouse monoclonal antibodies (12F7, Santa Cruz or 610154, Millipore). Monoclonal antibodies against PTH2 (TIP39; [Z-24] sc-134089, Santa Cruz), EPAS1 (HIF-2; NB100-132, Novus Biologicals) and LRRC32 (GARP; IMG-6354A Imgenex) were also used where indicated. Where appropriate, blots were stripped using Restore Western stripping reagent (Thermo Scientific) and probed using anti-GAPDH (ab9485, Abcam) to ensure equal loading.
Microarray analysis
Total RNA (500 ng) was amplified using the Illumina Total Prep RNA amplification kit (Applied Biosystems) according to the manufacturer's instructions. The biotinylated cRNA (1500 ng per sample) was applied to Illumina HumanWG-6 v3 expression bead chips and hybridized overnight at 58°C. Chips were washed, detected, and scanned according to the manufacturer's instructions and the scanner output imported into BeadStudio Version 3 software (Illumina). Statistical analysis of the data were performed using the lumi package23 for Bioconductor/R (www.r-project.org). Differentially expressed genes were identified as those showing > 1.5-fold change in expression relative to the control (untreated) sample with an adjusted P value < .005. Raw data have been deposited in the GEO database (accession no. GSE42071).
Validation of microarray results by quantitative RT-PCR
mRNA was reverse transcribed to cDNA in a reaction primed by oligo(dT)12–18 using Superscript III following the manufacturer's protocols (Invitrogen). cDNA was diluted to the equivalent of 1 ng of input total RNA per reaction and amplified in 40 cycles of PCR on a Stratgene MX3000P using TaqMan Gene Expression probes and primers, following the manufacturer's instructions (Applied Biosystems). The assay IDs were as follows: endothelial PAS (PER-ARNT-SIM) domain containing protein 1 (EPAS1, Hs01026142_m1), glycoprotein A repetitions predominant (GARP/LRRC32, Hs00194136_m1), parathyroid hormone 2 (PTH2, Hs01094670_g1), vasohibin 1 (VASH1, Hs00208609_m1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Hs99999905_m1). Ct values were normalized to GAPDH to allow comparison between samples.
Murine MK cultures
Littermate wild-type (WT) and LRP6−/− mouse24 fetuses were isolated between embryonic days 13 and 15 in accordance with guidelines approved by the Children's Hospital, Boston, animal care and use committee and the IACUC (protocol No. 08-05-1152). Single-cell suspensions, prepared by successive passage through 22- and 25-gauge needles, were cultured in Dulbecco modified Eagle medium (Gibco BRL) supplemented with 10% fetal bovine serum albumin, 2mM L-glutamax, 50 U/mL penicillin, 50 μg/mL streptomycin, and 0.1% tissue culture supernatant from a fibroblast cell line engineered to secrete recombinant human thrombopoietin (TPO).25 Between days 4 and 6, these fetal liver cultures contained 50% to 60% acetylcholinesterase-positive cells. TPO was added only at the initiation of liquid cultures.26 On the third day of MK culture, cells were placed on a 1.5% to 3.0% albumin step gradient and sedimented27 to obtain enriched populations of MKs in the pellet. MKs homogeneity was verified before treatment, analyzed by MK/platelet-specific markers. The cultures were 97% positive for GPIIbIIIa and 87% positive for GPIX (data not shown). MKs were then resuspended in Dulbecco modified Eagle medium with 10% fetal bovine serum albumin, 1% antibiotic, and 0.1 μg/mL recombinant human TPO alone, and in the presence of Wnt3a or Wnt5a ± 5 μg/mL DKK1, and cultured at 37°C and 5% CO2 before analysis.
MK cultures were examined on day 5 by phase contrast microscopy using a Nikon eclipse TS100 benchtop microscope (Nikon Instruments) at 40× magnification, digital images were collected on a Hamamatsu C2400 CCD camera (Hamamatsu) and analyzed using ImageJ Version 1.42q software. Mature MKs were identified by size (> 10 μm diameter) and distinguished from proplatelet-producing MKs because of the presence or absence of long proplatelet elongations (circularity ≥ 0.7). Our laboratory has invested a large effort to identify methods to quantitate proplatelet production. We recently established that round MKs do not adhere to coverslips at the same rate as proplatelet-producing MKs, leading to inaccurate quantitation with immunofluorescence microscopy. Thus, we focused our efforts on a live cell assay. For quantification of the percentage of proplatelet-producing MKs, 10 representative pictures were taken at 40× magnification per sample, and 4 separate samples were analyzed per condition tested. An average number of ∼35 cells were counted per sample and categorized as either round MKs or proplatelet-producing MKs. Quantifications from sample replicates were pooled such that ∼ 140 cells were counted in total per condition tested. Total cell counts were comparable across all conditions tested. Statistical significance was determined by 1-way ANOVA followed by Dunnett multiple comparison test to compare test samples with the control experiments.
Analysis of MK ploidy
Mouse fetal livers were prepared as previously described, and single-cell suspensions were cultured for 4 days either alone or in the presence of Wnt3a (600 ng/mL), Wnt5a (600 ng/mL), or DKK1 (5 μg/mL). Round MKs were then placed on a 1.5% to 3.0% albumin step gradient, sedimented, and resuspended in 100 μL citrate buffer (40mM Na-Citrate pH 7.4, 0.25mM sucrose). Coloring buffer (400 μL; 0.5% NP-40, 0.5mM EDTA) supplemented with 0.5 mg/mL RNase A and 20 μg/mL propidium iodide were added to the MK suspension and cells were measured for ploidy by flow cytometry (FACSCalibur, Becton Dickinson). Experiments were performed in triplicate. Error bars represent ± 1 SD.
Results
Wnt signaling pathways are present in MKs
To determine the specific components of the Wnt signaling pathway present in human MKs differentiated from umbilical cord blood-derived CD34+ progenitor cells, we used data from our previously published microarray analyses of the MK transcriptome and other mature blood cell lineages.21,22 Transcripts encoding the main components of the canonical and several components of the noncanonical Wnt signaling effectors were present in MKs (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These were confirmed using a PCR array specific for the Wnt signaling pathway (Figure 1B). Of note, DKK1, disheveled-associated activator of morphogenesis 1 (DAAM1), NEMO-like kinase (NLK), prickle-like protein 1 (PRICKLE1), and Van Gogh-like 1 and 2 (VANGL1, VANGL2) were identified as having MK-restricted expression in our previous microarray studies (Figure 1C).21 Together these analyses confirm that the main components of the canonical pathway are expressed in MKs, along with several noncanonical pathway components.
Canonical Wnt signaling is functional in the megakaryocytic cell line CHRF 288-11
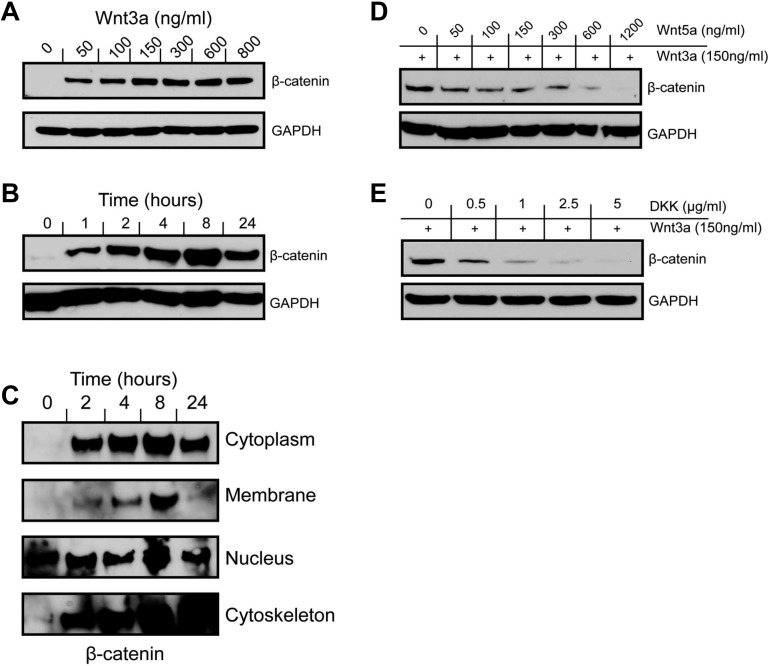
We investigated the function of the Wnt signaling pathway using the CHRF288-11 cell line as a model for MKs.28,29 When treated with Wnt3a for 24 hours, at doses between 50 and 800 ng/mL, β-catenin accumulation was observed in whole cell lysates, with a maximal effect observed at 150 ng/mL (Figure 2A). The response to Wnt3a (150 ng/mL) was time-dependent (Figure 2B), with an increase in β-catenin abundance after 1 hour and the response peaking after 8 hours. Analysis of the subcellular fractions of the CHRF288-11 cells showed that the canonical Wnt signal caused β-catenin to accumulate not only in the nucleus, but also in the cytoplasmic, membrane, and cytoskeletal fractions of the cell in a time-dependent manner (Figure 2C), indicating that β-catenin's activity in MKs may not limited to the regulation of gene expression. Again, β-catenin accumulation reached a maximum at 8 hours, after which a slight reduction was observed in the cytoplasm and nucleus, and a complete reduction in the membrane fraction. In contrast, β-catenin continued to accumulate in the cytoskeletal fraction over time. Nuclear accumulation of β-catenin suggests that it may function in its traditional role as a transcriptional regulator. Consistent with the observations of Soda et al in the TPO/UT7 cell line,13 we observed a detectable level of nuclear β-catenin in the untreated cell, indicating that there may be a minimal level of endogenous Wnt signaling in operation in these cells. Interestingly, costimulation of the noncanonical Wnt signaling pathway with low amounts of Wnt5a (50 ng/mL) inhibited the Wnt3a-mediated accumulation of β-catenin, suggesting that in MKs there may be crosstalk between these 2 pathways (Figure 2D). Inhibition by Wnt5a was also dose responsive, with maximal inhibition observed when Wnt3a-treated cells (150 ng/mL) were coincubated with higher doses of Wnt5a (600 and 1200 ng/mL). The canonical Wnt antagonist, DKK1, also showed a dose-dependent inhibition of Wnt3a-mediated β-catenin accumulation, confirming that the signaling observed is dependent on a canonical mechanism (Figure 2E).
Figure 2.
Canonical Wnt signaling is functional in MKs. Incubation of CHRF288-11 cells with Wnt3a results in a dose (A) and time (B; dose 150 ng/mL) dependent increase in cellular β-catenin levels as detected by Western blotting of whole cell lysates using an anti–β-catenin antibody. (C) Subcellular fractionation of cells treated with Wnt3a indicates that β-catenin accumulation occurs in subcellular fractions during a 24 hour period. (D) β-catenin accumulation in response to Wnt3a treatment is inhibited in a dose-dependent manner by the noncanonical agonist Wnt5a. (E) The canonical antagonist DKK1 inhibits β-catenin accumulation in a dose-dependent manner at an 8 hour time point. All blots are representative of at least 3 or more independent experiments.
Canonical and noncanonical Wnt ligands induce a transcriptional response in megakaryocytic cells
To determine whether Wnt3a and Wnt5a could elicit a transcriptional response in CHRF288-11 cells, we performed whole genome microarray analysis of cells treated with Wnt3a (150 ng/mL) or Wnt5a (1200 ng/mL), doses that we showed above induced or inhibited cellular β-catenin accumulation, respectively. To determine whether the crosstalk between the 2 ligands regulates gene transcription, we analyzed the transcriptional response of the cells when coincubated with both Wnt3a and Wnt5a. These studies identified that both Wnt3a and Wnt5a regulate the expression of a small number of genes in these cells (Figure 3A, supplemental Table 1), and revealed distinct, overlapping and antagonistic effects of Wnt3a and Wn5a.
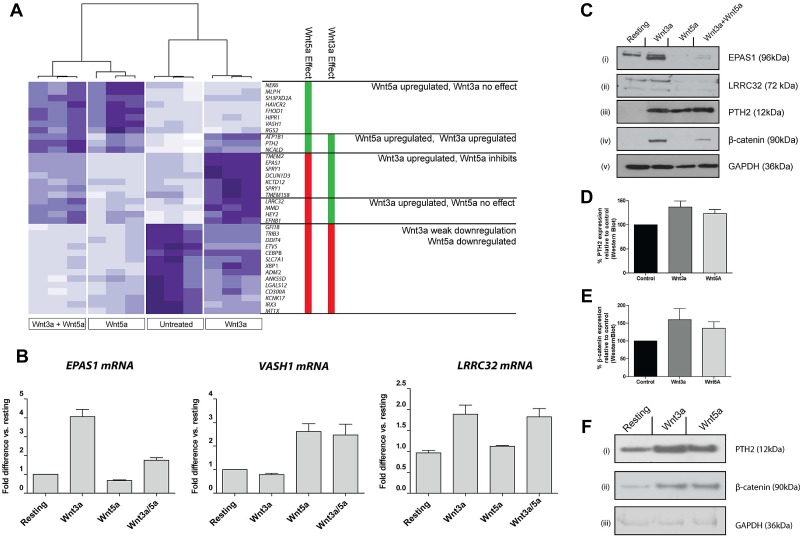
Figure 3.
Transcriptional response of MKs to signaling via Wnt3a and Wnt5a. (A) Heatmap representation of genes differentially expressed in CHRF288-11 cells in response to Wnt3a, Wnt5a, or both in combination. The data from 3 replicates for each experimental group are shown. Clustering of these data identifies subsets of genes that are regulated by either one or both of the ligands. Genes shown in bold are known targets of Wnt3a signaling. (B) Confirmation of observed patterns of expression by RT-PCR for transcripts encoding EPAS1, VASH1, and LRRC32 in response to treatment with Wnt ligands. Data are presented as mean ± SD (n = 3) of fold difference versus expression in untreated cells. (C) Confirmation of changes in protein expression levels of EPAS1, LRRC32, and PTH2 in response to treatment with Wnt ligands. (D-F) PTH2 and β-catenin expression in fetal liver derived murine MKs in response to Wnt3a or Wnt5a. Data shown in panels D and E represent mean detection level (± SD) as determined by Western blotting followed by densitometry. (F) Representative blots showing PTH2 (i), β-catenin (ii), and GAPDH (iii) expression in fetal liver MKs after Wnt treatment.
Using a cut-off of 1.5-fold change in gene expression with an adjusted P value less than .005, 36 genes were identified, which responded positively or negatively to treatment with Wnt3a, Wnt5a, or a combination of the 2 (supplemental Table 1). Cluster analysis identified distinct patterns of Wnt responses among these genes (Figure 3A), several of which are known to be responsive to Wnt3a in other cells, including hypoxia inducible factor 2α/Hif2α (EPAS1),30 monocyte to macrophage differentiation factor (MMD),31 sprouty homologue (SPRY),31 ephrin B1 (EFNB1),32 TMEM2,31 and TMEM158.33 Of note is the observation that the platelet receptor, LRRC32 (also known as GARP)22,34 was up-regulated after Wnt3a treatment. To date there has been little characterization of the genes regulated by Wnt5a. Here we show several genes respond to this noncanonical Wnt signal, including Vasohibin-1 (VASH1).
Consistent with the observations in Figure 2, many of the genes up-regulated by Wnt3a, including EPAS1, were inhibited by coincubation with Wnt5a. Others, including LRRC32, were unaffected. Conversely, all genes up-regulated by Wnt5a were either unaffected by coincubation with Wnt3a, or, as in the case of parathyroid hormone 2 (PTH2), were up-regulated by both. Several genes were found to be down-regulated by Wnt5a (> 1.5-fold); for most of these genes, weak down-regulation (< 1.5-fold) was also observed in the Wnt3a treated samples.
The microarray expression patterns for EPAS1 (Wnt3a up-regulated, inhibited by Wnt5a), VASH1 (Wnt5a up-regulated), and LRRC32 (Wnt3a up-regulated, not inhibited by Wnt5a) were replicated by RT-PCR (Figure 3B), and in the case of EPAS1 and LRRC32, by Western blotting (Figure 3C). In the case of LRRC32, up-regulation of the protein in response to Wnt3a appeared to be inhibited by Wnt5a, whereas the mRNA expression was unchanged. The expression pattern for PTH2, which was up-regulated in response to Wnt3a and Wnt5a, was confirmed at the protein level in both CHRF cells (Figure 3C) and in MKs derived from murine fetal liver (Figure 3D-F). Elevated β-catenin levels were also observed in response to Wnt signaling in these primary cells (Figure 3E-F). We also examined global gene expression in human cord blood derived MKs and found that the majority of genes regulated by Wnt signaling in the CHRF-288-11 cell line are also similarly regulated in response to Wnt signaling in primary MKs (supplemental Figure 2).
LRP6−/− fetal liver cells display impaired megakaryopoiesis
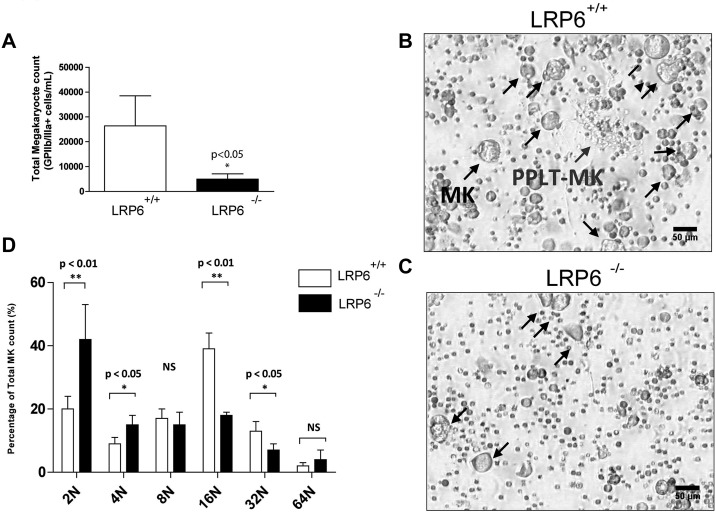
To establish the importance of canonical Wnt signaling in MK maturation, fetal liver megakaryopoiesis from LRP6−/− mice was compared with WT littermate controls. LRP6−/− mice are embryonic lethal and frequently die during late gestation (E15-E18), reflecting the predominant role of this receptor in transducing canonical Wnt signaling in embryonic development.24 Mouse fetal liver cells were isolated on embryonic day 13 and cultured in the presence of TPO to direct MK differentiation. Interestingly, LRP6−/− fetal liver cells generated significantly fewer MKs in culture (Figure 4A-C) and these were of lower ploidy (Figure 4D) than WT control fetal liver cells (Figure 4B-D). These results demonstrate a role for canonical Wnt signaling through LRP6 for MK maturation in culture. LRP6+/− embryos, by comparison, develop normally and circulating blood counts are not affected in adult mice, suggesting that a single copy of this gene is sufficient for normal hematopoietic development (data not shown). LRP5−/− mice have a WT phenotype,35 further underscoring the importance of LRP6 signaling in hematopoietic stem-cell development.
Figure 4.
Ex vivo analysis of fetal liver megakaryopoiesis in LRP6−/− mice. (A) Absolute numbers of MK cells (GPIIb/IIIa+) cells in MK cultures from fetal liver cells. Data shown are the mean ± SD from replicate cultures of LRP6+/+ (n = 5) and LRP6−/− (n = 4) cells. (B-C) Representative images from LRP6+/+ and LRP6−/− cultures, respectively. (D) Ploidy analysis of MKs derived from LRP6+/+ and LRP6−/− cultures. Data represent mean ± SD from replicate cultures of LRP6+/+ (n = 5) and LRP6−/− (n = 3) cells.
Wnt signaling is functional in primary MKs and regulates proplatelet formation
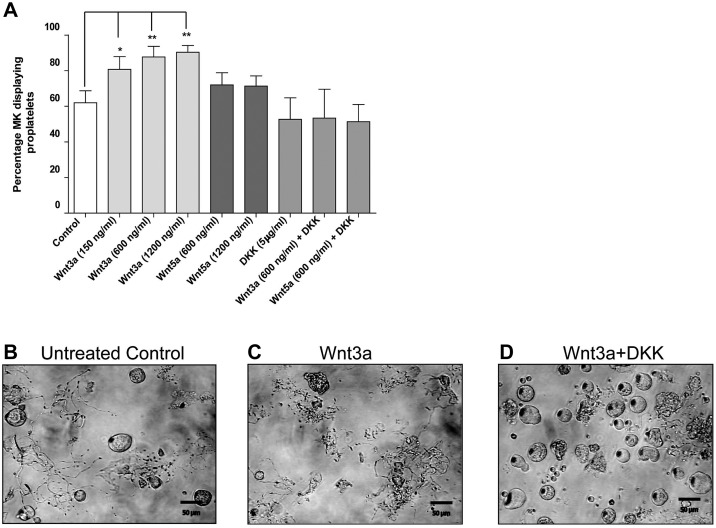
MKs generate platelets by remodeling their cytoplasm into long proplatelet extensions, which serve as assembly lines for platelet production. To establish which Wnt signaling factors are involved in this process, we analyzed the effect of Wnts on proplatelet production in a mouse fetal liver MK model (Figure 5A). Wnt3a was shown to significantly (P < .05) increase the number of MKs extending proplatelets at all concentrations tested, from a mean of 62% ± 6.7% (mean ± SD) in untreated to 80.75% ± 7.1%, 87.6% ± 6.02%, and 90.33% ± 3.78% when treated with 150 ng/mL, 600 ng/mL, and 1200 ng/mL Wnt3a, respectively. Representative data from control and Wnt3a treated samples are shown in panels 5B and 5C, respectively. The canonical antagonist DKK1 had no independent effect on proplatelet formation at 5 μg/mL (52.67% ± 12%), but did ablate the effect of Wnt3a (600 ng/mL), reducing the number of MKs extending proplatelets to levels observed in control samples (53.3% ± 16.2%; Figure 5A-D). The finding that Wnt3a can promote proplatelet formation coupled with the fact that this response can be abrogated by DKK1, demonstrate that canonical Wnt signaling controls proplatelet formation ex vivo and represents the first identification of extrinsic regulators of proplatelet formation.
Figure 5.
Wnt signaling stimulates proplatelet production in fetal liver MKs ex vivo. (A) Percentage of cultured MKs extending proplatelets in cells treated with Wnt3a, Wnt5a, and DKK1. Data are presented as mean ± SD from 3 individual experiments. P values and significance have been determined by 1-way ANOVA followed by Dunnett posttest to compare all groups with untreated cells. (B-D) Representative images of proplatelet-producing MKs in untreated, Wnt3a, and Wnt5a treated samples, respectively.
Discussion
We previously demonstrated a role for canonical Wnt signaling in the regulation of platelets, acting to inhibit activation.10 Here we demonstrate for the first time the presence and functional effects of the Wnt signaling pathways in MKs and identify the Wnt ligands Wnt3a and DKK1 as extrinsic regulators of proplatelet formation.
Wnt signaling has been found to regulate the formation and function of several blood-cell lineages and of hematopoietic progenitors. Indeed, Scheller et al have shown that constitutive activation of β-catenin in the hematopoietic system of the mouse blocked multilineage differentiation of HSCs resulting in severe anemia, thrombocytopenia, and a decrease in bone marrow cellularity.36 Deletion of β-catenin in the hematopoietic system, by comparison, was shown to result in no defects, possibly because of compensation by other catenin family members.37 Although the constitutive expression of β-catenin in HSCs inhibits the generation of all hematopoietic lineages, it is unclear what role the Wnt pathway plays in later stages of MK differentiation and proplatelet formation.
Through gene expression analyses of in vitro differentiated human MKs we identified the presence of key components of the canonical Wnt signaling pathway, as well as several components of the noncanonical pathway. Consistent with our observations in platelets,10 the transcript encoding the FZD6 receptor was expressed in MKs. Several additional FZD receptors were detected by either microarray or RT-PCR approaches, suggesting that MKs may be responsive to instruction from a variety of Wnt signals. Beyond the receptor, components of the axin/APC destruction complex were detected at the transcript level. Several transcripts encoding noncanonical Wnt signaling proteins, including NLK, DAAM1, VANGL2, and PRICKLE1, were found. Intriguingly, several of these latter transcripts were among the most MK-specific compared with purified populations of erythroblasts22 or other blood cells.21 MKs also express the noncanonical Wnt11 and Wnt5b, and the transcript encoding canonical antagonist, DKK1. It is possible that these proteins may be secreted from MKs and function in an autocrine or paracrine manner, modulating MK development or influencing other cells within the hematopoietic niche.7
These observations support a potential role for Wnt signaling in MK development and platelet formation, in addition to platelet function. To explore this, we used the recombinant ligands Wnt3a, Wnt5a, and DKK1 to investigate Wnt signaling in MKs. First, we established time and dose-dependent canonical Wnt signaling in MKs, using the human cell line CHRF288-11. This cell line is a model for studies of MK differentiation and gene expression patterns,29 and expresses Wnt signaling components at levels that are similar to that of MKs differentiated from CD34+ HPCs (supplemental Figure 1). Furthermore, in this model Wnt3a signaling was inhibited by Wnt5a, which acts as an antagonist of canonical Wnt signaling in human embryonic kidney 293 cells38 and HSCs.39 Similarly Wnt3a and Wnt5a have antagonistic roles in the development of HSCs into early lymphoid cells.4
Whole genome microarray analysis was used to further explore the role of Wnt signaling in the regulation of MKs, and the functional interaction between Wnt3a and Wnt5a signaling pathways. The canonical and noncanonical Wnt signaling pathways appear to play overlapping, diverse, and antagonistic roles in the regulation of gene expression in megakaryocytic cells, which may contribute to MK differentiation and maturation. Indeed, many of the “Wnt responsive” genes have known roles in hematopoiesis and megakaryopoiesis, suggesting that Wnt ligands and signaling pathways may modulate MK differentiation or commitment in vivo.
Several known Wnt3a responsive genes including EPAS130 showed increased expression after Wnt3a treatment. EPAS1, also known as hypoxia inducible factor 2α (HIF2α), is essential for normal blood development in mice, with EPAS1 deficient mice displaying pancytopenia.40 As with the antagonism between Wnt3a and Wnt5a observed at the level of β-catenin stabilization, the increase in expression of EPAS1 induced by Wnt3a was abrogated in the presence of Wnt5a. In contrast, the Wnt3a induction of the transcript encoding the recently identified platelet receptor LRRC32 (GARP)22 was not inhibited by Wnt5a coincubation. By Western blotting however, a modest increase in LRRC32 expression was detected with Wnt3a, whereas a substantial decrease in expression was observed with Wnt5a. Thus, LRRC32 may be regulated at multiple levels by Wnt5a and Wnt3a independently. (This leucine-rich repeat receptor is important for the development of regulatory T-cells,41 and as demonstrated in a recent zebrafish morpholino knockdown model, has a role in thrombocyte adhesion and thrombus formation.34 LRRC32 is also engaged in tethering TGF-β at the cell surface42 and may in part explain known interactions between the WNT and TGF signaling pathways43).
Several genes were uniquely up-regulated in response to Wnt5a, including VASH1, an inhibitor of VEGF and hypoxia induced angiogenesis.44 Wnt5a also reduced the expression of several transcription factors known to be involved in MK differentiation and/or hematopoiesis, including GFI1B and CEBPB. PTH2, up-regulated in response to both Wnt3a and Wnt5 independently, may be analogous to its homologue PTH, which is known to facilitate the interaction between HSCs and the osteoblastic niche.45
To further investigate the role of Wnt signaling in megakaryopoiesis, we examined fetal liver cells from LRP6−/− mice. LRP6−/− embryos exhibit severe developmental defects consistent with a significant reduction in canonical Wnt signaling.24 LRP6−/− mice die at birth, prohibiting the analysis of adult hematopoiesis, although peripheral blood counts from adult heterozygous LRP6+/− mice showed no hematopoietic defect (data not shown). Thus, we isolated embryonic fetal liver cells from the mice to investigate MK development ex vivo. The LRP6−/− cells generated 5-fold fewer MKs than LRP6+/+ cells. Of the few MKs which developed from the LRP6−/−, significantly fewer reached higher levels of ploidy (16N, 32N). Although it is probable that total hematopoiesis, and not just megakaryopoiesis, is affected, these data confirm a defect in MK maturation in these mice. Proplatelet formation was observed in some of the LRP6−/− MKs, although given the low frequency of MKs in these cultures, the effect of LRP6 knockout on the frequency of proplatelet formation was difficult to assess. Our results suggest that LRP6-dependent canonical Wnt signaling is required for MK proliferation and maturity, but is not essential per se for MK commitment. We note that a lower level of canonical Wnt signaling is still present via the LRP5 receptor, although its contribution alone is not sufficient for most embryonic developmental processes.
Wnt signaling was shown to be critical for the regulation of cell shape and polarity, specifically in the formation of protrusive structures from migrating cells, including neurite outgrowth.46 Because the LRP6−/− culture model did not allow accurate assessment of late MK differentiation and proplatelet formation, we used WT fetal liver cultures to assess the contribution of exogenous canonical and noncanonical Wnt signals to proplatelet formation. Strikingly, Wnt3a increased the number of mouse fetal liver primary MKs displaying proplatelets. This effect was completely inhibited by DKK1, consistent with a role for canonical Wnt signaling in proplatelet formation. Wnt5a showed no significant effect on proplatelet formation, suggesting that Wnt is mediating this effect solely through the canonical pathway. Although identification of the specific mechanisms underlying this process are beyond the scope of this paper, Wnt signaling regulates actin cytoskeleton and microtubule assembly and stabilization in different cell types,47,48 which are key steps in the formation of proplatelets.17,49,50 Furthermore, it has been demonstrated that constitutive activation of αIIbβ3–mediated outside-in signaling in human MKs negatively influences proplatelet formation.50 As we have previously shown that Wnt3a functions in both human and mouse platelets to inhibit αIIbβ3–mediated outside-in signaling,10 we can hypothesize that Wnt3a may be driving proplatelet formation in MKs by inhibiting αIIbβ3–mediated outside-in signaling.
Although the exact function of Wnt signaling in hematopoiesis is complex and not yet fully understood, it is clear that during their differentiation hematopoietic cells are exposed and respond to a multitude of Wnt ligands.7 Here, we have demonstrated that Wnt signals specifically regulate the expression of known MK and hematopoietic genes in vitro, and modulate MK proliferation, maturation, and proplatelet formation ex vivo. Taken together, these data reveal a novel regulatory pathway in megakaryopoiesis, and offer the first insights into the exogenous regulation of platelet formation in the mature megakaryocyte.
Supplementary Material
Acknowledgments
This work was supported by grants and fellowships provided by the Science Foundation Ireland (P.B.M., 10/IN.1/B3012); the Irish Research Council for Science, Engineering, and Technology (I.C.M., PD/2008/33), the Program for Research in Third-Level Institutions, administered by the Higher Education Authority of Ireland (D.J.F. and P.B.M.), the National Institute for Health Research, United Kingdom (P.B. and N.A.W.), and National Institutes of Health (NIH) Grant HL68130 (J.E.I.) and GM74241 (X.H.). J.E.I. is an American Society of Hematology Junior Faculty Scholar. J.N.T. is an American Society of Hematology Scholar. M.R.T. is a Marie Curie Intra-European Fellow (237296).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: I.C.M., J.N.T., M.R.T., B.T.M., X.H., D.J.F., J.E.I., and P.B.M. designed research; I.C.M., J.N.T., M.R.T., B.M.S., B.T.M., G.M., P.B., V.S., R.P.M., and C.B. performed experiments; I.C.M., J.N.T., M.R.T., B.T.M., B.M.S., G.M., A.R., R.P.M., N.A.W., J.E.I., and P.B.M. analyzed data; and I.C.M., J.N.T., J.E.I. and P.B.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patricia B. Maguire, School of Biomolecular and Biomedical Science, UCD Conway Institute, University College Dublin, Belfield, Dublin 4, Ireland; e-mail: patricia.maguire@ucd.ie.
References
- 1.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281(32):22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 2.Semenov MV, Habas R, Macdonald BT, He X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell. 2007;131(7):1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Kokolus K, Nemeth MJ. Non-canonical Wnt signaling pathways in hematopoiesis. Immunol Res. 2010;46(1-3):155–164. doi: 10.1007/s12026-009-8116-7. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra S, Baba Y, Garrett KP, Staal FJ, Gerstein R, Kincade PW. Contrasting responses of lymphoid progenitors to canonical and noncanonical Wnt signals. J Immunol. 2008;181(6):3955–3964. doi: 10.4049/jimmunol.181.6.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staal FJ, Sen JM. The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur J Immunol. 2008;38(7):1788–1794. doi: 10.1002/eji.200738118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemeth MJ, Bodine DM. Regulation of hematopoiesis and the hematopoietic stem cell niche by Wnt signaling pathways. Cell Res. 2007;17(9):746–758. doi: 10.1038/cr.2007.69. [DOI] [PubMed] [Google Scholar]
- 7.Malhotra S, Kincade PW. Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell Stem Cell. 2009;4(1):27–36. doi: 10.1016/j.stem.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DK, Nathan Grantham R, Trachte AL, Mannion JD, Wilson CL. Activation of the canonical Wnt/beta-catenin pathway enhances monocyte adhesion to endothelial cells. Biochem Biophys Res Commun. 2006;347(1):109–116. doi: 10.1016/j.bbrc.2006.06.082. [DOI] [PubMed] [Google Scholar]
- 9.Tickenbrock L, Schwable J, Strey A, et al. Wnt signaling regulates transendothelial migration of monocytes. J Leukoc Biol. 2006;79(6):1306–1313. doi: 10.1189/jlb.0905539. [DOI] [PubMed] [Google Scholar]
- 10.Steele BM, Harper MT, Macaulay IC, et al. Canonical Wnt signaling negatively regulates platelet function. Proc Natl Acad Sci U S A. 2009;106(47):19836–19841. doi: 10.1073/pnas.0906268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SY, Kim S, Yun-Choi HS, Jho EH. Wnt5a potentiates U46619-induced platelet aggregation via the PI3K/Akt pathway. Mol Cells. 2011;32(4):333–336. doi: 10.1007/s10059-011-0134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ono M, Matsubara Y, Shibano T, Ikeda Y, Murata M. GSK-3beta negatively regulates megakaryocyte differentiation and platelet production from primary human bone marrow cells in vitro. Platelets. 2011;22(3):196–203. doi: 10.3109/09537104.2010.541959. [DOI] [PubMed] [Google Scholar]
- 13.Soda M, Willert K, Kaushansky K, Geddis AE. Inhibition of GSK-3beta promotes survival and proliferation of megakaryocytic cells through a beta-catenin-independent pathway. Cell Signal. 2008;20(12):2317–2323. doi: 10.1016/j.cellsig.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szalai G, LaRue AC, Watson DK. Molecular mechanisms of megakaryopoiesis. Cell Mol Life Sci. 2006;63(21):2460–2476. doi: 10.1007/s00018-006-6190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115(12):3339–3347. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battinelli EM, Hartwig JH, Italiano JE., Jr Delivering new insight into the biology of megakaryopoiesis and thrombopoiesis. Curr Opin Hematol. 2007;14(5):419–426. doi: 10.1097/MOH.0b013e3282bad151. [DOI] [PubMed] [Google Scholar]
- 17.Italiano JE, Jr., Patel-Hett S, Hartwig JH. Mechanics of proplatelet elaboration. J Thromb Haemost. 2007;5(Suppl 1):18–23. doi: 10.1111/j.1538-7836.2007.02487.x. [DOI] [PubMed] [Google Scholar]
- 18.Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 19.Patel SR, Hartwig JH, Italiano JE., Jr The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115(12):3348–3354. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tijssen MR, Cvejic A, Joshi A, et al. Genome-wide analysis of simultaneous GATA1/2, RUNX1, FLI1, and SCL binding in megakaryocytes identifies hematopoietic regulators. Dev Cell. 2011;20(5):597–609. doi: 10.1016/j.devcel.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watkins NA, Gusnanto A, de Bono B, et al. A HaemAtlas: characterizing gene expression in differentiated human blood cells. Blood. 2009;113(19):e1–9. doi: 10.1182/blood-2008-06-162958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macaulay IC, Tijssen MR, Thijssen-Timmer DC, et al. Comparative gene expression profiling of in vitro differentiated megakaryocytes and erythroblasts identifies novel activatory and inhibitory platelet membrane proteins. Blood. 2007;109(8):3260–3269. doi: 10.1182/blood-2006-07-036269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du P, Kibbe WA, Lin SM. lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24(13):1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 24.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407(6803):535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 25.Villeval JL, Cohen-Solal K, Tulliez M, et al. High thrombopoietin production by hematopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood. 1997;90(11):4369–4383. [PubMed] [Google Scholar]
- 26.Lecine P, Villeval JL, Vyas P, Swencki B, Xu Y, Shivdasani RA. Mice lacking transcription factor NF-E2 provide in vivo validation of the proplatelet model of thrombocytopoiesis and show a platelet production defect that is intrinsic to megakaryocytes. Blood. 1998;92(5):1608–1616. [PubMed] [Google Scholar]
- 27.Drachman JG, Sabath DF, Fox NE, Kaushansky K. Thrombopoietin signal transduction in purified murine megakaryocytes. Blood. 1997;89(2):483–492. [PubMed] [Google Scholar]
- 28.Fugman DA, Witte DP, Jones CL, Aronow BJ, Lieberman MA. In vitro establishment and characterization of a human megakaryoblastic cell line. Blood. 1990;75(6):1252–1261. [PubMed] [Google Scholar]
- 29.Fuhrken PG, Chen C, Miller WM, Papoutsakis ET. Comparative, genome-scale transcriptional analysis of CHRF-288-11 and primary human megakaryocytic cell cultures provides novel insights into lineage-specific differentiation. Exp Hematol. 2007;35(3):476–489. doi: 10.1016/j.exphem.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Doubravska L, Simova S, Cermak L, Valenta T, Korinek V, Andera L. Wnt-expressing rat embryonic fibroblasts suppress Apo2L/TRAIL-induced apoptosis of human leukemia cells. Apoptosis. 2008;13(4):573–587. doi: 10.1007/s10495-008-0191-z. [DOI] [PubMed] [Google Scholar]
- 31.Klapholz-Brown Z, Walmsley GG, Nusse YM, Nusse R, Brown PO. Transcriptional program induced by Wnt protein in human fibroblasts suggests mechanisms for cell cooperativity in defining tissue microenvironments. PLoS One. 2007;2(9):e945. doi: 10.1371/journal.pone.0000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Railo A, Pajunen A, Itaranta P, et al. Genomic response to Wnt signalling is highly context-dependent–evidence from DNA microarray and chromatin immunoprecipitation screens of Wnt/TCF targets. Exp Cell Res. 2009;315(16):2690–2704. doi: 10.1016/j.yexcr.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Zirn B, Samans B, Wittmann S, et al. Target genes of the WNT/beta-catenin pathway in Wilms tumors. Genes Chromosomes Cancer. 2006;45(6):565–574. doi: 10.1002/gcc.20319. [DOI] [PubMed] [Google Scholar]
- 34.O'Connor MN, Salles II, Cvejic A, et al. Functional genomics in zebrafish permits rapid characterization of novel platelet membrane proteins. Blood. 2009;113(19):4754–4762. doi: 10.1182/blood-2008-06-162693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou CJ, Wang YZ, Yamagami T, Zhao T, Song L, Wang K. Generation of Lrp6 conditional gene-targeting mouse line for modeling and dissecting multiple birth defects/congenital anomalies. Dev Dyn. 2010;239(1):318–326. doi: 10.1002/dvdy.22054. [DOI] [PubMed] [Google Scholar]
- 36.Scheller M, Huelsken J, Rosenbauer F, et al. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7(10):1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 37.Cobas M, Wilson A, Ernst B, et al. Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med. 2004;199(2):221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4(4):e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci U S A. 2007;104(39):15436–15441. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA. The HIF family member EPAS1/HIF-2alpha is required for normal hematopoiesis in mice. Blood. 2003;102(5):1634–1640. doi: 10.1182/blood-2003-02-0448. [DOI] [PubMed] [Google Scholar]
- 41.Wang R, Wan Q, Kozhaya L, Fujii H, Unutmaz D. Identification of a regulatory T cell specific cell surface molecule that mediates suppressive signals and induces Foxp3 expression. PLoS One. 2008;3(7):e2705. doi: 10.1371/journal.pone.0002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106(32):13445–13450. doi: 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19(1):71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato Y, Sonoda H. The vasohibin family: a negative regulatory system of angiogenesis genetically programmed in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27(1):37–41. doi: 10.1161/01.ATV.0000252062.48280.61. [DOI] [PubMed] [Google Scholar]
- 45.Garrett RW, Emerson SG. The role of parathyroid hormone and insulin-like growth factors in hematopoietic niches: physiology and pharmacology. Mol Cell Endocrinol. 2008;288(1-2):6–10. doi: 10.1016/j.mce.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 46.Endo Y, Beauchamp E, Woods D, et al. Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3 and c-Jun N-terminal kinase-dependent mechanism. Mol Cell Biol. 2008;28(7):2368–2379. doi: 10.1128/MCB.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells. 1998;3(10):659–670. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 48.Salinas PC. Modulation of the microtubule cytoskeleton: a role for a divergent canonical Wnt pathway. Trends Cell Biol. 2007;17(7):333–342. doi: 10.1016/j.tcb.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Patel SR, Richardson JL, Schulze H, et al. Differential roles of microtubule assembly and sliding in proplatelet formation by megakaryocytes. Blood. 2005;106(13):4076–4085. doi: 10.1182/blood-2005-06-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bury L, Malara A, Gresele P, Balduini A. outside-in signaling generated by a constitutively activated integrin αIIbβ3 impairs proplatelet formation in human megakaryocytes. PLoS One. 2012;7(4):e34449. doi: 10.1371/journal.pone.0034449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.