Abstract
Many chemotherapeutic agents selectively target rapidly dividing cells, including cancer cells, by causing DNA damage that leads to genome instability and cell death. We used Drosophila melanogaster to study how mutations in key DNA repair genes affect an organism’s response to chemotherapeutic drugs. In this study, we focused on camptothecin and its derivatives, topotecan and irinotecan, which are type I topoisomerase inhibitors that create DNA double-strand breaks in rapidly dividing cells. Here, we describe two polymorphisms in Drosophila Cyp6d2 that result in extreme sensitivity to camptothecin but not topotecan or irinotecan. We confirmed that the sensitivity was due to mutations in Cyp6d2 by rescuing the defect with a wild-type copy of Cyp6d2. In addition, we showed that combining a cyp6d2 mutation with mutations in Drosophila brca2 results in extreme sensitivity to camptothecin. Given the frequency of the Cyp6d2 polymorphisms in publcly available Drosophila stocks, our study demonstrates the need for caution when interpreting results from drug sensitivity screens in Drosophila and other model organisms. Furthermore, our findings illustrate how genetic background effects can be important when determining the efficacy of chemotherapeutic agents in various DNA repair mutants.
Keywords: cytochrome p450, homologous recombination, double-strand breaks, chemotherapeutics
The complexities of human genetic disorders often require model systems to provide a better understanding of the disease mechanism. Drosophila melanogaster provides an excellent system for human disease research because of its genetic tractability and the presence of many homologs of human disease genes in the fly genome (Rubin 2000). As such, it has been used to the study the genetic mechanisms of cancer for nearly 40 years (Gateff and Schneiderman 1974; Gateff 1978), and multiple facets of carcinogenesis have been investigated in that time (reviewed in Rudrapatna et al. 2012). Drosophila also has proven to be an invaluable tool to research the effects of chemotherapeutic drugs (Boyd and Setlow 1976; Radcliffe et al. 2002; Jaklevic et al. 2006; Edwards et al. 2011; Gladstone and Su 2011) and the effects of mutations in key DNA repair genes (reviewed in Su 2011).
We have used mutagenic chemicals and radiation to better understand the functions of critical DNA repair proteins (Chan et al. 2010; Kane et al. 2012). Of particular interest to us is the topoisomerase I (Top1) poison, camptothecin, from which the chemotherapeutic drugs topotecan and irinotecan are derived (reviewed in Legarza and Yang 2006; Pommier 2006). Camptothecin and its derivatives stabilize the normally transient covalent link between DNA and Top1, thereby interfering with the relaxation of supercoiling that occurs during events requiring DNA unwinding, such as replication or transcription (Hsiang et al. 1985; Hsiang and Liu 1988; Koster et al. 2007). The classical model proposes that single-strand breaks at the sites of camptothecin-induced Top1-DNA links are converted into double-strand breaks (DSBs) after collision with a replication fork (Holm et al. 1989; Hsiang et al. 1989). Recent research has challenged this theory, however, proposing instead that the accumulation of regressed forks or supercoiled DNA is responsible for the toxic effects (Ray Chaudhuri et al. 2012).
Although cancer frequently involves defects in multiple genes or pathways, there are specific examples in which mutations in a single gene are associated with significant cancer risk. Perhaps one of the most well-known examples is the breast cancer susceptibility gene, brca2. Individuals inheriting a mutant copy of the gene exhibit a significant increase in breast and ovarian cancer risk (Wooster et al. 1995). The Brca2 protein functions in homologous recombination (HR) repair of DNA DSBs (reviewed in Thorslund and West 2007 and Boulton 2006). HR uses an intact DNA template to synthesize nucleotides lost on a broken homologous chromosome or sister chromatid. It is mediated by the recombinase, Rad51 (Sung 1994), which is recruited to the sites of DSBs by Brca2 (Davies et al. 2001). Similar to its mammalian homolog, Drosophila Brca2 interacts with DmRad51 (Brough et al. 2008) and plays a critical role in both mitotic and meiotic HR repair of DSBs in vivo (Klovstad et al. 2008).
We were interested in examining the role of Drosophila Brca2 in the repair of camptothecin-induced DNA damage. To do this, we treated two stocks of brca2 mutant flies with camptothecin. We were surprised to discover that one line of brca2 mutants was exceptionally sensitive to the drug. Further investigation revealed that these flies carried a second mutation in Cyp6d2, a cytochrome P450 gene, which, when combined with the brca2 mutation, resulted in synergistic hypersensitivity to camptothecin. We now report that many publicly available Drosophila stocks carry this mutation or a second, independent mutation in Cyp6d2 that also causes extreme sensitivity to camptothecin.
Materials and Methods
Fly culture conditions and stocks
Flies were kept at 25° with an alternating 12-hr light:12-hr dark cycle and fed a standard cornmeal agar diet. Fly stocks were acquired from the Bloomington Stock Center, with the exception of brca2KO (see below, Creation and isolation of mutants). For our mapping and sequencing studies, we used the sequence available on Flybase as our wild-type standard (Adams et al. 2000).
Creation and isolation of mutants
The brca247 mutation was created via an imprecise excision of P{SUPor-P}KG02287, located between brca2 and CG3746. The excision resulted in a deletion removing the first 2169 bp of brca2. The brca2KO mutant was a donation from Trudi Schüpbach’s laboratory and was created by ends-out HR (Klovstad et al. 2008).
The original scpt mutant was found in the same stock used to create brca247 but was created via a precise excision of the P-element. This mutant was later renamed cyp6d2SD. The other Cyp6d2 mutation, cyp6d2NT, was found in the P{GT1}CG42565BG02301 stock.
Sensitivity assays
Sensitivity assays were set up using five to eight virgin female flies and three male flies. The females were heterozygous for the mutation of interest, whereas males were either heterozygous or homozygous. Heterozygous flies were balanced by the CyO chromosome.
Parental flies were kept in vials for 3 d to lay eggs and were then transferred to new vials. The flies were then discarded after 2-3 additional days. Each set of vials was treated with mutagen or vehicle one day after the parents were removed. Camptothecin (Sigma-Aldrich) was dissolved in DMSO and then diluted in Tween 20/EtOH (5% ethanol, 1% Tween 20). Mechlorethamine, methyl methanesulfonate, hydroxyurea (Sigma-Aldrich) topotecan, irinotecan, and bleomycin (Enzo Life Sciences) were dissolved in water. Each sensitivity trial consisted of five to eight vials. Heterozygous and homozygous offspring were counted periodically until 18−20 d after the crosses were established. Percent survival was calculated by the following equation:
Sensitivity to ionizing radiation was characterized in a similar way. Parental flies (40−60 virgin females and 10−20 males) were allowed to lay eggs on grape agar plates for several days, with plates replaced every 10−14 hr. The plates were then supplemented with yeast paste and placed at 25°. Once larvae reached third instar stage, the grape agar plates were irradiated at a rate of 800 rads/min in a Gammator 1000 irradiator. After irradiation, the larvae were moved into fly bottles for further development. Adult flies were counted and sorted as described previously, and irradiated flies were compared with an unirradiated control.
Rescue sensitivity assays consisted of five to eight virgin female flies that were heterozygous for the mutation of interest (balanced by CyO) as well as one copy of the Cyp6d2 rescue construct (see below, Construction of the Cyp6d2 rescue stock). They were crossed to three males that were heterozygous or homozygous for the mutation tested, with no rescue construct. Vials were treated as described previously, and the progeny were sorted by wing type and eye color. Percent survival was calculated as described previously but separately for rescued and nonrescued flies.
Mapping
For complementation mapping, flies heterozygous for a defined deletion or insertion (over 2nd chromosome balancer CyO) were crossed to scpt homozygous flies in vials. Vials were treated as described previously for sensitivity assays, using the scpt lethal dose of 25 μM camptothecin. Flies were sorted by wing type to determine complementation.
Meiotic mapping used noncomplementing stocks carrying white+ markers at defined locations. Female flies heterozygous for the noncomplementing marked deletion or insertion (over a wild type 2nd chromosome to allow for meiotic recombination) were crossed to homozygous scpt mutant males in vials. Vials were treated with 25 μM camptothecin. Offspring were sorted by eye color, and the percent of red-eyed progeny was calculated.
Construction of the Cyp6d2 rescue stock
The full-length Cyp6d2 gene, including 402 bp upstream of the transcription start site and 285 bp downstream of the transcription termination site, was amplified by polymerase chain reaction (PCR) using Phusion polymerase (New England Biolabs) and the primers 5′-TCTAGAGGTACCGCGCTGACAATCCTACAAGC-3′ and 5′-AGATCTGCGGCCGCGATTCCGCAAGGTGGAGAAG-3′. The PCR product was digested with Acc65I and NotI and directionally cloned into pattB (gift of K. Basler). Purified plasmid (500 ng/μL) was injected into fly embryos <2 hr after egg laying containing an attP site on 3R: y1,M{vas-int}ZH2A,w*; M{3xP3-RFP,attP}ZH96E. Injected embryos were allowed to develop into adult flies at 25°. These adults were crossed to w1118 males or females, and the progeny sorted by eye color. We recovered at least one red-eyed fly in the progeny of approximately 6% of all surviving injected flies. The presence of the construct was confirmed by PCR and sequencing. Flies carrying the rescue construct were mated to mutant flies to generate stocks carrying both the mutation of interest and the rescue construct. These flies were used in rescue sensitivity assays as described above.
Allele-specific PCR
To identify stocks with specific Cyp6d2 mutations, allele-specific primers were each paired with the genomic primer downstream of Cyp6d2, 5′-ctctcgaattcagaacgagc-3′. The allele-specific primer, 5′-GGGTCCTAGGCACTGCAGAC-3′, was used to detect cyp6d2SD, with an annealing temperature of 60°. The allele-specific primer, 5′-CCCATCGCTTCGATTCAGAGAC-3′, was used to detect cyp6d2NT, with an annealing temperature of 56°. These pairs yield 391-bp and 452-bp products, respectively, when we amplified template DNA from the appropriate mutant but produce no product with a wild-type template. Phusion polymerase (New England Biolabs) was used for amplification.
Reverse-transcriptase PCR
To determine expression levels of Cyp6d2 in various backgrounds, 15 wandering 3rd instar larvae (5−6 d after egg laying) were isolated and frozen at −80°. RNA was purified using RNAqueous-4PCR (Ambion) and cDNA was synthesized using RETROscript (Ambion). Random decamers were used as primers for cDNA synthesis. Cyp6d2 cDNA was then amplified to detect transcripts in each genetic background. The following primers were used: XInt1 5′-CATTAGCTTAGCAATCGGTGG-3′; XInt2 5′-GGACATCTGCATCATGGAAACC-3′; XInt3 5′-CTTTTCCCATGCGAAGAGCTATGC-3′; F1 5′-CTCGCCAAATCATGACCAGC-3′; F2 5′-GCTAAGCTAATGAACCGCTTGG-3′; R1 5′-GCGGCATCGAAACGGAACTC-3′; rp49F 5′-CCATCCGCCCAGCATACAGG-3′; and rp49R 5′-CTCGTTCTCTTGAGAACGCAG-3′.
Results
Identification of a genetic modifier of camptothecin sensitivity
Drosophila Brca2 has been shown to play a critical role in HR repair of DNA DSBs (Brough et al. 2008; Klovstad et al. 2008). Along with its clinically approved analogs topotecan and irinotecan, camptothecin is traditionally thought to create DSBs in rapidly dividing cells via replication run-off (Strumberg et al. 2000), leaving a one-ended DSB (Tsao et al. 1993). Brca2-dependent HR repair could then be required to restart replication.
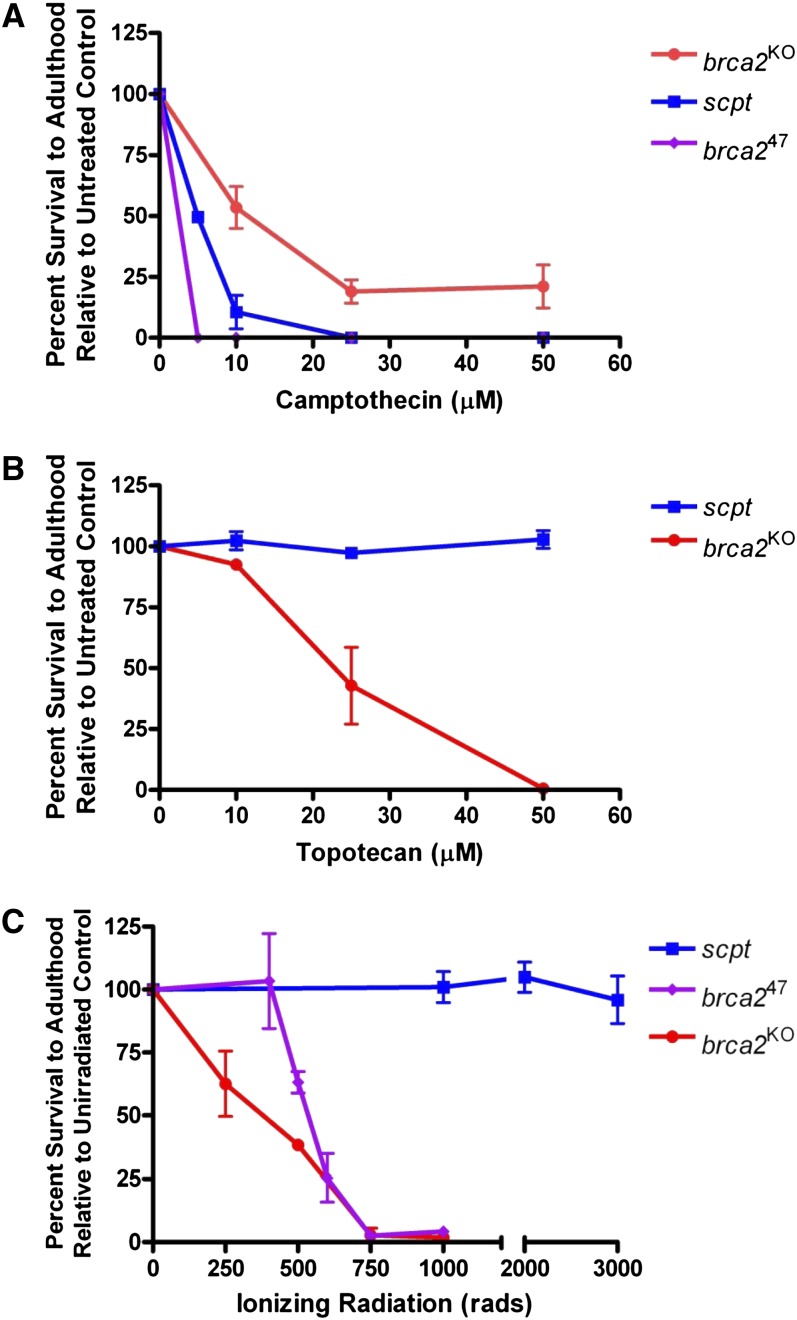
To test this hypothesis, we treated two independently derived brca2 mutants with camptothecin and quantified survival to adulthood. The first was a null allele (brca2KO) created by ends-out HR (Klovstad et al. 2008). The second (brca247) was created via an imprecise excision of a P-element 90 bp upstream of brca2 (P{SUPor-P}KG02287) and deletes the 5′ half of brca2. Both alleles were sensitive to camptothecin, suggesting that Brca2-mediated HR repair is important for repair of one-ended DSBs. Surprisingly, the brca247 mutant was significantly more sensitive to camptothecin than the brca2KO allele (Figure 1A). We suspected that the brca247 stock contained a second-site mutation that made it more sensitive than the brca2KO mutation. Such mutations are common in imprecise excision screens, and often occur near the site of the inserted element.
Figure 1 .
The scpt mutant is sensitive to camptothecin but not to topotecan or ionizing radiation. (A) Camptothecin sensitivity of the scpt mutant as well as two null alleles of brca2. All data points represent three independent trials of five to six vials each. (B) Topotecan sensitivity of the scpt mutant and brca2KO mutant. All data points represent three independent trials of five to six vials each. (C) Ionizing radiation sensitivity of the scpt mutant as well as two brca2 mutant alleles. All data points represent two to four independent trials of one bottle each. SDs are shown as error bars.
To confirm this, we created a precise excision of P{SUPor-P}KG02287. Sequencing of DNA from this stock revealed that the brca2 coding sequence was identical to wild-type sequence. Surprisingly, this precise excision stock also displayed high sensitivity to camptothecin, although not to the level of brca247 flies (Figure 1A). This suggested that the second-site mutation did not arise during the imprecise excision of the P-element but rather was present in the original stock used to create brca247. We named this mutation scpt (sensitive to camptothecin). Unlike brca2 mutants, scpt mutants were not sensitive to the camptothecin analog topotecan (Figure 1B) or to ionizing radiation (Figure 1C). Furthermore, scpt mutants were not sensitive to irinotecan, bleomycin, mechlorethamine (nitrogen mustard), methyl methanesulfonate, or hydroxyurea (data not shown).
The scpt mutation is present in multiple stocks
To understand the unusual specificity of the scpt mutation, we sought to identify the gene(s) mutated in this stock. Since scpt exhibited Mendelian segregation with the 2nd chromosome balancer CyO, we assumed the mutation was located on the 2nd chromosome. To map the mutation, we began by performing complementation tests using chromosome 2 deficiency stocks. We crossed deficiency stocks to scpt mutants and treated the offspring with 25 μM camptothecin, a lethal dose for scpt homozygotes. Our initial tests used the DrosDel (ED) Deficiency Collection (Ryder et al. 2004), available from the Bloomington Stock Center.
We were surprised to find that none of the 2nd chromosome fly stocks we used from this collection were able to complement the scpt mutation when treated with a lethal dose of camptothecin, regardless of the location of the deletion (Table 1). However, multiple stocks from the Exelixis and Bloomington Stock Center deficiency collections did complement the mutation at this dose. Therefore, we conclude that the progenitor stocks of the DrosDel collection as well as P{SUPor-P}KG02287 carry the scpt mutation.
Table 1. Cyp6d2 status of various stocks.
| Stocka | Cytological Location | Cyp6d2 Alleleb | Complementation Testc |
|---|---|---|---|
| P{SUPor-P} insertions | |||
| KG00490 (CG34370) | 58B1 | WT | Complements |
| KG01596 (whd) | 47A11 | WT | Complements |
| KG04872 (CG13322) | 49E1 | WT | Complements |
| KG06046 | 60F5 | WT | Complements |
| KG06805 (RabX1) | 59E2 | WT | ND |
| KG07568 (CG15704) | 53A4 | WT | Complements |
| KG00006 (CycB) | 59B2 | SD | Does not complement |
| KG02089 (Hrb87F) | 87F7 | SD | ND |
| KG02287 (CG4612, brca2) | 60D4 | SD | Does not complement |
| KG02463 (gce, Top1) | 13B6 | SD | ND |
| KG02566 (CG10880) | 40F1 | SD | Does not complement |
| KG05061 (Babos) | 58D4 | SD | Does not complement |
| KG06675 (CG9896) | 59C1 | SD | Does not complement |
| KG07633 (Egfr, CG30286) | 57E9 | SD | Does not complement |
| KG07401 (CG13511) | 58F4 | SD | Does not complement |
| KG07430 (Tim17b2) | 35D2 | SD | Does not complement |
| KG06763 | 35B1 | SD | Does not complement |
| KG07930 (Jheh3) | 55F8 | SD | Does not complement |
| Mi{MIC} insertions | |||
| MI02105 (grp) | 36A10 | NT | ND |
| MI02462 (Mad) | 23D3 | NT | ND |
| MI00056 (jbug) | 59A3 | WT | ND |
| MI02085 (Cyp6d2) | 58F4 | WT | ND |
| Mi{ET1} insertions | |||
| MB00453 (dp) | 25A1 | NT | ND |
| MB01292 (CG3746) | 58F4 | NT | Does not complement |
| MB05269 (Cyp6d2) | 58F4 | NT | Does not complement |
| MB05513 (CG13579) | 60D1 | NT | ND |
| P{GT1} insertions | |||
| BG02301 (CG42565) | 58F4 | NT | Does not complement |
| BG02743 (Hrb87F) | 87F7 | NT | ND |
| DrosDel deficiencies | |||
| Df(2L)ED284 | 25F2-26A3 | NT | Does not complement |
| Df(2L)ED1303 | 37E5-38C6 | NT | Does not complement |
| Df(2R)ED3683 | 55C2-56C4 | NT | Does not complement |
| Df(2R)ED3728 | 56D10-56E2 | NT | Does not complement |
| Df(2R)ED3923 | 57F6-57F10 | NT | Does not complement |
| Df(2R)ED3952 | 58B10-58E5 | NT | Does not complement |
| Df(2R)ED4061 | 60C8-60D13 | NT | Does not complement |
| Df(2R)ED4065 | 60C8-60E8 | NT | Does not complement |
| Df(2R)ED4071 | 60C8-60E8 | NT | Does not complement |
| Df(3R)ED4408 | 66A22-66C5 | NT | ND |
| Df(3R)ED10257 | 83A7-83B4 | NT | ND |
| Bloomington Stock Center deficiencies | |||
| Df(2R)BSC597 | 58A2-58F1 | WT | Complements |
| Df(2R)BSC598 (Cyp6d2) | 58F3-59A1 | Deleted | Does not complement |
| Df(2R)BSC599 | 59B1-60F5 | WT | Complements |
| Df(2R)BSC602 | 60C8-60E5 | WT | Complements |
| Df(2R)BSC603 | 60C7-60D1 | ND | Complements |
| Df(2R)BSC604 | 60D4-60E11 | ND | Complements |
| Df(2R)BSC605 | 60D8-60E8 | ND | Complements |
| Df(2R)BSC606 | 60D10-60E1 | WT | Complements |
| Df(2R)BSC607 | 60E4-60E8 | WT | Complements |
| Df(2R)BSC769 | 59B7-59D9 | WT | Complements |
| Df(2R)BSC784 | 59B4-59B6 | ND | Complements |
| Df(2R)BSC787 | 58F4-59B1 | ND | Complements |
| Exelixis deficiencies | |||
| Df(2R)Exel6044 | 37F2-38E3 | ND | Complements |
| Df(2R)Exel6079 | 59A3-59B1 | WT | Complements |
| Df(3R)Exel6178 | 90F4-91A5 | WT | ND |
ND, no data; WT, wild type.
Genes potentially affected by transposon insertions are shown.
WT: sequence matches Flybase at two loci of interest; SD: stock carries cyp6d2SD allele; NT: stock carries cyp6d2NT allele.
Stock tested for complementation (survival) with original scpt allele (precise excision of P{SUPorP}KG02287) at a dose of 25 μM camptothecin.
Because multiple DrosDel stocks were carriers, we wanted to see whether other chromosomes containing P{SUPor-P} elements also carried scpt. We performed complementation tests as before, using P{SUPor-P} stocks. Roughly two-thirds of the P{SUPor-P} stocks tested did not complement the scpt mutation (Table 1). Location of the P{SUPor-P} element did not correlate with complementation of scpt. Thus, we hypothesize that the progenitor stock used to create the P{SUPor-P} collection (Roseman et al. 1995) carried the mutation, although it appears to have been lost in a subset of these stocks.
Mapping the scpt mutation
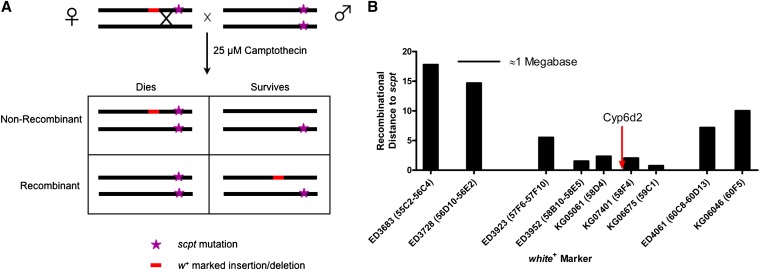
Because the DrosDel stocks and many P{SUPor-P} stocks carry the scpt mutation at an unknown location and a red eye marker (white+) at a defined location, we used traditional meiotic mapping to determine the location of scpt (Figure 2A). Unbalanced female flies heterozygous for the scpt, white+ chromosome (from either P{SUPor-P} or DrosDel stocks) were crossed to white-eyed homozygous scpt males, and the progeny were treated with 25 μM camptothecin. Lower-than-expected survival rates of red-eyed flies (<50%) indicated linkage of the white+ marker to scpt. We did this using multiple DrosDel and P{SUPor-P} stocks near the right end of chromosome 2 (Figure 2B). These tests indicated a location of interest near the cytological bands 58B−59C.
Figure 2 .
Meiotic mapping of the scpt mutation. (A) Cross scheme for meiotic mapping. Flies that inherit the white+ marker have red eyes. Flies that inherit two mutant copies of the scpt mutation do not survive treatment with 25 µM camptothecin. The percentage of surviving flies with red eyes should be equal to the recombination distance between the white+ marker and scpt. (B) Multiple meiotic mapping crosses identified a region containing the scpt mutation. Both DrosDel deficiencies (ED) and P{SUPor-P} elements (KG) were used. Distances on the x-axis are approximations of the physical distance between these markers. The region shown spans from cytological band 55C to 60F (the terminal quarter of chromosome 2R, or about 7 megabases).
We further refined the position of scpt with complementation tests using deficiencies from the Exelixis and Bloomington Stock Center collections. Based on our complementation tests (Table 1), we assumed that these deficiency collections did not carry scpt, and therefore noncomplementation indicated that the deficiency deleted the gene(s) mutated in scpt flies. Of the five deficiencies near the region of lowest recombination distance, only Df(2R)BSC598 did not complement the scpt mutation. This suggested that scpt was located in the 36.9-kb region deleted in this deficiency. Furthermore, we found that an overlapping deletion in Df(2R)BSC787 complemented scpt, allowing us to narrow down the scpt-containing region to approximately 20 kb. This region contains 13 genes, one of which is the cytochrome P450 gene, Cyp6d2.
We sequenced the coding regions of all 13 of these genes using flies from complementing P{SUPor-P} stocks (KG06046 and KG01596) and noncomplementing P{SUPor-P} stocks (KG02287 and KG06675). Amino acid changes we identified in the noncomplementing stocks are shown in supporting information, Table S1. The complementing P{SUPor-P} stocks matched the published Drosophila sequence found on FlyBase at almost all locations. Importantly, the Flybase sequenced stock, y1; Gr22b1 Gr22d1 cn1 CG33964R4.2 bw1 sp1; LysC1 MstProx1 GstD51 Rh61 (Adams et al. 2000) was not sensitive to camptothecin (data not shown).
The scpt mutation alters splicing of the Cyp6d2 transcript
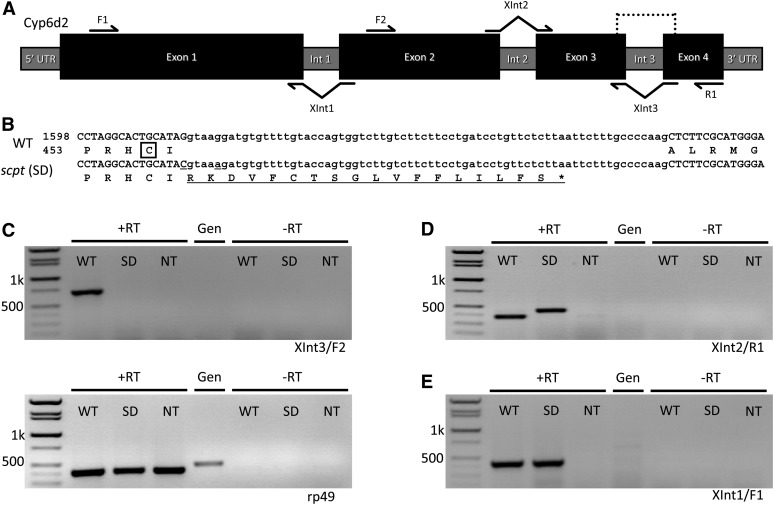
Two of the polymorphisms detected by sequencing were located near the exon three/intron three junction in Cyp6d2 (Figure 3, A and B). The first (G→C) is located at the terminal nucleotide of exon three and is also the first nucleotide of an alanine codon. The second (G→A) is located five nucleotides downstream of the exon−intron junction. If the two single-nucleotide polymorphisms (SNPs) did not affect splicing, the first SNP would change the alanine (A458) to a proline. Alignment of Drosophila Cyp6d2 and other P450 enzymes indicated that this change is in a highly conserved region identified as the heme-binding domain (Tijet et al. 2001). Alternatively, if the two SNPs combined to disrupt splicing of intron three, the alanine would become an arginine and a frameshift would result in a premature stop codon 17 amino acids downstream.
Figure 3 .
Mutations in Cyp6d2 affect transcription and RNA processing. (A) Diagram of the Cyp6d2 gene structure. Protein coding regions are represented by black boxes, whereas gray bars represent introns and UTRs. Dotted line indicates the region shown in B. Primers shown were used in RT-PCRs in C−E. (B) Nucleotide and amino acid alignment of wild-type (WT) and scpt mutant Cyp6d2. scpt SNPs in this region are underlined. The heme-binding cysteine is boxed in the WT protein. Failure to splice intron three (sequence in lower-case letters) results in a frameshift mutation and a premature stop codon (*). Total wild-type protein length is 512 amino acids. (C−E) Semiquantitative RT-PCR of wild-type and mutant larvae. Primers indicated below gels refer to those shown in (A). RT-PCR with (+RT) and without (−RT) reverse transcriptase was performed. Genomic (Gen) DNA template is also shown for comparison. Sizes of molecular weight markers are indicated. (C) The XInt3/F2 primer pair yields a PCR product of 709 bp if splicing of intron 3 is correct. The rp49 primer pair (control reaction) yields PCR products of 398 bp and a genomic product of 460 bp. (D) The XInt2/R1 primer pair yields a PCR product of 375 bp if introns 2 and 3 are properly spliced. Intron 3 length is 70 bp. (E) XInt1/F1 yields a PCR product of 440 bp if intron 1 is correctly spliced.
To distinguish between these two possibilities, we performed semiquantitative reverse transcription (RT)-PCR to analyze Cyp6d2 transcripts in wild-type and scpt mutant larvae. We synthesized total cDNA from RNA purifications and amplified DNA using primers that will anneal only to correctly spliced exons (Figure 3A). The pairing of a reverse primer that spans intron three with a forward primer in exon two resulted in no product for scpt mutant larvae, whereas both the scpt mutant and wild-type larvae had normal levels of the rp49 control transcript (Figure 3C). In addition, pairing a forward primer spanning intron two with a reverse primer in exon four produced a larger than expected product for scpt larvae (Figure 3D). These results are consistent with a splicing defect in scpt mutants and suggest that the intron three sequence is included with the final transcript. Sequencing confirmed that the cDNA templates isolated from the scpt mutant included intron three (data not shown). To demonstrate that splicing of other Cyp6d2 introns is normal in the scpt mutant, we paired a reverse primer that spans intron one with a forward primer in exon one. This produced equal length products for wild-type and scpt mutant larvae (Figure 3E). Based on these findings, we reasoned that the cyp6d2SD (splicing defective) allele was the best candidate for the scpt mutation.
To confirm that cyp6d2SD was the scpt mutation, we sought to rescue the camptothecin sensitivity with a wild-type copy of Cyp6d2 by using phiC31-mediated transgenesis (Bischof et al. 2007). To do this, we cloned and inserted wild-type Cyp6d2, along with several hundred base pairs of flanking sequence, into the vector pattB. The pattB-Cyp6d2 plasmid was injected into fly embryos carrying an attP site at cytological band 96E. Successful germline integration of the plasmid was detected in the following generation by screening for white+ flies.
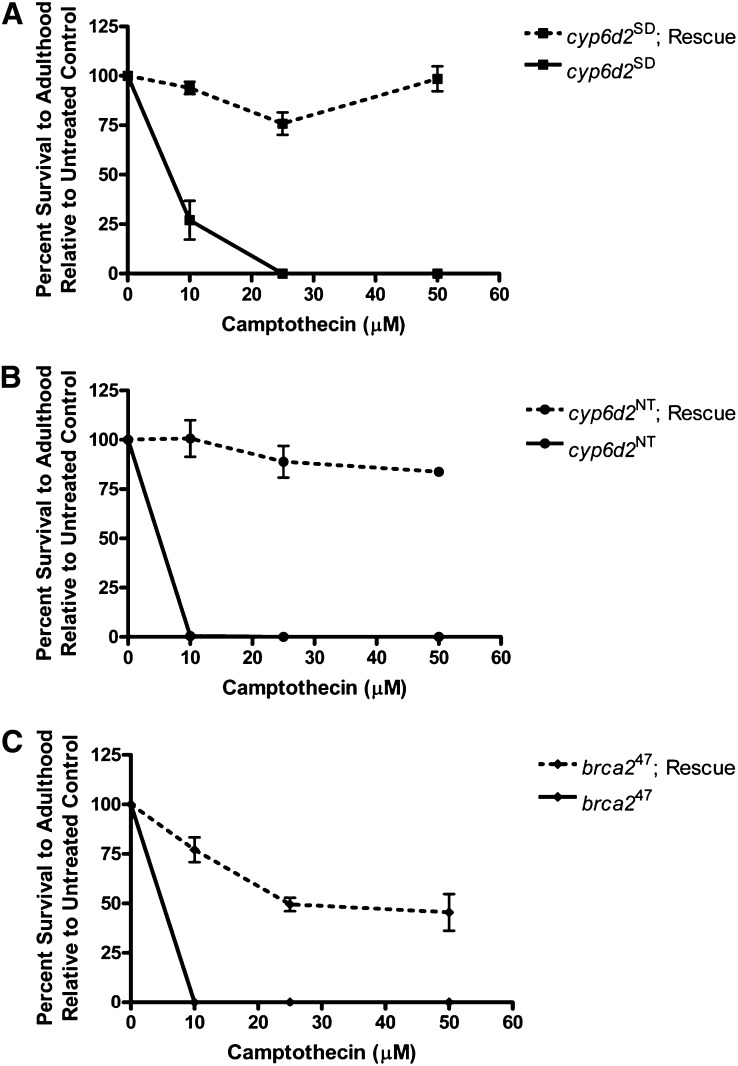
We crossed the attP-Cyp6d2 transgenic stock to our cyp6d2SD mutant flies to generate flies that carried both the mutation and the rescue construct and conducted sensitivity assays with these flies. cyp6d2SD homozygotes carrying one copy of the rescue construct showed nearly 100% survival at all doses tested (Figure 4A). We conclude that cyp6d2SD is the scpt mutation.
Figure 4 .
Rescue of camptothecin sensitivity by a single wild-type Cyp6d2 transgene. All data points represent three to four independent trials of five to six vials each. Error bars represent SDs. (A−B) Rescue of both cyp6d2 mutants. A precise excision of P{GT1}CG42565 was used as the cyp6d2NT mutant in (B). (C) Partial rescue of the brca247 allele.
A second, naturally occurring Cyp6d2 variant also sensitizes flies to camptothecin
During the course of examining Cyp6d2 sequences in other fly stocks, we discovered a second set of polymorphisms that produced a nearly identical camptothecin sensitivity phenotype (Figure 4B and Figure S2). In these stocks, consecutive asparagine residues (N438 and N439) were changed to aspartic acid and threonine respectively. Interestingly, semiquantitative RT-PCR revealed that this mutant produced little to no Cyp6d2 transcript (Figure 3, C−E). We named this mutant cyp6d2NT (no transcript). Sequence analysis showed that Mi{ET1} and P{GT1} insertion stocks, as well as DrosDel collection deficiencies carried the cyp6d2NT mutation (Table 1). The mutant phenotype of these flies was also completely rescued by the wild-type construct (Figure 4B).
We developed allele-specific primers to rapidly screen additional stocks for either of the cyp6d2 mutations (Figure S1; see Materials and Methods). For the cyp6d2NT allele, we used allele-specific primers specific to the N438D and N439T polymorphisms. Table 1 lists all stocks containing transposable elements and deficiencies that we have examined via complementation tests and/or PCR. Notably, multiple stocks with transposable elements or deficiencies on the X and 3rd chromosomes also were shown to carry the point mutations in Cyp6d2.
The brca247 phenotype results from defects in both HR and Cyp6d2
We reasoned that the brca247 mutant could be especially sensitive to camptothecin for two reasons: (1) it is unable to repair DSBs by HR and (2) it carries the cyp6d2SD mutation. To test this, we attempted to rescue the sensitivity phenotype of the brca247 mutant with the wild-type Cyp6d2 construct. Importantly, the construct provided only a partial rescue of the sensitivity (Figure 4C). Interestingly, the sensitivity of the Cyp6d2-rescued brca247 mutants is similar to the brca2KO stock (Figure 1A). These findings demonstrate that the brca247 mutant phenotype results from two mutations and strongly suggest that the rescue of the cyp6d2SD and cyp6d2NT mutant phenotypes by a wild-type copy of Cyp6d2 is not simply the result of overexpressing a detoxification gene.
Discussion
In this study, we have shown that many Drosophila stocks carry mutations in Cyp6d2 that render the flies hypersensitive to the drug camptothecin. We hypothesize that Cyp6d2 is a critical enzyme required for the breakdown and/or removal of camptothecin. In its absence, camptothecin levels remain high and cause significant cell death, most likely through the creation of one-ended DSBs. When combined with a mutation in the DSB repair gene, brca2, we observed a synergistic effect. Therefore, we hypothesize that at low doses of camptothecin, the resulting DSBs can be repaired through Brca2-dependent HR (Figure 1A).
These variants of Cyp6d2 illustrate a potential pitfall in the study of DNA repair genes in Drosophila. It is likely that many genes involved in detoxification or removal of harmful compounds are polymorphic. Such polymorphisms may skew drug sensitivity screens such that the results are misinterpreted. In an attempt to understand the role of HR proteins in one-ended DSB repair, we used camptothecin sensitivity assays to characterize different DNA repair mutants. We initially were misled to believe that brca2 mutants were exceptionally sensitive to camptothecin, more so than rad51 mutants (data not shown), suggesting a function for Drosophila Brca2 in camptothecin-induced damage repair outside of its well-defined role in HR. However, our results here, along with the observation that rad51 and brca2 mutants display similar sensitivities to topotecan (data not shown), suggest that this is not the case.
Cytochrome P450s and detoxification of xenobiotics
The cytochrome P450 superfamily, of which Drosophila Cyp6d2 is a member, is a group of metabolic enzymes with an unusually wide range of substrates (Coon et al. 1992). They are conserved throughout evolution (Coon et al. 1996) and are involved in the metabolism of many endogenous and exogenous compounds. P450 enzymes catalyze the addition of oxygen to a substrate via a heme cofactor (Coon et al. 1992; Hollenberg 1992). The additional oxygen atom may alter the stability of the substrate, leading to other molecular rearrangements (Coon et al. 1996; Bergé et al. 1998), or it may trigger conjugation by enzymes such as glutathione-S-transferases (reviewed in Tu and Akgül 2005). These processes lead to the detoxification and/or excretion of harmful compounds.
P450 activity is critically dependent on the heme ligand, which mediates the electron transfer reactions that ultimately lead to a modified substrate (Hollenberg 1992). The heme group is bound to the protein via a cysteine residue near its C-terminus and surrounded by conserved sequence (Tijet et al. 2001). Notably, the cyp6d2SD mutation is located very close to the heme-binding cysteine in Cyp6d2 (Figure 3B). In addition to altering the C-terminus of the protein sequence, this mutation also results in structural changes very close to the heme-binding cysteine. Because the cyp6d2SD mutant appears slightly more resistant to camptothecin than the cyp6d2NT mutant (Figure 4, A and B), we hypothesize that protein encoded by the cyp6d2SD allele has greatly reduced function and is a severe hypomorph.
The amino acid changes in cyp6d2NT must be closely linked to a noncoding change that affects the regulation of Cyp6d2. Because the camptothecin sensitivity in cyp6d2NT was successfully rescued by the transgene, such a change would have to be within the region contained in the rescue construct. Although there are no obvious changes in the promoter sequence that would be predicted to affect transcription initiation, the 3′ UTR of cyp6d2NT is significantly different from wild-type sequence (Figure S2).
Potential roles of Drosophila Cyp6d2
In Drosophila, chemical detoxification occurs in the Malpighian tubules, midgut and larval fat body. Consistent with a role in detoxification, the organ with the highest level of expression of Cyp6d2 is the larval fat body (Chintapalli et al. 2007; Yang et al. 2007). Temporally, the greatest expression levels of Cyp6d2 occur during the larval and prepupal stages (Chintapalli et al. 2007), which coincide with the days immediately after treatment in our sensitivity assays. Cyp6d2 expression was very high in hyperoxic conditions (Gruenewald et al. 2009), but it was not identified in a survey of multiple xenobiotic and insecticide screens (Giraudo et al. 2010). Therefore, its preferred substrates are unknown.
There are 83 functional P450 genes in the Drosophila genome, including 22 from the insect-specific CYP6 family (Tijet et al. 2001). Considering that P450 enzymes frequently have overlapping substrate specificity (Coon et al. 1992; Hollenberg 1992), it is reasonable to expect that another P450 enzyme could detoxify camptothecin in the absence of Cyp6d2. However, our data suggest that Cyp6d2 provides a nonredundant function in camptothecin detoxification. It is possible that camptothecin removal or breakdown could also involve multiple steps, with additional enzymes mediating other reactions. In this case, at least one of the reactions must require Cyp6d2.
The fact that scpt mutants are sensitive to camptothecin but not topotecan was an unexpected result. We propose three hypotheses to explain this. First, camptothecin may have alternate cytotoxicity separate from its inhibition of Top1. The initial discovery and chemical characterization of camptothecin was promising, but clinical trials were abandoned after issues of toxicity arose (Wall and Wani 1995; Legarza and Yang 2006). Later work prompted the development of topotecan and irinotecan (Kunimoto et al. 1987; Mattern et al. 1991). The camptothecin sensitivity we observed in fruit flies could be caused by the same mechanisms that resulted in the side effects observed in human clinical trials. This would suggest that Cyp6d2-mediated breakdown or removal of camptothecin prevents this toxicity from reaching lethal levels in flies.
Alternatively, a second hypothesis is that the active site of Cyp6d2 can accommodate camptothecin but not topotecan. The most likely explanation for such specificity would be the chemical properties of the two drugs. Camptothecin is a lipophilic molecule, whereas topotecan is hydrophilic. The human P450 enzymes most critical for drug detoxification, including Cyp3a4, tend to favor lipophilic substrates (Smith et al. 1997). Despite being structurally similar, the water-soluble topotecan (Pommier 2009) may not be a suitable substrate for Cyp6d2, which would explain why cyp6d2 mutants are not sensitive to the drug.
Our third hypothesis posits that lipophilic camptothecin may accumulate in the fat body (Zijlstra and Vogel 1988), which functions analogously to the liver in fruit flies and other insects (Buchon et al. 2009; Yang et al. 2007). In contrast, topotecan, being hydrophilic, may be more easily excreted, resulting in a shorter time of exposure to the drug. The observation that Cyp6d2 is highly expressed in the fat body (Yang et al. 2007), supports this model.
All of these hypotheses assume that Cyp6d2 is involved in camptothecin detoxification, but other possible explanations may exist. Recently, Cyp6d2 was shown to be upregulated 18 hr after ionizing radiation treatment, independent of p53 (van Bergeijk et al. 2012). This suggests that Cyp6d2 may have a role in p53-independent apoptosis. A defective apoptotic pathway could impair imaginal disc development in larvae, leading to the adult lethality that we observe.
Drosophila provides an excellent in vivo system to study the effects of DNA repair mutations and mutagens on genome stability and survival. Nevertheless, our studies suggest caution when using Drosophila as a tool for drug screening. Care must be taken in any analysis of phenotypes because of the possibility that genetic background effects, such as the cyp6d2 mutations, could be influencing the results.
Our study also has implications for the development of cancer chemotherapeutics. P450 enzymes and the polymorphisms that exist within them pose significant challenges in drug development (Guengerich 1999; Plant 2007; Maekawa et al. 2010). Point mutations in Drosophila P450 genes have previously been shown to dramatically affect drug detoxification (Amichot et al. 2004), suggesting fruit flies may be a valuable model for this research. Because many cancer chemotherapeutics damage DNA or target defective DNA repair mechanisms (Ferguson and Pearson 1996; Lawley and Phillips 1996), studying these drugs in Drosophila allows us to evaluate the influences of both detoxification and repair enzymes simultaneously.
Supplementary Material
Acknowledgments
We thank Alice Witsell for performing the injections to generate Cyp6d2 rescue flies. We also Trudi Schüpbach for the brca2KO stock used in this study, as well as Konrad Basler for the pattB plasmid and the Bloomington Stock Center for fly stocks. Finally, we thank Erik Dopman, Elyse Bolterstein, and Sergei Mirkin for helpful suggestions on the manuscript and experimental insight. This research was supported by National Science Foundation grant MCB0643253 and National Institutes of Health grant R01GM092866 to M.M.
Footnotes
Communicating editor: H. Salz
Literature Cited
- Adams M. D., Celniker S. E., Holt R. A., Evans C. A., Gocayne J. D., et al. , 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195 [DOI] [PubMed] [Google Scholar]
- Amichot M., Tarès S., Brun-Barale A., Arthaud L., Bride J.-M., et al. , 2004. Point mutations associated with insecticide resistance in the Drosophila cytochrome P450 Cyp6a2 enable DDT metabolism. Eur. J. Biochem. 271: 1250–1257 [DOI] [PubMed] [Google Scholar]
- Bergé J. B., Feyereisen R., Amichot M., 1998. Cytochrome P450 monooxygenases and insecticide resistance in insects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353: 1701–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J., Maeda R. K., Hediger M., Karch F., Basler K., 2007. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104: 3312–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton S. J., 2006. Cellular functions of the BRCA tumour-suppressor proteins. Biochem. Soc. Trans. 34: 633–645 [DOI] [PubMed] [Google Scholar]
- Boyd J. B., Setlow R. B., 1976. Characterization of postreplication repair in mutagen-sensitive strains of Drosophila melanogaster. Genetics 84: 507–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brough R., Wei D., Leulier S., Lord C. J., Rong Y. S., et al. , 2008. Functional analysis of Drosophila melanogaster BRCA2 in DNA repair. DNA Repair (Amst.) 7: 10–19 [DOI] [PubMed] [Google Scholar]
- Buchon N., Broderick N. A., Poidevin M., Pradervand S., Lemaitre B., 2009. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5: 200–211 [DOI] [PubMed] [Google Scholar]
- Chan S. H., Yu A. M., McVey M., 2010. Dual roles for DNA polymerase theta in alternative end-joining repair of double-strand breaks in drosophila ed. R.S. Hawley. PLoS Genet. 6: e1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J., Dow J. A. T., 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39: 715–720 [DOI] [PubMed] [Google Scholar]
- Coon M. J., Ding X. X., Pernecky S. J., Vaz A. D., 1992. Cytochrome P450: progress and predictions. FASEB J. 6: 669–673 [DOI] [PubMed] [Google Scholar]
- Coon M. J., Vaz A. D., Bestervelt L. L., 1996. Cytochrome P450 2: peroxidative reactions of diversozymes. FASEB J. 10: 428–434 [DOI] [PubMed] [Google Scholar]
- Davies A. A., Masson J. Y., McIlwraith M. J., Stasiak A. Z., Stasiak A., et al. , 2001. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol. Cell 7: 273–282 [DOI] [PubMed] [Google Scholar]
- Edwards A., Gladstone M., Yoon P., Raben D., Frederick B., et al. , 2011. Combinatorial effect of maytansinol and radiation in Drosophila and human cancer cells. Dis Model Mech 4: 496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson L. R., Pearson A. E., 1996. The clinical use of mutagenic anticancer drugs. Mutat. Res. 355: 1–12 [DOI] [PubMed] [Google Scholar]
- Gateff E., 1978. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science 200: 1448–1459 [DOI] [PubMed] [Google Scholar]
- Gateff E., Schneiderman H. A., 1974. Developmental capacities of benign and malignant neoplasms of Drosophila. Dev. Genes Evol. 176: 23–65 [DOI] [PubMed] [Google Scholar]
- Giraudo M., Unnithan G. C., Le Goff G., Feyereisen R., 2010. Regulation of cytochrome P450 expression in Drosophila: Genomic insights. Pestic. Biochem. Physiol. 97: 115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone M., Su T. T., 2011. Chemical genetics and drug screening in Drosophila cancer models. J. Genet. Genomics 38: 497–504 [DOI] [PubMed] [Google Scholar]
- Gruenewald C., Botella J. A., Bayersdorfer F., Navarro J. A., Schneuwly S., 2009. Hyperoxia-induced neurodegeneration as a tool to identify neuroprotective genes in Drosophila melanogaster. Free Radic. Biol. Med. 46: 1668–1676 [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., 1999. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu. Rev. Pharmacol. Toxicol. 39: 1–17 [DOI] [PubMed] [Google Scholar]
- Hollenberg P. F., 1992. Mechanisms of cytochrome P450 and peroxidase-catalyzed xenobiotic metabolism. FASEB J. 6: 686–694 [DOI] [PubMed] [Google Scholar]
- Holm C., Covey J. M., Kerrigan D., Pommier Y., 1989. Differential requirement of DNA replication for the cytotoxicity of DNA topoisomerase I and II inhibitors in Chinese hamster DC3F cells. Cancer Res. 49: 6365–6368 [PubMed] [Google Scholar]
- Hsiang Y. H., Liu L. F., 1988. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 48: 1722–1726 [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F., 1985. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 260: 14873–14878 [PubMed] [Google Scholar]
- Hsiang Y. H., Lihou M. G., Liu L. F., 1989. Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 49: 5077–5082 [PubMed] [Google Scholar]
- Jaklevic B., Uyetake L., Lemstra W., Chang J., Leary W., et al. , 2006. Contribution of growth and cell cycle checkpoints to radiation survival in Drosophila. Genetics 174: 1963–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane D. P., Shusterman M., Rong Y., McVey M., 2012. Competition between replicative and translesion polymerases during homologous recombination repair in Drosophila ed. R.S. Hawley. PLoS Genet. 8: e1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klovstad M., Abdu U., Schüpbach T., 2008. Drosophila brca2 is required for mitotic and meiotic DNA repair and efficient activation of the meiotic recombination checkpoint. PLoS Genet. 4: e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster D. A., Palle K., Bot E. S. M., Bjornsti M.-A., Dekker N. H., 2007. Antitumour drugs impede DNA uncoiling by topoisomerase I. Nature 448: 213–217 [DOI] [PubMed] [Google Scholar]
- Kunimoto T., Nitta K., Tanaka T., Uehara N., Baba H., et al. , 1987. Antitumor activity of 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxy-camptothec in, a novel water-soluble derivative of camptothecin, against murine tumors. Cancer Res. 47: 5944–5947 [PubMed] [Google Scholar]
- Lawley P. D., Phillips D. H., 1996. DNA adducts from chemotherapeutic agents. Mutat. Res. 355: 13–40 [DOI] [PubMed] [Google Scholar]
- Legarza K., Yang L.-X., 2006. New molecular mechanisms of action of camptothecin-type drugs. Anticancer Res. 26: 3301–3305 [PubMed] [Google Scholar]
- Maekawa K., Harakawa N., Yoshimura T., Kim S.-R., Fujimura Y., et al. , 2010. CYP3A4*16 and CYP3A4*18 alleles found in East Asians exhibit differential catalytic activities for seven CYP3A4 substrate drugs. Drug Metab. Dispos. 38: 2100–2104 [DOI] [PubMed] [Google Scholar]
- Mattern M. R., Hofmann G. A., McCabe F. L., Johnson R. K., 1991. Synergistic cell killing by ionizing radiation and topoisomerase I inhibitor topotecan (SK&F 104864). Cancer Res. 51: 5813–5816 [PubMed] [Google Scholar]
- Plant N., 2007. The human cytochrome P450 sub-family: transcriptional regulation, inter-individual variation and interaction networks. Biochim. Biophys. Acta 1770: 478–488 [DOI] [PubMed] [Google Scholar]
- Pommier Y., 2006. Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer 6: 789–802 [DOI] [PubMed] [Google Scholar]
- Pommier Y., 2009. DNA Topoisomerase I Inhibitors. Chemistry, Biology, and Interfacial Inhibition. 109: 2894–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe C. M., Silva E. A., Campbell S. D., 2002. A method for assaying the sensitivity of Drosophilareplication checkpoint mutants to anti-cancer and DNA-damaging drugs. Genome 45: 881–889 [DOI] [PubMed] [Google Scholar]
- Ray Chaudhuri A., Hashimoto Y., Herrador R., Neelsen K. J., Fachinetti D., et al. , 2012. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat. Struct. Mol. Biol. 19: 417–423 [DOI] [PubMed] [Google Scholar]
- Roseman R. R., Johnson E. A., Rodesch C. K., Bjerke M., Nagoshi R. N., et al. , 1995. A P element containing suppressor of hairy-wing binding regions has novel properties for mutagenesis in Drosophila melanogaster. Genetics 141: 1061–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., 2000. Comparative Genomics of the Eukaryotes. Science 287: 2204–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudrapatna V. A., Cagan R. L., Das T. K., 2012. Drosophila cancer models. Dev. Dyn. 241: 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder E., Blows F., Ashburner M., Bautista-Llacer R., Coulson D., et al. , 2004. The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics 167: 797–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. A., Ackland M. J., Jones B. C., 1997. Properties of cytochrome P450 isoenzymes and their substrates part 2: properties of cytochrome P450 substrates. Drug Discov. Today 2: 479–486 [Google Scholar]
- Strumberg D., Pilon A. A., Smith M., Hickey R., Malkas L., et al. , 2000. Conversion of topoisomerase I cleavage complexes on the leading strand of ribosomal DNA into 5′-phosphorylated DNA double-strand breaks by replication runoff. Mol. Cell. Biol. 20: 3977–3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T. T., 2011. Safeguarding genetic information in Drosophila. Chromosoma 120: 547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung P., 1994. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science 265: 1241–1243 [DOI] [PubMed] [Google Scholar]
- Thorslund T., West S. C., 2007. BRCA2: a universal recombinase regulator. Oncogene 26: 7720–7730 [DOI] [PubMed] [Google Scholar]
- Tijet N., Helvig C., Feyereisen R., 2001. The cytochrome P450 gene superfamily in Drosophila melanogaster: annotation, intron-exon organization and phylogeny. Gene 262: 189–198 [DOI] [PubMed] [Google Scholar]
- Tsao Y. P., Russo A., Nyamuswa G., Silber R., Liu L. F., 1993. Interaction between replication forks and topoisomerase I-DNA cleavable complexes: studies in a cell-free SV40 DNA replication system. Cancer Res. 53: 5908–5914 [PubMed] [Google Scholar]
- Tu C. P. D., Akgül B., 2005. Drosophila glutathione S-transferases. Methods Enzymol. 401: 204–226 [DOI] [PubMed] [Google Scholar]
- van Bergeijk P., Heimiller J., Uyetake L., Su T. T., 2012. Genome-wide expression analysis identifies a modulator of ionizing radiation-induced p53-independent apoptosis in Drosophila melanogaster. PLoS ONE 7: e36539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall M. E., Wani M. C., 1995. Camptothecin and taxol: discovery to clinic–thirteenth Bruce F. Cain Memorial Award Lecture. Cancer Res. 55: 753–760 [PubMed] [Google Scholar]
- Wooster R., Bignell G., Lancaster J., Swift S., Seal S., et al. , 1995. Identification of the breast cancer susceptibility gene BRCA2. Nature 378: 789–792 [DOI] [PubMed] [Google Scholar]
- Yang J., McCart C., Woods D. J., Terhzaz S., Greenwood K. G., ffrench-Constant R. H., Dow J. A. T., 2007. A Drosophila systems approach to xenobiotic metabolism. Physiol. Genomics 30: 223–231 [DOI] [PubMed] [Google Scholar]
- Zijlstra J. A., Vogel E. W., 1988. Metabolic inactivation of mutagens in Drosophila melanogaster. Mutat. Res. 198: 73–83 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.