Abstract
Background
Motor delays have been reported in retrospective studies of young infants who later develop Autism Spectrum Disorders (ASDs).
Objective
In this study, we prospectively compared the gross motor development of a cohort at risk for ASDs; infant siblings of children with ASDs (AU sibs) to low risk typically developing (LR) infants.
Methods
24 AU sibs and 24 LR infants were observed at 3 and 6 months using a standardized motor measure, the Alberta Infant Motor Scale (AIMS). In addition, as part of a larger study, the AU sibs also received a follow-up assessment to determine motor and communication performance at 18 months using the Mullen Scales of Early Learning.
Results
Significantly more AU sibs showed motor delays at 3 and 6 months than LR infants. The majority of the AU sibs showed both early motor delays and later communication delays.
Limitations
Small sample size and limited follow-up.
Conclusions
Early motor delays are more common in infant AU sibs than LR infants. Communication delays later emerged in 67–73% of the AU sibs who had presented with early motor delays. Overall, early motor delays may be predictive of future communication delays in children at risk for autism.
Keywords: Motor, Communication, Language, Autism, Infants, Early identification
1. INTRODUCTION
Autism Spectrum Disorders (ASDs) are defined by social and communication impairments, with some children also displaying repetitive behaviors and stereotyped interests (American Psychological Association, 2000). The rising prevalence of ASDs creates an urgent need for clinicians to identify ASD-related impairments (impairments associated with the genetic risk to develop ASDs) early in life so that children may receive earlier access to interventions, possibly leading to improved outcomes (Landa, Holman, O’Neill, & Stuart, 2011; Zwaigenbaum et al., 2009). ASDs can be reliably identified by 14 to 24 months based on nonverbal and verbal communication and social impairments (Charman et al., 2005; Chawarska, Klin, Paul, & Volkmar, 2007; Sullivan et al., 2007; Landa & Garrett-Mayer, 2006; Landa, Holman, & Garrett-Mayer, 2007). However, some studies suggest that there are no obvious social or communication deficits within the first six months of life (Young, Merin, Rogers, & Ozonoff., 2009; Yirmiya et al., 2007; Cassel et al., 2007). Multiple researchers have proposed that early signs of ASD-related impairments may first manifest within the motor system and present as a motor delay (Esposito, Venuti, Maestro, & Muratori, 2009; Ozonoff et al., 2008; Flanagan, Landa, Bhat, & Bauman, in press; Landa & Garrett-Mayer, 2006). Yet, very few studies have systematically examined early motor development and its links to later social communication development in infants at risk for autism. The present study addresses this gap by comparing the early gross motor development of infants at high risk for autism to a typically developing group of infants at low risk for autism within the first six months of life. Moreover, we examine the relation between motor performance during early infancy and communication development at 18 months using standardized tests of motor and communication performance in infants at high risk for autism.
1.1. Social communication delays in infants at risk for ASDs
Our group and others have recently identified various early markers for diagnosis of ASDs within the first and second year of life. These markers include disruptions in nonverbal communication such as infrequent initiation of and response to joint attention cues of others (Charman et al., 2005; Chawarska et al., 2007; Sullivan et al., 2007; Landa et al., 2007; Yoder, Stone, Walden, & Malesa, 2009), infrequent reciprocal social interaction, poor integration of eye gaze within such interactions, and infrequent shared positive affect (Chawarska et al., 2007; Landa et al., 2007). Verbal communication delays such as delayed onset of first words, reduced inventory of consonants, canonical syllables, and words have also been observed (Mitchell et al., 2006; Iverson & Wozniak, 2007; Landa et al., 2007; Paul, Fuerst, Ramsay, Chawarska, & Klin, 2010). Infants who received an ASD diagnosis by 36 months of age presented with verbal communication delays as early as 14 months (Landa et al., 2007). Infant siblings of children already diagnosed with autism, a group at high risk of developing ASDs, (Folstein et al., 1999; Landa et al., 2007; Zwaigenbaum et al., 2005) presented with communication delays within the first and second year even when they did not yet meet diagnostic criteria for ASDs (Landa & Garrett-Mayer, 2006; Paul et al., 2010). Taken together, these findings indicate the presence of significant non-verbal and verbal communication delays in infants at risk for autism in the first and second year of life.
1.2. Motor delays in infants at risk for ASDs
Although the majority of the studies focusing on motor functioning in ASDs have involved school-aged children and adolescents, motor delays have been implicated within the first few years of life in infants, toddlers, and preschoolers who later developed ASDs (Esposito et al., 2009; Teitelbaum, Teitelbaum, Nye, Fryman, & Maurer, 1998; Provost, Heimerl, & Lopez, 2007; Lloyd, MacDonald, & Lord, 2011). Adolescents and school-aged children with ASDs show deficits in gross motor and fine motor coordination, gait abnormalities, as well as poor static and dynamic balance documented by using standardized assessments as well as kinematic and dynamic analysis of movement and posture (Ghaziuddin & Butler, 1998; Green et al., 2002; Miyahara et al., 1997; Szatmari, Tuff, Finlayson, & Bartolucci, 1990; Vilensky, Damasio, & Maurer, 1981; Hallett et al., 1993; Rinehart et al., 2002; Minshew, Sung, Jones, & Furman, 2004). In addition, motor delays have been reported in infants who later developed ASDs via retrospective video analyses (Teitelbaum et al., 1998; Adrien et al., 1993; Ozonoff et al., 2008). In retrospective analyses, parents of children diagnosed with ASDs were either asked to recall their child’s motor problems during infancy or to provide videos of their children when they were at or below one year of age. These studies suggested delays in achieving motor milestones such as rolling, sitting, and crawling as well as atypical movement patterns such as movement asymmetries or abnormal reflexes. However, the interpretability of these findings are limited due to potential recall bias involving inaccurate memory about timing and quality of earlier developmental features as well as the lack of standardized contexts in which the movements were videotaped. Recent retrospective video analyses of motor development in autism have addressed some of these limitations by adding control groups and by closely matching the ages of infants across groups (Ozonoff et al., 2008; Esposito et al., 2009). Nevertheless, findings from these two studies are contradictory. One reported a marked delay in motor development in infants who later developed autism (Esposito et al., 2009) whereas the other reported a subtle delay comparable to infants who have general developmental delays (Ozonoff et al., 2008). Thus; there continue to be gaps in knowledge about motor impairment in infants at risk for ASDs. The current study builds on these findings from retrospective studies by conducting a prospective quantification of motor development of infants at risk for ASDs within the first six months of life using a standardized motor assessment, the Alberta Infant Motor Scale.
1.3. Early motor development facilitates communication development
Early motor development provides a foundation for future communication development. For example, non-verbal forms of communication include fine motor acts such as pointing, as well as gross motor acts such as head turning for looking (Gernsbacher, Stevenson, Khandakar, & Goldsmith, 2008). Moreover, gestural development is closely linked to language development (Iverson & Goldin-Meadow, 2005). Infants’ early rhythmic arm movements appeared to peak around the same time they began to babble (Iverson & Fagan, 2004). This relationship between early arm movements and babble onset was lacking in some infants who later developed ASDs (Iverson & Wozniak, 2007). Interestingly, infants who later developed autism were reported to have poor early manual motor skills which also correlated with their later speech fluency (Gernsbacher, Sauer, Geye, Schweigert, & Goldsmith, 2008). Gross motor skills that expand a child’s posture, movement and/or exploratory opportunities such as sitting and locomotion are also known to advance verbal and non-verbal communication in typically developing infants. For example, the transition to independent sitting was associated with greater variation in utterance production as seen by greater variation in consonant-vowel sounds and fewer single vowel sounds (Yingling, 1981; Iverson, 2010). Sitting posture frees an infant’s rib cage and allows infants to maintain subglottal pressure and produce advanced patterns of vocalization such as consistent consonant-vowel sounds (Yingling, 1981). In late infancy, infants initiate “social bids” to caregivers by “reaching out” with their arms to share their object-based play (Karasik, Tamis-LeMonda, & Adolph, 2011). Newly walking infants show greater moving bids to share objects with their caregivers versus the more stationary bids initiated by age-matched crawlers (Karasik et al., 2011). This suggests that walking allows infants to have a more controlled and reliable social interaction with their caregivers. Such relationships between motor development and verbal and non-verbal communication development have not been examined in infants at risk for ASDs. The present study builds on these past studies by taking a first step to examine the relationship between early motor development and later communication development in infants at risk for ASDs.
Infant siblings of children with autism, termed AU sibs, are a cohort at high risk to develop ASDs as well as related milder impairments representing an intermediate phenotype known as the Broader Autism Phenotype (Landa & Garrett-Mayer, 2006; Landa et al., 2007). While 18.7% of AU sibs may develop ASDs (Ozonoff, Young, Carter et al., 2011, Sumi, Taniai, Miyachi, & Tanemura, 2006), another 25–50% may develop general communication, motor, and social delays that comprise the Broader Autism Phenotype (Bailey, Phillips, & Rutter, 1996). In the present study, we conducted a prospective examination of gross motor performance of AU sibs as compared to low risk, typically developing infants termed LR infants at 3 and 6 months using the Alberta Infant Motor Scale. Moreover, we also examined the relation of early motor delays in AU sibs with their communication development at 18 months using the Receptive and Expressive Language Scales of the Mullen Scales of Early Learning (Mullen, 1995). We hypothesized that: a) a greater number of AU sibs will present with motor delays during infancy compared to LR infants at 3 and 6 months, and b) AU sibs who present with motor delays during infancy will also be more likely to exhibit communication delays by 18 months of age than AU sibs without early motor delays. It must be noted that due to inadequate follow-up we will not be discussing the ASD outcomes of the AU sibs and will limit ourselves to the relation between early motor development and 18-month communication outcomes.
2. MATERIALS & METHODS
2.1. Participants
Twenty-four infant siblings of children with ASDs (AU sibs; males=12, females=12, X2=0.0, p=1) and 24 typically developing infants with a low risk for autism defined by no family history of ASDs (LR infants; males=9, females=15; X2=1.56, p>0.1; a non-significant gender difference between groups) were administered the Alberta Infant Motor Scale (AIMS) at 6 months of age. 18 of the 24 infants per group also received the AIMS assessment at 3 months. The mean age (and standard deviation) of AU sibs and LR infants at the 3-month-visit was 3.7 (0.5) and 4.0(0.6) months, respectively, and at the 6-month-visit was 6.5 (0.6) and 6.5 (0.6), respectively. There was no significant age difference between groups (p>0.1) at either visit.
All infants assessed at 3 months of age were also observed at 6 months of age. As part of a larger study following the development of infants at risk for autism, AU sibs also received a follow-up developmental assessment at 18-months of age. For one AU sib, we included data from an assessment conducted at 14-months of age because this child did not come for an 18-month assessment visit. We were unable to conduct follow-up visits for the LR infants due to funding limitations. In total, we have 18-month communication outcomes on 16 of the 18 AU sibs observed at age 3 months. We also obtained 18-month communication outcomes on 21 of the 24 AU sibs observed at age 6 months.
2.2. Procedures
Participants were recruited through ASD advocacy groups, conferences, the autism center associated with our institute, and via mailed invitations to families identified through public birth announcements, and word of mouth. We term our comparison group as “low risk” because the general population is at a significantly lower risk to develop ASDs than younger siblings of children with ASDs (Bailey, Phillips & Rutter, 1996). The older siblings with autism met diagnostic criteria for autism on the Autism Diagnostic Observation Schedule (ADOS) (Lord, Rutter, DiLavore, & Risi, 1999) and the Autism Diagnostic Interview-Revised (ADI-R) (Lord, Rutter, & LeCouteur, 1994), and were judged to have autism by an expert clinician. Exclusion criteria for both groups were: low birth weight (< 2500 grams), gestational age (< 37 weeks), birth trauma, head injury, prenatal illicit drug or excessive alcohol exposure, known genetic disorder that would confer increased risk of ASDs (e.g., fragile X), or any orthopedic diagnoses. Infants were admitted to the study following informed parental written consent as approved by the Institutional Review Board at Johns Hopkins University or the University of Connecticut.
2.2.1
The Alberta Infant Motor Scale (AIMS; Piper & Darrah, 1994) was used to examine gross motor development. The AIMS is a valid and reliable tool to examine gross motor development of infants between birth and onset of walking in various at-risk populations including low- and high-risk preterm infants (Snyder, Eason, Philibert, Ridgway, McCaughey, 2008). It is comprised of subscales assessing postural development in supine, prone, sit, and stand with nominal ratings of 0 or 1 for the behaviors “not observed” versus “observed”. A summed score of “observed” behaviors is obtained for each subscale. A summation of all subscale totals provides a total raw score, which is then converted to a percentile rank. The first author, a pediatric physical therapist, administered the AIMS. An AIMS percentile rank between 0th–25th was considered a low motor performance whereas any score above the 25th percentile was considered age-expected or within normal limits (WNL) (Haastert, de Vries, Helders, & Jongmans, 2006). The primary coder was a graduate student with physical therapy training who was blinded to grouping. Intra- and inter-rater reliability was measured using intra-class correlations (ICCs) with a one-way random effects model. Three- and 6-month AIMS data of 12 randomly selected infants (6 per group) were used for intra-rater and inter-rater reliability coding. The primary coder established 99% intra-rater (p<0.001) and 98% inter-rater reliability (p<0.001) with the first author using approximately 120 minutes of data.
2.2.2
The Mullen Scales of Early Learning (MSEL; Mullen, 1995) assessment was used to assess communication outcome at 18 months. The MSEL is a standardized, valid, and reliable general developmental measure for ages birth to 68 months (Mullen, 1995) including Gross Motor, Fine Motor, Visual Reception, as well as Receptive and Expressive Language scales. Raw, standard (T scores), and age-equivalent scores can be generated for each scale. For this study, a licensed speech-pathologist or a licensed developmental psychologist administered the MSEL at the 18-month visit for 21 of the 24 AU sibs. As mentioned earlier, only one AU sib received the MSEL assessment at 14 months rather than 18 months. A communication delay was defined as a T score at or below 40, or at least 1 SD below the mean on the Receptive and/or Expressive Language scale. The MSEL can identify communication delays in children with ASDs between 14 to 18 months of age and some other studies using the MSEL also report communication delays in AU sibs who did not receive an ASD diagnosis by 18 months (Landa & Garrett-Mayer, 2006; Luyster, Kadlec, Carter, & Tager-Flusberg, 2008). Note that we applied the same criteria for motor delay for the Gross motor subscale of MSEL and only 3 AU sibs continued to show delayed motor development at 14 or 18 months based on this general measure of gross motor milestones (which does not include a refined assessment of postural/gross motor delay). Hence, we only discuss 18-month communication outcomes and not the motor outcomes.
2.3. Statistical Analysis
We conducted a Group (AU sibs, LR infants) x AIMS Subscale (supine score, prone score, sit score, and total score) Analysis of Variance (ANOVA) with “Group” as the between-subjects factor and “Scale” as the within-subjects factor. AIMS percentile scores were also compared using t-tests. These ANOVAs were done separately for each visit (3-month and 6-month) due to the discrepancy in number of infants between the two age points (3m data = 18 infants per group; 6m data = 24 infants per group). Greenhouse-Geisser corrections were applied if the sphericity assumption was violated and alternate F-ratios and p-values are reported. We assumed unequal variance during post-hoc testing, if the Levene’s test for equality of variance was significant. Post-hoc t-tests with p<0.05 were considered statistically significant. In order to examine how individual infants fit within the group trends, we also separated infants within each group into two categories of low (percentile score ≤ 25) versus within normal limits (WNL or percentile score > 25) motor performance as defined earlier. Chi-square analyses are reported for these categories. Lastly, we examined the association between the presence/absence of early motor delay (3-month and 6-month data) and communication outcome classification (yes/no communication delay) at 18 months in AU sibs using Fisher’s exact tests.
3. RESULTS
In this section, we address 3-month and 6-month performance of each group and then describe later communication performance
3.1. 3-month AIMS performance
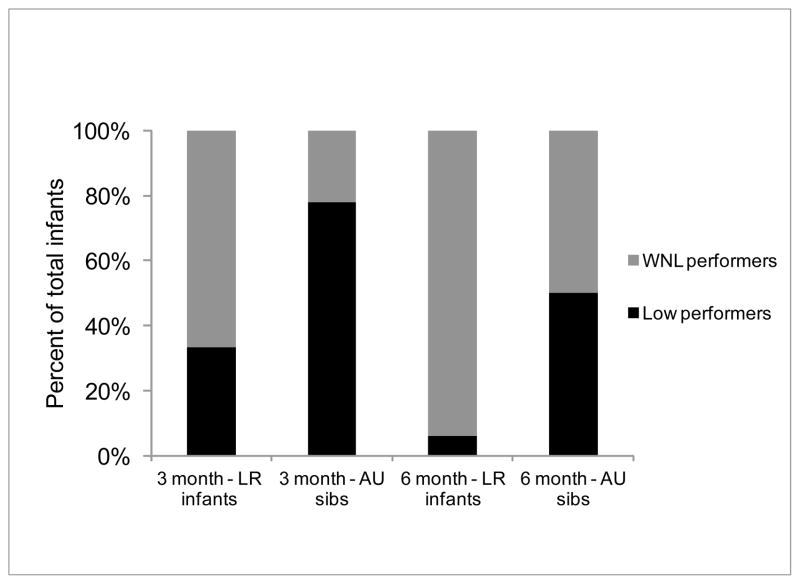
Average 3-month AIMS performance of both groups is presented in Table 1 and Figure 1. A greater number of infants in the AU sibs group showed delayed motor development at 3 months than the LR group. The Group x Subscale ANOVA revealed a main effect of Group (F(1,33)=7.68, p=0.009) and a Group x Subscale interaction (F(1.75,57.73)=5.79, p=0.007). Post-hoc t-tests revealed significant group differences for the prone (p=0.002), sit (p=0.005), total (p=0.002) raw scores and percentile scores (p=0.03) with AU sibs showing lower scores than LR infants. In terms of individual trends, 14 of the 18 3-month-old AU sibs (i.e., 78% of the AU sibs) were low performers (X2=5.56, p=0.02) and only 8 of the 24 LR infants (i.e., 33% of the LR infants) were low performers (X2=2.67, p=0.1). Overall, a significant number of infants had low motor performance in the AU sibs group and the majority of the WNL performers were found among the LR infants.
Table 1.
Mean (SD) of AIMS raw scores and percentile ranks of infant siblings of children with ASDs (AU sibs) and low risk (LR) infants at 3 (3m) and 6 months (6m).
| Age | Prone scores | Supine scores | Sit scores | Total scores | Percentile rank for Total Scores | |
|---|---|---|---|---|---|---|
| 3m | LR n=18 | 4.95 (1.75) | 4.78 (1.38) | 2.43 (1.27) | 14.22 (3.93) | 38.9 (26.2) |
| AU sibs n=18 | 3.33 (1.46)* | 3.89 (1.13)* | 1.44 (0.86)* | 10.67 (2.45)* | 21.4 (16.4)* | |
| 6m | LR n=24 | 11.46 (2.32) | 7.97 (0.95) | 7.06 (1.69) | 30.09 (4.28) | 52.21 (20.35) |
| AU sibs n=24 | 9.66 (3.97)* | 7.46 (0.98)* | 6.25 (2.58) | 26.58 (6.83)* | 34.25 (29.64)* | |
p<0.05
Figure 1. Categories of Motor Performance in AU sibs and LR infants at 3 and 6 months.
Proportion of low and within normal limit (WNL) performers among the infant siblings of children with ASDs (AU sibs) and low risk (LR) infants at 3 and 6 months. Note: AIMS percentile rank ≤ 25th percentile was defined as low motor performance.
3.2. 6-month AIMS performance
The average 6-month AIMS performance is presented in Table 1 and Figure 1. The AU sibs group was more delayed at 6 months of age than LR infants. The Group x Subscale ANOVA revealed a main effect of Group (F(1,46)=6.02, p=0.018) and a Group x Subscale interaction (F(1.59,73.5)=5.01, p=0.014). Post-hoc t-tests revealed significant group differences for prone scores (p=0.02), supine scores (p=0.04), total raw scores (p=0.02) and percentile scores (p=0.003) with AU sibs performing worse than LR infants. Twelve of the 24 6-month-old AU sibs (i.e., 50% of the AU sibs) were low performers (X2=0, p>0.1). In contrast, only 2 of the 24 LR infants (i.e., 8.3% of the LR infants) were low performers (X2=16.67, p<0.001). Overall, only 50% of the AU sibs continued to show motor delays at 6 months as compared the other AU sibs and the majority of the LR infants.
3.3. Relation between motor performance in infancy and 18-month communication performance
Tables 2 and 3 show the number of infants assessed at each age who were represented in the four identified categories: i) infants with both motor delays in infancy and a communication delay at 18 months; ii) infants with a communication delay at 18 months without motor delays in infancy; iii) infants with motor delays in infancy without a communication delay at 18 months; and iv) infants with no motor or communication delay.
Table 2.
Categories of infant siblings of children with ASDs (AU sibs) based on 3-month motor delay (MD) and 18-month communication delay (CD). These include infants with MD and CD, only MD, only CD, and no MD or CD.
| 3-month motor vs. 18-month communication performance (n=16) | Communication delay present at 18 months |
No communication delay at 18 months |
| Motor delay present at 3 months | 8 AU sibs had both, MD and CD | 4 AU sibs had MD but no CD |
| No motor delay at 3 months | 0 AU sibs had a CD but no MD | 4 AU sibs had no MD or CD |
Table 3.
Categories of infant siblings of children with ASDs (AU sibs) based on 6-month motor delay (MD) and 18-month communication delay (CD). These include infants with MD and CD, only MD, only CD, and no MD or CD.
| 6-month motor vs. 18-month communication performance (n=21) | Communication delay present |
No communication delay |
| Motor delay present | 8 AU sibs had both, MD and CD | 3 AU sibs had MD but not CD |
| No motor delay | 4 AU sibs had a CD but no MD | 6 AU sibs had no MD or CD |
3.3.1. Relation between motor delays identified at 3 months of age and communication outcomes at 18 months
The MSEL assessment was administered for 16 of the 18 AU sibs observed at 3 months of age. On comparing the 3-month and 18-month data, we observed four categories of infants (see Table 2). First, among the 16 AU sibs with 18-month communication outcomes, eight AU sibs (or 50%) had both - motor delays at 3 months using the AIMS and a communication delay at 18 months based on the MSEL Receptive or Expressive Language scale. Second, all infant AU sibs who met the criteria for a communication delay at 18 months had also exhibited a motor delay at 3 months. Third, four AU sibs had motor delays at 3 months but did not show a communication delay at 18 months. Lastly, four AU sibs showed no early motor or later communication delays. The Fisher’s exact test showed that significantly more AU sibs with an 18-month communication delay also had a motor delay at 3 months (p=0.04).
3.3.2. Relation between infants with motor delays identified at 6 months of age and communication outcomes at 18 months
The MSEL assessment was administered to 21 of the 24 AU sibs observed at 6 months of age (see Table 3). First, among these 21 AU sibs, eight AU sibs (or 38%) had both motor delays at 6 months as assessed by the AIMS and a communication delay at 18 months based on the MSEL Receptive or Expressive Language Scale. Second, four AU sibs showed a communication delay at 18 months without motor delays in infancy. Third, three AU sibs had motor delays at 6 months but did not show a communication delay at 18 months. Lastly, six AU sibs showed no motor or communication delay at both ages. The Fisher’s exact test showed a statistical trend for more AU sibs with an 18-month communication delay to also have a motor delay at 6 months (p=0.1).
4. DISCUSSION
Motor development in infancy was delayed in a large proportion of AU sibs based on the AIMS, with some of these motor delayed high-risk infants ‘catching up’ to age-expected levels of motor functioning by 6 months of age. In addition, motor performance during infancy, particularly early infancy, was linked to communication functioning at 18 months for the AU sibs. Hence, both of our hypotheses were supported. Specifically, a subset of 12 AU sibs scored poorly across the AIMS prone and supine subscales at 3 months and continued to show overall gross motor delays based on the low total scores at 6 months (see Figure 1). Lastly, a subset of eight AU sibs (38–50%) who showed early motor delays at 3 and 6 months continued to exhibit a risk for communication delay at the 18-month follow-up visit (see Tables 2 & 3).
Early motor delays as an indicator of early disruptions related to risk for ASD
Our finding that early motor delays are present in infant siblings of children with ASDs (AU sibs) is in agreement with what is known about AU sibs based on prospective studies of their general development. AU sibs who received an ASD diagnosis later in life showed gross motor delays by 14 months (Landa & Garrett-Mayer, 2006; Iverson & Wozniak, 2007). Specifically, delayed onset of walking was observed in some infants who developed ASDs by 24 months of age (Landa & Garrett-Mayer, 2006). Another prospective study, involving a case series of infant AU sibs later diagnosed with autism, reported delays in sitting and reaching at 6 months and a delay or poor quality of walking at 12 months (Bryson, Zwaigenbauam, Brian, et al., 2007). Atypical repetitive movements such as head shaking, hand flapping, and rocking on knees were reported by 18 months without obvious repetitive behaviors early on in life (Bryson, Zwaigenbaum, Brian, et al., 2007; Loh, Soman, Brian, et al., 2008). In addition, poor postural control, for example, head lag in a pull-to-sit task, has been identified in 6-month-old AU sibs than in low risk controls (Flanagan et al., 2012; Iverson & Wozniak, 2007). Taken together, delayed onset of motor skills, immature movement patterns, as well as movement abnormalities have been reported in infants at risk for ASDs. Our data extend the previous work to the first half year of life and confirm the increased presence of early motor delays or postural delays, in AU sibs compared to infants at low risk for ASDs using a different measure of motor functioning.
Our finding of more significant motor delays in AU sibs than in LR infants at 3 and 6 months of age is compatible with retrospective reports of motor delays in ASD infants that are of equal or greater severity than motor delays observed in infants with general developmental delay (Ozonoff et al., 2008; Esposito et al., 2009). Specifically, delays in supine, prone, and sitting postures in AU sibs were comparable to infants with idiopathic global developmental delays but more severe than those seen in typically developing infants (Ozonoff et al., 2008). However, a more recent study reported that infants who later developed ASDs showed greater movement asymmetries and immature supine postures than developmentally delayed and typically developing infants (Esposito et al., 2009). Overall, there is empirical support for the presence of motor delays in infants who later develop ASDs as well as later born siblings of children with ASDs. Moreover, our data showed that only a subset of AU sibs exhibited early motor delays and communication delays in the future. Therefore, motor delays may be a feature of ASD from early on and may present in infants who have a greater genetic risk for ASD. This issue warrants closer clinical consideration and further research.
4.1. How would early motor delay impact communication development in infants and children with ASDs?
We propose that there is empirical support for a motor-communication linkage in autism.55 Our data showed that motor performance of AU sibs during infancy was linked to their communication outcomes at 18 months. We acknowledge that motor delays found in our study are not ASD specific and that our sample size was small. However, past studies with large sample sizes report that motor delays at 18 months are highly predictive of ASDs at three years of age in toddlers at-risk for ASDs (Brian, Bryson, Garon, et al., 2008). Similarly, motor performance at age 6 months was predictive of communication and social functioning at 36 months (Flanagan et al., 2012). Likewise, motor performance in two-year-old children newly diagnosed with ASDs significantly correlated with outcomes at four years of age (Sutera, Pandey, Esser, et al., 2007). A recent prospective case-series on AU sibs identified a persistence of immature postures such as prone and crawl in the second year of life (Nickel, Thatcher, & Iverson, 2010). These researchers proposed that time spent in immature postures hinders AU sibs’ ability to communicate with their caregivers (Nickel et al., 2010) Slowed or uncoordinated head and arm movements may limit effective head turning, reaching, pointing, giving, and showing that are key components of initiation and response to joint attention bids of others (Gernsbacher et al., 2008a). We propose that understanding the limitations in planning and coordination of movement and posture are fundamental to a comprehensive understanding of the qualitative communication impairment of ASDs. More specifically, we propose that a developmentally important linkage exists between motor and communication impairments in autism.
4.2. Clinical implications for assessment and treatment
Based on our data, we propose that some infants at-risk for ASDs are at high risk for motor delays within the first half-year of life. It is important to note that for some AU sibs, motor delays may first become apparent after the first year. For example, for some infants, the delayed onset of walking may be the first missed motor milestone (Landa & Garrett-Mayer, 2006; Ozonoff et al., 2008; Esposito & Venuti, 2008). Thus, we recommend developmental surveillance of siblings of children with ASDs through the third birthday. Professionals working with families must monitor both the social communication as well as fine and gross motor development. Specifically, the Alberta Infant Motor Scale could be a useful assessment to monitor motor development of infant siblings of children with ASDs in the first year of life. In addition, recent data indicate the need for multi-developmental system surveillance throughout childhood due to later-emerging difficulties in siblings of children with ASDs that include motor, social communication, and cognitive systems (Gamliel, Yirmiya, Jaffe, Manor, & Sigman, 2009; Hilton, Zhang, Whilte, Klohr, & Constantino, 2011). We also recommend that motor delays be addressed through motor interventions to enhance motor development and movement-based social skills such as imitation and communicative gestures. Specifically, early low-cost, caregiver-provided enhanced object-based and postural experiences could significantly improve the motor functioning of infants at risk for ASDs (Lobo, Galloway, & Savelsbergh, 2004; Lobo & Galloway, 2008; Landa, 2008). Thus, motor experts such as occupational and physical therapists should be included as members of the early intervention team assessing infants and children at risk for ASDs. Most importantly, infants with early motor delays may be at risk for communication delays. Our current data do not claim that motor delays in infant sibs are autism-specific; however, their early motor delays are predictive of future communication delays.
4.3. Limitations
As an initial study on gross motor development, there are important limitations on the generalization of our results. First, we examined a relatively small sample of AU sibs. Second, we did not control for birth order. Several of our LR infants were first born which may have contributed to the greater incidence of motor delays among LR infants at 3 months. Parent handling and positioning can influence infant motor development (Lobo et al., 2004; Lobo & Galloway, 2008). Nevertheless, by 6 months of age, the majority of the LR infants had reached age-appropriate levels of motor performance. Third, we were unable to obtain follow-up assessments at the age of 18 months in LR infants. Lastly, we did not report on motor development beyond six months of age, nor did we provide diagnostic outcomes beyond 18 months. We do not have adequate diagnostic assessments due to funding limitations as well as lack of follow-up from families. Thus, future research involving larger sample sizes is required to evaluate the long-term outcomes and connections between early motor and later social communication impairments in infants at risk for ASDs.
4.4. Conclusions
Siblings of children with ASDs presented with significant motor delays within the first half year of life. These motor delays may affect early object exploration skills and learning (Lobo et al., 2004; Lobo & Galloway, 2008) as well as social bids involving object sharing with caregivers (Karasik et al., 2011). These early manual and social skills may ultimately play an important role in early lexical development (Iverson, 2010) and may contribute to future communication delays in at-risk infants. Our results suggest that motor delays early in life may provide a risk marker that is relevant for clinicians and early educators. Clinicians should consider evaluating the fine and gross motor development along with social communication development of infant siblings of children with ASDs during routine developmental follow-ups. Based on the aforementioned links between motor and communication development in ASDs, treating fine and gross motor impairments early on in life may facilitate forms of communication such as imitation skills, fine motor and whole body gestures, as well as joint attention, lack of which are considered hallmarks of ASDs.
Highlights.
Gross motor development of infant siblings of children with ASDs (AU sibs) was compared to low risk typically developing (LR) infants. AU sibs also received a follow-up assessment of communication development at 18 months.
Significantly more AU sibs showed motor delays at 3 and 6 months than LR infants.
AU sibs showed both early motor delays and later communication delays.
Early motor delays may be predictive of future communication delays in children at risk for autism.
Acknowledgments
We would like to thank the infants and families who participated in this study. We also thank the various undergraduate, graduate, and doctoral students as well as clinical research staff at the Kennedy Krieger REACH laboratory who contributed to this project through scheduling, data organization, data collection, and data processing: Ashley Faherty, Marguerite Adams, Julianna Finelli, Dana Herman, Christine Hess, James Mancini, Alison Marvin, Allison Nelson, Allison O’Neill, Amy Reese, Julie Rusyniak, and Melissa Warren. We thank Rajashree Kotejoshyer, a Master’s degree student in Allied Health at the University of Connecticut with an entry-level degree in physical therapy. During her time in our lab, Rajashree established AIMS coding reliability with the first author. In addition, she was the blinded coder for all AIMS data. AB and RL thank Cure Autism Now and Karma Foundation for the mentor based, Young Investigator Award awarded to AB. RL thanks the National Institutes of Mental Health for support of her research through grants MH59630 and 154MH066417.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV-TR) 4. Washington, DC: 2000. [Google Scholar]
- Adrien J, Lenoir P, Martineau J, Perrot A, Hameury L, Larmande C, et al. Blind ratings of early symptoms of autism based upon family home movies. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32(3):617–627. doi: 10.1097/00004583-199305000-00019. [DOI] [PubMed] [Google Scholar]
- Almeida K, Dutra M, Mello R, Reis A, Martins P. Concurrent validity and reliability of the Alberta Infant Motor Scale in premature infants. Journal de Pediatrica. 2008;84(5):442–448. doi: 10.2223/JPED.1836. [DOI] [PubMed] [Google Scholar]
- Bailey A, Phillips W, Rutter M. Autism: Towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. Journal of Child Psychology and Psychiatry. 1996;37(1):89–126. doi: 10.1111/j.1469-7610.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- Bhat A, Landa R, Galloway C. Current Perspectives on Motor Functioning in Infants, Children, and Adults With Autism Spectrum Disorders. Physical Therapy. 2011;91(7):1116–1129. doi: 10.2522/ptj.20100294. [DOI] [PubMed] [Google Scholar]
- Brian J, Bryson S, Garon N, Roberts W, Smith I, Szatmari P, Zwaigenbaum L. Clinical assessment of autism in high-risk 18-month-olds. Autism. 2008;12(5):433–456. doi: 10.1177/1362361308094500. [DOI] [PubMed] [Google Scholar]
- Bryson S, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, McDermott C. A prospective case series of high-risk infants who developed autism. Journal of Autism and Developmental Disorders. 2007;37(1):12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- Cassel T, Messinger D, Ibanez LV, Haltigan JD, Acosta S, Buchman AC. Early social and emotional communication in the infant siblings of children with autism spectrum disorders: An examination of the broad phenotype. Journal of Autism and Developmental Disorders. 2007;37(1):122–132. doi: 10.1007/s10803-006-0337-1. [DOI] [PubMed] [Google Scholar]
- Charman T, Taylor E, Drew A, Cocerill H, Brown J, Baird G. Outcome at 7 years of age of children diagnosed with autism at age 2: predictive valitdity of assessments conducted at 2 and 3 years of age and pattern of symptom change over time. Journal of Child Psychology and Psychiatry. 2005;46(5):500–513. doi: 10.1111/j.1469-7610.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Volkmar F. Autism spectrum disorder in the second year: Stability and change in syndrome expression. Journal of Child Psychology and Psychiatry. 2007;48(2):128–138. doi: 10.1111/j.1469-7610.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- Esposito G, Venuti P. Analysis of toddler’s gait after six months of independent walking to identify autism: A preliminary study. Perceptual and Motor Skills. 2008;106(1):259–269. doi: 10.2466/pms.106.1.259-269. [DOI] [PubMed] [Google Scholar]
- Esposito G, Venuti P, Maestro S, Muratori F. An exploration of symmetry in early autism spectrum disorders: An analysis of lying. Brain and Development. 2009;31(2):131–138. doi: 10.1016/j.braindev.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Flanagan J, Landa R, Bhat A, Bauman M. Head lag in infants at risk for autism: A preliminary study. American Journal of Occupational Therapy. 2012 doi: 10.5014/ajot.2012.004192. [DOI] [PubMed] [Google Scholar]
- Folstein S, Santangelo S, Gilman S, Piven J, Landa R, Lainhart J, Hein J, Wzorek M. Predictors of cognitive test patterns in autism families. Journal of Child Psychology and Psychiatry. 1999;40:1117–1128. [PubMed] [Google Scholar]
- Gamliel I, Yirmiya N, Jaffe D, Manor O, Sigman M. Developmental Trajectories in Siblings of Children with Autism: Cognition and Language from 4 Months to 7 Years. Journal of Autism and Developmental Disorders. 2009;39:1131–1144. doi: 10.1007/s10803-009-0727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaziuddin M, Butler E. Clumsiness in Autism and Asperger syndrome: A further report. Journal of Intellectual Disability Research. 1998;42(1):43–48. doi: 10.1046/j.1365-2788.1998.00065.x. [DOI] [PubMed] [Google Scholar]
- Green D, Baird G, Barnett A, Henderson L, Huber J, Henderson S. The severity and nature of motor impairment in Asperger’s syndrome: A comparison with specific developmental disorder of motor function. Journal of Child Psychology and Psychiatry. 2002;43(5):655–668. doi: 10.1111/1469-7610.00054. [DOI] [PubMed] [Google Scholar]
- Gernsbacher M, Stevenson J, Khandakar S, Goldsmith H. Why does joint attention look atypical in autism? Child Development Perspectives. 2008a;2(1):38–45. doi: 10.1111/j.1750-8606.2008.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernsbacher M, Sauer E, Geye H, Schweigert E, Goldsmith H. Infant and toddler oral- and manual-motor skills predict later speech fluency in autism. Journal of Child Psychology and Psychiatry. 2008b;49(1):43–50. doi: 10.1111/j.1469-7610.2007.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M, Lebiedowska M, Thomas S, Stanhope S, Denckla M, Rumsey J. Locomotion of autistic adults. Archives of Neurology. 1993;50(12):1304–1308. doi: 10.1001/archneur.1993.00540120019007. [DOI] [PubMed] [Google Scholar]
- Hilton C, Zhang Y, Whilte M, Klohr C, Constantino C. Motor impairment in sibling pairs concordant and discordant for autism spectrum disorders. Autism. 2011;1:12. doi: 10.1177/1362361311423018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert IC, de Vries LS, Helders PJM, Jongmans MJ. Early gross motor development of preterm infants according to the Alberta Infant Motor Scale. The Journal of Pediatrics. 2006;149:617–622. doi: 10.1016/j.jpeds.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Iverson J. Developing language in a developing body: the relationship between motor development and language development. Journal of Child Language. 2010;37(2):229–261. doi: 10.1017/S0305000909990432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson J, Fagan M. Infant vocal-motor coordination: precursors to speech-gesture systems. Child Development. 2004;75(4):1053–1066. doi: 10.1111/j.1467-8624.2004.00725.x. [DOI] [PubMed] [Google Scholar]
- Iverson J, Goldin-Meadow S. Gesture Paves Way for Language Development. Psychological Science. 2005;16(5):367–371. doi: 10.1111/j.0956-7976.2005.01542.x. [DOI] [PubMed] [Google Scholar]
- Iverson J, Wozniak R. Variation in vocal-motor development in infant siblings of children with autism. Journal for Autism and Developmental Disorders. 2007;37(1):158–170. doi: 10.1007/s10803-006-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb LG, Law JK, Landa R, Law PA. Onset patterns prior to 36 months in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010;0(11):1389–1402. doi: 10.1007/s10803-010-0998-7. [DOI] [PubMed] [Google Scholar]
- Karasik L, Tamis-LeMonda C, Adolph K. Transition from crawling to walking and infants’ actions with objects and people. Child Development. 2011;82(4):1199–1209. doi: 10.1111/j.1467-8624.2011.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R. Autism spectrum disorders in the first 3 years of life. In: Shapiro BK, Accardo PJ, editors. Autism Frontiers: Clinical Issues and Innovations. Baltimore: Paul H. Brookes Publishing Co; 2008. pp. 97–123. [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: A prospective study. Journal of Child Psychology and Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Landa R, Holman K, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007;64(7):853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Landa R, Holman K, O’Neill A, Stuart E. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: A randomized controlled trial. Journal of Child Psychology and Psychiatry. 2011;52(1):13–21. doi: 10.1111/j.1469-7610.2010.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo M, Savelsbergh G, Galloway J. General and Task-Related Experiences Affect Early Object Interaction. Child Development. 2004;75(4):1268–1281. doi: 10.1111/j.1467-8624.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- Lobo M, Galloway J. Postural and Object-Oriented Experiences Advance Early Reaching, Object Exploration, and Means – End Behavior. Child Development. 2008;79(6):1869–1890. doi: 10.1111/j.1467-8624.2008.01231.x. [DOI] [PubMed] [Google Scholar]
- Loh A, Soman T, Brian J, Bryson S, Roberts W, Szatmari S, Zwaigenbaum L. Stereotyped motor behaviors associated with autism in high-risk infants: A pilot videotape analysis of a sibling sample. Journal of Autism and Developmental Disorders. 2008;37(1):25–36. doi: 10.1007/s10803-006-0333-5. [DOI] [PubMed] [Google Scholar]
- Lloyd M, Macdonald M, Lord C. Motor skills of toddlers with autism spectrum disorders. Autism. 2011;15(3):1–18. doi: 10.1177/1362361311402230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised (ADI-R): A revised version of diagnostic interview for caregivers of individuals with pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Luyster R, Kadlec M, Carter A, Tager-Flusberg H. Language Assessment and Development in Toddlers with Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2008;38(8):1426–1438. doi: 10.1007/s10803-007-0510-1. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Brian J, Zwaigenbaum L, Roberts W, Szatmari P, Smith I, Bryson S. Early language and communication development of infants later diagnosed with autism spectrum disorder. Developmental and Behavioral Pediatrics. 2006;27(2):69–78. doi: 10.1097/00004703-200604002-00004. [DOI] [PubMed] [Google Scholar]
- Minshew N, Sung K, Jones B, Furman J. Underdevelopment of the postural control system in autism. Neurology. 2004;63(11):2056–2061. doi: 10.1212/01.wnl.0000145771.98657.62. [DOI] [PubMed] [Google Scholar]
- Miyahara M, Tsujii M, Hori M, Nakanishi K, Kageyama H, Sugiyama T. Brief report: Motor incoordination in children with Asprger’s Syndrome and learning disabilities. Journal of Autism and Developmental Disorders. 1997;27(5):595–603. doi: 10.1023/a:1025834211548. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Nickel L, Thatcher A, Iverson J. Postural Development in Infants with and without Risk for Autism Spectrum Disorders. Presented at the International Meeting for Autism Research; Philadelphia, PA. 2010. [Google Scholar]
- Ozonoff S, Young G, Goldring S, Greiss-Hess L, Herrera A, Steele J, Macari S, Rogers S. Gross motor development, movement abnormalities, and early identification of autism. Journal of Autism and Developmental Disorders. 2008;38(4):644–656. doi: 10.1007/s10803-007-0430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young G, Carter A, Stone W. Recurrence Risk for Autism Spectrum Disorders: A Baby Siblings Research Consortium Study. Pediatrics. 2011;128(3):488–495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Fuerst Y, Ramsay G, Chawarska K, Klin A. Out of the mouths of babes: Vocal production in infant siblings of children with ASD. Journal of Child Psychology Psychiatry. 2010 doi: 10.1111/j.1469-7610.2010.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Darrah J. Motor assessment of the developing infant. Saunders Publishing; Philadelphia, PA: 1994. [Google Scholar]
- Provost B, Lopez B, Heimerl S. A comparison of motor delays in young children: Autism spectrum disorder, developmental delay, and developmental concerns. Journal of Autism and Developmental Disorders. 2007;37(2):321–328. doi: 10.1007/s10803-006-0170-6. [DOI] [PubMed] [Google Scholar]
- Rao P, Faherty A, Landa R. Differences in imitative synchronicity in children with high functioning autism and children without autism spectrum disorder. Presented at the International Meeting for Autism Research; Philadelphia, PA. 2010. [Google Scholar]
- Rinehart N, Bradshaw J, Brereton A, Tonge B. A clinical and neurobehavioural review of high-functioning autism and Asperger’s disorder. Australian and New Zealand Journal of Psychiatry. 2002;36(6):762–770. doi: 10.1046/j.1440-1614.2002.01097.x. [DOI] [PubMed] [Google Scholar]
- Snyder P, Eason J, Philibert P, Ridgway A, McCaughey T. Concurrent validity and reliability of the Alberta Infant Motor Scale in infants at dual risk for motor delays. Physical and Occupational Pediatric Therapy. 2008;28(3):267–82. doi: 10.1080/01942630802224892. [DOI] [PubMed] [Google Scholar]
- Sullivan M, Finelli J, Marvin A, Garrett-Mayer E, Bauman M, Landa R. Response to joint attention in toddlers at risk for autism spectrum disorder: A prosepctives study. Journal of Autism and Developmental Disorders. 2007;37(1):37–48. doi: 10.1007/s10803-006-0335-3. [DOI] [PubMed] [Google Scholar]
- Sumi S, Taniai H, Miyachi T, Tanemura M. Sibling risk of pervasive developmental disorder estimated by means of an epidemiological survey in Nagoya, Japan. Journal of Human Genetics. 2006 doi: 10.1007/s10038-006-0392-7. [DOI] [PubMed] [Google Scholar]
- Sutera S, Pandey J, Esser E, Rosenthal M, Wilson L, Barton M, Green J, Fein D. Predictors of optimal outcome in toddlers diagnosed with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(1):98–107. doi: 10.1007/s10803-006-0340-6. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Tuff L, Finlayson A, Bartolucci G. Asperger’s Syndrome and Autism: Differences in behavior, cognition, and adaptive functioning. Journal of American Academy of Child and Adolescent Psychiatry. 1990;29(1):130–136. doi: 10.1097/00004583-199001000-00021. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Teitelbaum O, Nye J, Fryman J, Maurer R. Movement analysis in infancy may be useful for early diagnosis of autism. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(23):13982–13987. doi: 10.1073/pnas.95.23.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilensky J, Damasio A, Maurer R. Gait disturbances in patients with autistic behavior: A preliminary study. Archives of Neurology. 1981;38(10):646–649. doi: 10.1001/archneur.1981.00510100074013. [DOI] [PubMed] [Google Scholar]
- Yingling J. Unpublished Doctoral Dissertation. University of Denver; 1981. Temporal features of infant speech: A description of babbling patterns circumscribed by postural achievement. [Google Scholar]
- Yirmiya N, Gamliel I, Pillowsky T, Feldman R, Baron-Cohen S, Sigman M. The development of siblings of children with autism at 4 and 14 months: social engagement, communication, and cognition. Journal of Child Psychology and Psychiatry. 2006;47(5):511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Young GS, Merin N, Rogers S, Ozonoff S. Gaze behavior and affect at 6 months: predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science. 2009;12(5):798–814. doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder P, Stone WL, Walden T, Malesa E. Predicting social impairment and ASD diagnosis in younger siblings of children with autism spectrum disorder. Journal for Autism Developmental Disorders. 2009;39(10):1381–1391. doi: 10.1007/s10803-009-0753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Neuroscience. 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Lord C, Rogers S, Carter A, Carver L, Yirmiya N. Clinical assessment and management of toddlers with autism spectrum disorders: Insights from studies of high-risk infants. Pediatrics. 2009;123(5):1383–1391. doi: 10.1542/peds.2008-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]