Abstract
Hydrogen sulfide (H2S) has been known for hundreds of years because of its poisoning effect. Once the basal bio-production became evident its pathophysiological role started to be investigated in depth. H2S is a gas that can be formed by the action of two enzymes, cystathionine gamma-lyase and cystathionine beta-synthase, both involved in the metabolism of cysteine. It has several features in common with the other two well known “gasotransmitters” (nitric oxide and carbon monoxide) in the biological systems. These three gasses share some biological targets; however, they also have dissimilarities. For instance, the three gases target heme-proteins and open KATP channels; H2S as NO is an antioxidant, but in contrast to the latter molecule, H2S does not directly form radicals. In the last years H2S has been implicated in several physiological and pathophysiological processes such as long term synaptic potentiation, vasorelaxation, pro- and anti-inflammatory conditions, cardiac inotropism regulation, cardioprotection, and several other physiological mechanisms. We will focus on the biological role of H2S as a molecule able to trigger cell signaling. Our attention will be particularly devoted on the effects in cardiovascular system and in cardioprotection. We will also provide available information on H2S-donating drugs which have so far been tested in order to conjugate the beneficial effect of H2S with other pharmaceutical properties.
Keywords: Hydrogen sulfide, Cardioprotection, Gasotransmitter, Ischemic preconditioning, Nitric oxide
1. Introduction
Hydrogen sulfide (H2S) has been known for hundreds of years as a poisoning and toxic pollutant. It is a colorless gas with a high solubility in water. H2S is permeable to plasma membranes as its solubility in lipophilic solvents is fivefold greater than in water. Therefore, the gas can easily diffuse through the cells and reach intracellular compartments. In the literature several massive intoxications are reported and in the non-scientific literature poisoning by this gas is referred to as “sulphuratted hydrogen” intoxications. These fatal episodes often occurred in sewers and swamps as the main biological source is represented by anaerobic bacterial digestion of organic substrates [1] but it is also produced through inorganic reactions in volcanic gases, natural gas and well waters [2]. Chemical and enzymatic transformation of the sulfur compounds of foodstuff such as mushrooms, garlic and onions are also responsible for H2S production in human gut [3]. High concentrations of H2S lead to the inhibition of mitochondrial electron transport chain at the level of cytochrome oxidase c representing a high impact occupational and environmental hazard [4]. In fact, while low micromolar doses can reversibly bind the cytochrome c, acute H2S poisoning may lead to death through respiratory paralysis and pulmonary edema. These conditions are often reported in autopsies of individuals killed by hydrogen sulfide poisoning [5]. In the organism H2S is rapidly oxidized to elemental sulfur, sulfur oxide (SO2), and sulfates such as sulfuric acid or it can be hydrolyzed to hydrosulfide and sulfide ions [6].
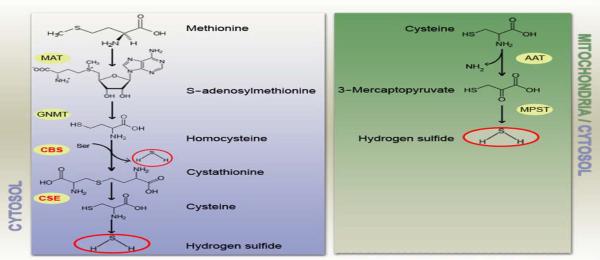
At physiological pHs in an aqueous solution, about one third of H2S remains undissociated. In recent years the biological role of H2S has been re-evaluated because of its low dose effects on eukaryotic cells. In mammalian cells low levels of H2S are detected with different technical approaches including colorimetric assay and polarographic probes and recently the order of magnitude of basal H2S concentration has been reset to low nanomolar range [7] and [8]. Interestingly, it was already reported in the past that low doses of the potentially dangerous hydrogen sulfide could be of some benefits in the balneologic treatment of hypertension with thermal and mud baths [9] and [10]. However, only in the last years the non-toxic action of H2S started to be investigated in depth. Although early evidence of a basal production of H2S in animal tissue were published almost a century ago [11] only recently it has been demonstrated that H2S is produced as a side product by constitutively expressed enzymes involved in the metabolism of cysteine: cystathionine beta-synthase (CBS) and cystathionine gamma-lyase (CSE) [12], [13] and [14] (Fig. 1). Moreover, H2S donors as well as inhibitors of the basal production have been tested in different experimental models showing surprising data about its physiological role with different experimental approaches (Table 1). In this review we summarize the literature about the biological properties of hydrogen sulfide with particular interest on the cardiovascular effects and the potential role in cardioprotection. We, finally, overview the recent findings about novel H2S-donating drugs that started to be tested on animal model and in vitro experimental settings.
Fig. 1.
Enzymatic pathways of H2S production in mammalian cells. On the left panel the cytosolic pathway leading to the formation of H2S from methionine. On the right panel formation of H2S in mitochondria from cysteine. MAT: methionine adenosyltransferase. GNMT: glycine N-methyltransferase. CBS: cystathionine β-synthase. CSE: cysthationine γ-lyase. AAT: aspartate aminotransferase. MPST: 3-mercaptopyruvate sulfur transferase.
Mancardi et al.
Table 1.
Effects of H2S donors on different experimental settings.
| Cell type | Experimental model | Concentration of H2S donors | Effect of H2S or it donors | Refs |
|---|---|---|---|---|
| Male Spargue–Dawley rat | NaSh 0.1–1 μM | Cytoprotection against ischemia/reperfusion | [67] | |
| Langendorff perfusion | ||||
| PAEC (porcine pulmonary arterial endothelial cell) | ACS6 10 μM NaSH 1–40 μM | Inhibition of O2̇− | [95] | |
| Male Wistar rat, LAD occlusion | NaSH 3 mg/kg | AAR reduction | [69] | |
| Rat cardiomyocyte | NaSH 100 μM | Delayed cardioprotection against lethal ischemia | [71] | |
| Rat aortic vascular smooth muscle (primary and immortalized) | S-Didofenac 10–100 μM | Decrease cell survival | [96] | |
| - Miocardial 1/R murine model and mitochondria isolation | − 50 μg/kg | Respiration rate in mitochondria was increase 2.2 fold | [66] | |
| - Mitochondria isolation from murine heart | −10 μM | Recovery of respiration after 39' of hypoxia | ||
| Male Wistar rat | NaSH 100 μl/kg | Inhibition aspirin-induced leukocyte adherence | [50] | |
| VSMCs | Male SHR rat | 5×10−5, 1×104, 5×10−4 mol/L | Decrease blood pressure Inhibition | [54] |
| Ang II-induced VSMCs proliferation | ||||
| Male Wistar rat | ||||
| - Hippocampus | NaSH 50–160 μM | Induction of long term potentiation | [87] | |
| - Portal vein | EC50 = 160 μM | Induction of relaxation | [28] | |
| - Thoracic aorta | EC50 > 1 mM | |||
| Isolated pregnant rat uterine strips in vitro | L–Cysteine, NaSH 10−3 M | Decreases in utherine spontaneous contractillity | [52] | |
| Mice, hepatic 1/R injury | 1K11001 (Na2S) 0.3 mg/kg | Attenuation hepatic 1/R injury | [72] |
The cell type, the experimental model, the concentration of H2S, the main effect, and the bibliographic reference number are reported.
2. Biological effects
Unfortunately, the best known effect of this gas with a characteristic smell of rotten eggs is the binding to cytochrome c oxidase which is responsible for its toxicity [15]. This enzyme catalyzes the oxidation of ferrocytochrome c by oxygen which represents the terminal acceptor of the electron transport chain in mitochondria. It can therefore interacts with other gaseous molecules such as carbon monoxide (CO), nitric oxide (NO), hydrogen cyanide (HCN), and H2S [16]. Yet, akin NO, H2S can behave either as a substrate or inhibitor of cytochrome c oxidase [17] and [18] leading to the oxidation of the gaseous molecule and the reduction of the enzyme. Nevertheless, the mitochondrial enzyme inhibition of the gas is non-competitive to the binding of cytochrome c with oxygen [19]. Inevitably, the reaction is coupled with oxygen consumption and with the formation of ferryl enzyme intermediates [19]. The degree of mitochondrial impairment changes according to experimental settings also depending on the tissues and species considered: in intact cells mitochondrial respiration is decreased of 50% by 30 μM H2S [20] whereas 10 μM is sufficient to half-inhibit respiration of isolated mitochondria [21]. Interestingly, low concentrations (< 20 μM) of sodium hydrosulfide (NaSH) stimulate mitochondrial oxygen consumption and augment membrane potential, while higher concentrations inhibit cytochrome c oxidase lowering oxygen consumption [22]. It is still not clear whether endogenously produced H2S could significantly inhibit respiration in vivo. The idea that H2S could be of some physiological importance arose when it became clear that it was naturally produced in several organisms by constitutively expressed enzymes and that it is present in mammalian blood at nanomolar concentration [7]. The normal blood level of H2S is reported to be between ~ 10 μM in Wistar [23] and ~ 50 μM in Sprague–Dawley rats [24]. In different tissues higher levels of H2S are detected: for example in brain tissue concentrations ranging ~ 50–160 μM are reported [25]. However, in contrast with NO, which is generated from both from endothelial and smooth muscle cells (SMCs), it is not clear whether H2S is produced only in SMCs [24] or in both endothelium and SMCs as later reported [26]. In fact, no evidence of the presence of CSE has been detected in rat aortic endothelium by RT-PCR [24] whereas significant levels of CSE have been reported in two immortalized endothelial cell lines [26].
Among the biological effects described in the literature several findings concern the Central Nervous System in which the main source on H2S is CBS. However, recent data indicate that enzymatic production by 3-mercaptopyruvate sulfurtransferase maintains elevate H2S levels in CBS−/− mice brain [27]. Abe and Kimura postulated a role of H2S in the long term synaptic potentiation in the hippocampus [28]. The same group showed later that H2S acts synergistically with NO in inducing vasorelaxation of ileum, portal vein and thoracic aorta [25]. In a model of isolated porcine irides H2S reverses carbachol-induced contraction while has no effect on basal contraction [29]. Moreover, inhibition of KATP channels with 100–300 μM glibenclamide blocks H2S action on pre-constricted irides [29]. Thus, this report demonstrates the involvement of intracellular [K+] in mediating H2S-induced SMCs relaxation. As regards to gene regulation H2S has been reported to regulate expression of cytochrome c oxidase, vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF) and receptors for TGF-β [30]. Because of its action on KATP channels H2S inhibits pancreatic insulin secretion decreasing ATP levels and thus opening the KATP channels in INS-1E cells [31].
3. H2S as the novel gasotransmitter
The arising findings on the biological properties stimulated several groups toward the research on the mechanism of action of H2S and very soon it was entitled as the third “gasotransmitter” besides NO and CO because of the biochemical similarities with the “siblings” molecules. These three molecules share diffusibility through biological membranes, biological effects at low doses and toxicity at high concentrations. Alike NO and CO hydrogen sulfide's bimodal activity does not require a receptor to initiate the intracellular signaling. The main physiological effects of gasotransmitters are mediated by the bound to hemoglobin. The three gaseous molecules are produced at different basal concentrations by constitutively expressed enzymes [32]: CO is produced by heme oxygenase (HO), H2S by CSE and CBS while NO is formed by the activity of the three isoforms of Nitric Oxide Synthase (nNOS, eNOS, iNOS). As regards to the substrates CO is formed through the metabolism of heme groups to biliverdin while the activity of NOSs is prevalently based on the metabolism of l-arginine. CBS and CSE are probably not the only enzymes able to form H2S in mammalian tissue but the proposed alternative enzymatic sources have limited physiological relevance [33]. The two main enzymes involved in the production of H2S are two pyridoxal 5′-phosphate-dependent enzymes with a preferential expression of CBS in the CNS and CSE in liver, kidney and blood vessels with a decreasing activity in tail artery, aorta and mesenteric arteries respectively [34]. CBS catalyzes the reaction of l-serine and l-homocysteine to l-cystathionine and H2O through a β-replacement between l-serine, l-cysteine, cysteine thioethers and other β-substituted α-l-amino acids and mercaptans. The replacement of serine by cysteine leads to the formation of H2S instead of H2O (Fig. 1). CSE catalyzes the conversion of l-cystathionine into l-cysteine, 2-oxobutanoate and NH3 and, as a side activity, induces the elimination of l-homoserine forming H2O, 2-oxobutanoate and NH3. CSE can also react with l-cystine, producing thiocysteine, pyruvate and NH3, and with l-cysteine producing pyruvate, NH3 and H2O [35]. Despite of the toxicity at high concentration CO has been reported to play a physiological role in regulating synaptic transmission and vascular function at low doses [36] and [37]. It has also been shown that inhibition of cytochrome c oxidase by CO can result in mitochondrial Reactive Oxygen Species (ROS) production and exert anti-inflammatory effect [38]. NO physiological activities have been widely investigated and data unequivocally show a key role in the regulation of vascular tone, platelet aggregation, smooth muscle relaxation, and synaptic function. Some authors suggest a potential role as gasotransmitter for hydrogen cyanide as well but this hypothesis will require further investigation to be confirmed [16]. The three gasotransmitters have been demonstrated to be synaptic modulator without having conventional receptors on the membrane of communicating neurons [39]. The endogenously produced gases diffuse through vessels wall and hemoglobin is thought to be a common target for CO, NO, and H2S forming respectively carboxyhemoglobin [40], nytrosylhemoglobin [41], and sulfhemoglobin [42]. It appears, therefore, clear that the biological behavior of hydrogen sulfide has a lot in common with the two gaseous transmitter so far identified [32]. Interestingly, NO and H2S might collaborate in regulating the vascular homeostasis although unequivocal results about the interplay of the two molecules are still lacking. Some authors reported a synergistic effect of H2S in NO-induced vasodilation [34] whereas others reported the contrary [25]. It has been shown that sodium nitroprusside induces over-expression of H2S generating enzymes in rat vascular tissues suggesting an increased H2S production NO-mediated [43]. Moreover, H2S seems to have a potent antioxidant effect scavenging peroxynitrite and ameliorating cultured neuronal cell survival after ONOO− challenge [44]. NO and H2S might also interact at molecular level forming a yet unidentified nitrosothiol with potential physiological relevance as suggested by Whiteman et al. [45].
The normo-physiological levels of H2S in the blood are reported to range from 10 to 300 μM with the exception of one study reporting the value of 5 μM measured with a polarographic probe [46]. However, sulfide concentrations of whole blood, plasma, or BSA decay as its consumption is proportional to protein concentration but independent from oxygen partial pressure as demonstrated by saturation with N2 that does not affect decay rate [47]. It has been suggested that blood levels of H2S are undetectable in the vertebrate using a modified polarographic sensor [47]. In order to address H2S presence in different tissues exogenous H2S absorption has been tested in homogenates of brain, heart and liver showing that brain absorption is much slower than those of heart and liver [48]. The vasorelaxant effect of H2S has been demonstrated in different experimental models although it is not clear whether it is produced at the endothelial level. The effect on vessels seems to be dependent on oxygen concentration [46] and vasodilation is partially mediated by the opening of KATP sensitive channels at least in some vascular districts [24]. In fact, the gas can easily diffuse through SMCs and increase the K+ permeability leading to hyperpolarization and relaxation of SMCs. In vivo, an intravenous bolus injection of H2S determines a transient fall in blood pressure that can be mimicked by the KATP channel opener Pinacidil [24]. However, the in vivo hypotensive effect might be also attributed to an inhibition of Angiotensin-Converting-Enzyme as it has recently been shown [49]. Endogenous formation of H2S from CSE contributes to maintain normal blood pressure as demonstrated in CSE−/− mice that show higher systemic pressure compared to the wild type animals thus suggesting a pivotal contribution of this enzyme for the normal vessels tone [26]. At the vascular level the beneficial effect of H2S is also based on a marked decrease in neutrophil adhesion. H2S contributes also to reduce vascular infiltration acting as an anti-inflammatory agent [50]. This effect can be reversed in a model of hemorrhagic shock where the anti-inflammatory response can be turned into a pro-inflammatory effect [51]. Sodium hydrosulfide relaxant action was also shown in an in vitro model of pregnant rat uterus strips where it induced significant decreases in uterine spontaneous contractility in a dose-dependent manner [52] (Table 1).
4. Hydrogen sulfide and the cardiovascular system
Although definitive evidence supporting the hypothesis of a basal enzymatic production of H2S are still questioned the contribution of H2S to the cardiovascular homeostasis is gaining more significance [26]. In the past H2S was believed to impair the cardiovascular function relatively to its toxic effect [53]. However, the role of hydrogen sulfide in maintaining the vascular tone is nowadays well established. It was at first demonstrated that exogenously applied NaSH could induce relaxation in smooth muscle of rat thoracic aorta [25] and subsequently that in vivo administration of H2S induces a transient but significant fall in blood pressure [24]. Therefore, experimental data suggest that the hypotensive effect of H2S is a consequence of the relaxation of blood vessels SMCs triggered by opening of KATP channels increasing K+ currents and hyperpolarizing membrane of SMCs of peripheral vessels [24]. Also in an ex vivo model of human internal mammary artery rings NaSH was effective in inducing relaxation. It has also been shown that in Spontaneous Hypertensive Rats (SHRs) production of H2S is markedly decreased in the thoracic aorta. Hypertension in these animals can be reversed by exogenous H2S administration which also leads to a decrease in vascular collagen accumulation [54] (Table 1). However, endogenous H2S was showed to regulate basal tone by inhibition of CSE with DL-propargylglycine [55]. This was the first report on the presence and on the activity of H2S generating enzymes in humans. In contrast with NO, H2S vasorelaxant effect is not mediated by soluble guanylyl cyclase (sGC) as demonstrated by the inhibition of sCG with ODQ which does not prevent H2S-induced vasorelaxation [24]. It is also likely that this effect is mediated by inward Ca2+ currents and by an increase of intracellular Ca2+ concentration [34]. In an in vitro model of perfused rat mesenteric artery beds (MABs) either NaSH or bubbled H2S solution induce a significant relaxation proportionally with the concentration though the results are different when compared to data obtained in rat aortic tissue [56]. In fact, NaHS vasodilates the pre-contracted MABs but the extent of relaxation is significantly lower than that of bubbled H2S solution [56]. Therefore, it is reasonable to believe that the fall in blood pressure observed by several authors is dependent on the general relaxation of peripheral resistance blood vessel which is well represented by MABs [56].
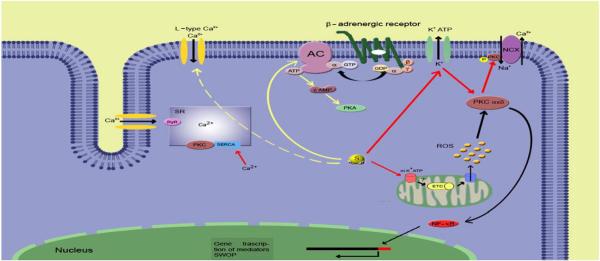
4.1. H2S as a preconditioning agent
Although more than twenty years have passed since its first description, ischemic preconditioning (IP) still remains one of the most potent cardioprotective mechanism against ischemia/reperfusion (I/R) injury [57]. It consists of brief sub-lethal episodes of ischemia conferring protection against a subsequent prolonged ischemic insult. Several triggers, mediators and effectors of IP have been so far identified [58] and [59]. Among the others NO and its redox sibling molecule HNO− have also been proven to induce cardioprotection in the isolated rat heart [60] despite of the contradictory dynamics of NO [41] and the orthogonal properties of the two molecules [61]. Both classical ischemic preconditioning and NO-induced cardioprotection is mediated by the opening of mitochondrial KATP channels, PKCε translocation, and ROS production [62]. Since all of these mediators are also activated by H2S a potential role in IP for the third gasotransmitter can be suggested (Fig. 2). The hypothesis that H2S can function as a preconditioning agent is also supported by the fact that respiratory inhibition with this gas is different from hypoxia. In fact, while oxygen deprivation leads to massive cell loss, H2S induces suspended animation which confers protection against potentially lethal hypoxia [63]. Suspended animation is a fascinating phenomenon consisting in lowering metabolic rate and increasing resistance to low oxygen concentration pretreating mice with H2S [64]. However, these studies are unique and H2S has been shown to be protective against myocardial ischemia/reperfusion injury only when given before hypoxia [65]. An increasing number of studies provide evidence that both exogenous and endogenous H2S exert protective effects against myocardial I/R injuries in different experimental settings [66], [67], [68] and [69]. NaSH pre-treatment significantly reduces cardiomyocytes death after in vitro I/R simulation and attenuates both duration and scores of arrhythmias during the initial 10 min of reperfusion after a low-flow ischemia in isolated rat hearts [70]. Accordingly to these data, NaSH shows beneficial effects in Langendorff-perfused hearts after a 30 min left coronary artery ligation followed by 120 min reperfusion [68]. Further support to the hypothesis that H2S could play a pivotal role as a preconditioning agent is provided by in vivo models of I/R in which pre-treatment with sodium hydrosulfide triggered the so called second window of protection [69]. Thus, being the late preconditioning phase initiate by protein expression H2S can also regulate gene transcription (Fig. 1). In this study it was also proved that the action of hydrogen sulfide is mediated by mitochondrial KATP sensitive channels. In fact, 5-hydroxydecanoate (5-HD) was used instead of glibenclamide ensuring a selective blockage of mitochondrial population of KATP channels [69]. However, it is not clear whether H2S-induced cardioprotection involves NO release and/or the mediation of PKC [71]. Interestingly, H2S was proved to be effective also in the protection against hepatic I/R injury in a mouse model inhibiting the progression of apoptosis and increasing the expression of heat shock protein-90 (HSP-90) and Bcl-2 [72]. Similar results concern the cytoprotective effect of NaSH against oxidative stress on a cardiac cell line. In this model H2S upregulates mitochondrial Bcl-2 and activates the Akt pathway [73]. The cardioprotective effect of H2S donors is confirmed by an in vivo murine model of pharmacological preconditioning with IK1001 which confers protection against ischemic injury in term of decrease in infarct size, serum troponin-I levels, and oxidative stress [74]. Moreover, IK1001 induces up-regulation of Trx-1 and Trx-2, increased expression of HSP-90, HSP-70, Bcl-2, and Bcl-xL suggesting a potential mediation of antioxidant and anti-apoptotic signaling in the cytoprotective mechanisms [74]. The same group proposed that both endogenous and exogenous H2S can be useful in the treatment of heart failure using a transgenic mouse model in which the constitutive expression of CSE was upregulated in the heart [75]. Both in the transgenic mice and in IK1001 treated mice structural and functional deterioration of left ventricle were reduced and performance preserved after occlusion of the left coronary artery [75]. Consistently with these results, administration of Na2S improves resuscitation after cardiac arrest in mice and this effect is probably due to cell survival signaling pathway as suggested by phosphorylation of Akt and caspase-3 activation [76].
Fig. 2.
Proposed signaling pathways of H2S in cardioprotection: representation of the putative cascade of reactions triggered by molecular H2S. The yellow solid line indicates the direct inhibitory pathway to the β-adrenergic receptors and the dashed yellow line indicates the indirect inhibitory action on L-type Ca2+ channels. Red lines: direct activation of membrane and mitochondrial KATP channels with consequent activation/translocation of PKC. Black lines represent mechanism of early and late cardioprotection (SWOP). NCX: Na+/Ca2+ exchanger. SERCA: Sarco/Endoplasmatic Reticulum Ca2+-ATPase. RyR: Ryanodine Receptor. PKC: Protein Kinase C. PKA: Protein Kinase A. AC: Adelynate Cyclase. ROS: Reactive Oxygen Species. ETC: electron transport chain. mPTP: mitochondrial Permeability Transition Pore. SWOP: second window of protection.
Mancardi et al.
5. H2S and redox state
The chemical dynamic of H2S is strictly related to the redox state of cells. In physiological conditions at 37 °C and at pH 7.4 around 82.5% is present in the form of HS− and a small amount of S2− while the rest remains undissociated. However, taking in considerations the pKa1 of the reaction (6.755) the level of H2S at standard conditions (20 °C, pH 7.4) is significantly higher (30%) [77] and [78]. Under acidic conditions acid-labile sulfur can be released by the iron-sulfur core of mitochondrial enzymes and it has been measured in brain tissue of rats, bovines, and humans [79], [80] and [81]. On the other hand, physiological pH of mitochondria is too high to facilitate acid-labile sulfur release by this kind of storage [48]. Another form of storage is represented by the so called bound sulfur in the cytosolic fraction and H2S can be released under reducing conditions [82]. Olson's group proposed a model in which constitutively produced H2S diffuses from cytoplasm to mitochondria where it is rapidly oxidized [83]. The amount of H2S being oxidized is therefore proportional to the oxygen partial pressure (pO2) and biologically available H2S is the result of production minus oxidation [8]. Moreover, CBS and CSE activity is influenced by the redox environment [84] and [85] thus contributing to regulate intracellular concentration of H2S. Recently, a mitochondrial pathway for sulfide oxidation has been described in two different models [86]. In the mitochondrial matrix of rat liver and body-wall tissue of Arenicola marina, a sulfur dioxygenase converts persulfide to sulfite with consumption of oxygen. The persulfides are produced by sulfide by quinone oxidoreductase and a second molecule of persulfide is added by sulfur transferase leading to the formation of thiosulfate [86]. Interestingly, H2S consumption in purified mitochondria is increased when O2 is added and decreased when mitochondria are either denatured by heat or gassed with nitrogen [8]. In neurons H2S protective effect against oxidative stress is mediated by increased glutathione (GSH) concentration and by activating K and Cl− ATP channels [87]. Therefore, the antioxidant role of H2S seems to be mainly effective through intracellular GSH formation which buffers the redox imbalance. However, it has been shown that H2S increases mRNA levels of heme oxygenase-1 and it may act synergistically with CO in regulating vascular structural remodeling [88]. The ability of H2S to counteract redox imbalance has also been shown in aspirin-induced gastric toxicity [89]. In this model, the same pathway seems to mediate the antioxidant effect of H2S involving GSH formation, HO-1 up-regulation, and isoprostane synthesis inhibition.
6. Pharmacological implications
Recently, an increasing number of diseases have been related to an imbalance of endogenous H2S production [35]. The endogenous release of H2S from mammalian tissues is likely to occur in a slow and constant rate and it appears to be involved in several processes such as neuromodulation [28], hypertension [90], inflammation [33] and [91], hemorrhagic shock [92] and edema [93]. Pharmacological studies are either focused on the regulation of basal production of H2S or to investigate the role of exogenously applied donors. Until now, the majority of H2S donors used for biological researches have been restricted to sulfide salts. The most commonly used is NaHS, which have a fast releasing rate in aqueous solution producing one third of hydrogen sulfide compared to the concentration of the salt. However, to mimic the naturally occurring enzymatic release the researchers focused on organic compounds able to release the gasotransmitter over a prolonged period of time. In this contest, a novel water-soluble compound (GYY4137) was reported to slowly release H2S both in vitro and in vivo after being injected intravenously or intraperitonealy in anesthetized rats [94]. In these set of experiments it was demonstrated that GYY4137 acts as vasodilator and antihypertensive both in normal and spontaneously hypertensive rats [94]. The increasing volume of data regarding physiological and pathophysiological effects of hydrogen sulfide suggest future implications for sulfide-related compounds in the treatment of hypertension and ischemic pathologies. Pharmacological studies are also directed towards the inhibition of H2S production and/or H2S donors. In fact, an inhibitor of phosphodiesterase 5 (Sildenafil) was conjugated with an H2S releasing group and tested on pulmonary artery endothelial cells [95]. The H2S-donating derivative of Sildenafil (ACS6) releases more H2S than NaSH because of the three sulfur atoms bound to the molecule and the delivery rate reaches the peak after 2 h [95]. It was shown that superoxide formation is inhibited by hydrogen sulfide and that ACS6 can also increase cAMP representing a potential treatment for respiratory distress syndrome [95]. Another type of hydrogen sulfide releasing moiety was tested on smooth muscle cells: an H2S-releasing derivative of the nonsteroidal anti-inflammatory drug, diclofenac (S-diclofenac) inhibited cell proliferation and the authors postulated a potential role in the treatment of vascular occlusive disorders [96]. With other perspectives, S-diclofenac was used to revert the potentially lethal gastrointestinal toxicity of NSAIDs showing an enhanced anti-inflammatory activity and a diminished gastrointestinal toxicity [97]. Moreover, sulfide releasing sartans are reported to be cytoprotective against oxidative stress induced by hydrogen peroxide when compared to the non-sulfurate molecule [98]. Consistently, in the same cell line the effect of 30 μM of H2S induces an up-regulation of the Akt-GSK3β-ERK 1/2 pathway and the activation of Bcl2 with an anti-apoptotic effect [73]. Therefore, pro-survival and anti-apoptotic pathways might be activated simultaneously leading to an increased cell survival after oxidative stress. In another study, a new dithiolethione-containing aspirin (ACS14) was tested on rats showing inhibition of thromboxane comparable to aspirin while prostacyclin formation was decreased [89]. Thus, ACS14 maintains beneficial effects of parent compound whereas it appears to be less detrimental for the gastrointestinal mucosa. Interestingly, in an animal model of diabetes inhibition of H2S production by pancreatic islets lowered glycemia and increased serum level of insulin supporting the hypothesis that insulin release is impaired because of abnormal pancreatic production of H2S [99]. It was also found that NaSH exerts an anti-atherogenic effect downregulating ICAM-1 expression in a transgenic mouse model and the results is supported by data obtained in human umbilical vein endothelial cells (HUVECs) [100]. In fact, in HUVECs treated with NaSH ICAM-1 expression is suppressed via the NF-kβ pathway [100]. In rainbow trout endogenous hydrogen sulfide contributes to adrenergic stimulation enhancing catecholamine secretion in a Calcium-dependent manner [101]. In an avian model has been demonstrated that ventilation with high doses of hydrogen sulfide induces an increase of CO2 pulmonary receptors drive to the central respiratory neurons [102]. This effect is probably mediated by inhibition of carbonic anhydrase [102]. Hydrogen sulfide decreases oxygen demand as demonstrated by longer survival of mice exposed to 5% oxygen when pre-treated with H2S [63]. This condition is useful in ischemic/hypoxic tissues treatment. In fact, the possibility of protecting myocytes against hypoxia/reoxygenation injuries has been proved in vitro[71], ex vivo[68], and in vivo models [69] and [103] reducing both necrosis and apoptosis. The mechanism of action is probably related to the opening of the KATP channels as demonstrated by the loss of protection with co-infusion of channel blockers [69] and [103]. Further investigation reported an involvement of the ERK-AkT pathway in sulfide-preconditioned rat heart and a role of H2S in the classical preconditioning [104]. At the level of the gastrointestinal tract the enterobacterial flora is responsible for the production of H2S. However, excessive entry of sulfide in the circulation and potential toxicity on intestinal epithelium is prevented by regulatory enzymes activity [3] and [105]. Vascular production of H2S has been related to endotoxin-induced inflammation [106] and it was later demonstrated that higher rate of H2S production corresponds to an up-regulation of CSE expression in liver and kidney [107].
7. Conclusion
Despite of the early clues of some beneficial effects hydrogen sulfide has been neglected for a long time as a potential biological actor. Neither its high diffusibility nor its strong ability to bind respiratory enzymes served to stimulate studies about its physiological role until recent years. On the other hand only when modern tools, such as polarographic probes, have replaced non-specific colorimetric techniques used to determine sulfide concentrations it became possible to detect the basal concentration. The basal endogenous presence of H2S suddenly stimulated the scientific community to investigate the biological dynamics of this molecule with evident similarities to NO and CO. It was soon demonstrated that H2S has a lot in common with the other two gasotransmitters both in terms of chemical activities and biological targets [32]. In the last years H2S have been implicated in long term synaptic potentiation [28], vasorelaxation [25], pro- and anti-inflammatory processes [50] and [51], cardiac inotropism regulation [108], cardioprotection [66], [68] and [69], and several other physiological processes [35], [44], [99] and [100]. Benefits from H2S might also be attributed to its antioxidant properties and in contrast with NO it does not form radicals directly [44] and [109]. Nevertheless, it is clear that H2S plays a pivotal role in the basal regulation of vessels tone as well as other basic process such as neuromodulation and inflammation. Contemporarily, novel H2S-donating drugs have been tested in order to conjugate the beneficial effect of H2S with other pharmaceuticals [66], [73], [89], [95] and [96]. However, a definite agreement on the concentrations level in different tissues is still lacking. Yet it is uncertain the ability of blood to transport and deliver sulfides. Further studies are also required in order to establish the mechanisms of action of this extremely diffusible molecule in different cell types. So far only the a role for plasmalemmal and mitochodrial K+ channel activation has been unequivocally demonstrated as a part of H2S's activity on cells [69]. Electrophysiological variations as well as ionic channels activity are still to be elucidated to better understand the effect of H2S on excitable tissues. Finally, but not of less importance, the picture of all the potential biochemical reactions is still foggy and it needs to be clarified for a sufficient comprehension of the possible interactions of sulfides with plasma proteins and intracellular enzymes.
Acknowledgments
We would like to thank Regione Piemonte, “Compagnia di San Paolo”, Torino, the Istituto Nazionale per la Ricerca Cardiovascolare (INRC) and the Italian Ministry of University and Research (MIUR) for their financial contributions to the project.
References
- [1].Lederer A. Some observations on the formation of hydrogen sulphide in sewage. Am. J. Public Health (N Y) 1913;3:552–561. doi: 10.2105/ajph.3.6.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bates MN, Garrett N, Shoemack P. Investigation of health effects of hydrogen sulfide from a geothermal source. Arch. Environ. Health. 2002;57:405–411. doi: 10.1080/00039890209601428. [DOI] [PubMed] [Google Scholar]
- [3].Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- [4].Beauchamp RO, Jr., Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- [5].Yalamanchili C, Smith MD. Acute hydrogen sulfide toxicity due to sewer gas exposure. Am. J. Emerg. Med. 2008;26(518):e5–e7. doi: 10.1016/j.ajem.2007.08.025. [DOI] [PubMed] [Google Scholar]
- [6].Gunina AI. The metabolism of hydrogen sulfide (H2S35) injected subcutaneously. Bull. Exp. Biol. Med. 1957;43:176–179. [Google Scholar]
- [7].Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- [8].Olson KR. Hydrogen sulfide and oxygen sensing: implications in cardiorespiratory control. J. Exp. Biol. 2008;211:2727–2734. doi: 10.1242/jeb.010066. [DOI] [PubMed] [Google Scholar]
- [9].Popovich A, Gavrilishina GE. Use of hydrogen sulfide baths from Konopkivka spring in the overall treatment of hypertension. Vrach Delo. 1979:36–37. [PubMed] [Google Scholar]
- [10].Sabo GP. Hydrogen sulfide carbon dioxide mud baths as a new balneologic factor. Sov. Med. 1950;9:26–27. [PubMed] [Google Scholar]
- [11].Sluiter E. The production of hydrogen sulphide by animal tissues. Biochem. J. 1930;24:549–563. doi: 10.1042/bj0240549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Erickson PF, Maxwell IH, Su LJ, Baumann M, Glode LM. Sequence of cDNA for rat cystathionine gamma-lyase and comparison of deduced amino acid sequence with related Escherichia coli enzymes. Biochem. J. 1990;269:335–340. doi: 10.1042/bj2690335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Swaroop M, Bradley K, Ohura T, Tahara T, Roper MD, Rosenberg LE, Kraus JP. Rat cystathionine beta-synthase. Gene organization and alternative splicing. J. Biol. Chem. 1992;267:11455–11461. [PubMed] [Google Scholar]
- [14].Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Roth SH, Skrajny B, Reiffenstein RJ. Alteration of the morphology and neurochemistry of the developing mammalian nervous system by hydrogen sulphide. Clin. Exp. Pharmacol. Physiol. 1995;22:379–380. doi: 10.1111/j.1440-1681.1995.tb02024.x. [DOI] [PubMed] [Google Scholar]
- [16].Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- [17].Nicholls P, Kim JK. Sulphide as an inhibitor and electron donor for the cytochrome c oxidase system. Can. J. Biochem. 1982;60:613–623. doi: 10.1139/o82-076. [DOI] [PubMed] [Google Scholar]
- [18].Nicholls P, Kim JK. Oxidation of sulphide by cytochrome aa3. Biochim. Biophys. Acta. 1981;637:312–320. doi: 10.1016/0005-2728(81)90170-5. [DOI] [PubMed] [Google Scholar]
- [19].Petersen LC. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim. Biophys. Acta. 1977;460:299–307. doi: 10.1016/0005-2728(77)90216-x. [DOI] [PubMed] [Google Scholar]
- [20].Leschelle X, Goubern M, Andriamihaja M, Blottiere HM, Couplan E, Gonzalez-Barroso MD, Petit C, Pagniez A, Chaumontet C, Mignotte B, Bouillaud F, Blachier F. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochim. Biophys. Acta. 2005;1725:201–212. doi: 10.1016/j.bbagen.2005.06.002. [DOI] [PubMed] [Google Scholar]
- [21].Yong R, Searcy DG. Sulfide oxidation coupled to ATP synthesis in chicken liver mitochondria. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2001;129:129–137. doi: 10.1016/s1096-4959(01)00309-8. [DOI] [PubMed] [Google Scholar]
- [22].Goubern M, Andriamihaja M, Nubel T, Blachier F, Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007;21:1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- [23].Mason J, Cardin CJ, Dennehy A. The role of sulphide and sulphide oxidation in the copper molybdenum antagonism in rats and guinea pigs. Res. Vet. Sci. 1978;24:104–108. [PubMed] [Google Scholar]
- [24].Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- [26].Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3- Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- [28].Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Monjok EM, Kulkarni KH, Kouamou G, McKoy M, Opere CA, Bongmba ON, Njie YF, Ohia SE. Inhibitory action of hydrogen sulfide on muscarinic receptorinduced contraction of isolated porcine irides. Exp. Eye Res. 2008;87:612–616. doi: 10.1016/j.exer.2008.09.011. [DOI] [PubMed] [Google Scholar]
- [30].Deplancke B, Gaskins HR. Hydrogen sulfide induces serum-independent cell cycle entry in nontransformed rat intestinal epithelial cells. FASEB J. 2003;17:1310–1312. doi: 10.1096/fj.02-0883fje. [DOI] [PubMed] [Google Scholar]
- [31].Yang W, Yang G, Jia X, Wu L, Wang R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J. Physiol. 2005;569:519–531. doi: 10.1113/jphysiol.2005.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- [33].Li L, Bhatia M, Moore PK. Hydrogen sulphide—a novel mediator of inflammation? Curr. Opin. Pharmacol. 2006;6:125–129. doi: 10.1016/j.coph.2005.10.007. [DOI] [PubMed] [Google Scholar]
- [34].Zhao W, Wang R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- [35].Szabo C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. 870 D. Mancardi et al. / Biochimica et Biophysica Acta 1787 (2009) 864-872. [DOI] [PubMed] [Google Scholar]
- [36].Koehler RC, Traystman RJ. Cerebrovascular effects of carbon monoxide. Antioxid. Redox Signal. 2002;4:279–290. doi: 10.1089/152308602753666334. [DOI] [PubMed] [Google Scholar]
- [37].Durante W, Johnson FK, Johnson RA. Role of carbon monoxide in cardiovascular function. J. Cell Mol. Med. 2006;10:672–686. doi: 10.1111/j.1582-4934.2006.tb00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zuckerbraun BS, Chin BY, Bilban M, d'Avila JC, Rao J, Billiar TR, Otterbein LE. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]
- [39].Boehning D, Snyder SH. Novel neural modulators. Annu. Rev. Neurosci. 2003;26:105–131. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- [40].Wang R. Resurgence of carbon monoxide: an endogenous gaseous vasorelaxing factor. Can. J. Physiol. Pharmacol. 1998;76:1–15. doi: 10.1139/cjpp-76-1-1. [DOI] [PubMed] [Google Scholar]
- [41].Mancardi D, Ridnour LA, Thomas DD, Katori T, Tocchetti CG, Espey MG, Miranda KM, Paolocci N, Wink DA. The chemical dynamics of NO and reactive nitrogen oxides: a practical guide. Curr. Mol. Med. 2004;4:723–740. doi: 10.2174/1566524043359854. [DOI] [PubMed] [Google Scholar]
- [42].Arp AJ, Childress JJ, Vetter RD. The sulphide-binding protein in the blood of the vestimentiferan tubeworm, Riftia pachyptila, is the extracellular hemoglobin. J. Exp. Biol. 1987;128:139–158. [Google Scholar]
- [43].Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can. J. Physiol. Pharmacol. 2003;81:848–853. doi: 10.1139/y03-077. [DOI] [PubMed] [Google Scholar]
- [44].Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite `scavenger'? J. Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- [45].Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem. Biophys. Res. Commun. 2006;343:303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- [46].Koenitzer JR, Isbell TS, Patel HD, Benavides GA, Dickinson DA, Patel RP, Darley-Usmar VM, Lancaster JR, Jr., Doeller JE, Kraus DW. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1953–H1960. doi: 10.1152/ajpheart.01193.2006. [DOI] [PubMed] [Google Scholar]
- [47].Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1930–R1937. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- [48].Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain, Antioxid. Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- [49].Laggner H, Hermann M, Esterbauer H, Muellner MK, Exner M, Gmeiner BM, Kapiotis S. The novel gaseous vasorelaxant hydrogen sulfide inhibits angiotensin-converting enzyme activity of endothelial cells. J. Hypertens. 2007;25:2100–2104. doi: 10.1097/HJH.0b013e32829b8fd0. [DOI] [PubMed] [Google Scholar]
- [50].Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- [51].Mok YY, Moore PK. Hydrogen sulphide is pro-inflammatory in haemorrhagic shock. Inflamm. Res. 2008;57:512–518. doi: 10.1007/s00011-008-7231-6. [DOI] [PubMed] [Google Scholar]
- [52].Sidhu R, Singh M, Samir G, Carson RJ. L-cysteine and sodium hydrosulphide inhibit spontaneous contractility in isolated pregnant rat uterine strips in vitro. Pharmacol. Toxicol. 2001;88:198–203. doi: 10.1034/j.1600-0773.2001.d01-104.x. [DOI] [PubMed] [Google Scholar]
- [53].Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- [54].Zhao X, Zhang LK, Zhang CY, Zeng XJ, Yan H, Jin HF, Tang CS, Du JB. Regulatory effect of hydrogen sulfide on vascular collagen content in spontaneously hypertensive rats. Hypertens. Res. 2008;31:1619–1630. doi: 10.1291/hypres.31.1619. [DOI] [PubMed] [Google Scholar]
- [55].Webb GD, Lim LH, Oh VM, Yeo SB, Cheong YP, Ali MY, El Oakley R, Lee CN, Wong PS, Caleb MG, Salto-Tellez M, Bhatia M, Chan ES, Taylor EA, Moore PK. Contractile and vasorelaxant effects of hydrogen sulfide and its biosynthesis in the human internal mammary artery. J. Pharmacol. Exp. Ther. 2008;324:876–882. doi: 10.1124/jpet.107.133538. [DOI] [PubMed] [Google Scholar]
- [56].Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- [57].Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- [58].Rastaldo R, Penna C, Cappello S, Mancardi D, Pagliaro P, Losano G. Ischemic postconditioning: an effective strategy of myocardial protection? G. Ital. Cardiol. (Rome) 2006;7:464–473. [PubMed] [Google Scholar]
- [59].Rastaldo R, Pagliaro P, Cappello S, Penna C, Mancardi D, Westerhof N, Losano G. Nitric oxide and cardiac function. Life Sci. 2007;81:779–793. doi: 10.1016/j.lfs.2007.07.019. [DOI] [PubMed] [Google Scholar]
- [60].Pagliaro P, Mancardi D, Rastaldo R, Penna C, Gattullo D, Miranda KM, Feelisch M, Wink DA, Kass DA, Paolocci N. Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning. Free Radic. Biol. Med. 2003;34:33–43. doi: 10.1016/s0891-5849(02)01179-6. [DOI] [PubMed] [Google Scholar]
- [61].Wink DA, Miranda KM, Katori T, Mancardi D, Thomas DD, Ridnour L, Espey MG, Feelisch M, Colton CA, Fukuto JM, Pagliaro P, Kass DA, Paolocci N. Orthogonal properties of the redox siblings nitroxyl and nitric oxide in the cardiovascular system: a novel redox paradigm. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2264–H2276. doi: 10.1152/ajpheart.00531.2003. [DOI] [PubMed] [Google Scholar]
- [62].Penna C, Mancardi D, Raimondo S, Geuna S, Pagliaro P. The paradigm of postconditioning to protect the heart. J. Cell Mol. Med. 2008;12:435–458. doi: 10.1111/j.1582-4934.2007.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Blackstone E, Roth MB. Suspended animation-like state protects mice from lethal hypoxia. Shock. 2007;27:370–372. doi: 10.1097/SHK.0b013e31802e27a0. [DOI] [PubMed] [Google Scholar]
- [64].Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- [65].Pan TT, Neo KL, Hu LF, Yong QC, Bian JS. H2S preconditioning-induced PKC activation regulates intracellular calcium handling in rat cardiomyocytes. Am. J. Physiol. Cell Physiol. 2008;294:C169–C177. doi: 10.1152/ajpcell.00282.2007. [DOI] [PubMed] [Google Scholar]
- [66].Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ji Y, Pang QF, Xu G, Wang L, Wang JK, Zeng YM. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur. J. Pharmacol. 2008;587:1–7. doi: 10.1016/j.ejphar.2008.03.044. [DOI] [PubMed] [Google Scholar]
- [68].Johansen D, Ytrehus K, Baxter GF. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury—evidence for a role of K ATP channels. Basic Res. Cardiol. 2006;101:53–60. doi: 10.1007/s00395-005-0569-9. [DOI] [PubMed] [Google Scholar]
- [69].Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock. 2006;26:154–161. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- [70].Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J. Pharmacol. Exp. Ther. 2006;316:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- [71].Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J. Mol. Cell Cardiol. 2006;40:119–130. doi: 10.1016/j.yjmcc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- [72].Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am. J. Physiol., Heart Circ. Physiol. 2008;295:H801–H806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mancardi D, Pagliaro P, Penna C. Annual Main Meeting of the Society for Experimental Biology. Vol. 150A. Elsevier; Marseille, France: 2008. p. S126. [Google Scholar]
- [74].Calvert JW, Jha S, Gundewar S, Duranski MR, Lefer DJ. Vol. 118. New Orleans, LA: 2008. pp. S_1446–S_1447. [Google Scholar]
- [75].Calvert JW, Gundewar S, Jha S, Elrod JW, Lefer DJ. Vol. 118. New Orleans, LA: 2008. p. S_441. [Google Scholar]
- [76].Minamishima S, Yu JD, Bougaki M, Adrie C, Minamishima YA, Lefer DJ, Ichinose F. Vol. 118. New Orleans, LA: 2008. pp. S_1446–S_1447. [Google Scholar]
- [77].Dorman DC, Moulin FJ, McManus BE, Mahle KC, James RA, Struve MF. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol. Sci. 2002;65:18–25. doi: 10.1093/toxsci/65.1.18. [DOI] [PubMed] [Google Scholar]
- [78].Dombkowski RA, Russell MJ, Olson KR. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R678–R685. doi: 10.1152/ajpregu.00419.2003. [DOI] [PubMed] [Google Scholar]
- [79].Goodwin LR, Francom D, Dieken FP, Taylor JD, Warenycia MW, Reiffenstein RJ, Dowling G. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J. Anal. Toxicol. 1989;13:105–109. doi: 10.1093/jat/13.2.105. [DOI] [PubMed] [Google Scholar]
- [80].Savage JC, Gould DH. Determination of sulfide in brain tissue and rumen fluid by ion-interaction reversed-phase high-performance liquid chromatography. J. Chromatogr. 1990;526:540–545. doi: 10.1016/s0378-4347(00)82537-2. [DOI] [PubMed] [Google Scholar]
- [81].Warenycia MW, Goodwin LR, Benishin CG, Reiffenstein RJ, Francom DM, Taylor JD, Dieken FP. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem. Pharmacol. 1989;38:973–981. doi: 10.1016/0006-2952(89)90288-8. [DOI] [PubMed] [Google Scholar]
- [82].Ogasawara Y, Isoda S, Tanabe S. Tissue and subcellular distribution of bound and acid-labile sulfur, and the enzymic capacity for sulfide production in the rat. Biol. Pharm. Bull. 1994;17:1535–1542. doi: 10.1248/bpb.17.1535. [DOI] [PubMed] [Google Scholar]
- [83].Olson KR, Dombkowski RA, Russell MJ, Doellman MM, Head SK, Whitfield NL, Madden JA. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J. Exp. Biol. 2006;209:4011–4023. doi: 10.1242/jeb.02480. [DOI] [PubMed] [Google Scholar]
- [84].Banerjee R, Zou CG. Redox regulation and reaction mechanism of human cystathionine-beta-synthase: a PLP-dependent hemesensor protein. Arch. Biochem. Biophys. 2005;433:144–156. doi: 10.1016/j.abb.2004.08.037. [DOI] [PubMed] [Google Scholar]
- [85].Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- [86].Hildebrandt TM, Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008;275:3352–3361. doi: 10.1111/j.1742-4658.2008.06482.x. [DOI] [PubMed] [Google Scholar]
- [87].Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid. Redox Signal. 2006;8:661–670. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- [88].Qingyou Z, Junbao D, Weijin Z, Hui Y, Chaoshu T, Chunyu Z. Impact of hydrogen sulfide on carbon monoxide/heme oxygenase pathway in the pathogenesis of hypoxic pulmonary hypertension. Biochem. Biophys. Res. Commun. 2004;317:30–37. doi: 10.1016/j.bbrc.2004.02.176. [DOI] [PubMed] [Google Scholar]
- [89].Sparatore A, Perrino E, Tazzari V, Giustarini D, Rossi R, Rossoni G, Erdman K, Schroder H, Soldato PD. Pharmacological profile of a novel H(2)S-releasing aspirin. Free Radic. Biol. Med. 2009;46:586–592. doi: 10.1016/j.freeradbiomed.2008.11.013. [DOI] [PubMed] [Google Scholar]
- [90].Zhong G, Chen F, Cheng Y, Tang C, Du J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J. Hypertens. 2003;21:1879–1885. doi: 10.1097/00004872-200310000-00015. [DOI] [PubMed] [Google Scholar]
- [91].Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- [92].Mok YY, Atan MS, Yoke Ping C, Zhong Jing W, Bhatia M, Moochhala S, Moore PK. Role of hydrogen sulphide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulphide biosynthesis. Br. J. Pharmacol. 2004;143:881–889. doi: 10.1038/sj.bjp.0706014. D. Mancardi et al. / Biochimica et Biophysica Acta 1787 (2009) 864-872 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bhatia M, Sidhapuriwala J, Moochhala SM, Moore PK. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br. J. Pharmacol. 2005;145:141–144. doi: 10.1038/sj.bjp.0706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Characterization of a novel, water-soluble hydrogen sulfidereleasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- [95].Muzaffar S, Jeremy JY, Sparatore A, Del Soldato P, Angelini GD, Shukla N. H(2)S-donating sildenafil (ACS6) inhibits superoxide formation and gp91(phox) expression in arterial endothelial cells: role of protein kinases A and G. Br. J. Pharmacol. 2008;155:984–994. doi: 10.1038/bjp.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Baskar R, Sparatore A, Del Soldato P, Moore PK. Effect of S-diclofenac, a novel hydrogen sulfide releasing derivative inhibit rat vascular smooth muscle cell proliferation. Eur. J. Pharmacol. 2008;594:1–8. doi: 10.1016/j.ejphar.2008.07.029. [DOI] [PubMed] [Google Scholar]
- [97].Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Antiinflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic. Biol. Med. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- [98].Mancardi D, Tullio F, Raimondo S, Rastaldo R, Penna C, Pagliaro P. In: Elsevier, editor. 28th Annual meeting, European Section of the International Society of Heart Research; Athens, Greece: Addendum, Elsevier; 2008. [Google Scholar]
- [99].Wu L, Yang W, Jia X, Yang G, Duridanova D, Cao K, Wang R. Pancreatic islet overproduction of H(2)S and suppressed insulin release in Zucker diabetic rats. Lab. Invest. 2009;89:59–67. doi: 10.1038/labinvest.2008.109. [DOI] [PubMed] [Google Scholar]
- [100].Wang Y, Zhao X, Jin H, Wei H, Li W, Bu D, Tang X, Ren Y, Tang C, Du J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2009;29:173–179. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- [101].Perry SF, McNeill B, Elia E, Nagpal A, Vulesevic B. Hydrogen sulfide stimulates catecholamine secretion in rainbow trout (Oncorhynchus mykiss) Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R133–R140. doi: 10.1152/ajpregu.00185.2008. [DOI] [PubMed] [Google Scholar]
- [102].Klentz RD, Fedde MR. Hydrogen sulfide: effects on avian respiratory control and intrapulmonary CO2 receptors. Respir. Physiol. 1978;32:355–367. doi: 10.1016/0034-5687(78)90123-8. [DOI] [PubMed] [Google Scholar]
- [103].Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC, Huang SH, Tan CS, Whiteman M, Lu J, Moore PK. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J. Appl. Physiol. 2007;102:261–268. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]
- [104].Hu Y, Chen X, Pan TT, Neo KL, Lee SW, Khin ES, Moore PK, Bian JS. Cardioprotection induced by hydrogen sulfide preconditioning involves activation of ERK and PI3K/Akt pathways. Pflugers Arch. 2008;455:607–616. doi: 10.1007/s00424-007-0321-4. [DOI] [PubMed] [Google Scholar]
- [105].Furne J, Springfield J, Koenig T, DeMaster E, Levitt MD. Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: a specialized function of the colonic mucosa. Biochem. Pharmacol. 2001;62:255–259. doi: 10.1016/s0006-2952(01)00657-8. [DOI] [PubMed] [Google Scholar]
- [106].Hui Y, Du J, Tang C, Bin G, Jiang H. Changes in arterial hydrogen sulfide (H(2)S) content during septic shock and endotoxin shock in rats. J. Infect. 2003;47:155–160. doi: 10.1016/s0163-4453(03)00043-4. [DOI] [PubMed] [Google Scholar]
- [107].Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- [108].Yong QC, Pan TT, Hu LF, Bian JS. Negative regulation of beta-adrenergic function by hydrogen sulphide in the rat hearts. J. Mol. Cell Cardiol. 2008;44:701–710. doi: 10.1016/j.yjmcc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- [109].Lefer DJ. A new gaseous signaling molecule emerges: cardioprotective role of hydrogen sulfide. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17907–17908. doi: 10.1073/pnas.0709010104. [DOI] [PMC free article] [PubMed] [Google Scholar]