Abstract
Background
RV dysfunction frequently occurs and independently prognosticates in left-sided HF. It is not clear which right ventricular (RV) afterload measure has the greatest impact on RV function and prognosis. We examined the determinants, prognostic role and response to treatment of pulmonary arterial capacitance (PAC, ratio of stroke volume over pulmonary pulse pressure), in relation to pulmonary vascular resistance (PVR) in heart failure (HF).
Methods and Results
We reviewed 724 consecutive patients with HF who underwent right heart catheterization between 2000 and 2005. Changes in PAC were explored in an independent cohort of 75 subjects treated for acute decompensated HF. PAC showed a strong inverse relation with PVR (r=−0.64) and wedge pressure (r=−0.73), and provides stronger prediction of significant RV failure than PVR (AUC ROC 0.74 vs 0.67 respectively, p = 0.003). During a mean follow-up of 3.2 ± 2.2 years, both lower PAC (p<0.0001) and higher PVR (p<0.0001) portend more adverse clinical events (all-cause mortality and cardiac transplantation). In multivariate analysis, PAC (but not PVR) remains an independent predictor (Hazard ratio =0.92 [95% confidence interval: 0.84–1.0, p=0.037]). Treatment of HF resulted in a decrease in PVR (270±165 to 211±88 dynes·sec·cm−5, p=0.002), a larger increase in PAC (1.65±0.64 to 2.61±1.42 ml/mmHg, p<0.0001), leading to an increase in pulmonary arterial time constant (PVR × PAC) (0.29±0.12 to 0.37±0.15 sec, p<0.0001).
Conclusions
PAC bundles the effects of PVR and left sided filling pressures on RV afterload, explaining its strong relation with RV dysfunction, poor long-term prognosis, and response to therapy.
Keywords: heart failure, hemodynamics, pulmonary arterial capacitance, pulmonary vascular resistance
There has been increasing recognition regarding the importance of right ventricular (RV) dysfunction as an independent determinant of poor prognosis and exercise intolerance in patients with left-sided HF 1–7. In particular, RV function correlates better with exercise capacity than left ventricular function 4, 8. There are several potential mechanisms that may contribute to deteriorating RV that may ultimately become dysfunctional in association with LV failure. First, it can suffer from the same cardiomyopathic process as the left ventricle. Second, decreased coronary perfusion, LV dilatation in a limited pericardial compartment (ventricular interdependence) and septal dysfunction can all alter RV systolic and diastolic properties. Finally, LV failure can directly increase RV afterload through a “passive” (pulmonary venous pressure elevation) and “reactive” (pulmonary vasoconstriction and remodeling) component, thus putting the afterload-sensitive RV at increased risk of failure even in the absence of intrinsic damage 9–10. It is however unclear whether PVR is the best reflection of RV afterload in the setting of left-sided HF.
Pulmonary arterial capacitance (PAC), defined as the ratio of stroke volume (SV) over pulmonary pulse pressure (PP), is a recently proposed determinant of RV afterload. It represents the distensibility of the pulmonary arterial tree and has shown to be a better predictor of mortality than PVR in pulmonary arterial hypertension (PAH) 11–12. Likewise,has the importance of compliance, as a predictor of adverse events, been demonstrated in the systemic circulation. In healthy individuals and in patients with PAH, it has been demonstrated that PVR and PAC are inversely related in such way that their product forms a constant 13. This even holds true during treatment for pulmonary arterial hypertension 14–16. This implies that early in the course of PAH, small increases in PVR are accompanied by large decreases in PAC, likely accounting for the superior prognostic ability of the latter. In PAH however, the primary pathology lies in the remodeling of the small arteries and arterioles whereas in heart failure the primary pathology is localized in the left ventricle with subsequent rise in filling pressures. Remodeling or constriction of small pulmonary arteries is a possibility but not a requisite. Very recently, Tedford et al. described the influence of wedge pressure and age on the relationship between PAC and PVR 17.
The aims of this study were: 1) To determine relationships between PAC and various clinical, hemodynamic and echocardiographic variables. 2) To investigate whether the inverse relationship between PAC and PVR remains valid in the setting of (left-sided) heart failure 3) To test which afterload measure (PVR or PAC) best correlates with RV failure 4) To test which afterload measure (PVR or PAC) best predicts outcomes. 5) To determine whether PAC is modifiable during treatment of ADHF.
Methods
Study Population
We reviewed clinical information for all consecutive patients aged 18 years or older with advanced chronic (> 6 months) HF who underwent right heart catheterization between January 1, 2000, and August 31, 2005, in the Cleveland Clinic cardiac catheterization laboratory. The primary indication for right heart catheterization was worsening HF symptoms or as part of a workup for heart transplant candidacy. Patients were excluded if they had a diagnosis of PAH, were on chronic inotropic drug infusions or had complex congenital heart disease.
To explore whether PAC could be modified during treatment, we examined a separate cohort of consecutive ADHF patients admitted to the Cleveland Clinic for hemodynamically-tailored therapy between December 1, 2010 and September 30, 2011. We included all patients whose pulmonary capillary wedge pressure (PCWP) decreased 10 mmHg or more during treatment upon serial measurements. The Cleveland Clinic Institutional Review Board has approved this study.
Hemodynamic assessment
Right heart catheterization was done in the catheterization laboratory at rest in the supine position. The procedure was performed by a heart failure fellow in the presence of a member of the heart failure staff cardiologist. The internal venous jugular approach was commonly used. A balloon-tipped catheter was used to obtain mean right atrial (RA) pressure, pulmonary artery (PA) systolic and diastolic pressures, as well as pulmonary capillary wedge pressure (PCWP). All measurements were obtained at end expiration at steady state with the patient in a supine position. Pulmonary capillary wedge pressure was calculated by the computer as the integrated mean. Mixed central venous blood gas was sampled from the PA. Cardiac output was measured by assumed Fick equation. PVR and systemic vascular resistance (SVR), cardiac index, stroke volume (SV), and transpulmonary gradient were calculated using the standard formulas. Systolic and diastolic blood pressures were obtained using a digital sphygmomanometer at the time of the procedure. PAC is directly related to the volume of forward flow (i.e., stroke volume) and inversely proportional to the PA pulse pressure (i.e. difference between PA systolic and diastolic pressures), according to the following equation: PAC = Stroke volume / pulse pressure and expressed in ml/mmHg. The product of resistance and capacitance (PVR × PAC) is referred to as the pulmonary arterial time constant τ.
Besides the hemodynamic variables, demographic characteristics, medical history, medical treatment, implanted devices, and echocardiographic parameters (if done within 30 days before the catheterization procedure) were also collected. Left ventricular (LV) ejection fraction was measured by biplane Simpson’s method, mitral and tricuspid regurgitation were semi-qualitatively graded by color-flow Doppler in the conventional parasternal long-axis and apical 4-chamber images: minimal: 1+ (jet area/left atrial area <10%); moderate: 2+ (jet area/left atrial area 10% to 20%); moderate-severe: 3+ (jet area/left atrial area 20% to 45%); or severe: 4+ (jet area/left atrial area > 45%). RV systolic dysfunction was visually assessed on a scale of 0 to 4, with 0 being normal and 4 being severely hypokinetic RV wall motion. RV failure was defined as grade 3 or 4 hypokinetic motion.
The duration of follow up was defined as the interval from the index right heart catheterization date to December 31, 2006. Manual chart review for all-cause mortality and transplantation was performed, and review of the Social Security Death Index confirmed the death status. All cause mortality and cardiac transplantation were combined as the pre-specified primary outcome for survival analysis.
For the second set of patients with serial assessment, two sets of hemodynamics (on admission and at time of pulmonary artery catheter removal) were used for this analysis. The hemodynamic values were documented in the chart by other cardiologists who were unaware of the present study, after careful leveling of the transducer. In addition, demographics and treatment data were abstracted from the chart.
Statistical Methods
The data are expressed as mean ± standard deviation for continuous data and as a percentage for categorical data. Comparisons are made with the use of the student t-test or Wilcoxon rank sum test. A paired t-test was used to detect changes in PVR, PAC or τ after treatment. The distribution of PAC was asymmetric and therefore expressed as median and inter-quartile range. Spearman correlation coefficients were used to assess the univariate relationships between PVR/PAC and clinical, hemodynamic and echocardiographic variables. To demonstrate the relationship between PVR and PAC, a non-linear curve was fitted according to the formula y = cte/x (hyperbola formula). Receiver operating characteristic (ROC) curves were constructed to examine the association between different afterload measures and coinciding RV failure. The Cox proportional hazards regression model was used to determine which variables were significant predictors for all cause mortality and cardiac transplantation during the mean 3.2 ± 2.2 years follow-up period. A multivariate model was constructed using well-known predictors of adverse outcomes in heart failure (age, NYHA class, RV function, cardiac index and glomerular filtration rate) in addition to one of the afterload parameters. Study population was stratified into PAC and PVR quartiles to facilitate identification of high and low risk patients by Kaplan-Meier survival analysis. Statistical significance was set at a two-tailed probability level of less than 0.05. All analyses were performed with SAS version 9.1 and JMP version 5.1 (SAS Institute Inc, Cary, NC). The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Baseline characteristics
A total of 724 patients, for whom hemodynamic data were available, underwent right heart catheterization between January 1, 2000 and August 31, 2005. The mean and median PAC was respectively 3.07 ± 2.05 mL/mmHg and 2.5 (interquartile range 1.60 – 3.96) mL/mmHg. Table 1 shows the baseline characteristics of our study cohort stratified according to those above versus below median PAC levels. Patients with reduced PAC were older, had more impaired hemodynamics and impaired cardiac performance by echocardiography (available in 650 patients, 90%).
Table 1.
Baseline patient characteristics stratified according to median pulmonary arterial capacitance (PAC) levels.
| Total Population (n=724) |
PAC > 2.5 ml/mmHg (n=362) |
PAC ≤ 2.5 ml/mmHg (n=362) |
p-value | |
|---|---|---|---|---|
| Baseline Clinical Data | ||||
| Age (years) | 55 ± 11 | 53 ± 12 | 56 ± 11 | < 0.0001 |
| Gender (% male) | 77 | 77 | 78 | 1.00 |
| Ischemic Etiology (%) | 50 | 49 | 51 | 0.43 |
| Body Mass Index (kg/m2) | 28 ± 5 | 29 ± 6 | 27 ± 5 | 0.0036 |
| Diabetes Mellitus (%) | 28 | 24 | 31 | 0.031 |
| Hemodynamic Data | ||||
| Heart Rate (bpm) | 81 ± 19 | 78 ± 18 | 84 ± 19 | <0.0001 |
| Mean arterial pressure (mmHg) | 85 ± 13 | 84 ± 12 | 87 ± 13 | 0.0053 |
| Mean RA pressure (mmHg) | 8.9 ± 5.9 | 6.6 ± 5.1 | 11.2 ± 5.7 | <0.0001 |
| Mean PA pressure (mmHg) | 30 ± 11 | 22 ± 8 | 37 ± 9 | <0.0001 |
| Pulse Pressure (mmHg) | 24 ± 10 | 17 ± 6 | 31 ± 9 | <0.0001 |
| PCWP (mmHg) | 19 ± 9 | 14 ± 7 | 25 ± 7 | <0.0001 |
| Transpulmonary gradient (mmHg) | 10.4 ± 5.9 | 8.1 ± 4 | 12.6 ± 7 | <0.0001 |
| Fick Cardiac index (L/min/m2) | 2.3 ± 0.6 | 2.6 ± 0.6 | 2.0 ± 0.5 | <0.0001 |
| Stroke Volume | 59 ± 20 | 70 ± 20 | 48 ± 14 | <0.0001 |
| PVR (dynes*s*cm−5) | 208 ± 141 | 138 ±72 | 279 ± 158 | <0.0001 |
| SVR (dynes*s*cm−5) | 1496 ± 468 | 1324 ± 394 | 1670 ± 473 | <0.0001 |
| RVSWI (mL*mmHg/m2) | 578 ± 263 | 507 ± 231 | 648 ± 275 | <0.0001 |
| Echocardiographic Data | ||||
| LV ejection fraction (%) | 19 ± 9 | 21 ± 10 | 17 ± 8 | <0.0001 |
| LV end-diastolic diameter (mm) | 6.6 ± 1.1 | 6.5 ± 1.1 | 6.7 ± 1.1 | 0.018 |
| RV function class ≥3+ (%) | 48 | 29 | 66 | <0.0001 |
| RV systolic pressure (mmHg) | 46 ± 17 | 34 ± 10 | 58 ± 13 | <0.0001 |
| Mitral regurgitation ≥3+ (%) | 33 | 23 | 43 | <0.0001 |
| Tricuspid regurgitation ≥3+ (%) | 19 | 12 | 25 | <0.0001 |
| Medications | ||||
| ACE inhibitor or ARBs (%) | 86 | 87 | 84 | 0.04 |
| Beta-blockers (%) | 69 | 72 | 66 | 0.11 |
| Loop diuretics (%) | 92 | 89 | 95 | 0.004 |
| Aldosterone antagonists (%) | 37 | 34 | 39 | 0.14 |
| Device Data | ||||
| ICD (%) | 38 | 38 | 38 | 0.93 |
| CRT-D (%) | 9 | 7 | 10 | 0.18 |
| Laboratory Data | ||||
| eGFR (mL/min/m2, n=664) | 71 ± 28 | 74 ± 28 | 68 ± 27 | 0.008 |
| BNP (pg/mL, n= 207) | 730 ± 872 | 476 ± 818 | 981 ± 845 | <0.0001 |
| Hemoglobin (g/dL, n=667) | 13.4 ± 1.8 | 13.6 ± 1.6 | 13.1 ± 1.9 | 0.0004 |
Abbreviations: PAC = pulmonary arterial capacitance; bpm = beats per minute; RA = right atrial; PA = pulmonary arterial; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; SVR = systemic vascular resistance; RVSWI = right ventricular stroke work index; LV = left ventricular; RV = right ventricular; ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; ICD = implantable cardioverter defibrillator; CRT-D = cardiac resynchronization therapy with defibrillator; eGFR = estimated glomerular filtration rate; BNP = B-type natriuretic peptide.
Correlations between PAC, PVR and other variables
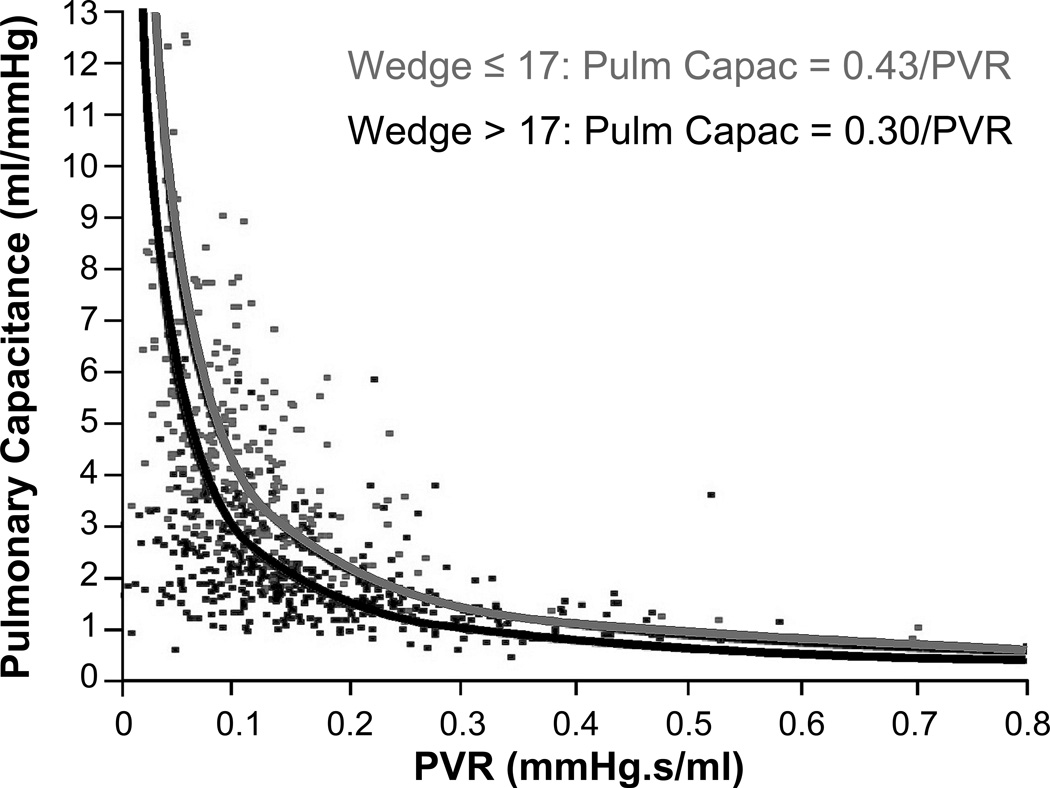
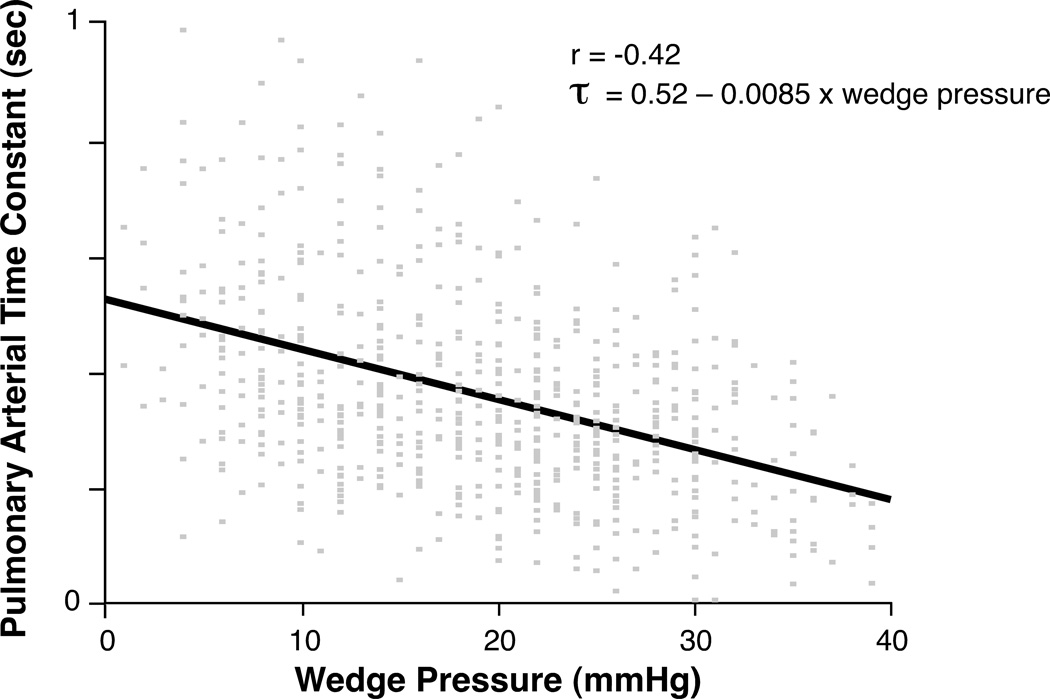
Table 2 illustrates correlations between PAC, PVR and various clinical, hemodynamic, and echocardiographic variables in our study cohort. PAC highly correlates with PCWP (r = − 0.73, p<0.0001) and mean PAP (r = − 0.81, p<0.0001). The correlation coefficients of PAC were larger (in absolute magnitude) than those of PVR when correlated with different measures of RV failure such as RV dysfunction class (r = − 0.46 vs. r = 0.32), tricuspid insufficiency (r = − 0.37 vs. r = 0.25) and right atrial pressure (r = − 0.51 vs r = 0.31). As expected, there is a strong, although not perfect, negative correlation between the 2 afterload measures PAC and PVR (r = − 0.66, p<0.0001). Figure 1 plots PAC as a function of PVR and demonstrates their hyperbolic relationship. However, the exact shape of the hyperbola, and thus the pulmonary arterial time constant τ = PVR × PAC, decreases with increasing wedge pressure (r = −0.42, p < 0.0001) (Figure 1 and 2). When dichotomized at a wedge pressure of ≤ or > 17 mmHg, the mean pulmonary arterial time constant τ decreases from 0.43 sec to 0.30 sec. Of note, the correlation between mean pulmonary pressure (mPap) and τ (r = −0.11) is much lower than the correlation between wedge pressure and τ.
Table 2.
Spearman correlation coefficients between PAC (=pulmonary arterial capacitance) and PVR (=pulmonary vascular resistance) on one hand and various clinical, hemodynamic and echocardiographic variables on the other.
| PAC | PVR | |||
|---|---|---|---|---|
| Correlation coefficient |
p-value | Correlation coefficient |
p-value | |
| Baseline Clinical Data | ||||
| Age (years) | −0.21 | <0.0001 | 0.16 | <0.0001 |
| Body Mass Index (kg/m2) | 0.20 | <0.0001 | −0.15 | <0.0001 |
| NYHA class | −0.24 | <0.0001 | 0.12 | 0.0009 |
| Hemodynamic Data | ||||
| Heart Rate (bpm) | −0.22 | <0.0001 | 0.09 | 0.01 |
| Mean arterial pressure (mmHg) | −0.09 | 0.019 | 0.05 | 0.16 |
| Mean RA pressure (mmHg) | −0.51 | <0.0001 | 0.31 | <0.0001 |
| Mean PA pressure (mmHg) | −0.81 | <0.0001 | 0.62 | <0.0001 |
| Pulse Pressure (mmHg) | −0.84 | <0.0001 | 0.53 | <0.0001 |
| PCWP (mmHg) | −0.73 | <0.0001 | 0.31 | <0.0001 |
| Transpulmonary gradient (mmHg) | −0.44 | <0.0001 | 0.88 | <0.0001 |
| Fick Cardiac index (L/min/m2) | 0.55 | <0.0001 | −0.40 | <0.0001 |
| Stroke Volume | 0.67 | <0.0001 | −0.45 | <0.0001 |
| PAC (ml/mmHg) | −0.64 | <0.0001 | ||
| PVR (dynes*s*cm−5) | −0.64 | <0.0001 | ||
| SVR (dynes*s*cm−5) | −0.49 | <0.0001 | 0.42 | <0.0001 |
| RVSWI (mL*mmHg/m2) | −0.25 | <0.0001 | 0.30 | <0.0001 |
| Echocardiographic Data | ||||
| LV ejection fraction (%) | 0.29 | <0.0001 | −0.19 | <0.0001 |
| LV end-diastolic diameter (mm) | −0.10 | 0.014 | 0.04 | 0.31 |
| RV systolic dysfunction class | −0.46 | <0.0001 | 0.32 | <0.0001 |
| Mitral regurgitation severity | −0.19 | <0.0001 | 0.19 | <0.0001 |
| Tricuspid regurgitation severity | −0.37 | <0.0001 | 0.25 | <0.0001 |
| Laboratory Data | ||||
| eGFR (mL/min/m2, n=664) | 0.17 | <0.0001 | −0.12 | 0.002 |
| BNP (pg/mL, n= 207) | −0.57 | <0.0001 | 0.35 | <0.0001 |
| Hemoglobin (g/dL, n=667) | 0.13 | 0.0007 | −0.03 | 0.39 |
Abbreviations as in Table 1.
Figure 1. Influence of wedge pressure on the hyperbolic relationship between PAC and PVR.
Pulmonary arterial capacitance (PAC) and pulmonary vascular resistance (PVR) relate in such way that their product forms a constant: PAC × PVR = cte which equals PAC = cte/PVR, the formula of a hyperbola. The value of the cte represents the pulmonary arterial time constant. In the total cohort, the cte which best fits the datapoints is 0.35. However, the value of the pulmonary arterial time constant and so the shape of the hyperbola, depends on wedge pressure. The higher the wedge pressure, the lower the time constant as illustrated (H0 2 hyperbola’s are similar: p < 0.0001)
Figure 2. Pulmonary arterial time constant as a function of wedge pressure.
The pulmonary arterial time constant τ (sec) is the product of the pulmonary vascular resistance (PVR), expressed in mmHg × sec/ml, times pulmonary arterial capacitance expressed in ml/mmHg. Spearman’s correlation coefficient between wedge pressure and time constant is −0.42. The pulmonary arterial time constant is predicted by the formula τ = 0.52 – 0.0085 × wedge pressure.
Association of RV failure with different afterload measures
RV failure, as defined by echocardiographic dysfunction grade 3 or more, was very frequent (312/650 patients (48%) with available echocardiograms). The association of PAC with RV failure (AUC ROC = 0.74) was superior to the association of PVR (AUC ROC = 0.67, p = 0.003) with RV failure. In comparison, the AUC ROC for wedge pressure is 0.71 (p = 0.11 with PAC). Patients with PAC below the median (< 2.5 ml/mmHg) and PVR above the median (> 170 dynes∙sec∙cm−5 = 2.1 WU) have an Odds ratio (OR) of 6.45 (4.35 – 9.67, p<0.0001) of having RV failure compared to patients with high PAC (above median) and low PVR (below median). If PVR was high with preserved PAC (above median), the OR was 1.94 (1.14 – 3.28, p=0.02). However, when PAC was low with preserved PVR (below median), the OR was 4.45 (2.58 – 7.80, p<0.0001) again underscoring the importance of PAC.
Predictive capacity of PAC and PVR for adverse events
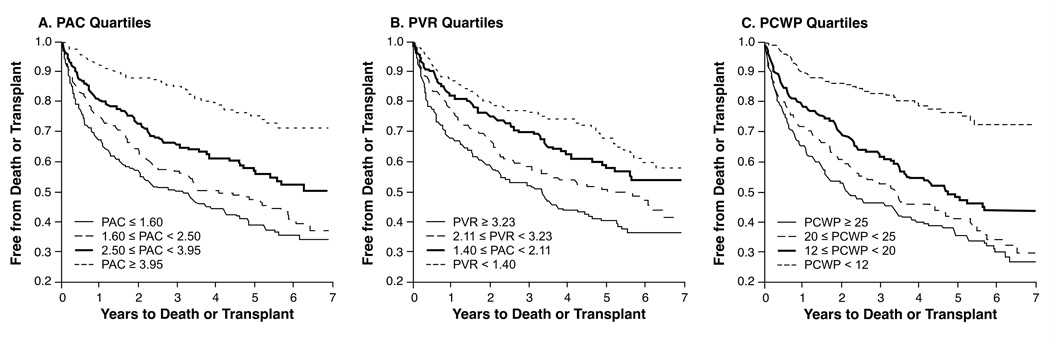
The follow-up period ended at December 31, 2006, after a median follow-up of a little more than 3 years (1130 days [IQR 382–1883 days]). A total of 224 deaths (31%) and 163 transplants (23%) had occurred. Overall, decreasing quartiles of PAC, PVR, and PCWP were all associated with increasing rates of death or transplantation (Figure 3, log-rank p<0.0001). In univariate Cox proportional hazard modeling, PAC was a stronger predictor of all-cause mortality or transplantation than PVR (C-statistic 0.69 vs. 0.64, p = 0.02), while the C-statistic for PCWP was 0.67 (p = 0.21 with PAC). In multivariable analysis, PAC (but not PVR) remained an independent predictor of adverse clinical events even after adjusting for various clinical, hemodynamic, and echocardiographic variables including age, NYHA class, Fick CI, RV function and eGFR (Table 3). Fick cardiac index, eGFR, and RV systolic function however, were the strongest predictors of death or transplantation.
Figure 3. Clinical outcomes according to pulmonary arterial capacitance (PAC) and pulmonary vascular resistance (PVR) quartiles.
A. According to PAC quartiles. B. According to PVR quartiles. C. According to PCWP quartiles. Log-rank < 0.0001 for both.
Table 3.
Multivariate Cox proportional hazard model for death or transplantation.
| predictors | Hazard ratio* for death or transplant |
p-value |
|---|---|---|
| NYHA (class) | 1.09 (0.85–1.40) | 0.50 |
| Age (years) | 0.97 (0.92–1.16) | 0.53 |
| Fick (L/min/m2) | 0.56 (0.44–0.72) | <0.0001 |
| PAC (ml/mmHg) | 0.92 (0.84–1.0) | 0.037 |
| RV function (class) | 1.18 (1.06–1.31) | 0.0019 |
| eGFR (ml/min/1.73m2) | 0.90 (0.85–0.94) | <0.0001 |
Abbreviations as in Table 1.
Hazard Ratio per 1 increment for NYHA class, Fick index, PAC and RV function. Per 10 increment for age and eGFR.
When PAC was exchanged for PVR in this model. The p-value of PVR was only 0.69.
The influence of treatment on PAC, PVR and τ
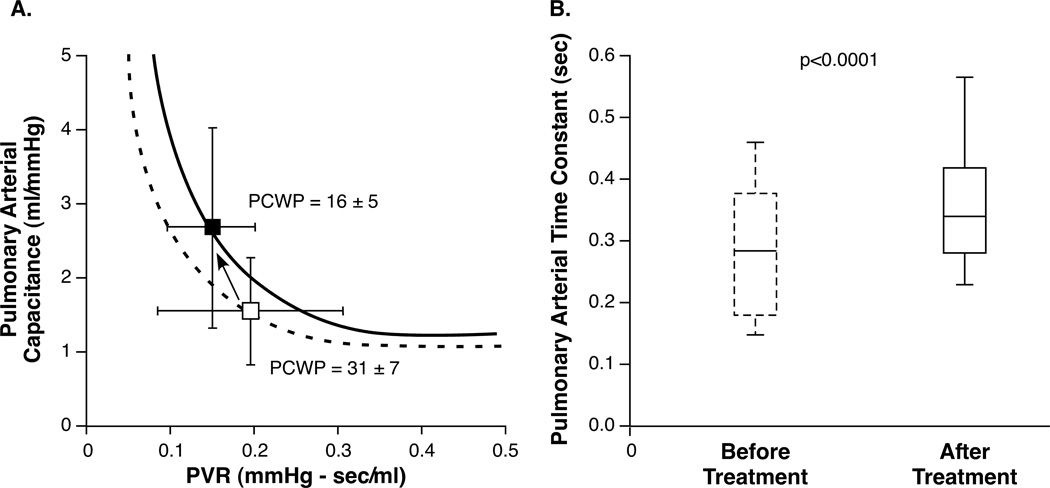
A total of 208 patients were admitted for hemodynamically tailored therapy between December 1, 2010 and September 30, 2011. 180 patients had a PCWP documented at baseline and at time of catheter removal. 75 of those had a decrease in PCWP > 10 mmHg. Table 4 shows demographic characteristics, hemodynamic variables and received treatment during admission for this cohort. Figure 4 demonstrates how during treatment, PVR decreases (0.20 ± 0.12 to 0.16 ± 0.07 mmHg∙sec/ml, p = 0.002) (270±165 to 211±88 dynes·sec·cm−5) and how PAC increases even more (1.65 ± 0.64 to 2.61 ± 1.42 ml/mmHg, p < 0.0001) resulting in a higher pulmonary arterial time constant τ in most patients ( 0.29 ± 0.12 to 0.37 ± 0.15, p < 0.0001).
Table 4.
Demographics, hemodynamics and medical treatment of 75 patients admitted with ADHF.
| Treatment Cohort (n=75) |
|
|---|---|
| Baseline Clinical Data | |
| Age (years) | 57 ± 13 |
| Gender (% male) | 78 |
| Ischemic Etiology (%) | 47 |
| Body Mass Index (kg/m2) | 29 ± 6 |
| Diabetes Mellitus (%) | 44 |
| Left Ventricular EF (%) | 24 ± 13 |
| Atrial Fibrillation (%) | 28 |
| Baseline Hemodynamic Data | |
| Heart Rate Baseline (bpm) | 87 ± 18 |
| Mean arterial pressure Baseline (mmHg) | 82 ± 13 |
| Mean RA pressure Baseline (mmHg) | 18 ± 6 |
| Mean PA pressure Baseline (mmHg) | 43 ± 9 |
| PCWP Baseline (mmHg) | 31 ± 7 |
| Fick Cardiac index Baseline (L/min/m2) | 1.84 ± 0.64 |
| Follow-up Hemodynamic Data | |
| Heart Rate FU (bpm) | 86 ± 15 |
| Mean arterial pressure FU (mmHg) | 75 ± 8 |
| Mean RA pressure FU (mmHg) | 9 ± 9 |
| Mean PA pressure FU (mmHg) | 29 ± 6 |
| PCWP FU (mmHg) | 16 ± 5 |
| Fick Cardiac index FU (L/min/m2) | 2.67 ± 0.83 |
| Medications | |
| ACE inhibitor or ARBs (%) | 72 |
| Beta-blockers (%) | 72 |
| Loop diuretics (%) | 99 |
| Aldosterone antagonists (%) | 56 |
| Nitroprusside (%) | 75 |
| Hydralazine (%) | 74 |
| Isosorbide Dinitrate (%) | 66 |
| Milrinone (%) | 28 |
| Dobutamine (%) | 24 |
| IABP (%) | 7 |
| Device Data | |
| ICD (%) | 59 |
| CRT-D (%) | 21 |
| Laboratory Data | |
| Creatinine (mg/dl) | 1.52 ± 0.69 |
Abbreviations as in Table 1.
FU = follow-up (time of catheter removal)
Figure 4. PAC, PVR and τ before and after treatment for ADHF.
A. PVR and PAC before (open box) and after treatment (closed box). Because PCWP (pulmonary capillary wedge pressure) decreases, the shift in PVR and PAC does not completely follow the original hyperbola. B. Pulmonary arterial time constant increases in patients whose PCWP decreases (> 10 mmHg) during treatment.
Discussion
The key finding of this study is that PAC, an integrated measure of RV afterload, may be superior to PVR in explaining RV failure and in predicting adverse outcomes in the setting of left-sided heart failure. The reason for this resides in the quality of PAC to combine the effects of PVR and pulmonary capillary wedge pressure on RV afterload. Furthermore, treatment with intensive medical therapy is associated with a favorable shift in PAC.
The “afterload” concept is daily used by clinicians while treating patients with left-, right- or biventricular failure. Although conceptualized as the load the ventricle experiences during contraction, afterload is much more difficult to measure and quantify. Pressures do not account for flow and because the heart is a pulsatile, and not a continuous-flow pump, resistance is not sufficient to describe afterload. The most widely accepted model to describe the hydraulic afterload is the so-called “3-element windkessel model” which combines the resistance “R” of the small arteries and arterioles with the elastic properties (compliance or capacitance “C”) of the whole arterial system and with the characteristic impedance “Z” of the blood and proximal artery 18–19. PVR bundles R and Z and is the most often used measure of RV afterload in clinical practice. The attraction of this model lies in the fact that the parameters are fairly easy to measure, have physiologic meaning and are straightforward to interpret.
The capacitance or compliance of the pulmonary circulation is dependent on vessel wall elasticity but also on vessel wall diameter and the latter in an exponential way. In other words, when the diameter of the pulmonary vessels increases, compliance will decrease to a much larger aspect. In addition and different from the systemic circulation, compliance is distributed over the entire pulmonary arterial bed owing to the large number of peripheral vessels. The latter 2 particularities form the base of the constant product of PVR and PAC (pulmonary arterial time constant τ) between individuals and during treatment observed in PAH patients 14. Indeed, whenever PVR increases, elevations in intravascular pressure will result, leading to stiffer arteries and reduced compliance. Even so, when part of the pulmonary arterial tree is lost due to an embolus, resistance will increase and compliance will decrease 20. This interdependence of PVR and PAC is different from the systemic circulation where resistance and compliance are anatomically more separated and can evolve independent from each other (e.g. systolic hypertension of the elderly due to a decrease in compliance of the proximal arteries).
In contrast to PAH, where an increase in PVR constitutes the primary pathology, elevations in PVR are only optional in left-sided heart failure and the result of either vasoconstriction or smooth muscle proliferation secondary to elevated left-sided filling pressures (“backward failure”). Also in contrast to PAH, wedge pressure is highly variable within and between patients, changes during treatment and is a major determinant of pulmonary intravascular pressure and consequently of PAC. Because increases in wedge pressure lower PAC but do not necessarily increase PVR, the pulmonary arterial time constant decreases with increasing wedge pressure. The hyperbolic relationship between PVR and PAC is still recognized, but the exact position is dependent on wedge pressure (Figure 1).
We hypothesize that the observed superior prognostic ability of PAC over PVR is explained by the representation of two hemodynamic effects (those of PVR and wedge pressure) in one measure, namely PAC. This adds to the classic explanation in PAH, where PAC is believed to be more sensitive especially early in the disease process when small increases in PVR result in large decreases in PAC 11, 14. Because the heart failure population is very heterogeneous, the absolute strength of outcome prediction of PAC is less evident than in the PAH population where patients mainly die from RV failure.
The overall better performance of PAC does not alter the specific importance of increased PVR in a subgroup of patients. It was recently demonstrated that patients with “reactive” or so-called “out-of-proportion pulmonary hypertension” (defined as mPAP > 25 mmHg, PCWP > 15 mmHg and PVR > 3WU) have a particularly worse prognosis 10. Moreover, the “passive” (increase in wedge pressure) component to RV afterload is more easily correctable by medical treatment or even heart transplantation than the “reactive” component. PVR will keep playing an important role, for example in determining eligibility for heart transplantation.
The above discussion concentrated on how PAC is influenced by increased vessel wall diameter either due to increased PVR or wedge pressure. The question remains how the quality of the vessel wall itself influences PAC and whether inter-individual variations are clinically meaningful as is the case in the systemic circulation. The remarkable consistency and similarity of τ in patients with PAH made authors believe that there is little or no structural change in the elastic properties of the pulmonary arterial circulation 13. Very recently, Tedford et al. found a small but significant influence of age on τ 17. There is indeed some evidence that structural changes in the pulmonary arterial circulation occur with increasing age 21–22. Similar to our study, they found a very significant influence of PCWP on τ, a relationship described by the formula τ = 0.46 – 0.0063 × wedge pressure which is remarkably similar than the formula found in the present study (τ = 0.52 – 0.0085 × wedge pressure). However, in our study, there is still considerable variation in τ for every given wedge pressure (see figure 2) which might be explained in part by inaccurate measurements (e.g. catheter wip), the effect of large v-waves on the wedge pressure (calculated as the integrated mean) and age, as discussed. In addition it remains possible that other unappreciated factors influence the inverse PVR – PAC relationship. The finding that PCWP augments the pulsatile load on the “afterload sensitive” right ventricle and that this translates in more RV failure and ultimately worse outcomes may serve as an extra argument to screen for and treat elevated filling pressures.
Study Limitations
This study has several limitations. First, it is a retrospective study with well-known inherent limitations. Second, we arbitrarily defined a reasonable time frame with regards to clinical, laboratory, and echocardiographic data in relation to the catheterization procedure. Third, there are currently no “gold standard” for RV dysfunction, and in our paper this was adjudicated by a very crude visual echocardiographic assessment, while other commonly described echocardiographic estimates of RV function (such as tricuspid annular plane systolic excursion) were not available. However, if incorrectly done it would likely attenuate the association with RV afterload measures and therefore weaken our results. Moreover, despite the rough nature of this assessment, RV failure remained one of the strongest prognostic factors, even after multivariate adjustment. We were also unable to determine if treatment that do not directly alter pulmonary vascular tone have any effect on PAC since treatment goals of reducing PCWP were achieved by a variety of medications and procedures. Finally, the hemodynamic values were obtained from the electronic medical record as original tracings were not available for retrospective analyses, and oxygen consumption rates were assumed rather than measured. Nevertheless, we believe that the high standard of care in a catheterization laboratory run by cardiologists specialized in heart failure, should limit misinterpretation or inaccuracy in measurements.
Conclusion
We conclude that in heart failure with reduced ejection fraction, RV afterload is best represented by PAC because this measure combines a hyperbolic inverse relationship with PVR with a linear inverse relation with wedge pressure. These properties account for its superior predictive ability. PAC is an independent predictor of all-cause mortality and heart transplantation in this patient group and is modifiable by therapy. Further prospective studies are needed to determine the practical utility and implications of this finding.
Clinical Perspectives.
In heart failure with reduced ejection fraction, right ventricular afterload is difficult to quantify and appreciate. Pulmonary arterial capacitance (PAC) is an integrated measure of right ventricular afterload that combines a hyperbolic inverse relationship with pulmonary vascular resistance with a linear inverse relation with wedge pressure. In our large series of patients with advanced heart failure, we observed incremental prognostic value of PAC reduction with adverse long-term outcomes, and improvement in PAC following vasodilator therapy in decompensated states. These findings validated the clinical relevance of PAC, and support the evolving concept of “reserve” in right ventricular forward flow that may provide a more integrated view of ventricular interdependence at the bedside. Improvement in PAC with vasodilator therapy further illustrates the importance of maintaining or improving PAC rather than simply focusing on lowering either pressure or resistance.
Sources of Funding
Dr. Dupont is supported by a research grant from the Belgian American Educational Foundation (BAEF). Dr. Tang is supported by National Institutes of Health grants R01HL103931, P20HL113452 and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439-06).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Karatasakis GT, Karagounis LA, Kalyvas PA, Manginas A, Athanassopoulos GD, Aggelakas SA, Cokkinos DV. Prognostic significance of echocardiographically estimated right ventricular shortening in advanced heart failure. Am J Cardiol. 1998;82:329–334. doi: 10.1016/s0002-9149(98)00344-0. [DOI] [PubMed] [Google Scholar]
- 2.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: Report of a national heart, lung, and blood institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 3.Polak JF, Holman BL, Wynne J, Colucci WS. Right ventricular ejection fraction: An indicator of increased mortality in patients with congestive heart failure associated with coronary artery disease. J Am Coll Cardiol. 1983;2:217–224. doi: 10.1016/s0735-1097(83)80156-9. [DOI] [PubMed] [Google Scholar]
- 4.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–1153. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 5.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 6.Juilliere Y, Barbier G, Feldmann L, Grentzinger A, Danchin N, Cherrier F. Additional predictive value of both left and right ventricular ejection fractions on long-term survival in idiopathic dilated cardiomyopathy. Eur Heart J. 1997;18:276–280. doi: 10.1093/oxfordjournals.eurheartj.a015231. [DOI] [PubMed] [Google Scholar]
- 7.de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, Lablanche JM. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–954. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 8.Baker BJ, Wilen MM, Boyd CM, Dinh H, Franciosa JA. Relation of right ventricular ejection fraction to exercise capacity in chronic left ventricular failure. Am J Cardiol. 1984;54:596–599. doi: 10.1016/0002-9149(84)90256-x. [DOI] [PubMed] [Google Scholar]
- 9.Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol. 1999;34:1802–1806. doi: 10.1016/s0735-1097(99)00408-8. [DOI] [PubMed] [Google Scholar]
- 10.Aronson D, Eitan A, Dragu R, Burger AJ. Relationship between reactive pulmonary hypertension and mortality in patients with acute decompensated heart failure. Circ Heart Fail. 2011;4:644–650. doi: 10.1161/CIRCHEARTFAILURE.110.960864. [DOI] [PubMed] [Google Scholar]
- 11.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol. 2006;47:799–803. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 12.Mahapatra S, Nishimura RA, Oh JK, McGoon MD. The prognostic value of pulmonary vascular capacitance determined by doppler echocardiography in patients with pulmonary arterial hypertension. J Am Soc Echocardiogr. 2006;19:1045–1050. doi: 10.1016/j.echo.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Lankhaar JW, Westerhof N, Faes TJ, Marques KM, Marcus JT, Postmus PE, Vonk-Noordegraaf A. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2006;291:H1731–H1737. doi: 10.1152/ajpheart.00336.2006. [DOI] [PubMed] [Google Scholar]
- 14.Lankhaar JW, Westerhof N, Faes TJ, Gan CT, Marques KM, Boonstra A, van den Berg FG, Postmus PE, Vonk-Noordegraaf A. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur Heart J. 2008;29:1688–1695. doi: 10.1093/eurheartj/ehn103. [DOI] [PubMed] [Google Scholar]
- 15.Bonderman D, Martischnig AM, Vonbank K, Nikfardjam M, Meyer B, Heinz G, Klepetko W, Naeije R, Lang IM. Right ventricular load at exercise is a cause of persistent exercise limitation in patients with normal resting pulmonary vascular resistance after pulmonary endarterectomy. Chest. 2011;139:122–127. doi: 10.1378/chest.10-0348. [DOI] [PubMed] [Google Scholar]
- 16.de Perrot M, McRae K, Shargall Y, Thenganatt J, Moric J, Mak S, Granton JT. Early postoperative pulmonary vascular compliance predicts outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Chest. 2011;140:34–41. doi: 10.1378/chest.10-1263. [DOI] [PubMed] [Google Scholar]
- 17.Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ, Kass DA. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation. 2012;125:289–297. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westerhof N, Elzinga G, Sipkema P. An artificial arterial system for pumping hearts. J Appl Physiol. 1971;31:776–781. doi: 10.1152/jappl.1971.31.5.776. [DOI] [PubMed] [Google Scholar]
- 19.Westerhof N, Lankhaar JW, Westerhof BE. The arterial windkessel. Med Biol Eng Comput. 2009;47:131–141. doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 20.Saouti N, Westerhof N, Helderman F, Marcus JT, Stergiopulos N, Westerhof BE, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Rc time constant of single lung equals that of both lungs together: A study in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;297:H2154–H2160. doi: 10.1152/ajpheart.00694.2009. [DOI] [PubMed] [Google Scholar]
- 21.Mackay EH, Banks J, Sykes B, Lee G. Structural basis for the changing physical properties of human pulmonary vessels with age. Thorax. 1978;33:335–344. doi: 10.1136/thx.33.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris P, Heath D, Apostolopoulos A. Extensibility of the human pulmonary trunk. Br Heart J. 1965;27:651–659. doi: 10.1136/hrt.27.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]