Summary
The transcription factor Sox2 maintains the pluripotency of early embryonic cells and regulates the formation of several epithelia during fetal development. Whether Sox2 continues to play a role in adult tissues remains largely unknown. We here show that Sox2 marks adult cells in several epithelial tissues where its expression has not previously been characterized, including the stomach, cervix, anus, testes, lens and multiple glands. Genetic lineage tracing and transplantation experiments demonstrate that Sox2-expressing cells continuously give rise to mature cell types within these tissues, documenting their self-renewal and differentiation potentials. Consistent with these findings, ablation of Sox2+ cells in mice results in a disruption of epithelial tissue homeostasis and lethality. Developmental fate mapping reveals that Sox2+ adult stem cells originate from fetal Sox2+ tissue progenitors. Thus, our results identify Sox2 expression in numerous adult ectodermal and endodermal stem cell compartments, which are critical for normal tissue regeneration and survival.
Introduction
It remains an unresolved question whether the self-renewal of pluripotent, fetal and adult stem cells is controlled by the same or by different molecules. Recent findings show that expression of the polycomb group protein Bmi-1 (Sangiorgi and Capecchi, 2008, 2009), the HMG box transcription factor Sox9 (Furuyama et al.), the telomere subunit Tert (Breault et al., 2008; Montgomery et al.) and the G-protein coupled receptor Lgr5 (Barker et al.; Barker et al., 2007; Jaks et al., 2008) mark various types of adult stem cells. While these observations indicate molecular commonalities among different somatic stem cells, these regulators are mostly absent in pluripotent stem cells. Another previous study documented that depletion of the zinc finger transcription factor Zfx1 (Galan-Caridad et al., 2007) affects the self-renewal of both embryonic and hematopoietic stem cells, suggesting common mechanisms between pluripotent stem cells and this mesodermal stem cell type. However, Zfx1 is also expressed in differentiated cells and a role in endodermal and ectodermal stem cell compartments has not yet been described. Lastly, several reports have claimed that the pluripotency genes Oct4 and Nanog are expressed in adult stem cells but analyses of reporter animals and genetic knockout studies failed to confirm these findings (Lengner et al., 2007). In contrast, accumulating evidence suggests that Sox2, which maintains pluripotency in concert with Oct4 and Nanog, may play additional roles in fetal and adult progenitors.
Sox2 belongs to a large family of SRY-related HMG box transcription factors that are important during development and cellular differentiation (Lefebvre et al., 2007). Sox2 is initially expressed in the inner cell mass (ICM) and extraembryonic ectoderm of blastocysts (Avilion et al., 2003). Embryos deficient for Sox2 lack a pluripotent ICM and fail early in development, while deletion of Sox2 in embryonic stem cells (ESCs) results in their inappropriate differentiation into trophectoderm-like cells (Masui et al., 2007). Forced expression of Sox2, in combination with Oct4, Klf4 and c-Myc, endows somatic cells with pluripotency, giving rise to induced pluripotent stem cells (Takahashi and Yamanaka, 2006). Collectively, these results underline the importance of Sox2 in both the establishment and maintenance of pluripotent stem cells.
Upon exit from pluripotency, Sox2 signaling is critical for the formation of several endodermal and ectodermal tissues during fetal development including the nervous system (Bylund et al., 2003; Ellis et al., 2004; Graham et al., 2003), lens epithelium (Kamachi et al., 1998), anterior foregut endoderm (Que et al., 2007) and its derivatives as well as sensory cells of the taste bud (Okubo et al., 2006), inner ear (Kiernan et al., 2005) and retina (Mansukhani et al., 2005; Taranova et al., 2006). More recently, Sox2 expression has also been reported in some adult tissues such as progenitors of the brain (Brazel et al., 2005; Ellis et al., 2004) and retina (Taranova et al., 2006), trachea (Que et al., 2009), tongue epithelium (Okubo et al., 2009) and dermal papilla of the hair follicle (Driskell et al., 2009), as well as in putative progenitors of pituitary gland (Fauquier et al., 2008). Importantly, a systematic survey of Sox2 expression in adult tissues has not yet been performed. Furthermore, except for neural stem cells (Suh et al., 2007), genetic fate mapping data to assess the lineage relationship between fetal and adult Sox2+ cells as well as that of Sox2+ adult cells and their putative progeny are missing.
In this study, we have developed mouse models to (i) systematically evaluate the Sox2 expression pattern, (ii) determine the lineage relationship of Sox2+ cells, and (iii) assess the functional importance of Sox2-expressing cells in adult tissues.
Results
Widespread Sox2 expression in adult epithelial tissues
In order to systematically evaluate the expression patterns of Sox2 in adult tissues, we generated Sox2-GFP indicator mice from embryonic stem cells (ESCs) targeted with a previously characterized knock-in construct (Ellis et al., 2004) (Figure 1A). Consistent with other reports (Avilion et al., 2003; Ellis et al., 2004), we detected strong GFP expression in blastocysts, neural progenitor cells (NPCs) and ESC cultures (Figure 1B), indicating that the knock-in allele faithfully reports Sox2 expression in previously documented Sox2+ cell types.
Figure 1. Widespread Sox2-GFP expression in epithelial tissues of mice.
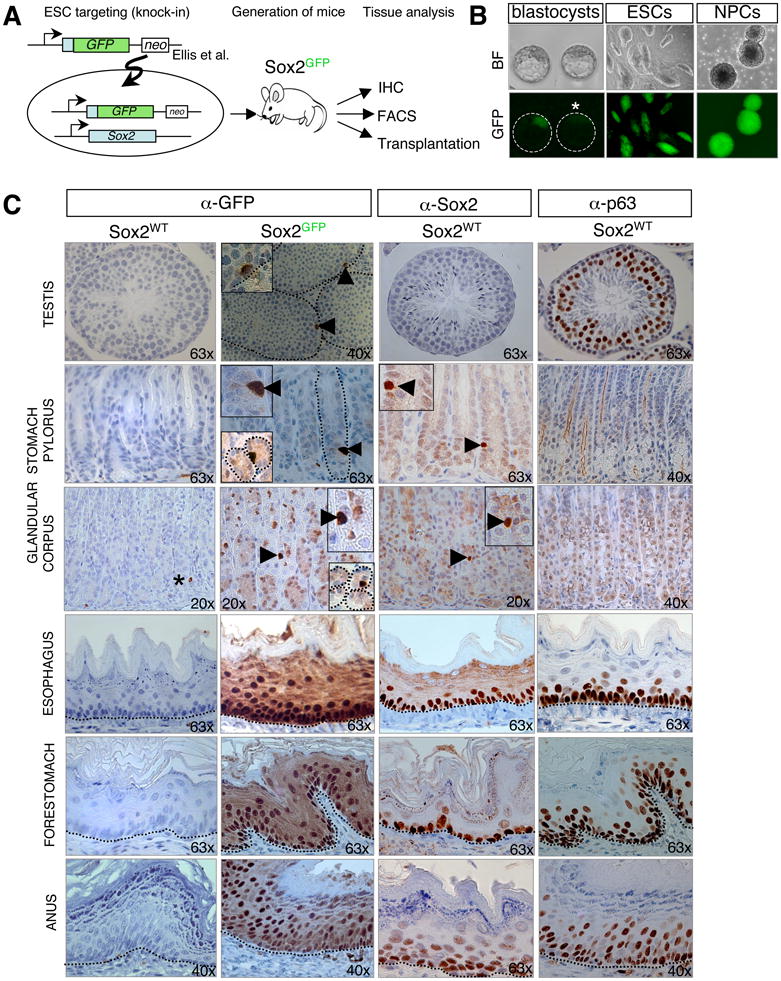
(A) Experimental outline for the generation and analysis of Sox2GFP reporter mice. IHC, immunohistochemistry; FACS, fluorescence-activated cell sorting. (B) Sox2-GFP expression in targeted embryonic stem cells (ESCs), the inner cell mass of blastocysts (asterisk marks GFP-negative control blastocyst), and neonatal neural progenitor cells (NPCs). BF, brightfield. (C) IHC for GFP, Sox2 and the basal cell marker p63 on paraffin-embedded sections of adult testis, pylorus and corpus of the glandular stomach, esophagus, forestomach, and anus of wild type (Sox2WT) and Sox2GFP mice. Inserts also show cross-sections of corpus and pylorus. Asterisk marks unspecific antibody staining of blood vessels. Dashed lines outline gastric units (glandular stomach) or the basement membrane (testes, esophagus, forestomach, anus). Original magnifications as indicated. See also Suppl. Figure 1 and 2.
We next assessed GFP expression in adult tissues of Sox2-GFP mice by performing immunohistochemistry (IHC) on isolated, paraffin-embedded tissues. In agreement with previous observations, Sox2-GFP signal was detected in the subventricular zone of the brain, neural retina, trachea and bronchiolar epithelium of the lungs, tongue and dermal papillae of the hair follicles (Suppl. Figure 1 and data not shown). In addition, we identified here for the first time Sox2-GFP expression in the seminiferous and lens epithelium, glandular stomach as well as in squamous epithelia lining the esophagus, forestomach, anus and cervix (Figure 1C and Suppl. Figures 1, 3D).
In accordance with the observation that Sox2-GFP expression is enriched within phenotypically immature basal cells in adult squamous epithelia, its expression patterns resemble those of the basal progenitor cell marker p63 in these tissues (Yang et al., 1998) (Figure 1C and Suppl. Figures 1, 3D). Sox2-GFP signal in the testes was confined to individual and rare cells adjacent to the basement membrane, consistent with the previously defined location of stem cells (Yoshida) (Figure 1C). Single Sox2+ cells were also detected in gastric units of the glandular stomach, composed of the so-called corpus and pylorus compartments that have been suggested to contain stem cells (Bjerknes and Cheng, 2002; Karam and Leblond, 1993) (Figure 1C). Moreover, we identified rare Sox2+ cells within the mitotically active epithelial layer of the lens (Suppl. Figure 1), which constitutes a stem cell niche, in ganglion cells adjacent to kidney, prostate, uterus and oral cavity and in salivary, tracheal and cervix-associated glands where it may mark stem or progenitor cells (Suppl. Figure 2A, B).
No GFP signal was seen in liver, kidney, heart, small intestine, colon, pancreas, or bone marrow (data not shown). The specificity of the Sox2-GFP IHC staining was confirmed by FACS analysis (Suppl. Figure 2C) and IHC with a Sox2-specific antibody (Figure 1C and Suppl. Figure 1). While FACS analysis showed GFP-positive cells in all analyzed Sox2-GFP+ tissues, IHC for Sox2 failed to yield a signal in testes. This may indicate that Sox2 protein levels are lower in testes compared with other tissues or, alternatively, that Sox2 mRNA is not translated into protein. In support of the latter possibility, explanted germline stem cells have recently been shown to produce Sox2 mRNA but no protein (Imamura et al., 2006). The expanded expression domain of Sox2- GFP signal compared with Sox2 signal across squamous epithelia (Figure 1C) is likely caused by the long half-life of GFP. Together, our results show that Sox2 exhibits widespread expression in immature-appearing cells of multiple self-renewing epithelial tissues where it has not been previously detected in the adult.
Sox2 marks bona fide adult stem cells
To test whether Sox2-expressing cells contain in fact adult stem cells, we devised a genetic lineage tracing approach. Briefly, we engineered ESCs to harbor a tamoxifen-inducible Cre allele (CreER-T2) in the endogenous Sox2 locus (Figure 2A, B). Additional targeting of a ROSA26-lox-STOP-lox (lsl)-EYFP reporter allele (Srinivas et al., 2001) into these cells and subsequent exposure to the tamoxifen analog 4-OHT gave rise to around 20% EYFP+ cells in treated but not in untreated cells, thus validating the inducibility and specificity of the system (Figure 2C). Mice were generated from these ESCs to establish a stable colony of Sox2-CreER; ROSA26-lsl-EYFP animals for further analysis. Untreated Sox2-CreER; ROSA26-lsl-EYFP animals and mice treated with solvent (corn oil) alone showed extremely rare and thus negligible spontaneous labeling events in lung, stomach and brain (data not shown).
Figure 2. Genetic lineage tracing identifies Sox2+ stem cells in testes, glandular stomach and lens.
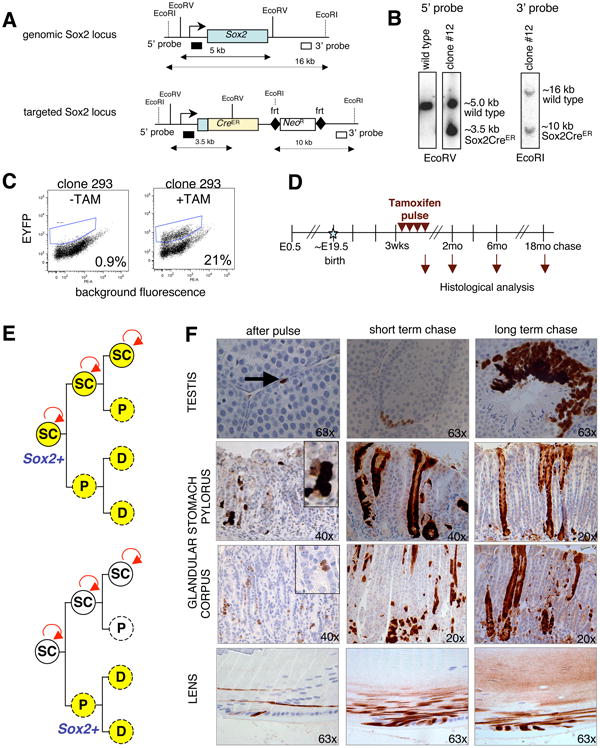
(A) Targeting strategy for generating Sox2-CreER ESCs and mice. Restriction sites, southern blot analysis probes and expected restriction fragment lengths are indicated. (B) Southern blot analyses with 5′ and 3′ probes, as indicated in (A), to verify correct targeting. (C) ESCs carrying the Sox2-CreER and ROSA26-lsl-EYFP alleles were analyzed by FACS for EYFP expression 2 weeks after exposure to 4-OHT. (D) Experimental outline for pulse-chase experiment. Tamoxifen (TAM) was given intraperitoneally on 4 consecutive days to adult Sox2-CreER, ROSA26-lsl-EYFP mice (“pulse”). Animals were sacrificed at the indicated time points and tissues analyzed by FACS and IHC for EYFP. E, embryonic day; wks, weeks; mo, months. (E) Possible outcomes of lineage tracing assay. If Sox2-expressing cells are stem cells (SC), they should activate the EYFP reporter upon TAM administration and give rise to permanently labeled Sox2+ stem cells as well as differentiated progeny (top image). In contrast, Sox2+ progenitors (P) and derivative differentiated cells (D) would only be transiently labeled (indicated by dashed circle, bottom image). (F) IHC for EYFP on lineage tracing samples. Note individual cells or small patches of EYFP+ cells immediately after the pulse, which gradually expand over the subsequent months to span the entire epithelium. Short-term chase reflects 1mo (testes) and 2mo (stomach, lens), respectively. The long-term chase periods shown are 6mo for testes and lens, and 15mo for stomach. Original magnifications as indicated. See also Suppl. Figure 3.
In order to permanently label Sox2-expressing cells and their progeny in vivo, we initially injected tamoxifen intraperitoneally on 4 consecutive days into a cohort of 3-6 week-old Sox2-CreER; ROSA26-lsl-EYFP animals (Figure 2D). Tissues were isolated immediately after the treatment (“pulse”) or at different time points thereafter (“chase”). If Sox2 expression marks stem cells, we would expect to find permanent labeling of both the putative Sox2+ stem cells as well as differentiated progeny (Figure 2E). However, if Sox2+ cells are short-lived progenitors, we expect to see transient labeling of cells, which should disappear over time due to their replenishment by unlabeled stem cells.
We first followed the fate of Sox2-expressing cells in testes since the differentiation structure of the seminiferous epithelium is relatively simple with single spermatogonia (As), located at the basement membrane, continuously giving rise to more committed chained spermatogonia and ultimately mature sperm inside the tubules (Yoshida). Immediately after the pulse, individual cells were labeled adjacent to the basement membrane, consistent with the seen Sox2-GFP expression pattern (Figure 2F, top row). After one month of chase, small chains of spermatogonia were EYFP+ and after three months of chase, entire sectors spanning from spermatogonia to mature sperm were labeled. These results demonstrate that individual Sox2+ cells give rise to more cells of their own, defined as immature spermatogonia lining the seminiferous epithelium, as well as to differentiated progeny that continuously produce sperm cells, thus identifying Sox2+ cells as bona fide stem cells in testes. We noted that the labeling efficiency in testes was very low with, on average, only few labeled tubules detected per testis section. We speculate that this is a consequence of the observed low transcriptional activity of Sox2 in testes (Suppl. Figure 2C).
We next focused on the glandular stomach to follow the fate of Sox2+ cells. Analysis of Sox2-CreER; ROSA26-lsl-EYFP lineage tracing mice immediately after the 4-day administration of tamoxifen showed the appearance of individual dispersed EYFP+ cells as well as small patches of EYFP+ cells, consistent with the notion that some rare Sox2+ cells had expanded over the 4-day pulse period (Figure 2F, middle rows). Importantly, 15-22 months after the pulse, entire glands were fully labeled in both the corpus and pylorus, suggesting that Sox2-expressing cells can self-renew as well as give rise to the mature cell types of the glandular stomach (Figure 2F, Suppl. Figure 3A). It is worth mentioning that fully labeled corpus glands were observed less frequently than fully labeled pyloric glands at all time points and especially soon after the pulse (1-3 months), in agreement with a reported slower turnover rate of corpus cells (3-194 days) compared with pyloric cells (1-60 days) (Barker et al.).
Similar to the labeling patterns in glandular stomach and testes, individual EYFP+ cells were detected in the lens epithelium after the tamoxifen pulse (Figure 2F, bottom row). Following the chase, basal cells remained labeled and also gave rise to mature lens fibers inside the lens body, consistent with their identity as stem cells.
Next, we followed the fate of Sox2+ cells in squamous epithelia including tongue, esophagus, forestomach, anus and cervix. Analysis of these tissues immediately after the 4-day pulse identified individual EYFP+ cells or patches of labeled cells in the basal and suprabasal layers of the epithelia but not in the differentiated layers, which is in agreement with the observed expression patterns of Sox2 (Suppl. Figure 3B,D). However, when analyzing mice 1-3 months after the pulse, we observed clonal areas of EYFP+ cells stretching from the basal layer to the stratified epithelium. This clonal pattern remained stable at 15-22 months of chase, suggesting that basal Sox2+ cells contain long-term stem cells that maintain these tissues.
Of note, bronchiolar epithelial cells maintained stable EYFP+ clones over several months as well. EYFP+ cells were never found in the alveoli of the lungs, indicating that Sox2+ cells do not contain the precursors for alveolar cells under steady state conditions (Suppl. Figure 3C, bottom panels). Moreover, we detected EYFP+ cells within the acini and ducts of sublingual glands after the pulse, which remained labeled over long term, suggestive of putative stem cells (Lombaert and Hoffman) (Suppl. Figure 3C, top panels).
To exclude the possibility that the observed labeling patterns were due to marking of transient postnatal progenitor cells, we repeated pulse-chase experiments on 6-month-old animals and analyzed tissue sections 12 months later. Consistent with the prior results, we detected fully labeled EYFP+ tissue clones in the testes, glandular stomach and squamous epithelia including tongue, esophagus, forestomach and cervix (Suppl. Figure 3E). Of note, we noticed a lower frequency of labeling in older mice compared with young animals, either due to a decrease in Sox2+ stem cells and/or reduced efficiency of the lineage tracing system with age.
Sox2+ gastric stem cells are multipotent and self-renew
In contrast to Sox2+ squamous epithelia, lens and testes, which harbor unipotent stem cells, gastric glands of the stomach are thought to contain multipotent stem cells that can, depending on the region, produce 3-4 common cell types throughout life (Barker et al.). These are the so-called mucus cells, parietal cells, enteroendocrine cells and chief cells with the latter cell type only found in the corpus. To further characterize Sox2+ stomach cells and their differentiated progeny, we first performed double labeling experiments with antibodies recognizing the major differentiated cell types of the stomach. This analysis revealed that Sox2-GFP+ cells were negative for all tested differentiation markers in pylorus and corpus (Figure 3A and Suppl. Figure 4A), supporting the notion that they are uncommitted stem cells. In contrast, analysis of EYFP+ glands descending from lineage-traced Sox2+ cells 6 months after the pulse showed co-staining of EYFP+ cells with markers of enteroendocrine cells, mucus cells, parietal cells and chief cells, respectively (Figure 3B and Suppl. Figure 4B). These data corroborate the conclusion that Sox2+ cells are multipotent stem cells in both the pylorus and corpus of the glandular stomach. Our observation that entirely labeled glands were still detectable up to 460 days after the pulse indicates that Sox2+ stem cells have self-renewal potential (Suppl. Figure 3A, E).
Figure 3. Self-renewal and multipotency of Sox2+ stomach stem cells.
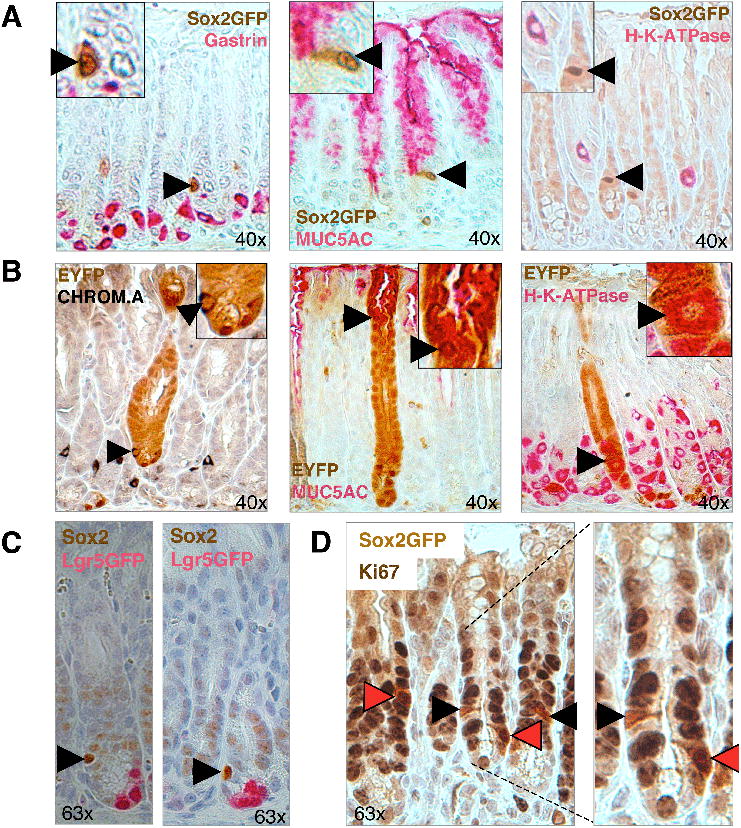
(A) Co-staining for Sox2-GFP and gastric markers on pylorus sections. Sox2-GFP+ cells (arrowheads) do not express differentiated cell markers gastrin (enteroendocrine cells), MUC5AC (mucus cells) or H-K-ATPase (parietal cells). (B) Co-staining of lineage tracing sections of Sox2-CreER; ROSA26-lsl-EYFP mice with differentiation markers in glandular stomach. Co-staining (arrowhead) was seen between EYFP and Chromogranin A (enteroendocrine cells) and MUC5AC (mucus cells) in the pylorus and with H-K-ATPase (parietal cells) in the corpus-pylorus transition zone. (C) Co-localization analysis for Sox2 and Lgr5-EGFP in pylorus. Sox2 (arrowhead) and Lgr5-EGFP (pink) IHC signals appear to mark different cells in pyloric glands. (D) Co-localization analysis between proliferation marker Ki67 and Sox2-GFP. Black arrowheads depict Ki67+ cells, red arrowheads depict Ki67+Sox2-GFP+ cells. Original magnifications as indicated. See also Suppl. Figure 4.
A previous study has shown that Lgr5+ cells function as multipotent stem cells of the pylorus (Barker et al.), raising the question of whether there is overlap in the expression patterns of Sox2 and Lgr5. Lgr5-GFP+ cells were confirmed to be present at a frequency of 3-4 cells at the base of each gland, while we observed between 1 and 2 Sox2-GFP+ cells above the base of each gland (Figure 1C, 3C). Interestingly, IHC for both Sox2 and GFP on pyloric sections of Lgr5-GFP-ires-CreER mice showed no apparent overlap in the expression of these two markers, suggesting that Lgr5 and Sox2 mark different types of stem cells in the pylorus (Figure 3C). However, we cannot exclude the existence of double-positive cells expressing low levels of the respective other marker that was beyond the threshold for IHC detection.
Co-labeling experiments of Sox2-GFP pyloric sections for GFP and the proliferation marker Ki67 further showed that roughly 50% of the Sox2+ cells are actively cycling (n=45 Sox2-GFP nuclei), indicating that half of the Sox2+ cells may be quiescent under homeostatic conditions (Figure 3D). Analysis of Sox2-CreER; ROSA26- lsl-EYFP mice receiving only a single dose of tamoxifen after one day and one week, respectively, confirmed our observation that some Sox2+ cells are cycling and give rise to EYFP+ patches of cells after a week while some Sox2+ cells appear to be slow-cycling and remain as singly labeled EYFP+ cells (Suppl. Figure 4C).
Sox2+ testis cells reconstitute spermatogenesis in germ cell-depleted, infertile mice
To confirm that Sox2 expression marks stem cells with an independent assay, we tested whether Sox2+ testis cells could reconstitute spermatogenesis in mice carrying a mutation in the c-kit gene (designated c-kitW/Wv) that ablates spermatogonial stem cells, thus rendering animals infertile (Brinster and Zimmermann, 1994).
In order to track donor cells, we crossed Sox2-GFP animals with ROSA26-lacZ mice, which allowed us to detect transplanted donor cells by X-gal staining in recipients (Figure 4A). FACS analysis of testes cells isolated from 2-week-old male mice showed that roughly one third of Sox2-GFP+ cells were found within the c-kit− population, which has previously been shown to be enriched for testis-repopulating cells (Shinohara et al., 2000), whereas two thirds of Sox2-GFP+ cells were found within the c-kit+ population (Suppl. Figure 5A). This observation indicates heterogeneity within the Sox2-expressing spermatogonial cell population and is consistent with a previous study analyzing c-kit and Oct4-GFP expression in testes (Ko et al., 2009).
Figure 4. Sox2-GFP+ testis cells reconstitute spermatogenesis in infertile mice.
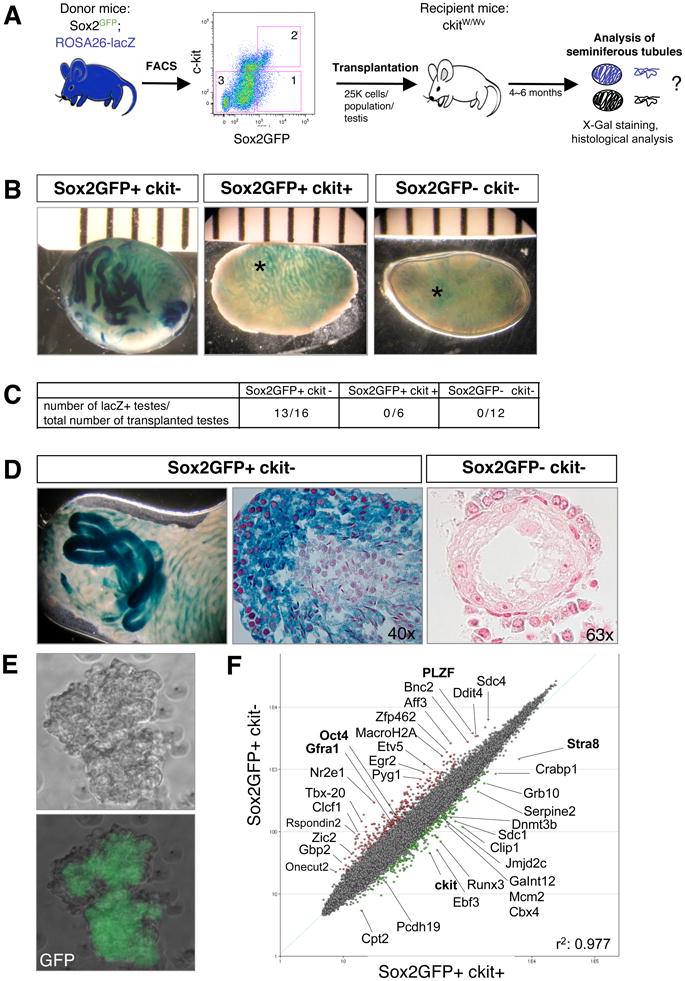
(A) Experimental outline. Sox2GFP animals were crossed with ROSA26-lacZ animals to enable tracking of transplanted donor cells. Sox2GFP+ckit− cells (1), Sox2GFP+ckit+ cells (2), and Sox2GFP−ckit− cells (3) from the testes of 2-week-old animals were transplanted into the vas deferens of germ cell-depleted, infertile ckitW/Wv recipient mice and testes analyzed for beta-gal activity after 6 months. (B) X-gal stain of transplanted ckitW/Wv testis 6 months after transplantation with the indicated cell populations. Light blue signal (asterisk) reflects background staining of the interstitium. (C) Table summarizing repopulation experiments. (D) Whole mount X-gal staining (left image) and paraffin sections of repopulated seminiferous tubules counterstained with neutral red (center image). Note the presence of immature spermatogonia at periphery and mature sperm in center of tubules transplanted with Sox2GFP+ckit− cells and lack of spermatogenesis upon transplantation of Sox2GFP−ckit− cells (right image). (E) Scatter plot of gene expression profiles comparing Sox2GFP+ckit− and Sox2GFP+ckit+ testis cells. Green dots depict genes with 2-fold and higher expression in Sox2GFP+ckit+ cells, red dots depict genes 2-fold and higher expression in Sox2GFP+ckit− cells. (pairwise analysis, two-fold change, t-test P=0.05, Benjamini and Hochberg correction.) Previously characterized spermatogonial stem cell genes are shown in bold. (F) Phase contrast and GFP images of a spermatogonial stem cell line derived from Sox2-GFP mice (passage 10). See also Suppl. Figure 5.
Sorted Sox2-GFP+c-kit−, Sox2-GFP-c-kit− and Sox2-GFP+c-kit+ cells were then transplanted into the seminiferous tubules of c-kitW/Wv mice (Suppl. Figure 5B). When we examined transplanted testes 4-6 months later, only Sox2-GFP+c-kit− cells gave rise to X-gal positive clones (13/16 testes) that, upon histological analysis, showed repopulation of seminiferous tubules and differentiation into mature sperm, whereas both Sox2-GFP+c-kit+ cells and Sox2-GFP-c-kit− cells generally failed to initiate spermatogenesis (0/7 testes and 0/12 testes analyzed, respectively) (Figure 4B-D). Our data demonstrate that only Sox2-GFP+c-kit− cells contain testis-repopulating potential and corroborate the finding that these Sox2-GFP+ spermatogonial cells qualify as authentic stem cells.
In further agreement with our interpretation that Sox2-GFP+ testis cells qualify as stem cells, we discovered that a spermatogonial stem cell (SSC) line established from unfractionated Sox2-GFP testis cells expressed Sox2-GFP in all resultant colonies (Figure 4E and Suppl. Figure 5C).
Finally, we wondered whether a molecular comparison of the Sox2-GFP+c-kit+ and Sox2-GFP+c-kit− spermatogonial cells would uncover genes that could explain the exclusive repopulation potential of the latter cell population. To this end, we sorted individual testis cell populations and subjected extracted and amplified RNA to array analysis. Indeed, we discovered that well-established spermatogonial stem cell regulators such as PLZF, Oct4, GFRo1 (Yamada et al., 2006), which have previously been shown to be important for spermatogenesis, were expressed more abundantly in the Sox2-GFP+c-kit− compared with the Sox2-GFP+c-kit+ population (Figure 4F).
Fetal Sox2+ cells give rise to adult Sox2+ stem cell compartments
Given that Sox2 is already expressed and plays important roles in fetal development, we wondered whether Sox2+ cells emerging in the embryo are the precursors for the observed Sox2-expressing tissues in the adult. We first re-evaluated Sox2 expression in E15.5 fetuses by IHC. Consistent with previous observations, we saw Sox2 signal in the developing brain, stomach, inner ear, eye, vibrissae (Suppl. Figure 6) and in esophagus, trachea and lungs (data not shown). In addition, we noticed Sox2 signal within ganglia and in Schwann cells surrounding nerves as well as in scattered cells within the epidermis (data not shown).
To determine whether these fetal Sox2+ cells give rise to the corresponding Sox2+ adult tissue, we injected pregnant females carrying Sox2-CreER; ROSA26-lsl-EYFP embryos with tamoxifen at E13.5 or E14.5 and analyzed their tissues as adults (Figure 5A). Similar to results obtained with adult labeling, we detected EYFP expression in the fore- and glandular stomach, esophagus, tongue, lens, lungs, trachea, and brain (Figure 5B and data not shown). Moreover, we saw EYFP expression in rare crypts of the duodenum, which was never seen in adults and is in agreement with reported Sox2 expression at the boundary between fetal glandular stomach and duodenum (Que et al., 2007). In further contrast to adult labeling patterns, we observed EYFP+ cells in the submandibular gland (Figure 5B). We also noticed that the labeled stomach samples usually contained clusters of 3-10 adjacent gastric units, suggesting that fetal or early postnatal progenitors had undergone clonal expansion following their genetic marking. Together, these results show that early Sox2+ fetal progenitors are the precursors for Sox2+ adult stem cells and that some tissues (duodenum, submandibular gland) appear to express Sox2 only transiently during fetal development.
Figure 5. Fetal Sox2+ progenitors give rise to adult Sox2+ stem cells.
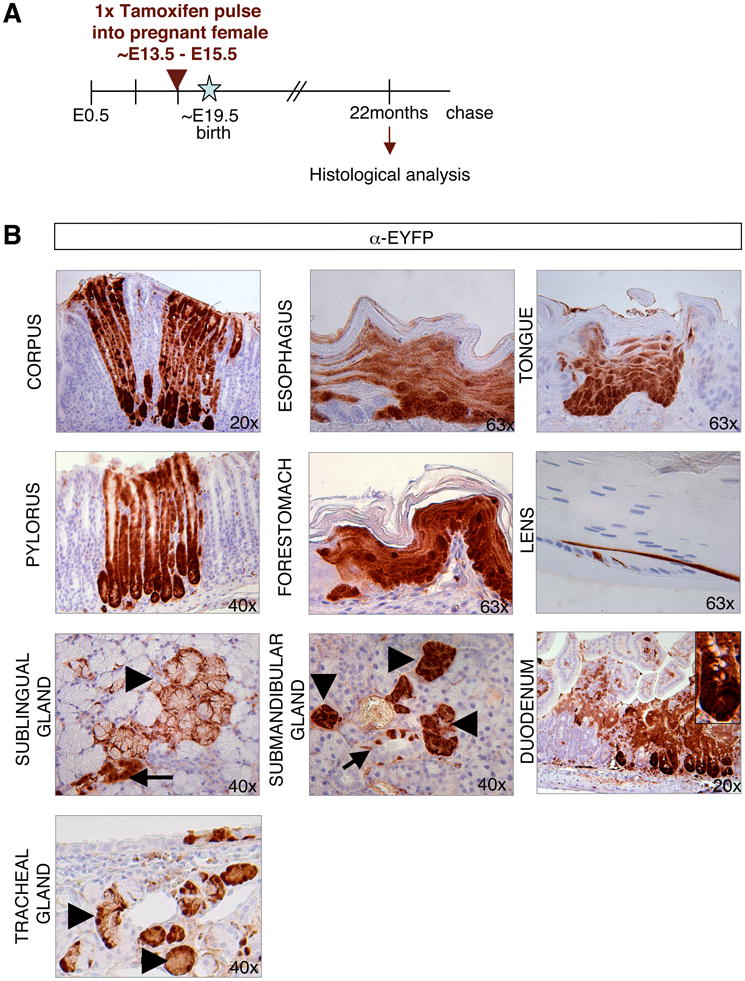
(A) Experimental strategy for embryonic lineage tracing. (B) IHC for EYFP in Sox2-CreER; ROSA26-lsl-EYFP mice that received one pulse of tamoxifen at E14.5 and were analyzed after 22 months. Labeled cells were detected in corpus and pylorus sections of the glandular stomach, forestomach esophagus, tongue, lens, ducts (arrow) and acini (arrowhead) of salivary glands, duodenum and tracheal glands. Original magnifications as indicated. See also Suppl. Figure 6.
Ablation of Sox2+ cells disrupts tissue homeostasis and causes lethality
Since Sox2 expression marks stem cells in many epithelial tissues, we next wished to test if Sox2+ cells are essential for tissue maintenance and ultimately the viability of mice. To address this question, we generated Sox2-TK mice from ESCs in which a truncated thymidine kinase gene (deltaTK) has been inserted into the endogenous Sox2 locus (Figure 6A, B). Exposure of any Sox2+, dividing cells to the drug gancyclovir (GCV) is expected to induce cell death. Indeed, treatment of Sox2-TK ESCs with GCV resulted in massive apoptosis within 4 days, hence validating the system (Figure 6C).
Figure 6. Ablation of Sox2+ cells disrupts tissue homeostasis and causes lethality.
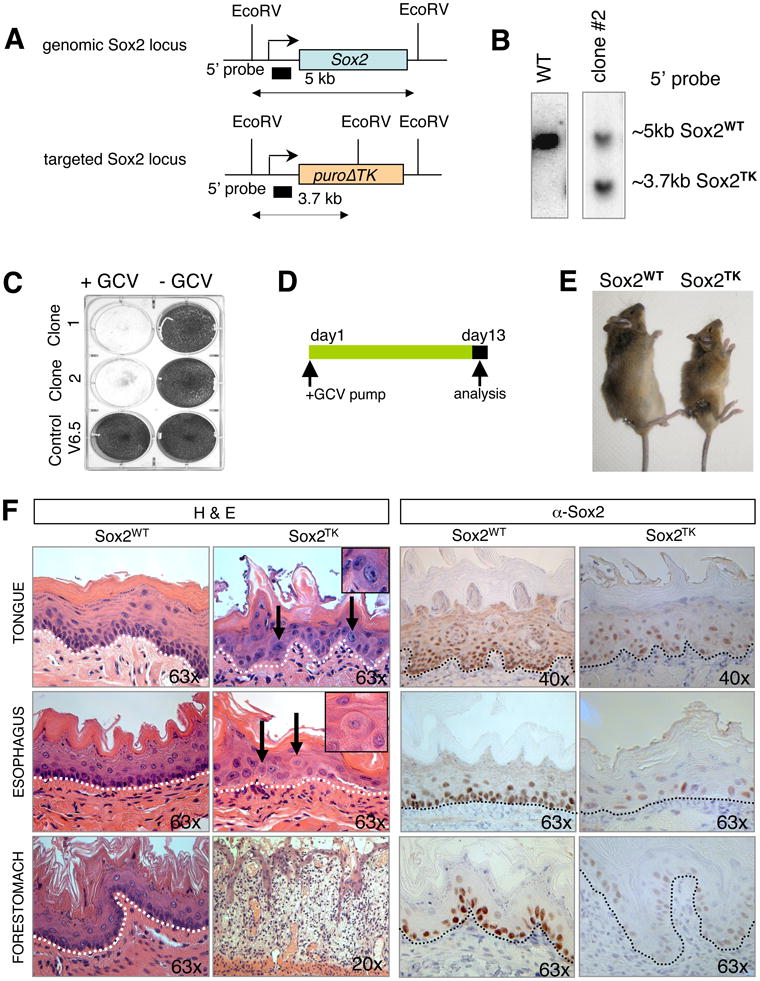
(A) Targeting strategy to generate Sox2TK mice. Restriction sites, Southern blot analysis probes and expected restriction fragment lengths are indicated. (B) Southern blot analysis to confirm correct integration of Sox2TK allele in ESCs. (C) Crystal violet stain of Sox2TK and control (V6.5) ESCs after 4 days of gancyclovir (GCV) treatment. (D) Experimental outline. ALZET pumps, releasing a constant dose of GCV over 2 weeks, were implanted into Sox2TK mice and control Sox2WT littermates. Animals were then sacrificed and tissues analyzed histologically. (E) Morbid Sox2TK mouse next to Sox2WT littermate after 13 days of GCV treatment. (F) H&E staining of paraffin-embedded sections from tongue, esophagus, and forestomach of GCV-treated Sox2TK mice shows loss of basal layer (dashed line) in tongue, esophagus and forestomach (second column) compared with control mice (left column). Note the appearance of atypical cells (arrows) in all tissues and severe inflammation as well as edema formation in the forestomach. GCV-treated WT animals remained unaffected (left column). IHC for Sox2 confirmed almost complete absence of Sox2+ cells in Sox2TK mice with very rare Sox2+ cells remaining (right column). Original magnifications as indicated.
To deliver GCV to tissues in vivo, we subcutaneously implanted ALZET osmotic pumps into the flanks of Sox2-TK mice, which systemically release a constant dose of GCV over two weeks (Figure 6D). Seven days after implantation, animals became morbid and died within another week. Treated mice appeared smaller in size, which may be the result of dehydration and a failure to resorb food (Figure 6E). Indeed, a body-wide tissue analysis of morbid mice after 13 days of GCV treatment suggested that the development of ulcers of the stomach and oral mucosa are likely the causes of death.
The detailed histological analysis of morbid mice revealed that the Sox2+ layers closest to the basement membrane were absent in GCV-treated animals while the stratified layers of the forestomach, tongue and esophagus remained unaffected, coinciding with the lack of Sox2 expression in those cells (Figure 6F). The absence of immunohistochemical signal for Sox2 in tissue sections of treated animals confirmed that most Sox2+ cells had in fact been eliminated (Figure 6F). We further detected severe inflammation and occasionally edema formation as well as atypical cells in forestomach and tongue (insets in Figure 6F). Because of these secondary effects, we cannot exclude the possibility that a destruction of niche cells in the Sox2-TK model contributed to the observed loss of epithelia. However, we failed to observe any obvious phenotypes in the testes, lungs and glandular stomach by H&E staining 13 days after GCV treatment, possibly because of a low turnover of Sox2+ stem cells in those tissues or inefficient cell ablation. These data demonstrate that some Sox2+ cells are essential for normal tissue maintenance and ultimately the survival of mice.
Rescue of morbidity upon withdrawal of gancyclovir
Lastly, we wanted to test whether the lethal phenotype caused by ablation of Sox2+ cells in vivo is reversible. We therefore administered GCV for only 7 days until mice became morbid and then surgically removed the pumps (Figure 7A). Histological analysis of those mice showed a severe disruption of tissue integrity as was seen before in animals induced for two weeks (data not shown). Notably, discontinuation of GCV treatment resulted in a gradual recovery of animals over the subsequent weeks, indicating that the morbid phenotype is indeed reversible (Figure 7B). Sox2 immunohistochemistry on tissue sections showed residual Sox2+ cells immediately after GCV treatment, which became more abundant after 7 days of recovery (Suppl. Figure 7). Examination of tissue sections of tongue, esophagus and forestomach 3 months after withdrawal of GCV showed normal epithelialization of tissues with intact basal and differentiated cell layers (Figure 7B, Suppl. Figure 7). We surmise that residual Sox2+ cells escaped GCV-mediated apoptosis and are responsible for regenerating the destroyed epithelia. Alternatively, non-ablated suprabasal cells may have repopulated the basal layer to regenerate epithelia.
Figure 7. Rescue of morbidity upon withdrawal of gancyclovir.
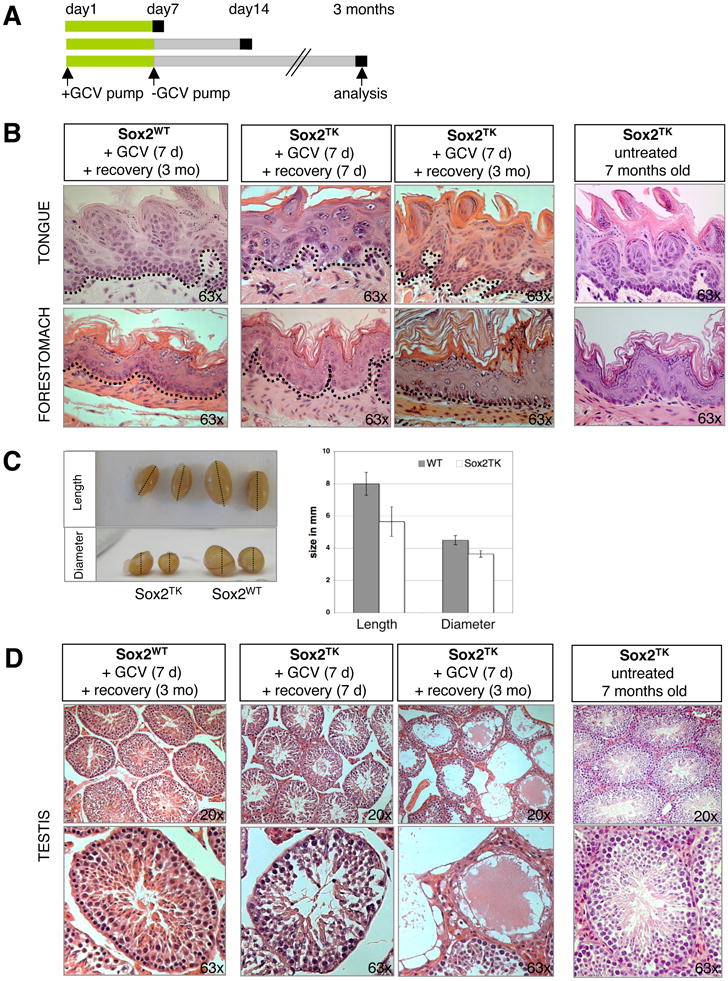
(A) Experimental strategy. GCV was given for 7 consecutive days to 3- or 10-week old mice. Treated Sox2TK mice and Sox2WT controls were then allowed to recover. Tissues were analyzed 7 days (7d) and 3 months (3mo) after removal of GCV pumps. (B) Histological analysis of Sox2TK tongue and forestomach after 7 days of GCV treatment followed by 7 days (second column) or 3 months (third column) of recovery. GCV-treated WT mice and 7 month-old untreated Sox2TK mice showed no abnormalities in tissue architecture (first and last columns, respectively). (C) 3 months after GCV treatment, testes from Sox2TK mice exhibited a significant reduction in size compared with treated control littermates. (D) Histological analysis of Sox2TK testes after 7d of GCV treatment followed by 7d or 3mo, respectively, of recovery. After 7d of recovery, the seminiferous tubules of Sox2TK testes showed a mild phenotype with less sperm (second column) compared to GCV-treated Sox2WT animals (left column). This phenotype was exacerbated after 3mo when entire atrophic tubules were seen (third column). No phenotypic abnormalities were seen in testes of age-matched untreated Sox2TK mice (right column). Original magnifications as indicated. See also Suppl. Figure 7.
Three months after GCV treatment, the testes of Sox2-TK mice appeared smaller in size compared to those of GCV-treated control mice (Figure 7C). Consistent with this finding, histological analysis showed an increased number of atrophic tubules that were completely devoid of spermatogonia and mature sperm, suggesting that the phenotype of ablating Sox2+ stem cells manifests later compared with that in forestomach and tongue (Figure 7D). This delayed phenotype is probably due to a slower turnover rate of Sox2+ stem cells in testes compared to forestomach, esophagus and tongue and demonstrates that Sox2+ testis cells are essential for continuous spermatogenesis.
Discussion
In this study, we have identified expression of the stem cell factor Sox2 in several adult epithelial tissues where it has not previously been characterized, including the testes, fore- and glandular stomach, anus, cervix, esophagus, lens as well as glands associated with oral cavity, trachea and cervix. Importantly, we provide unequivocal fate mapping evidence that Sox2+ cells contain long-term stem cells in some of these tissues. We further assign a critical requirement for Sox2+ cells in tissue homeostasis by showing that their ablation causes a fatal disruption of epithelial integrity.
To our knowledge, Sox2 is the only transcriptional regulator that is commonly expressed between ESCs, fetal progenitors and a number of adult stem cells and may therefore point towards molecular similarities in the regulation of pluripotent and different adult stem cells. Our developmental lineage tracing data show that Sox2+ adult stem cells originate from early Sox2+ epithelial progenitors in fetal development. These results suggest that Sox2 expression plays key roles at multiple stages of pre- and postnatal development. While it is initially required for the establishment of pluripotent founder cells within the embryo, it subsequently controls the formation of fetal ectodermal and endodermal primordia as well as fetal germ cells and eventually becomes confined to some derivative adult tissues where it marks unipotent and multipotent stem cells.
Recent elegant fate mapping studies have found that Lgr5 expression marks stem cells in several epithelial tissues (Barker et al., 2007; Jaks et al., 2008) including the pyloric stomach (Barker et al.), raising the question of whether the same or different stem cells are labeled compared with Sox2+ cells. Based on our co-expression results, we conclude that Sox2 expression labels a different and more rare population of cells in the glandular stomach than Lgr5 expression. Another pronounced difference between Lgr5+ and Sox2+ stem cells is the finding that Lgr5+ cells are seen in the intestine and pyloric stomach whereas Sox2+ cells are found in both the pylorus and corpus compartment of the adult glandular stomach but not in the intestine. A previous study has identified a rare population of Villin-expressing cells in antral glands that may coincide with the Sox2-expressing cells described here. In contrast to our study and the report by Barker et al., however, lineage tracing experiments showed that these Villin+ cells only give rise to entirely labeled gastric units in response to interferon treatment. Future studies will be needed to conclusively establish whether Villin+, Lgr5+ and Sox2+ cells are different types of stem cells that independently replenish the stomach or whether these cells are hierarchically related with each other. The notion of distinct stomach stem cells is reminiscent of recently identified stem cell markers in the small intestine that seem to label different cell populations (Barker et al., 2007; Montgomery et al.; Sangiorgi and Capecchi, 2008).
In the testis, we detected rare individual Sox2+ cells that may represent As spermatogonia, which are thought to be the most primitive cells in testes (Yamada et al., 2006). This interpretation is supported by our findings that (i) Sox2-GFP+c-kit− testis cells successfully restore spermatogenesis upon transplantation into infertile mice, (ii) lineage tracing analysis permanently labels clones containing immature spermatogonia and mature sperm, (iii) ablation of Sox2+ cells results in loss of spermatogenesis, and (iv) derivation of spermatogonial stem cell lines selects for Sox2-expressing cells. The observation that Sox2 is also expressed in c-kit+ testis cells that do not reconstitute spermatogenesis and in some transit-amplifying cells of squamous epithelia including forestomach, esophagus, tongue, anus and cervix suggests that in these tissues Sox2 is not exclusively expressed in stem cells. It is noteworthy that Sox2 expression was seen in basal cells of squamous epithelia lining inner organs (anus, cervix, esophagus, forestomach, tongue) but was absent from the interfollicular epidermis of the skin, which is organized in a similar fashion. This may either reflect functional differences of these epithelia or their distinct developmental origin despite a remarkably similar structure.
Lastly, we have identified Sox2+ stem cells in the lens epithelium that gives rise to secondary lens fibers throughout adult life, as well as in ganglia, salivary glands and glands associated with trachea and cervix. Additional experiments are required to unequivocally test whether Sox2+ cells in those tissues also harbor stem cell activity.
In summary, our results establish Sox2 as a widespread marker of pluripotent and many adult stem/progenitor cell types, and suggest that common target genes and pathways may be activated that are crucial for maintaining their self-renewal and differentiation potentials. Given the recent recognition that Sox2 can act as an oncogene in squamous cell carcinoma of the lung and esophagus (Bass et al., 2009), it should further be interesting to investigate whether the Sox2-expressing cells, identified here, can act as tumor initiating cells and whether Sox2 expression itself may be oncogenic in these tissues.
Experimental Procedures
Mice
V6.5 ESCs were targeted with knock-in constructs containing either CreERT2 (Feil et al., 1997), puro-delta-TK (Chen and Bradley, 2000) or EGFP (Ellis et al., 2004) alleles under the control of endogenous Sox2 regulatory elements using standard protocols. Correct insertion of constructs was verified by Southern blot, and correctly targeted clones were injected into BDF1 blastocysts and transferred into pseudo-pregnant females. Resultant chimeric mice were bred with 129SvJae mice and germline offspring were bred to establish stable lines. All animal experiments were approved by the IACUC committee and are conform to the regulatory standards.
Tamoxifen induction
Sox2-CreER; ROSA26-lsl-EYFP ES cell clones were treated with one dose of 100nM 4-OH Tamoxifen (Calbiochem), and cells were analyzed by FACS after two weeks. 2mg Tamoxifen (Sigma), dissolved in corn oil, was given daily to 3-6 week old Sox2-CreER; ROSA26-lsl-EYFP animals on 4 consecutive days if not otherwise stated. Mice were analyzed by IHC on day 5 (pulse time point), and at different time points thereafter. Sox2-CreER; ROSA26-lsl-EYFP littermates injected with corn oil alone were used as controls. Sox2WT; ROSA26-lsl-EYFP animals were used as staining control. For lineage tracing in the embryo, a single pulse of 2mg TAM plus 1mg Progesteron was given to pregnant females at E14.5 and resultant offspring were analyzed at 22 months of age.
Tissue preparation and immunohistochemistry
Tissues were harvested, fixed in 10% Formalin or Bouins solution overnight and then stored in PBS until further processing. H&E stain and IHC were performed following standard protocols. Antibodies used were GFP/YFP (Living colors, JL-8, Clontech); Sox2 (AB5603, Millipore) and p63 (sc-8431, Santa Cruz), Ki67 (ab15580, Abcam) MUC5AC (ab3649, Abcam); Gastrin (sc-7783, Santa Cruz); H-K-ATPASE (D032-3H, MBL) and Chromogranin A (ab15160, Abcam).
Gancyclovir treatment
Cultured Sox2TK ESC clones were treated with 2uM GCV (Cytovene, Roche). For in vivo treatments, GCV was dissolved in 0.9% saline and filled into ALZET osmotic pumps. 7d or 14d pumps were used to release a constant dose of 2mg/day. Mice were anesthetized and pumps were inserted subcutaneously. For recovery experiments, 7d GCV pumps were inserted subcutaneously and surgically removed after 7 days.
Spermatogonial cell transplantation
Testes from 10-20 Sox2GFP; ROSA26-lacZ+ donor mice were dissected at the age of 2 weeks to harvest single cell suspensions. Sox2GFP+ckit-, Sox2GFP+ckit+ and Sox2GFP-ckit- populations were sorted by FACS and transplanted into the vas deferens of germ cell-depleted, infertile c-kitW/Wv recipient mice. Briefly, 25,000 cells were transplanted per testis. Leuprolide (Sigma) (0.77mg per g bodyweight) was injected right after transplantation to support engraftment. Transplanted mice were analyzed 6 months after transplantation for repopulation of seminiferous tubules by X-gal staining and by histological examination.
Supplementary Material
Acknowledgments
We thank Laura Prickett-Rice and Kat Folz-Donahoe for assistance with FACS, Nimet Maherali and Matthias Stadtfeld for helpful discussions and critical reading of the manuscript. K.A. and K.H. were supported by a Seed Grant from the Harvard Stem Cell Institute. Additional support to K.H. was from the NIH Director's Innovator Award, the Kimmel and V Foundations for Cancer Research and MGH startup funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production processerrors may be discovered which could affect the content, and all legal disclaimers that apply to the journalpertain.
References
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes & development. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell stem cell. 7:656–670. doi: 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van den Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nature genetics. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. Multipotential stem cells in adult mouse gastric epithelium. American journal of physiology. 2002;283:G767–777. doi: 10.1152/ajpgi.00415.2001. [DOI] [PubMed] [Google Scholar]
- Brazel CY, Limke TL, Osborne JK, Miura T, Cai J, Pevny L, Rao MS. Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging cell. 2005;4:197–207. doi: 10.1111/j.1474-9726.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- Breault DT, Min IM, Carlone DL, Farilla LG, Ambruzs DM, Henderson DE, Algra S, Montgomery RK, Wagers AJ, Hole N. Generation of mTert-GFP mice as a model to identify and study tissue progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10420–10425. doi: 10.1073/pnas.0804800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- Chen YT, Bradley A. A new positive/negative selectable marker, puDeltatk, for use in embryonic stem cells. Genesis. 2000;28:31–35. doi: 10.1002/1526-968x(200009)28:1<31::aid-gene40>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci U S A. 2008;105:2907–2912. doi: 10.1073/pnas.0707886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochemical and biophysical research communications. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nature genetics. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- Galan-Caridad JM, Harel S, Arenzana TL, Hou ZE, Doetsch FK, Mirny LA, Reizis B. Zfx controls the self-renewal of embryonic and hematopoietic stem cells. Cell. 2007;129:345–357. doi: 10.1016/j.cell.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Imamura M, Miura K, Iwabuchi K, Ichisaka T, Nakagawa M, Lee J, Kanatsu-Shinohara M, Shinohara T, Yamanaka S. Transcriptional repression and DNA hypermethylation of a small set of ES cell marker genes in male germline stem cells. BMC Dev Biol. 2006;6:34. doi: 10.1186/1471-213X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Collignon J, Lovell-Badge R, Kondoh H. Development. Vol. 125. Cambridge, England: 1998. Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction; pp. 2521–2532. [DOI] [PubMed] [Google Scholar]
- Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. The Anatomical record. 1993;236:259–279. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Ko K, Tapia N, Wu G, Kim JB, Bravo MJ, Sasse P, Glaser T, Ruau D, Han DW, Greber B, et al. Induction of pluripotency in adult unipotent germline stem cells. Cell Stem Cell. 2009;5:87–96. doi: 10.1016/j.stem.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Dumitriu B, Penzo-Mendez A, Han Y, Pallavi B. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. The international journal of biochemistry & cell biology. 2007;39:2195–2214. doi: 10.1016/j.biocel.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengner CJ, Camargo FD, Hochedlinger K, Welstead GG, Zaidi S, Gokhale S, Scholer HR, Tomilin A, Jaenisch R. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell stem cell. 2007;1:403–415. doi: 10.1016/j.stem.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IM, Hoffman MP. Epithelial stem/progenitor cells in the embryonic mouse submandibular gland. Frontiers of oral biology. 14:90–106. doi: 10.1159/000313709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansukhani A, Ambrosetti D, Holmes G, Cornivelli L, Basilico C. Sox2 induction by FGF and FGFR2 activating mutations inhibits Wnt signaling and osteoblast differentiation. J Cell Biol. 2005;168:1065–1076. doi: 10.1083/jcb.200409182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proceedings of the National Academy of Sciences of the United States of America. 108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Clark C, Hogan BL. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells. 2009;27:442–450. doi: 10.1634/stemcells.2008-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Pevny LH, Hogan BL. Sox2 is required for development of taste bud sensory cells. Genes & development. 2006;20:2654–2659. doi: 10.1101/gad.1457106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL. Development. Vol. 134. Cambridge, England: 2007. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm; pp. 2521–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nature genetics. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 lineage tracing identifies a self-renewing pancreatic acinar cell subpopulation capable of maintaining pancreatic organ homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7101–7106. doi: 10.1073/pnas.0902508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci U S A. 2000;97:8346–8351. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D'Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, Pevny LH. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes & development. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Yamamoto Y, Nagasawa M, Hara A, Kodera T, Kojima I. In vitro transdifferentiation of mature hepatocytes into insulin-producing cells. Endocr J. 2006;53:789–795. doi: 10.1507/endocrj.k06-116. [DOI] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Molecular cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yoshida S. Stem cells in mammalian spermatogenesis. Development, growth & differentiation. 52:311–317. doi: 10.1111/j.1440-169X.2010.01174.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


