Abstract
Objective
To determine if the magnitude of the acute injury response to shock-wave lithotripsy (SWL) depends on the number of SWs delivered to the kidney, as SWL causes acute renal oxidative stress and inflammation which are most severe in the portion of the kidney within the focal zone of the lithotripter.
Materials and Methods
Pigs (7–8 weeks old) received 500, 1000 or 2000 SWs at 24 kV from a lithotripter to the lower pole calyx of one kidney. At 4 h after treatment the kidneys were removed, and samples of cortex and medulla were frozen for analysis of the cytokine, interleukin-6, and for the stress response protein, heme oxygenase-1 (HO-1). Urine samples taken before and after treatment were analysed for the inflammatory cytokine, tumour necrosis factor-α. For comparison, we included previously published cytokine data from pigs exposed to sham treatment.
Results
Treatment with either 1000 or 2000 SWs caused a significant induction of HO-1 in the renal medulla within the focal zone of the lithotripter (F2, 1000 SWs, P < 0.05; 2000 SWs, P < 0.001). Interleukin-6 was also significantly elevated in the renal medulla of the pigs that received either 1000 or 2000 SWs (P < 0.05 and <0.001, respectively). Linear dose–response modelling showed a significant correlation between the HO-1 and interleukin-6 responses with SW dose (P < 0.001). Urinary excretion of tumour necrosis factor-α from the lithotripsy-treated kidney increased only for pigs that received 2000 SWs (P < 0.05).
Conclusion
The magnitude of renal oxidative stress and inflammatory response in the medulla increased with the number of SWs. However, it is not known if the HO-1 response is beneficial or deleterious; determining that will inform us whether SWL-induced renal injury can be assessed by quantifying markers of oxidative stress and inflammation.
Keywords: shock wave lithotripsy, kidney, oxidative stress, heme oxygenase, inflammation
Introduction
Shock wave lithotripsy (SWL) is the preferred treatment for uncomplicated kidney stones of ≤ 2 cm located in the upper urinary tract, because it is noninvasive and has a high success rate for stone removal [1]. Weighing against these benefits, SWL also induces a predictable pattern of acute renal injury. This injury includes rupture of blood vessels in the medulla and cortex, accompanied by intraparenchymal bleeding, oxidative stress, inflammation, and an impairment of renal haemodynamics [2]. Resolution of the acute injury results in scar formation and the loss of functional renal tissue [2].
Recent clinical reports implicating SWL in the onset of hypertension [3], exacerbation of stone disease [4–6], and an increased incidence of diabetes [7], have renewed debate about the long-term safety of SWL. Given the necessary relationship between acute injury and chronic adverse effects, attention has been directed at gaining a more complete understanding of acute SWL induced injury. To this end, the effects of SWL on renal morphology, lesion size, and renal function have been studied in settings where treatment variables, such as SW delivery rate [8,9], dose [10,11], and energy (discharge voltage), have been varied [12]. Incremental increases in any of these treatment settings resulted in increased (i.e. dose-related) tissue injury.
However, much less is known about the oxidative stress and inflammatory response to SWL. Support for the involvement of oxidative stress in renal injury after SWL has come from studies showing that inhibitors of oxidative stress reduced the severity of tissue injury [13] or decreased urinary levels of markers of tubular damage after SWL [14,15], and that delivering more SWs to the kidney produced greater free radical activity, which can lead to oxidative stress [16].
While earlier studies focused on oxidative injury to the renal cortex [16], our recent work showed that acute oxidative stress and inflammation after SWL are highly localized to the renal medulla within the focus (F2) of the lithotripter [17]. In particular, both interleukin-6 (IL-6) and heme oxygenase-1 (HO-1) increased in the renal medulla, but did not change significantly in the cortex. Accordingly, we focused the present study solely on the renal medulla and determined whether the magnitude of acute renal oxidative stress and inflammation after SWL depends on the number of SWs delivered to the kidney. Our approach was to examine the dose-response relationship of SWs to changes in levels of HO-1, IL-6, and TNF-α in the shocked kidney.
Materials and Methods
The experimental protocol used in this study was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine and Methodist Hospital. Female farm pigs, 7–8 weeks old (10–15 kg, Hardin Farms, Danville, IN, USA), were assigned to receive either 500, 1000 or 2000 SWs at 120 SWs/min and 24 kV from an HM3 lithotripter (Dornier, Wessling, Germany). Procedures for the surgical placement of the vascular and ureteric catheters were described previously, as has the protocol for use of the lithotripter [17]. Before SWL, and at intervals of 500 SWs, the position of the SW focus (on a lower pole) was verified by injecting a small amount of contrast medium (Renografin 60%, Bracco Diagnostics, Princeton, NJ, USA) through the ureteric catheter into the urinary collection system of the treated kidney.
Plasma and urine samples were collected throughout the experiment. Aliquots of urine designated for cytokine analysis were frozen on dry ice, and stored at −80 °C. At 4 h after treatment the kidneys were perfused with cold isotonic saline and removed. Transverse tissue sections were cut from the lower and upper poles of both kidneys. The sections were then divided into cortex and medulla before snap-freezing in liquid nitrogen and storage at −80 °C.
Renal homogenates for analysis of cytokines were prepared as previously described [17]. Briefly, frozen renal tissue was weighed, then homogenized in two volumes of ice-cold buffer containing 10 mM HEPES, 10 mM KCl, 0.1 mM EGTA, 1 mM dithiothreitol, and 0.25 mM phenyl methylsulphonyl fluoride. The homogenate was centrifuged twice at 3000 g for 15 min at 4 °C. Protein was assayed using the Coomassie Plus assay (Pierce, Rockford, IL, USA) and aliquots of the final supernatant were stored at −80 °C.
Renal microsomes were prepared for analysis of HO-1 by Western blot. Frozen kidney tissue was weighed, then homogenized in three volumes of ice-cold 20 mM potassium phosphate buffer (pH 7.4) containing 135 mM KCL, 0.1 mM EDTA, Complete Protease Inhibitor Cocktail (Roche, Indianapolis, IN, USA), 1 mM sodium orthovanadate, and 0.1 mM phenyl methylsulphonyl fluoride. After low-speed centrifugation to remove large particles, homogenates were centrifuged at 100 000 g for 1 h at 4 °C. The microsomal pellet was resuspended in 20 mM potassium phosphate buffer (pH 7.4) containing 1 mM KCL, 10 mM EDTA, and protease inhibitors. After assay of protein, aliquots were stored at −80 °C.
Effective renal plasma flow was estimated by measuring the renal clearance of para-amino hippuric acid using a colorimetric assay, as previously described [10]. TNF-α was quantified in urine using a Quantikine Porcine TNF-α ELISA kit (R & D Systems, Minneapolis, MN, USA). IL-6 was measured in renal homogenates with a Quantikine Porcine IL-6 ELISA kit (R & D Systems).
For Western blot, renal microsomal protein (25 μg) prepared in sample buffer was separated on a 10% PAGE, followed by electrophoretic transfer to a polyvinylidene fluoride membrane. After blocking in 10 mM Tris-buffered saline with 0.05% Tween and 5% milk, the membrane was incubated with a rabbit polyclonal anti-HO-1 antibody (1: 2000; Stressgen SPA-895: Assay Designs, Ann Arbor, MI, USA), followed by incubation with a donkey antirabbit IgG-horseradish peroxidase-conjugated antibody (1: 20 000; Jackson Immunoresearch, West Grove, PA, USA). Bands were detected by enhanced chemiluminescence (Pierce). Membranes were stripped and probed for β-actin using a mouse monoclonal anti-β-actin-peroxidase conjugate antibody (1: 60 000; Abcam, Piscataway, NJ, USA). Band intensities were quantified by densitometry (Quantity One, Bio-Rad, Hercules, CA, USA).
For comparison, we included data for urinary TNF-α excretion and tissue IL-6 levels of pigs receiving sham treatment, from a published study on the effect of SWL on renal oxidative stress and inflammation [17]. That study was carried out with the same lithotripter, pigs of the same size and with the same protocol as the present experiment. Western blots generated to examine the dose–response effect of SWs on HO-1 included samples from the sham and 2000 SW groups used in the previous study, as well as samples from other pigs in these groups which had not been analysed for HO-1.
ANOVA was used to compare mean HO-1 values from the four groups, with posthoc comparisons between each of the SWL groups and the sham group after a significant overal ANOVA (P < 0.05). As both IL-6 and TNFα values were highly skewed, we used nonparametric methods to analyse these data. The Kruskal-Wallis test was used to derive the overall P values when comparing differences in medians of the four groups. After significant overall P values, posthoc comparisons were conducted using the Wilcoxon test with Monte Carlo simulations to obtain P values from the nonparametric test rather than relying on asymptotic results. A linear dose-response model was also used to test for trends in HO-1 and IL-6 values in the four groups; the criterion for statistical significance was P < 0.05.
Results
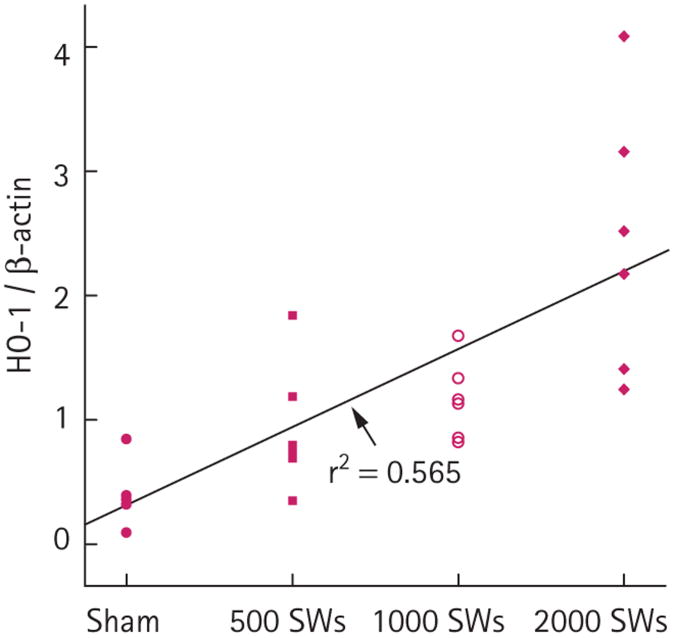
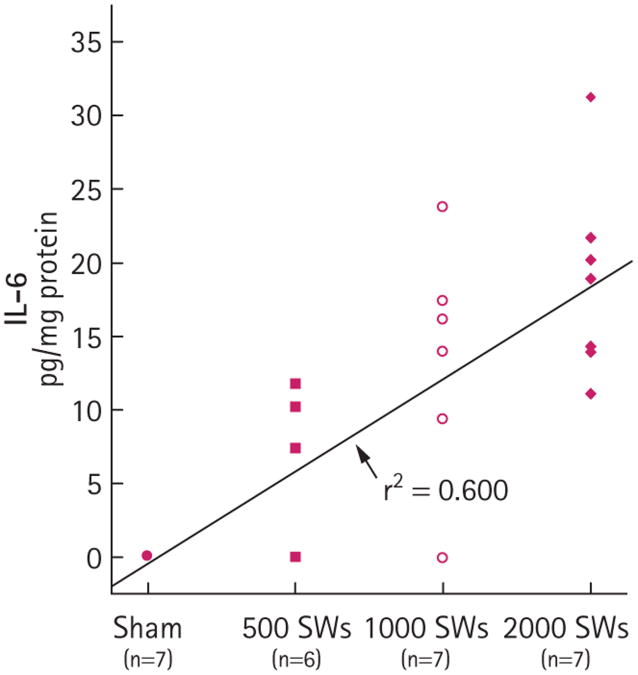
Body weights, and baseline measurements of blood pressure and effective renal plasma flow were not significantly different among the treatment groups (Table 1). HO-1 in the medulla within F2 was significantly higher in the groups receiving either 1000 or 2000 SWs than in the sham group (Fig. 1). Although there was a trend for HO-1 to increase in the medulla after 500 SWs, this was not significant compared to sham pigs (P = 0.153). Similarly, IL-6 levels in the medulla within F2 increased with SW number (Fig. 2), with significant increases above sham levels for groups receiving 1000 or 2000 SWs (the values for sham were previously reported [17] and are included here for comparison). This effect was specific to regions of the medulla within F2, as there was no significant increase of tissue IL-6 in either the upper pole of the treated kidney or the contralateral kidney (data not shown). There was a trend to increased IL-6 levels after 500 SWs (P = 0.071). When fitted to a linear dose-response model, the HO-1 (Fig. 1) and IL-6 (Fig. 2) data showed a statistically significant dose-response relationship (P < 0.001) with SW number.
Table 1. Mean (SEM) body weight, blood pressure, and effective renal plasma flow (ERPF) of the four treatment groups.
| Treatment | Body weight, kg | Blood pressure, mmHg | ERPF mL/min |
|---|---|---|---|
| Sham | 14.0 (0.5) | 65.7 (1.3) | 56.5 (3.4) |
| 500 SW | 15.8 (1.0) | 60.4 (2.0) | 48.0 (6.5) |
| 1000 SW | 14.6 (1.0) | 63.4 (2.5) | 54.6 (8.2) |
| 2000 SW | 14.4 (0.6) | 68.0 (2.6) | 47.8 (3.5) |
Fig. 1.
HO-1 in the left lower pole medulla (treated kidney, F2 for SWL) for sham and SWL-treated pigs at 4 h after treatment (six pigs in each group). For 1000 SWs vs sham, P < 0.05. For 2000 SWs vs sham, P < 0.001. For the dose–response effect, tissue HO-1 vs SW dose, P < 0.001.
Fig. 2.
Tissue IL-6 levels in the left lower pole medulla (treated kidney, F2 for SWL) of sham- and SWL-treated pigs at 4 h after treatment: 1000 SWs vs sham, P < 0.05; 2000 SWs vs sham, P < 0.001. For the dose–response effect, tissue IL-6 vs SW dose, P < 0.001.
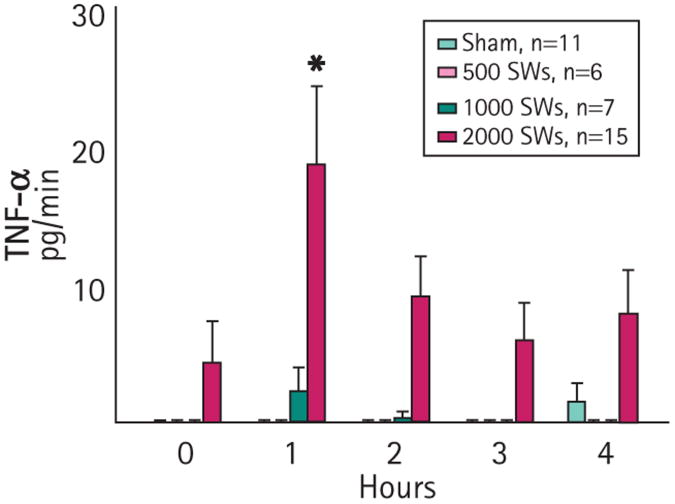
The urinary excretion of TNF-α from the treated kidney for the groups receiving either 500 or 1000 SWs did not change significantly from sham values at any sample time (Fig. 3), whereas the urinary excretion of TNF-α by pigs receiving 2000 SWs increased significantly by 1 h after SWL, and declined thereafter [17]. Preliminary measurements showed that TNF-α excretion at 30 min was no different from 1 h after SWL, indicating that there was no peak of TNF-α excretion in the urine at <1 h after treatment (data not shown).
Fig. 3.
Urine TNF-α excretion from treated kidneys of sham- and SWL-treated pigs. *P < 0.01 vs sham. Values are the mean (SEM).
Discussion
To our knowledge, this is the first study to show a dose-related effect of SWs on oxidative stress and inflammation within renal tissue. In particular, the data indicate that HO-1 and IL-6 levels in the medulla within F2 correlated with the number of SWs delivered. That this injury response begins early in treatment and increases with SW dose is supported by the observations that haematuria, indicative of blood vessel damage, was observed after a mean (SEM) of 287 (121) SWs for all groups receiving SWs, and that the highly significant correlation to dose–response effect for IL-6 and HO-1 estimates that for each increase in dose of SWs (sham, 500, 1000 and 2000 SWs), HO-1 (measured as the HO-1/β-actin ratio) will increase by 0.64 arbitrary units and IL-6 will increase by 5.40 pg/mg protein. By contrast, urinary TNF-α excretion was increased only in the group receiving 2000 SWs (a clinical dose), suggesting that a threshold of injury must be reached before TNF-α appears in the urine.
Impairment of cardiovascular function resulting in hypoxia/ischaemia can influence systemic and tissue (e.g. renal) cytokine and stress protein levels [18,19]. Therefore, it was important to confirm that the baseline cardiovascular and renal vascular status of the pigs in all groups were similar, to interpret that our results implicate SW number as a causative factor for the changes observed after SWL.
The SW dose-related increase of IL-6 and the elevated urinary excretion of TNF-α after 2000 SWs provide evidence of an inflammatory response to SWL. While the increase of HO-1 indicates a concomitant state of oxidative stress, the role played by HO-1 in the tissue response to SWL remains unclear. Most studies indicate that HO-1 protects tissue from injury, but under certain conditions it can be injurious to tissue [19,20]. Future studies on the time course of the inflammatory and oxidative stress responses, and on the effects of modulation of HO-1 before SWL on tissue injury, will clarify these issues.
Oxidative stress has been documented in animals after SWL [14,15,21]. In dose– response studies of SWL, Munver et al. [13] and Delvecchio et al. [16] examined the relationship between SW number and free radical activity; the latter is commonly implicated in oxidative injury. In their studies, microdialysate was collected every 1000 SWs from the renal cortex of pigs treated with a total of 10 000 SWs. The level of conjugated dienes, a marker of lipid peroxidation mediated by free radical activity, increased progressively to nearly 100 times that of the untreated kidney. Pretreatment with allopurinol, an antioxidant, completely abolished the SW-induced production of conjugated dienes [13]. A second study using the same protocol showed that the treated pole of the treated kidney registered the highest levels of conjugated dienes, while levels in the untreated pole of the treated kidney were ≈50% lower [16]. Our recent work [17] extended the knowledge of SWL-induced oxidative stress and inflammation by establishing that the greatest acute renal injury is localized to the medulla within F2, and that the pattern of injury is distinct from ischaemia-reperfusion-induced acute renal injury.
Several variables have been used to assess renal injury after SWL. An early study of acute injury found that delivering more SWs from a Dornier HM2 lithotripter caused more subcapsular haematomas and increased the number and/or size of intraparenchymal haemorrhages in dog kidneys [22]. This result was verified in other species with a variety of lithotripters [2]. The acute parenchymal injury induced by SWL results in scar formation. Neuerberg et al. [23] reported a correlation between SW number and the size of fibrotic lesions in rat kidneys several months after SWL.
More recently, haemorrhagic lesion size and changes in renal function have been shown to depend on SW dose [10–12]. The present findings add oxidative stress and inflammation to the list of dose-dependent effects of SWL and expand the ability to assess renal injury after SWL.
Discharge voltage and SW rate also induce dose-related effects on lesion size and renal function after SWL [8,9, 12], and the renal papilla and medulla are probably the most sensitive to injury by SWs [24]. The present results and our earlier study [17] support this notion. As lower SW rates and discharge voltages cause less renal injury than do higher SW rates and voltages [25], it has been recommended that both variables be used at lower settings in the clinic [25]. However, it is not known if these strategies for renal protection prevent the acute renal oxidative stress and inflammatory response to SWL.
Two mechanisms, direct and indirect, contribute to the renal injury from SWL. The primary injury results from direct, SW-induced trauma to small blood vessels and tubules in the renal parenchyma. Secondarily, SW-induced renal vasoconstriction coupled with the rupture of arterioles produces localized ischaemia and a rich milieu for oxidative stress and inflammation to develop. While studies suggest that ischaemia or an ischaemia-reperfusion phenomenon induces much of the secondary injury caused by SWs [21,26], our recent work implicates other factors [17]. For example, heme and one of its breakdown products, iron, are probably present in higher concentrations in areas of haemorrhage after SWL, and could thereby injure cells and induce HO-1 synthesis [27]. Renal cells (e.g. tubular epithelial, vascular endothelial, resident macrophages) or infiltrating immune cells (e.g. macrophages) could up-regulate HO-1 after renal injury from SWs. In this regard, Basireddy et al. [28] provided evidence for a role for heme in inducing HO-1 in renal epithelial cells by showing dose-dependent induction of HO-1 on the apical and basolateral surfaces of Madine-Darby canine kidney II cells incubated with varying concentrations of hemin under hypoxic conditions. Other investigators reported that heme up-regulates HO-1 in porcine aortic endothelial cells [29] and human monocytes, in a dose- and time-dependent manner [30]. Further work is needed to determine which cell types induce HO-1 and the role of heme/iron in SWL-induced injury.
Questions about the safety of SWL have arisen after recent reports that implicate SWL-induced tissue injury in causing such chronic conditions as diabetes, hypertension, and exacerbation of stone disease [1]. To deal with these issues, it is necessary to better understand the conditions in which SWL might cause such effects, to continue to develop treatment protocols that protect the kidney during treatment, and to understand the mechanism of SW-induced injury and how the kidney responds to injury. To that end, it is essential to determine if the increase in HO-1 after SWL treatment is ultimately beneficial or deleterious to the SW-treated kidney. Thus, a more thorough knowledge of the action of the HO-1 response on SWL-induced injury might lead to ways to modulate that response, and possibly provide additional protection to tissue during SWL.
What's known on the subject? and What does the study add?
Oxidative stress and inflammation are tissue- and cell-level components of shock wave lithotripsy (SWL)-induced acute renal injury, which we recently showed to be localized principally to the medulla within the focal zone of the lithotripter. This study reports that the magnitude of the oxidative stress and inflammation observed in the medulla after SWL is dependent on the number of shock waves delivered to the kidney, indicating that this is a sensitive measure of renal injury caused by shock waves.
Acknowledgments
The authors express appreciation to Dr Lynn R. Willis for review of the manuscript, to Dr Alex Robling for support on technical aspects of this study, and to Cynthia Johnson for laboratory support. This project was funded in part by PHS grants P01-DK43881 and R01-DK67133.
Abbreviations
- SW(L)
shock wave (lithotripsy)
- IL-6
interleukin-6
- HO-1
heme oxygenase-1
Footnotes
Conflict of Interest: None declared.
References
- 1.McAteer JA, Evan AP. The acute and long-term adverse effects of shock wave lithotripsy. Semin Nephrol. 2008;28:200–13. doi: 10.1016/j.semnephrol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evan AP, Willis LR, Lingeman JE, McAteer JA. Renal trauma and the risk of long-term complications in shock wave lithotripsy. Nephron. 1998;78:1–8. doi: 10.1159/000044874. [DOI] [PubMed] [Google Scholar]
- 3.Janetschek G, Frauscher F, Knapp R, Hofle G, Peschel R, Bartsch G. New onset hypertension after extracorporeal shock wave lithotripsy. age related incidence and prediction by intrarenal resistive index. J Urol. 1997;158:346–51. doi: 10.1016/s0022-5347(01)64475-6. [DOI] [PubMed] [Google Scholar]
- 4.Parks JH, Worcester EM, Coe FL, Evan AP, Lingeman JE. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int. 2004;66:777–85. doi: 10.1111/j.1523-1755.2004.00803.x. [DOI] [PubMed] [Google Scholar]
- 5.Parks JH, Coe FL, Evan AP, Worcester EM. Urine pH in renal calcium stone formers who do and do not increase stone phosphate content with time. Nephrol Dial Transplant. 2009;24:130–6. doi: 10.1093/ndt/gfn420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evan AP, Lingeman JE, Coe FL, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005;67:576–91. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 7.Krambeck AE, Gettman MT, Rohlinger AL, Lohse CM, Patterson DE, Segura JW. Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of followup. J Urol. 2006;175:1742–7. doi: 10.1016/S0022-5347(05)00989-4. [DOI] [PubMed] [Google Scholar]
- 8.Evan AP, McAteer JA, Connors BA, Blomgren PM, Lingeman JE. Renal injury during shock wave lithotripsy is significantly reduced by slowing the rate of shock wave delivery. BJU Int. 2007;100:624–7. doi: 10.1111/j.1464-410X.2007.07007.x. [DOI] [PubMed] [Google Scholar]
- 9.Connors BA, Evan AP, Blomgren PM, et al. Extracorporeal shock wave lithotripsy at 60 shock waves/min reduces renal injury in a porcine model. BJU Int. 2009 Mar 26; doi: 10.1111/j.1464-410X.2009.08520.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willis LR, Evan AP, Connors BA, et al. Shockwave lithotripsy. dose-related effects on renal structure, hemodynamics, and tubular function. J Endourol. 2005;19:90–101. doi: 10.1089/end.2005.19.90. [DOI] [PubMed] [Google Scholar]
- 11.Connors BA, Evan AP, Blomgren PM, et al. Reducing shock number dramatically decreases lesion size in a juvenile kidney model. J Endourol. 2006;20:607–11. doi: 10.1089/end.2006.20.607. [DOI] [PubMed] [Google Scholar]
- 12.Connors BA, Evan AP, Willis LR, Blomgren PM, Lingeman JE, Fineberg NS. The effect of discharge voltage on renal injury and impairment caused by lithotripsy in the pig. J Am Soc Nephrol. 2000;11:310–8. doi: 10.1681/ASN.V112310. [DOI] [PubMed] [Google Scholar]
- 13.Munver R, Delvecchio FC, Kuo RL, Brown SA, Zhong P, Preminger GM. In vivo assessment of free radical activity during shock wave lithotripsy using a microdialysis system: the renoprotective action of allopurinol. J Urol. 2002;167:327–34. [PubMed] [Google Scholar]
- 14.Li B, Zhou W, Li P. Protective effects of nifedipine and allopurinol on high energy shock wave induced acute changes of renal function. J Urol. 1995;153:596–8. [PubMed] [Google Scholar]
- 15.Strohmaier WL, Koch J, Balk N, Wilbert DM, Bichler KH. Limitation of shockwave-induced renal tubular dysfunction by nifedipine. Eur Urol. 1994;25:99–104. doi: 10.1159/000475260. [DOI] [PubMed] [Google Scholar]
- 16.Delvecchio F, Auge BK, Munver R, et al. Shock wave lithotripsy causes ipsilateral renal injury remote from the focal point. the role of regional vasoconstriction. J Urol. 2003;169:1526–9. doi: 10.1097/01.ju.0000049648.13715.4b. [DOI] [PubMed] [Google Scholar]
- 17.Clark DL, Connors BA, Evan AP, Willis LR, Handa RK, Gao S. Localization of renal oxidative stress and inflammatory response after lithotripsy. BJU Int. 2009;103:1562–8. doi: 10.1111/j.1464-410X.2008.08260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonventre JV. Pathophysiology of ischemic acute renal failure. Inflammation, lung-kidney cross-talk, and biomarkers. Contrib Nephrol. 2004;144:19–30. [PubMed] [Google Scholar]
- 19.Hill-Kapturczak N, Chang SH, Agarwal A. Heme oxygenase and the kidney. DNA Cell Biol. 2002;21:307–21. doi: 10.1089/104454902753759726. [DOI] [PubMed] [Google Scholar]
- 20.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999;13:1800–9. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
- 21.Sarica K, Kosar A, Yaman O, et al. Evaluation of ischemia after ESWL: detection of free oxygen radical scavenger enzymes in renal parenchyma subjected to high-energy shock waves. Urol Int. 1996;57:221–3. doi: 10.1159/000282918. [DOI] [PubMed] [Google Scholar]
- 22.Delius M, Enders G, Xuan ZR, Liebich HG, Brendel W. Biological effects of shock waves: kidney damage by shock waves in dogs – dose dependence. Ultrasound Med Biol. 1988;14:117–22. doi: 10.1016/0301-5629(88)90178-0. [DOI] [PubMed] [Google Scholar]
- 23.Neuerburg J, Daus HJ, Recker F, et al. Effects of lithotripsy on rat kidney: evaluation with MR imaging, histology, and electron microscopy. J Comput Assist Tomogr. 1989;13:82–9. doi: 10.1097/00004728-198901000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Evan AP, Willis LR, McAteer JA, et al. Kidney damage and renal functional changes are minimized by waveform control that suppresses cavitation in shock wave lithotripsy. J Urol. 2002;168:1556–62. doi: 10.1016/S0022-5347(05)64520-X. [DOI] [PubMed] [Google Scholar]
- 25.McAteer JA, Evan AP, Williams JC, Jr, Lingeman JE. Treatment protocols to reduce renal injury during shock wave lithotripsy. Curr Opin Urol. 2009;19:192–5. doi: 10.1097/mou.0b013e32831e16e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen TD, Durrani AF, Brown SA, Ferraro R, Preminger GM. Lipid peroxidation induced by shockwave lithotripsy. J Endourol. 1998;12:229–32. doi: 10.1089/end.1998.12.229. [DOI] [PubMed] [Google Scholar]
- 27.Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol. 2007;18:414–20. doi: 10.1681/ASN.2006080894. [DOI] [PubMed] [Google Scholar]
- 28.Basireddy M, Lindsay JT, Agarwal A, Balkovetz DF. Epithelial cell polarity and hypoxia influence heme oxygenase-1 expression by heme in renal epithelial cells. Am J Physiol Renal Physiol. 2006;291:F790–5. doi: 10.1152/ajprenal.00402.2005. [DOI] [PubMed] [Google Scholar]
- 29.Bernardini C, Zannoni A, Turba ME, et al. Heat shock protein 70, heat shock protein 32, and vascular endothelial growth factor production and their effects on lipopolysaccharide-induced apoptosis in porcine aortic endothelial cells. Cell Stress Chaperones. 2005;10:340–8. doi: 10.1379/CSC-98R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang D, Reuter S, Buzescu T, August C, Heidenreich S. Heme-induced heme oxygenase-1 (HO-1) in human monocytes inhibits apoptosis despite caspase-3 up-regulation. Int Immunol. 2005;17:155–65. doi: 10.1093/intimm/dxh196. [DOI] [PubMed] [Google Scholar]