Abstract
C1 catecholamine neurons reside within the rostroventrolateral medulla (RVLM), an area that plays an integral role in blood pressure regulation through reticulospinal projections to sympathetic preganglionic neurons in thoracic spinal cord. In a previous investigation we mapped the efferent projections of C1 neurons, documenting supraspinal projections to cell groups in the preautonomic network that contribute to the control of cardiovascular function. That light microscopic study also revealed putative local circuit connections within RVLM. In this investigation we tested the hypothesis that RVLM C1 neurons elaborate a local circuit synaptic network that permits communication between C1 neurons giving rise to supraspinal and reticulospinal projections. A replication defective lentivirus vector that expresses enhanced green fluorescent protein (EGFP) under the control of a synthetic dopamine beta hydroxylase (DβH) promoter was used to label C1 neurons and their processes. Confocal fluorescence microscopy demonstrated thin varicose axons immunopositive for EGFP and tyrosine hydroxylase that formed close appositions to C1 somata and dendrites throughout the rostrocaudal extent of the C1 area. Dual label electron microscopic analysis revealed axosomatic, axodendritic and axospinous synaptic contacts with C1 and non-C1 neurons with a distribution recapitulating that observed in the light microscopic analysis. Labeled boutons were large, contained light axoplasm, lucent spherical vesicles, and formed asymmetric synaptic contacts. Collectively these data demonstrate that C1 neurons form a synaptic network within the C1 area that may function to coordinate activity among projection-specific subpopulations of neurons. The data also suggest that the boundaries of RVLM should be defined on the basis of function criteria rather than the C1 phenotype of neurons.
Keywords: RVLM, lentivirus vector, dopamine-beta-hydroxylase synthetic promoter, ultrastructure, synaptology, phenotypically defined anterograde tracing
Keywords: Abbreviations: AP, Arterial pressure, DβH, Dopamine beta hydroxylase, EGFP, Enhanced green fluorescent protein, ENK, Enkephalin, LV, Lentivirus, NA, Nucleus ambiguus, NPY, Neuropeptide Y, PLP, Paraformaldehyde-lysine-periodate fixative, RVLM, Rostroventrolateral medulla, SND, Sympathetic nerve discharge, TEM, Transmission electron microscopy, TH, Tyrosine hydroxylase
Studies over the past thirty years have established that the caudal brainstem exerts a profound influence over cardiovascular function (Dampney, 1994, Spyer, 1994, Guyenet, 2006, Schreihofer and Sved, 2011). Although evidence for a “vasomotor center” in the medulla is apparent in early literature, contemporary understanding of the central neural basis of cardiovascular control finds its roots in the 1980s in studies that localized a “pressor area” to a circumscribed region in the rostral ventrolateral medulla (RVLM). These investigations demonstrated that electrical or neurochemical stimulation of RVLM neurons in experimental animals increased arterial blood pressure (AP) while injection of GABA into this region produced a dose-dependent fall in AP and bradycardia (Dampney and Moon, 1980, Ross et al., 1984c). These seminal observations form the foundation for a voluminous literature that has subsequently refined our understanding of the function of this region and placed it within a larger polysynaptic network responsible for adaptive cardiovascular responses to behavioral and environmental challenges. It has also become increasingly apparent that altered function of the RVLM, or the circuitry that it functions within, can contribute to hypertension (Sved et al., 2003, Osborn, 2005, Guyenet, 2006). Thus, characterization of the synaptology through which RVLM exerts regulatory control over cardiovascular function has become increasingly important for defining and treating the pathogenesis that underlies hypertension (Sved et al., 2003).
A large literature has established that the RVLM is composed of a heterogeneous population of neurons distinguished by their projection targets and neurochemical phenotype. The seminal studies of Ross and colleagues (e.g., (Ross et al., 1984a, Ross et al., 1984c) mapped the area of brainstem in which chemical or electrical stimulation elicits a pressor response and demonstrated that the pressor region contained neurons of the C1 catecholamine cell group. Ross and colleagues were careful to note that non-catecholamine neurons contribute to RVLM reticulospinal projections, an important observation that has been confirmed and refined in a number of studies. In this regard, Jeske and McKenna reported that the adrenergic enzyme PNMT is present in ~50% of neurons retrogradely labeled from multiple levels of thoracic spinal cord (Jeske and McKenna, 1992) and it is now well established that both C1 and non-catecholamine RVLM neurons are barosensitive (Sved et al., 1994, Stornetta et al., 2001, Stornetta et al., 2002). Juxtacellular labeling of RVLM neurons after electrophysiological analysis has established that reticulospinal C1 and non-C1 neurons are distinguished by axon conduction velocity (Schreihofer and Guyenet, 1997) while neurochemical and circuit tracing studies have demonstrated that the nucleus is topographically organized with respect to neuronal phenotype and projection patterns. Notably, C1 reticulospinal neurons influencing sympathetic outflow are differentially concentrated in the rostral portion of C1 cell column in an area coextensive with the site that produces the most robust pressor response when stimulated chemically or electrically (Ross et al., 1984c, Tucker et al., 1987, Pyner and Coote, 1998, Schreihofer and Guyenet, 2000, Schreihofer and Sved, 2011) while C1 neurons in caudal RVLM colocalize neuropeptide Y (NPY) and project predominantly to supraspinal targets (Tucker et al., 1987). Despite these differences in projection targets and phenotype between rostral and caudal C1 cells, both populations respond to similar stimuli. Thus, the heterogeneity documented in these and other investigations raise the possibility that integrated output of the nucleus may rely upon local circuit connections between neurochemically-distinct and projection-specific populations of C1 neurons.
The goal of the present study was to test the hypothesis that C1 neurons elaborate a local circuit plexus within ventrolateral medulla that may function to synchronize activity of C1 neurons distinguished by projection targets. Toward this end we characterized the synaptology of local circuit C1 axonal arbors differentially labeled by phenotypically defined, lentivirus-mediated reporter expression driven by a synthetic DβH promoter. In an earlier report we used this technology to map the efferent projections of C1 neurons and demonstrate that they give rise to thin varicose axons that arborize within the immediate vicinity of the C1 population (Card et al., 2006). In the present study, we have expanded upon that observation using confocal microscopic analysis and transmission electron microscopy (TEM) to demonstrate that C1 neurons elaborate a local circuit synaptic network involving C1 and non-C1 neurons throughout the rostrocaudal extent of the C1 column.
Experimental Procedures
Animals
Adult male Harlan Sprague-Dawley rats weighing 230 to 440 grams at the onset of the experiment were used in the analysis. Photoperiod (12 hours light; light on at 0700) and temperature (22–25°C) were standardized and animals had free access to food and water throughout the experiment. Animal experiments were conducted in a laboratory approved for Biosafety Level 2+ experiments. Experimental procedures conformed to regulations stipulated in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Pittsburgh IACUC, Recombinant DNA Committee, and Division of Environmental Health and Safety.
Lentivirus vector
An HIV-1-based lentivirus vector (LV) expressing enhanced green fluorescent protein (EGFP) under the control of a synthetic DβH promoter was used to differentially label C1 neurons and their processes. This synthetic promoter contains 8 copies of a noradrenaline-specific regulatory element (PRSX8) that is activated by the homeodomain transcription factor Phox2 and drives transgene expression to a higher degree than the native DβH promoter (Hwang et al., 2001). The vector was aliquoted and stored at −80° C. Individual aliquots with a titer of 1 × 108 TU/ml were thawed and kept on ice during the surgery. The pipette was loaded with fresh vector for each injection and unused vector was inactivated with Chlorox and discarded. Further details on the construction and characterization of the vector have been published (Card et al., 2006).
Lentivirus vector injections
Nineteen animals were included in the study. Eleven of these animals were used in our initial proof-of-principal study using this vector (Card et al., 2006). Tissue from those animals had been stored at −20° C in cryoprotectant (Watson et al., 1986) to preserve antigenicity and was processed for dual immunofluorescence localization of EGFP and TH. The remaining eight animals were injected with the vector and processed for ultrastructural localization of TH and EGFP using immunoperoxidase and immunogold labels. The procedures for stereotaxic injection of vector in these animals duplicated those described in our previous study (Card et al., 2006). Briefly, following isoflurane induced anesthesia the head was secured in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) with the incisor bar set at 11.0 mm below the interaural line. The dorsal neck musculature was dissected free from the occipital bone and a portion of the bone and the dura mater was removed to expose the floor of the fourth ventricle. 100 nl of vector was injected through a glass pipette inserted into the caudal brainstem at a 20° angle using coordinates calculated from the caudal limit of the area postrema (Card et al., 2006). The LV vector was injected using a Picopump (World Precision Instruments, Inc., Sarasota, FL) and the pipette was left in situ for 5 minutes following completion of the infusion. The pipette was removed slowly, the incision sutured, and a subcutaneous injection of Ketofen (2 mg/kg) was administered. Animals recovered from anesthesia under a heat lamp and were returned to their home cage in the BSL 2+ laboratory where they lived for the balance of the experiment.
Preparation of tissue
Seven days after vector injection the animals were anesthetized with an overdose of sodium pentobarbital and perfused transcardially with buffered aldehyde solutions. Animals included in the light microscopic immunofluorescence analysis were perfused with phosphate buffered physiological saline (PBS) followed by paraformaldehyde-lysine-periodate fixative (PLP) (McLean and Nakane, 1974). Animals included in the ultrastructural analysis were perfused with 250 ml of PBS followed by 500 ml of 2% paraformaldehyde and 0.5% TEM grade glutaraldehyde (Sigma Aldrich, St. Louis, MO) in PBS. For the light microscopic analysis the brainstem was postfixed in PLP, cryoprotected in phosphate buffered sucrose solutions, and sectioned at 35 μm/section using a freezing microtome (Leica Instruments, Germany) fitted with a freezing stage (Physitemp, Clifton NJ). Brainstems processed for TEM analysis were postfixed in the paraformaldehyde-glutaraldehyde fixative for 6 to 12 hours at 4°C, sectioned serially in the coronal plane at 50 μm/section using a vibratome (Technical Products International, Inc., St. Louis MO), and collected serially in 24-well plates containing PBS.
Immunohistochemical reagents
EGFP was detected with a rabbit polyclonal antiserum (Lot 71B1; Molecular Probes; Eugene, OR). This IgG fraction was purified by ion exchange chromatography from an antiserum generated against green fluorescent protein isolated from the jellyfish Aequorea victoria. Specificity of this antibody in our hands was been documented (Billig et al., 2000). C1 neurons were identified with a mouse monoclonal antibody generated against tyrosine hydroxylase (TH; Chemicon, Temecula, CA). Although antisera to phenylethanolamine N-methyl transferase (PNMT) are more selective for the C1 population, we selected TH for this analysis because our parametric studies demonstrated that the TH antibody produces a more complete and robust staining of dendritic arbors than can be achieved with antisera generated against PNMT. Specificity of the TH antibody for catecholamine neurons was previously established (Ross et al., 1984b, Madden et al., 1999, Rinaman, 2001). Both antisera were diluted to 1:10,000 for immunoperoxidase localizations and 1:1,000 for immunofluorescence.
Immunoperoxidase localizations utilized species appropriate affinity purified secondary antibodies (Jackson ImmunoReseach Laboratories, Inc.; West Grove, PA) and Vectastain Elite avidin-biotin reagents (Vector Laboratories; Burlingame, CA). Fluorescence labeling was achieved with Alexafluor 488 and CY3 conjugated affinity purified secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.; West Grove, PA).
Immunofluorescence localizations
Coronal sections at a frequency of 210 μm through the rostrocaudal extent of the brain stem were processed for dual immunofluorescence localization of EGFP and TH. Technical details regarding this processing have been published (Card et al., 2006). Processed sections were mounted onto Superfrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA), dehydrated in graded alcohols, cleared in xylenes, and coverslipped with Cytoseal 60 (Richard-Allan Scientific, Kalamazoo, MI).
TEM immunocytochemical localizations
Alternate vibratome sections through caudal brainstem were processed for light and TEM localization of EGFP and TH. The light microscopic localizations were used as reference for the sections processed for TEM analysis. In each case, the EGFP reporter was visualized with immunoperoxidase labeling using the Molecular Probes rabbit polyclonal antibody at a dilution of 1:10,000; TH was localized using the Chemicon mouse monoclonal antibody at 1:10,000 and silver-intensified immunogold localization. Immunogold labeling was achieved using donkey anti-mouse secondary antibodies conjugated to 5 nm gold particles (AutoProbe One GAM; Amersham Biosciences, Arlington Heights, IL). Gold particles were enhanced with silver using the Inten-SEM Silver Enhancement Kit (Amersham Biosciences) according to the procedure described by Sesack and colleagues (Sesack et al., 2006). Sections were then postfixed in 1% osmium tetroxide for 30 minutes, washed in repeated changes of 0.1 M phosphate buffer, and dehydrated in a graded ethanol series. The sections were then passed through multiple changes of acetone followed by sequential changes of increasing concentrations of Epon-Araldite plastic resin diluted in acetone (1:1 for 6 hours; 3:1 overnight). After a final change in 100% resin for 10 hours the sections were flat-embedded between acrylic sheets and the plastic was polymerized overnight at 60°C.
Immunofluorescence analysis
Each section was initially examined with an Olympus BX51 epifluorescence microscope to determine the distribution of EGFP+ immunopositive cells and processes and the equivalence of staining to that observed in our published study. We then examined a subset of sections that sampled rostral, intermediate, and caudal levels of the C1 area (figure 1A) using a 20× water objective and a Leica TCS SP5 II confocal microscope (Leica Microsystems, Buffalo Grove, IL). Stacks of 24 optical planes were obtained from 1 to 3 areas within each section. We also obtained a collapsed projection image of each stack. The projection image from each stack was analyzed using Adobe Photoshop software. Potential appositions (axosomatic and axodendritic) between dual labeled (EGFP+ and TH+) axons and TH+ profiles (both single and double labeled with EGFP) were confirmed using the Photoshop channel tool in individual optical planes.
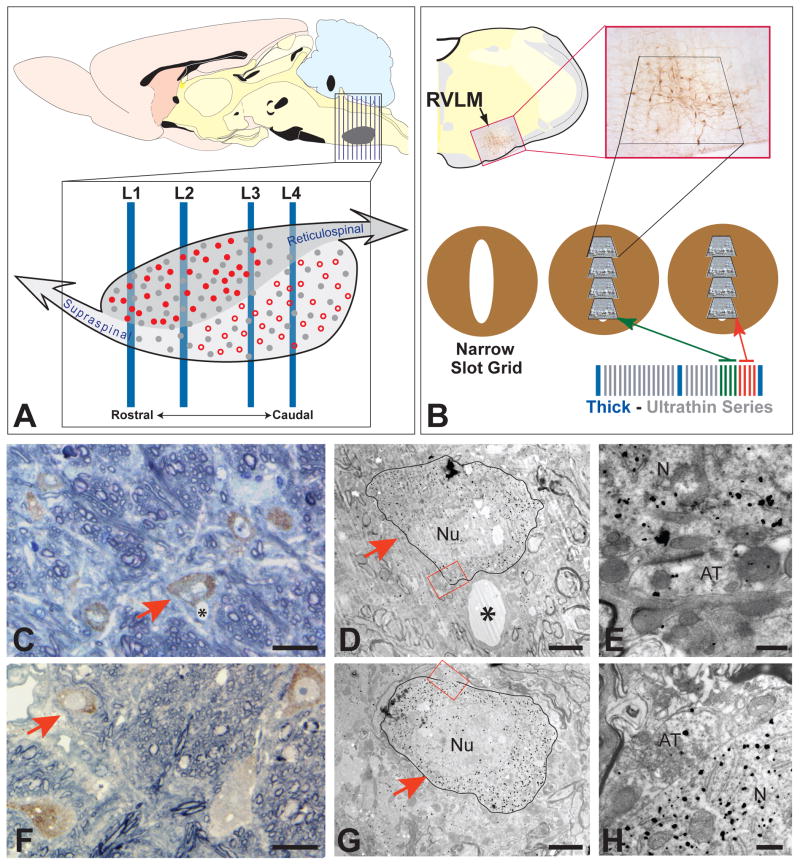
Figure 1.
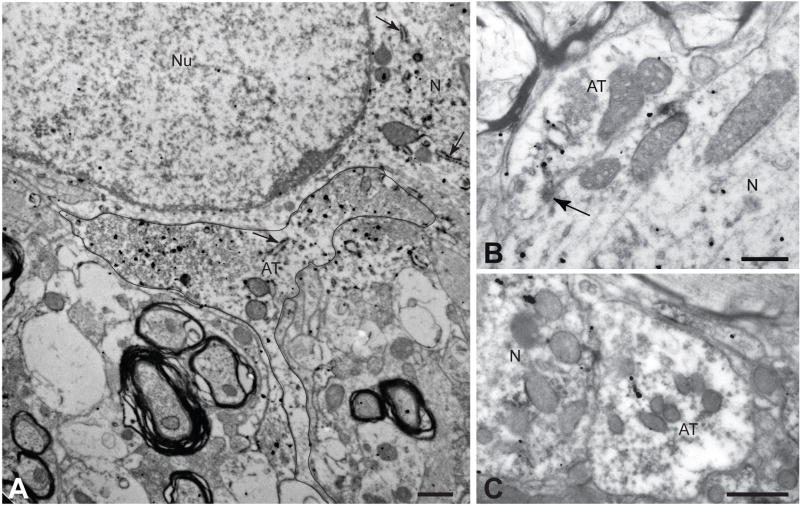
The experimental design incorporated in the TEM analysis and data typical of that collected for each case is illustrated. The relative position of the C1 cell group in the brainstem, as well as the four levels of analysis (L1, L2, L3 & L4) selected for TEM sampling, are illustrated schematically in figure 1A. The approximate location of the C1 column is shown by the gray area in the sagittal schematic at the top of A; vertical lines extending through the rostrocaudal extent of the nucleus in that diagram illustrate the extent of coronal vibratome sections collected for analysis. The representation of the C1 cell column shown in the expansion at the bottom of A schematically illustrates the two projection-specific and phenotypically-defined populations of neurons known to populate this region. Filled red circles represent C1 reticulospinal neurons that are concentrated at rostral levels of the nucleus. The open red circles represent C1 neurons that colocalize neuropeptide Y, are concentrated caudally in cell column, and project to supraspinal targets. The filled grey circles represent non-C1 neurons that contribute to both pathways. In figure 1B neurons immunopositive for TH illustrate the position of the C1 column in the ventrolateral medulla and delineate the area cut from the vibratome section for TEM analysis. The lower portion of the schematic illustrates the sectioning strategy incorporated in the analysis to ensure precise localization of labeled profiles within the area of C1. Series of ultrathin sections bracketed by toluidine blue stained thick sections were collected on formvar-coated narrow slot copper grids. Landmarks (e.g., blood vessels, labeled neurons) visible in the thick sections were then identified in the adjacent ultrathin sections and the position of labeled contacts was recorded. Figures C – E and F – H provide two examples of the correlation between light and TEM observations that this approach enabled. The red arrows identify the same labeled neurons in toluidine blue stained sections and the same cells are outlined in adjacent ultrathin sections. The red boxed areas in figures D and G define the location of the axosomatic contacts shown in figures E and H, respectively. See text for a more detailed description. The schematic images in figures A and B are adapted from the Swanson rat brain atlas (Swanson, 1998). AT = axon terminal, N = neuron cytoplasm, Nu = nucleus; asterisks identify blood vessels. Marker bars = 15 μm for A & F, 3 μm for D & G, and 0.5 μm for E & H.
TEM analysis
Figure 1 illustrates the approach used for the collection of TEM data. Flat embedded tissue from four coronal planes sampling the rostrocaudal extent of the C1 area (figure 1A) was prepared for ultrastructural analysis in the following manner. The location of immunopositive cells and processes in ventrolateral medulla was determined by examining transilluminated flat embedded sections using a stereomicroscope (Olympus SZX10 Research Stereomicroscope). The acrylic sheets were then pealed from the polymerized resin of infiltrated tissue and the area containing the immunopositive profiles was cut from the section and glued to a blank stub. The sample was trimmed to a trapezoid (figure 1B) using a Leica Ultracut E ultramicrotome and excess resin was cut from the face of the block using a diamond knife (Diatome, Hatfield, PA). Series of ultrathin sections (~600 angstroms) bracketed by 0.35 μm thick sections were cut from the top 10 μm of each sample (figure 1B). The thick sections were mounted on microscope slides and counter stained with Toluidine blue with mild heating. Immediately adjacent ultrathin sections were cut and collected on formvar coated thin slot grids (figure 1B) and stored in serial order in grid boxes.
Toluidine blue stained thick sections were examined for immunopositive profiles using a light microscope. Those that contained the largest concentration of immunopositive profiles were photographed and the images were used as a reference for ultrastructural analysis of adjacent thin sections (figures 1C and 1F). In this fashion we were able to define the precise position of areas analyzed at the ultrastructural level with respect to both the dorsoventral and mediolateral dimensions of the C1 column. Ultrathin sections were analyzed unstained using a transmission electron microscope (TEM; Morgagni, FEI, Hillsboro, OR) equipped with a CCD camera (Advanced Microscopy Techniques, Danvers, MA). Insertion of objective apertures into the path of the electron beam provided the necessary contrast for identification of landmarks and labeled profiles. The area shown to contain immunopositive profiles in the adjacent thick section was scanned systematically at 14,000× magnification. Landmarks (e.g., blood vessels; figure 1C & D) visible in the thick sections were located in the thin sections to ensure that all regions containing immunopositive profiles were included in the TEM analysis. This approach ensured comprehensive sampling of each of the four vibratome sections selected for analysis and also allowed precise determination of the area within the C1 column that was sampled. Selected immunopositive profiles exhibiting the best morphology were photographed at higher magnification. These included immunopositive perikarya photographed in the low magnification scans of the thin sections (figures 1D and 1G) as well as high magnification images of appositions and synapses involving labeled profiles (figures 1E and 1H). This approach also allowed us to identify the same profiles in serial series of ultrathin sections. This was particularly valuable since the intensity of labeling of profiles decreased the deeper the section was with respect to the surface of the vibratome section. Thus, we could identify a heavily labeled profile in a superficial section and then identify the same, more lightly labeled profile in deeper ultrathin sections to gain further insight into its morphology and synaptology.
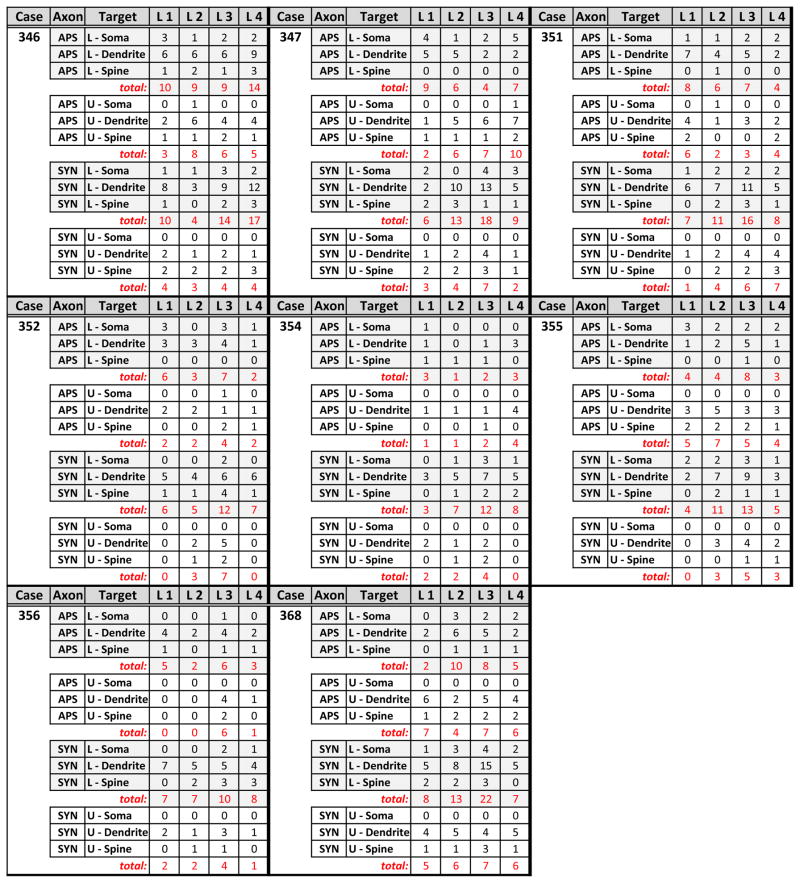
Quantitative data was obtained from one ultrathin section taken from each vibratome section that contained the most extensive immunocytochemical labeling. Each immunopositive profile encountered in a systematic 14,000× scan of the section was classified and recorded on an excel spreadsheet. These included labeled axons, somata, dendrites and spines involved in synaptic contacts or appositions. The data were compiled within Table 1 and figure 5 to demonstrate the rostrocaudal distribution of labeled profiles in the C1 column.
Table 1. Ultrastructural Analysis of Relations of Labeled Profile.
Quantitative data showing the number of contacts (appositions and synapses) between EGFP/TH+ axons and postsynaptic profiles for the 8 cases included in the TEM analysis is presented. The data were collected from one ultrathin section from each case containing robust immunocytochemical labeling. In each case, the full extent of the ultrathin section was systematically examined and all contacts involving EGFP/TH+ axons were classified according to their postsynaptic target, which included immunopositive somata, dendrites or spines of C1 neurons (TH alone or with EGFP labeling) or unlabeled (U) somata, dendrites or spines. Contacts were characterized as either synapses (SYN) or appositions (APS) in which the labeled axon directly contacted the postsynaptic element but did not display a synapse. Contacts were further subdivided based upon their rostrocaudal distribution in the C1 cell column (L1, L2, L3 & L4; see figure 1A).
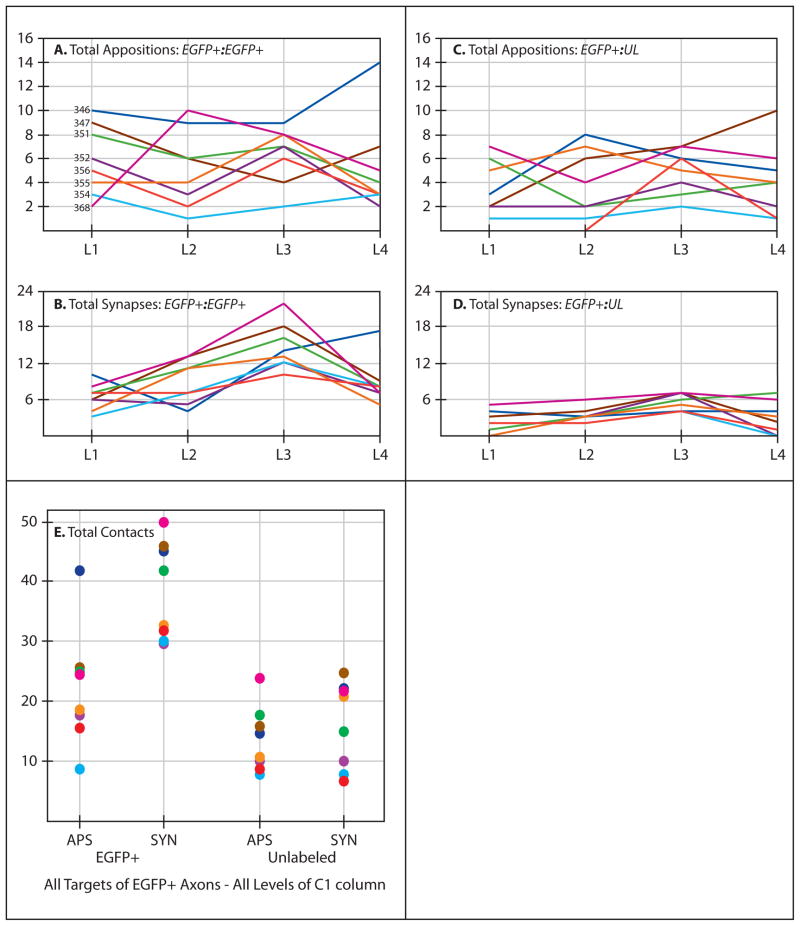
Figure 5.
Graphical representation of the data from Table 1 demonstrates that appositions and synapses of C1 axons with C1 somata, dendrites, and spines were observed throughout the rostrocaudal extent of the C1 column (A & B). Contacts between labeled axons and non-C1 neurons were also present at all four levels of the rostrocaudal axis (C & D). The numbers in A identify the eight cases included in the TEM analysis and color coding for each case is maintained throughout all of the figures. L1 – L4 on the X axis of figures A through D refers to the four levels of analysis of the C1 column defined in figure 1A. Figure E presents the total number of contacts in all for levels of analysis for each case classified according to postsynaptic target on the X axis (EGFP+ or Unlabeled) and type of contact (apposition or synapse). Note that there was a higher prevalence of synaptic contacts observed between EGFP+ pre- and postsynaptic elements.
RESULTS
Systematic examination of EGFP localization confirmed the differential expression of the reporter within C1 neurons and their processes. Three issues should be highlighted in evaluating these data. First, light and TEM analyses demonstrated that greater than 96% of EGFP immunopositive neurons in the ventrolateral medulla were also immunopositive for TH. The small number of EGFP+ neurons that did not contain TH likely resulted from leaky expression at the core of the injection due to high titers of the vector (e.g., see figures 2A and 2a″). Nevertheless, the prominent differential expression of EGFP in C1 neurons confirms the pattern of expression documented in our earlier study (Card et al., 2006) and is consistent with the restricted expression of the Phox2a transcription factor, which drives activity of the synthetic DβH promoter within C1 neurons (Card et al., 2006, Card et al., 2010). Second, the use of TH rather than PNMT raises the possibility that the EGFP reporter was expressed in a subset of A1 neurons that slightly overlap the caudal pole of RVLM. Although we cannot totally exclude this possibility we are well versed in evaluating the RVLM-A1 transition (Card et al., 2006) and eliminated any case in which EGFP+ neurons were observed at the level of, or caudal to, the obex. However, it should be emphasized that dendrites of A1 neurons extend into RVLM and it is therefore possible that some of the TH+ dendrites that we identified arose from A1 neurons. Third, two cases included in the light microscopic analysis also exhibited immunopositive neurons within the adjacent nucleus ambiguus (NA). This resulted from injections in which vector spread to the NA, whose cholinergic neurons also express Phox2a (Tiveron et al., 1996, Card et al., 2010). It is well established that axons of NA neurons leave the brainstem to innervate the esophagus. However, Schreihofer and Guyenet identified a cell exhibiting vagal motor neuron morphology whose axon collateralized in the area of the dorsal RVLM (see figure 8 of Schreihofer and Guyenet, 2003). Thus, it is possible that EGFP+ ambiguus neurons may have contributed labeled axons to the RVLM in the two cases where vector spread to the NA. We did not observe such collaterals in the light microscopic analysis of our material and the data collected from these animals not differ from that of other cases in which EGFP was not expressed in NA neurons. Therefore, we included these two animals in the analysis. While acknowledging these three caveats it is apparent that the majority of EGFP+/TH+ profiles observed in our analysis reflect labeling of C1 neurons, dendrites, and axons in RVLM.
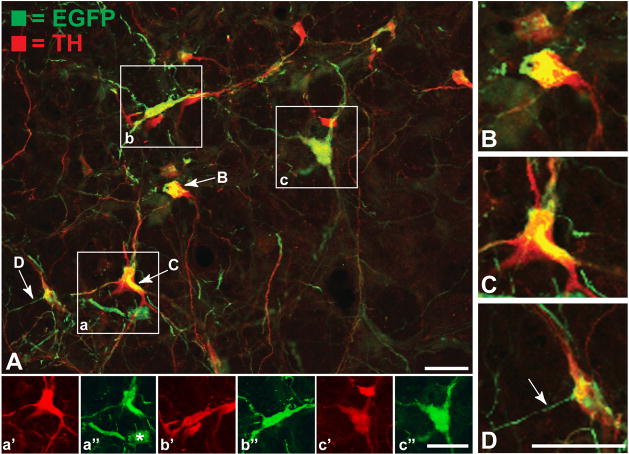
Figure 2.
Representative confocal images of immunofluorescence localizations of EGFP (green) and tyrosine hydroxylase (red) in the C1 column are illustrated. Figure 2A is a collapsed projection image of a stack of 24 confocal optical planes obtained near the vector injection site in the rostral portion of RVLM corresponding to L2 in figure 1A. Figures 2B through 2E are higher magnification images of subfields of the stack taken from individual optical planes; the position of each image in the stack is identified in figure 2A. Thin varicose EGFP+ process resembling axons are present within the field and many of the varicose expansions of these axons form intimate appositions with C1 neurons. Axosomatic contacts were distinguished by extremely large varicosities that conformed to the shape of labeled somata. Numerous examples of EGFP+ axons contacting dendrites of C1 neurons were also observed. The boxed areas in figure 2A (labeled a, b & c) are shown in the insets at the bottom of the figure. The red and green color channels for each of the boxed regions are shown separately to illustrate the extent of EGFP and TH colocalization. Quantitative assessment demonstrated that >96% of EGFP+ neurons also exhibited TH immunoreactivity. A small number of neurons exhibited EGFP labeling with no detectable TH (e.g., asterisk labeled cell in 2a′). Such cells were found in the vicinity of the injection site where concentrations of vector were highest. Marker bar = 100 μm for A; the magnification of figure B – D are the same with the marker bar in D = 50 μm; the magnification for the insets below figure 2A is the same with the marker bar in 2c′ = 50 μm.
It is also important to note that our prior published data using this vector demonstrated that it is only expressed in Phox2-containing neurons at the site of the injection. This is important since RVLM is innervated by other Phox2a-containing catecholamine neurons (Sun and Guyenet, 1986, Blessing et al., 1987, Van Bockstaele et al., 1989, Madden et al., 1999, Card et al., 2010). Thus, we can exclude the possibility that EGFP+ axons in the C1 column resulted from retrograde transport and expression of the vector in distant catecholamine afferent neurons.
Immunofluorescence analysis
EGFP+ neurons reliably sampled the C1 area in the eight cases analyzed from our prior investigation (Card et al., 2006). Consistent with the findings of that report EGFP expression was of variable intensity within different neurons, intensely labeling the entire somatodendritic compartment of the majority of neurons while producing relatively light labeling of the soma and primary dendrites in a minority. Importantly, only a subset of TH neurons expressed the reporter.
In every case we observed thin varicose EGFP+ axons arborizing within an area coextensive with the C1 neurons. These fibers branched to form terminal arbors with varicosities forming intimate associations with somata and dendrites of TH+ profiles, many of which also contained EGFP immunoreactivity. Figure 2 illustrates a projection stack of images typical of those observed in the confocal analysis. Examination of the 24 optical planes within the stack revealed intimate appositions between these fibers and neurons expressing TH, with or without the reporter. Putative boutons formed large axosomatic appositions with a shape that conformed to that of the apposed somata (e.g., figures 2B–D). In some instances a single fiber gave rise to multiple contacts with the same soma. Similar appositions occurred between EGFP/TH+ varicosities and dendrites containing EGFP and/or TH immunoreactivity. Such contacts were observed on dendrites of all sizes and individual fibers often formed multiple contacts with the same dendrite. Notably, varicose axonal arbors were also observed in areas that did not contain TH+ neurons, consistent with the possibility that labeled axons also contacted non-catecholaminergic neurons.
Some insight into the origin and distribution of EGFP+ axons within the C1 column was apparent in two cases in which EGFP+ neurons were concentrated within a restricted portion of the ventrolateral medulla. For example, the small number of EGFP+ neurons observed in case NI24 were concentrated in the rostral portion of the column, but thin varicose EGFP+ axons were observed in caudal extent of the C1 cell group. It is important to note that shrinkage of dendrites can produce beaded dendrites that resemble axons and we therefore conducted the TEM analysis to confirm the presence of EGFP+ axons within RVLM and to define their synaptology.
Ultrastructural analysis
The TEM analysis confirmed the presence of EGFP/TH+ axons coextensive with the distribution of C1 neurons. Immunopositive boutons were present throughout the rostrocaudal extent of the nucleus and formed synaptic connections with TH+ somata and dendrites as well as unlabeled profiles. All profiles exhibiting EGFP immunoperoxidase reaction product also contained silver intensified immunogold labeling indicative of TH immunoreactivity, emphasizing the high degree (>96%) of colocalization of these markers. TH immunogold labeling was only considered to be specific if the number of gold particles in a profile exceeded the background label. The nucleus of TH neurons served as an index of background labeling since TH is confined to the cell cytoplasm (figures 1C & F).
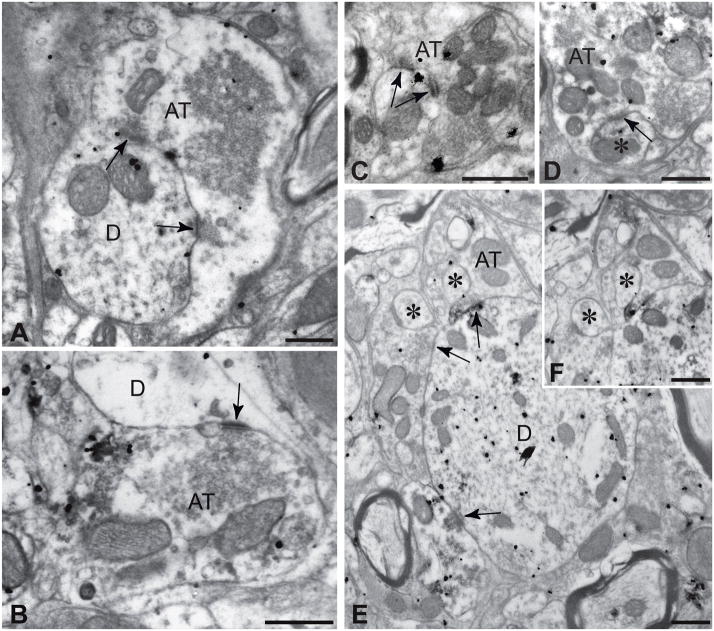
Immunopositive boutons exhibited the same ultrastructural features previously documented for PNMT+ terminals in RVLM (Milner et al., 1988a, Milner et al., 1989a, Aicher et al., 2001). Each axonal varicosity contained electron lucent axoplasm and concentrations of 40 nm lucent spherical vesicles (figures 3 & 4). Vesicles were typically concentrated within large aggregates in the center of the terminal and in smaller clusters at the presynaptic membrane of synaptic contacts. All synaptic contacts were asymmetric with prominent postsynaptic densities. These features are shown in figures 3 and 4, which are of lightly labeled terminals forming axosomatic (figures 3A–C), axodendritic (figures 4A–C & E), and axospinous (figures 4D & E) contacts. It was also notable that a subset of EGFP+ boutons formed multiple synaptic contacts with individual postsynaptic elements, especially when those contacts involved somata and proximal dendrites.
Figure 3.
Electron micrographs of three representative examples of axosomatic contacts between labeled profiles in the C1 column are illustrated. The immunogold label reflects localization of TH and the flocculent electron dense immunoperoxidase label commonly affiliated with cytoplasmic membrane profiles (arrows in 3A) is reflective of EGFP immunoreactivity. The black line traces the plasma membrane of an EGFP/TH+ axon that expands to form a contact with a double labeled C1 neuron. Figures 3B and 3C are through portions of labeled terminals forming axosomatic contacts. The terminal in 3B forms an asymmetric axosomatic synaptic contacts (black arrow) typical of those formed by labeled profiles. Note that the terminals exhibit lucent axoplasm, aggregates of lucent spherical vesicles within the terminal as well as in presynaptic aggregates opposite thickened postsynaptic densities. AT = axon terminal, N = neuron cytoplasm, Nu = nucleus. Marker bars = 0.5 μm.
Figure 4.
Electron micrographs demonstrating asymmetric axodendritic (A – C) and axospinous (D – F) synaptic contacts (arrows) between labeled profiles are illustrated. In each case the morphology of terminals recapitulated that observed for axosomatic contacts and all synapses were asymmetric. Axodendritic contacts were observed on all portions of the dendritic tree, including small distal dendrites (C). Although spines (asterisks) were not prolific within our sample, those that were encountered commonly received asymmetric synaptic contacts (D – F). Figure 4F is from a serial section adjacent to the ultrathin section that produced the image in 4E. AT = axon terminal, D = dendrite, asterisks identify spines. Marker bars = 0.5 μm.
The size, distribution, and morphology of axosomatic contacts conformed to the putative appositions documented in the immunofluorescence analysis. Axosomatic boutons were typically quite large and often formed multiple synaptic contacts with the same soma. Figure 3A illustrates a bouton typical of those observed terminating upon C1 neurons. The outlined presynaptic axon expands dramatically to generate a large varicose bouton that forms a continuous apposition with the postsynaptic neuron. Examples of sections through smaller portions of such terminals (figures 3B & C) revealed asymmetric axosomatic synaptic contacts with labeled neurons.
We also observed EGFP/TH+ boutons synapsing upon profiles that were not TH immunopositive. These profiles were observed among areas of neuropil containing many densely labeled cells and dendrites, supporting the conclusion that the postsynaptic cells and processes represent the non-catecholaminergic population of neurons known to reside within the C1 column.
Morphometric analysis demonstrated that EGFP/TH+ axons synapsed upon C1 neurons and their processes throughout the rostrocaudal extent of the C1 cell group. Table 1 illustrates the number of labeled axons that formed either appositions or synapses with somata, dendrites or spines in a single robustly labeled ultrathin section from each case. The same data are shown in graphical representations in figure 5. Figures 5A and B demonstrate that appositions and synapses of labeled axons upon labeled somata, dendrites and spines were distributed through the rostrocaudal extent of the C1 column in each case. When these contacts were subdivided based upon the postsynaptic target (soma, dendrite, spine) it was apparent that the majority of contacts were axodendritic (Table 1). Appositions and contacts between EGFP+ axon terminals and unlabeled postsynaptic targets were similarly distributed across the rostrocaudal extent of the C1 cell column and terminated predominantly upon dendrites (Table 1 and figure 5C & D). A particularly interesting aspect of the morphometric analysis related to the prevalence of synaptic contacts between EGFP+ axons and postsynaptic targets that were either EFGP+ (C1) or unlabeled (non-C1). When these classes of synaptic contacts were grouped across the four rostrocaudal planes sampled in the analysis, the prevalence of synaptic contacts upon C1 postsynaptic targets was higher than observed on unlabeled postsynaptic profiles (figure 5E). These may be related to the tendency, noted above, for EGFP+ axons to form multiple synaptic contacts with EGFP+ postsynaptic targets.
Collectively the data show that a) appositions and synapses between EGFP+ axons and EGFP+ postsynaptic profiles are present throughout the rostrocaudal extent of the C1 cell column, b) EGFP+ axons also form appositions and synaptic contacts with unlabeled (non-C1) postsynaptic profiles at all rostrocaudal levels of the C1 cell column, and c) synaptic contacts between EGFP+ pre- and postsynaptic profiles were observed more often than those between EGFP+ axons and unlabeled postsynaptic targets.
DISCUSSION
The data reported in this correlative light and electron microscopic investigation reveals a local circuit arborization of C1 axons that is presynaptic to both C1 and non-C1 neurons throughout the C1 cell column. Light microscopic data revealed thin varicose axons that were labeled by the EGFP reporter of C1 neurons and arborized in an area coextensive with the C1 cell group. Importantly, restricted labeling of C1 neurons in either the rostral or caudal RVLM revealed axons that projected into portions of the C1 column that did not contain infected neurons. The light microscopic data were complemented by ultrastructural localizations demonstrating labeled axonal varicosities that exhibited morphology consistent with that previously shown for adrenergic terminals within RVLM (Milner et al., 1989a, Aicher et al., 2001) and formed asymmetric synaptic contacts with both C1 and non-C1 neurons in all regions of the C1 cell column. The data are also consistent with the findings of Lipski and colleagues in which fills of barosensitive bulbospinal neurons in rostral RVLM revealed local collaterals terminating in RVLM (Lipski et al., 1995). Collectively, these data provide strong evidence for a local circuit plexus that coordinates activity throughout the C1 column.
The fact that the C1 local circuit plexus arborizes throughout the C1 cell column is important in light of studies that have demonstrated that C1 neurons are organized topographically with respect to neurochemical phenotype and efferent projections. Several studies combining retrograde tract tracing with phenotypic characterization have documented this topography. Such studies have shown that C1 reticulospinal neurons are differentially concentrated in the rostral portion of the C1 column, in an area coextensive with the site that produces the most robust pressor response when stimulated (Ross et al., 1984c, Tucker et al., 1987, Pyner and Coote, 1998, Schreihofer and Guyenet, 2000, Abbott et al., 2012). Other studies have shown that C1 and non-C1 neurons are further subdivided on the basis of peptide content. For example, enkephalin+ (ENK) neurons are present in the ventrolateral medulla (Khachaturian et al., 1983) and a subset of these neurons colocalize PNMT (Ciccatelli et al., 1989, Milner et al., 1989b). Quantitative analysis of preproenkephalin mRNA in neurons retrogradely labeled from thoracic cord demonstrated that ~20% of C1 and ~71% of non-C1 reticulospinal neurons are enkephalinergic (Stornetta et al., 2001). Varying degrees of colocalization of C1 neurons with calbindin (Granata and Chang, 1994, Goodchild et al., 2000), substance P (Lorenz et al., 1985, Pilowsky et al., 1986, Milner et al., 1988b), cocaine- and amphetamine-regulated transcript (Dun et al., 2002, Burman et al., 2004), and neuropeptide Y (Härfstrand et al., 1987, Minson et al., 1994, Stornetta et al., 1999) have also been reported.
The caudally concentrated C1 neurons that colocalize NPY are of particular interest because of their major contribution to supraspinal projections. Quantitative analysis in rat revealed that NPY contributes ~1% of the monosynaptic input to the IML (Llewellyn-Smith et al., 1990) and colocalization studies revealed that C1-NPY neurons at caudal levels of the nucleus are a small subset of the reticulospinal projection (Minson et al., 1994, Stornetta et al., 1999). Quantitative analysis has also shown that ~9% of reticulospinal C1 neurons contain NPY mRNA, while ~96% of C1 neurons projecting to hypothalamus contained NPY mRNA (Stornetta et al., 1999). Interestingly, Tucker and colleagues reported that C1 neurons project to either spinal cord or hypothalamus, but rarely to both (Tucker et al., 1987). Collectively, these data have been interpreted as evidence that the reticulospinal C1 neurons differ phenotypically from those that project to hypothalamus and that the two populations are largely segregated by their projection targets. The data presented in our study suggest that the local circuit connections of C1 neurons may function to coordinate activity within the distributed network of CNS neurons innervated by the projection specific subsets of C1 neurons.
It is well established that both C1 and non-C1 reticulospinal RVLM neurons exhibit cardiac- and respiratory-related activity (McAllen, 1985, 1986b, c, a, 1987, Haselton and Guyenet, 1989, Guyenet, 1990). Some investigations suggest that this activity is pacemaker related (Sun et al., 1988a, Sun et al., 1988b, Guyenet, 1990, Kangrga and Loewy, 1995) while others have advanced a “network hypothesis” in which individual action potentials are driven by excitatory postsynaptic potentials (Barman and Gebber, 1987, Lipski et al., 1996). Both theories are consistent with an integrative function for C1 neurons that would enable coordinated activity throughout the C1 column as well as within the larger network of central neurons devoted to control of cardiovascular function. A role for adrenergic stimulation in coordinating this activity within RVLM was demonstrated by Piquet and Schlichter (Piquet and Schlichter, 1998). These investigators recorded from regularly-discharging RVLM neurons in slice preparations obtained from young adult rats and demonstrated that this neuronal activity was labile; e.g., >80% of regularly-discharging neurons became spontaneously quiescent during the recording period. They further demonstrated that superfusion of noradrenaline increased the excitability of >80% of these neurons, a response that was concentration dependent, reversible, and blocked by adrenergic receptor blockers. These findings are consistent with the prior demonstrations that central catecholamines are involved in the generation of the 10 Hz rhythm in sympathetic nerve discharge (SND) (Orer et al., 1996) and that intracisternal injection of the glutamate receptor antagonist does not desynchronize SND in rat or cat (Gebber et al., 1989). In addition, Milner and colleagues report that a2A-adrenoreceptor immunoreactivity is present in the soma and proximal dendrites of C1 and non-C1 perikarya neurons (Milner et al., 1999). Considered with data documenting localization of other adrenoreceptor subtypes in RVLM (Nicholas et al., 1996, MacDonald et al., 1997, Piquet and Schlichter, 1998) it is clear that catecholamines are influential in determining the activity of RVLM neurons and the local plexus of C1 terminals described here may be contribute to that activity.
The full extent of the local circuit plexus within RVLM cannot be determined on the basis of our data. However, it is useful to note that data presented in Table 1 and figure 5 were collected from a single ultrathin section from each case that approximated 600 angstroms in thickness. The fact that synaptic profiles were observed as frequently as they were in sections so thin indicates that the synaptic connections between C1 neurons in the nucleus are relatively common. Nevertheless, greater insight into the extent of the local circuit plexus awaits a larger sample that permits quantitative assessment of the number of C1 axon terminals that occur within RVLM.
In recent years the RVLM has become increasingly defined on the basis of the C1 phenotype. However, it is now apparent that the reticulospinal projection is heterogeneous with respect to phenotype and that caudal C1 neurons project supraspinally. Furthermore, selective lesion of C1 neurons does not eliminate the ability of RVLM to regulate arterial pressure. These observations suggest a larger role for the C1 cell group in the central control of cardiovascular function and also argue that the RVLM should be defined on the basis of its influence upon hemodynamics rather than the distribution of the C1 population. This conforms with classic literature that defined RVLM as a vasomotor center and contemporary efforts to more tightly define the boundaries of the RVLM based upon the ability of resident neurons to alter arterial pressure (Schreihofer and Sved, 2011). This view also conforms to the increasing evidence for functional segregation of the ventrolateral medulla based upon differential projections. This is clear from the documented differential projections of rostral and caudal C1 neurons demonstrated by a number of laboratories. Additionally, a recent paper from Stornetta and colleagues demonstrated that cholinergic neurons at the medial aspect of RVLM project differentially to areas involved in sensory transduction but not to SPGs in the IML (Stornetta et al., 2012). Given these insights, we support a restrictive definition of RVLM in which neurons are baroreceptive, give rise to a reticulospinal projection to IML, and produce elevations in arterial pressure when stimulated. We interpret the local circuit synaptic plexus among C1 neurons in the ventrolateral medulla as part of the distributed network targeting other CNS cell groups influential in the regulation of cardiovascular function (Card et al., 2006). In this context, the coordinated activity achieved among neurons in this network would contribute to adaptive behavioral and physiological responses to challenges encountered in the environment.
Highlights.
Phenotypically defined anterograde tracing defines projections of C1 neurons.
C1 catecholamine neurons in ventrolateral medulla elaborate local circuit axons.
Axons of C1 neurons arborize throughout the rostrocaudal extent of the C1 column.
Ultrastructural analysis reveals synaptic contacts between C1 and non-C1 neurons.
Acknowledgments
These data were presented at the 2011 Society for Neuroscience meeting. Funding for the investigation was provided from NIH grants HL093134 (JPC) and HL33610 (MR). We gratefully acknowledge the expert technical assistance of Karina Steren.
Abbreviations
- AP
Arterial pressure
- DβH
Dopamine beta hydroxylase
- EGFP
Enhanced green fluorescent protein
- ENK
Enkephalin
- LV
Lentivirus
- NA
Nucleus ambiguus
- NPY
Neuropeptide Y
- PLP
Paraformaldehyde-lysine-periodate fixative
- RVLM
Rostroventrolateral medulla
- SND
Sympathetic nerve discharge
- TEM
Transmission electron microscopy
- TH
Tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abbott SB, Kanbar R, Bochorishvili G, Coates MB, Stornetta RL, Guyenet PG. C1 neurons excite locus coeruleus and A5 noradrenergic neurons along with sympathetic outflow in rats. Journal of Physiology. 2012;590:1–19. doi: 10.1113/jphysiol.2012.232157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher SA, Kraus JA, Sharma S, Patel A, Milner TA. Selective distribution of mu-opioid receptors in C1 adrenergic neurons and their afferents. Journal of Comparative Neurology. 2001;433:23–33. doi: 10.1002/cne.1122. [DOI] [PubMed] [Google Scholar]
- Barman SM, Gebber GL. Lateral tegmental field neurons of cat medulla: A source of basal activity of ventrolateral medullospinal sympathoexcitatory neurons. Journal of Neurophysiology. 1987;57:1410–1424. doi: 10.1152/jn.1987.57.5.1410. [DOI] [PubMed] [Google Scholar]
- Billig I, Foris JM, Enquist LW, Card JP, Yates BJ. Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains of pseudorabies virus. Journal of Neuroscience. 2000;20:7446–7454. doi: 10.1523/JNEUROSCI.20-19-07446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW, Hedger SC, Joh TH, Willoughby JO. Neurons in the area postrema are the only catecholamine-synthesizing cells in the medulla or pons with projections to the rostral ventrolateral medulla (C1-area) in the rabbit. Brain Research. 1987;419:336–340. doi: 10.1016/0006-8993(87)90604-4. [DOI] [PubMed] [Google Scholar]
- Burman KJ, Sartor DM, Verberne AJ, Llewellyn-Smith IJ. Cocaine- and amphetamine-regulated transcript in catecholamine and noncatecholamine presympathetic vasomotor neurons of rat rostral ventrolateral medulla. Journal of Comparative Neurology. 2004;476:19–31. doi: 10.1002/cne.20198. [DOI] [PubMed] [Google Scholar]
- Card JP, Lois J, Sved AF. Distribution and phenotype of Phox2a-containing neurons in the adult Sprague-Dawley rat. Journal of Comparative Neurology. 2010;518:2202–2220. doi: 10.1002/cne.22327. [DOI] [PubMed] [Google Scholar]
- Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. Journal of Comparative Neurology. 2006;499:840–859. doi: 10.1002/cne.21140. [DOI] [PubMed] [Google Scholar]
- Ciccatelli S, Millhorn DE, Hokfelt T, Goldstein M. Evidence for the occurrence of an enkephalin-like peptide in adrenaline and noradrenaline neurons of the rat medulla oblongata. Experimental Brain Research. 1989;74:631–640. doi: 10.1007/BF00247366. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Moon EA. Role of ventrolateral medulla in vasomotor response to cerebral ischemia. American Journal of Physiology. 1980;239:H348–H358. doi: 10.1152/ajpheart.1980.239.3.H349. [DOI] [PubMed] [Google Scholar]
- Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiological Reviews. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- Dun SL, Ng YK, Brailoiu GC, Ling EA, Dun NJ. Cocaine- and amphetamine-regulated transcript peptide-immunoreactivity in adrenergic C1 neurons projecting to the intermediolateral cell column of the rat. Journal of Chemical Neuroanatomy. 2002;23:123–132. doi: 10.1016/s0891-0618(01)00147-8. [DOI] [PubMed] [Google Scholar]
- Gebber GL, Barman SM, Zviman M. Sympathetic activity remains synchronized in the presence of glutamate antagonist. American Journal of Physiology. 1989;256:R722–R732. doi: 10.1152/ajpregu.1989.256.3.R722. [DOI] [PubMed] [Google Scholar]
- Goodchild AK, Llewellyn-Smith IJ, Sun Q-J, Chalmers J, Cunningham AM, Pilowsky PM. Calbindin-immunoreactive neurons in the reticular formation of the rat brainstem: catecholamine content and spinal projections. Journal of Comparative Neurology. 2000;424:547–562. doi: 10.1002/1096-9861(20000828)424:3<547::aid-cne11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Granata AR, Chang HT. Relationship of calbindin D-28k with afferent neurons to the rostral ventrolateral medulla in the rat. Brain Research. 1994;645:265–277. doi: 10.1016/0006-8993(94)91660-8. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. Role of the ventral medulla oblongata in blood pressure regulation. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. pp. 145–191. [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nature Reviews Neuroscience. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Härfstrand A, Fuxe K, Terenius L, Kalia M. Neuropeptide Y-immunoreactive perikarya and nerve terminals in the rat medulla oblongata: Relationship to cytoarchitecture and catecholamine cell groups. Journal of Comparative Neurology. 1987;260:20–35. doi: 10.1002/cne.902600103. [DOI] [PubMed] [Google Scholar]
- Haselton JR, Guyenet PG. Electrophysiological characterization of putative C1 adrenergic neurons in the rat. Neuroscience. 1989;30:199–214. doi: 10.1016/0306-4522(89)90365-5. [DOI] [PubMed] [Google Scholar]
- Hwang D-Y, Carlezon WA, Isacson O, Kim K-S. A high-efficiency synthetic promotoer that drives transgene expression selectively in noradrenergic neurons. Human Gene Therapy. 2001;12:1731–1740. doi: 10.1089/104303401750476230. [DOI] [PubMed] [Google Scholar]
- Jeske I, McKenna KE. Quantitative analysis of bulbospinal projections from the rostral ventrolateral medulla - contribution of C1-adrenergic and nonadrenergic neurons. Journal of Comparative Neurology. 1992;324:1–13. doi: 10.1002/cne.903240102. [DOI] [PubMed] [Google Scholar]
- Kangrga IM, Loewy AD. Whole-cell recordings from visualized C1 adrenergic bulbospinal neurons: ionic mechanisms underlying vasomotor tone. Brain Research. 1995;670:215–232. doi: 10.1016/0006-8993(94)01282-m. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Lewis ME, Watson SJ. Enkephalin systems in the diencephalon and brainstem of the rat. Journal of Comparative Neurology. 1983;220:310–320. doi: 10.1002/cne.902200305. [DOI] [PubMed] [Google Scholar]
- Lipski J, Kanjhan R, Kruszewska B, Rong W. Properties of presympathetic neurones in the rostral ventrolateral medulla in the rat: an intracellular study in vivo. Journal of Physiology. 1996;490:729–744. doi: 10.1113/jphysiol.1996.sp021181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Minson JB, Morilak DA, Oliver JR, Chalmers J. Neuropeptide Y-immunoreactive synapses in the intermediolateral cel column of rat and rabbit thoracic spinal cord. Neuroscience Letters. 1990;108:243–248. doi: 10.1016/0304-3940(90)90648-s. [DOI] [PubMed] [Google Scholar]
- Lorenz RG, Saper CB, Wong DL, Ciaranello RD, Loewy AD. Co-localization of substance P- and phenylethanolamine N-methytransferase-like immunoreactivity in neurons of ventrolateral medulla that project to the spinal cord: Potential role in control of vasomotor tone. Neuroscience Letters. 1985;55:255–260. doi: 10.1016/0304-3940(85)90444-6. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Kobilka BK, Scheinin M. Gene targeting - homing in on a2-adrenoceptor-subtype function. Trends in Pharmacological Sciences. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Ito S, Rinaman L, Wiley RG, Sved AF. Lesions of the C1 catecholaminergic neurons of the ventrolateral medulla in rats using anti-DβH-saporin. American Journal of Physiology. 1999;46:R1063–R1075. doi: 10.1152/ajpregu.1999.277.4.R1063. [DOI] [PubMed] [Google Scholar]
- McAllen RM. Mediation of fastigial pressor response and a somatosympathetic reflex by ventral medullary neurones in the cat. Journal of Physiology. 1985;368:423–433. doi: 10.1113/jphysiol.1985.sp015866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen RM. Action and specificity of ventral medullary vasopressor neurons in the cat. Neuroscience. 1986a;18:51–59. doi: 10.1016/0306-4522(86)90178-8. [DOI] [PubMed] [Google Scholar]
- McAllen RM. Identification and properties of subretrofacial bulbospinal neurones: a descending cardiovascular pathway in the cat. Journal of the Autonomic Nervous System. 1986b;17:151–164. doi: 10.1016/0165-1838(86)90090-1. [DOI] [PubMed] [Google Scholar]
- McAllen RM. Location of neurones with cardiovascular and respiratory function, at the ventral surface of the cat’s medulla. Neuroscience. 1986c;18:43–49. doi: 10.1016/0306-4522(86)90177-6. [DOI] [PubMed] [Google Scholar]
- McAllen RM. Central respiratory modulation of subretrofacial neurones in the cat. Journal of Physiology. 1987;388:533–545. doi: 10.1113/jphysiol.1987.sp016630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. Journal of Histochemistry and Cytochemistry. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Milner TA, Morrison SF, Abate C, Reis DJ. Phenylethanolamine N-methyltransferase-containing terminals synapse directly on sympathetic preganglionic neurons in the rat. Brain Research. 1988a;448:205–222. doi: 10.1016/0006-8993(88)91258-9. [DOI] [PubMed] [Google Scholar]
- Milner TA, Pickel VM, Abate C, Joh TH, Reis DJ. Ultrastructural characterization of substance P-containing neurons in the rostral ventrolateral medulla in relation to neurons containing catecholamine synthesizing enzymes. Journal of Comparative Neurology. 1988b;270:427–446. doi: 10.1002/cne.902700311. [DOI] [PubMed] [Google Scholar]
- Milner TA, Pickel VM, Morrison SF, Reis DJ. Adrenergic neurons in the rostral ventrolateral medulla: ultrastructure and synaptic relations with other transmitter-identified neurons. In: Ciriello J, et al., editors. Progress in Brain Research. Vol. 81. New York: Elsevier Science Publishers B. V; 1989a. pp. 29–47. [DOI] [PubMed] [Google Scholar]
- Milner TA, Pickel VM, Reis DJ. Ultrastructural basis for interactions between central opioids and catecholamines: I. Rostral ventrolateral medulla. Journal of Neuroscience. 1989b;9:2114–2130. doi: 10.1523/JNEUROSCI.09-06-02114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Rosin DL, Lee A, Aicher SA. Alpha2A-adrenergic receptors are primarily presynaptic heteroreceptors in the C1 area of the rat rostral ventrolateral medulla. Brain Research. 1999;821:200–211. doi: 10.1016/s0006-8993(98)00725-2. [DOI] [PubMed] [Google Scholar]
- Minson JB, Llewellyn-Smith IJ, Pilowsky PM, Chalmers JP. Bulbospinal neuropeptide Y-immunoreactive neurons in the rat: comparison with adrenaline-synthesizing neurons. Journal of the Autonomic Nervous System. 1994;47:233–243. doi: 10.1016/0165-1838(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Hokfelt T, Pieribone VA. The distribution and significance of CNS adrenoceptors examined with in situ hybridization. Trends in Pharmacological Sciences. 1996;17:245–255. doi: 10.1016/0165-6147(96)10022-5. [DOI] [PubMed] [Google Scholar]
- Orer HS, Zhong S, Barman SM, Gebber GL. Central catecholaminergic neurons are involved in expression of the 10-Hz rhythm in SND. American Journal of Physiology. 1996;270:R333–R341. doi: 10.1152/ajpregu.1996.270.2.R333. [DOI] [PubMed] [Google Scholar]
- Osborn JW. Hypothesis: Set-points and long-term control of arterial pressure. A theoretical argument for a long-term arterial pressure control system in the brain rather than the kidney. Clinical and Experimental Pharmacology and Physiology. 2005;32:384–393. doi: 10.1111/j.1440-1681.2005.04200.x. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Minson J, Hodgson AH, Howe P, Chalmers J. Does substance P coexist with adrenaline in neurons of the rostral ventrolateral medulla in the rat? Neuroscience Letters. 1986;71:293–298. doi: 10.1016/0304-3940(86)90636-1. [DOI] [PubMed] [Google Scholar]
- Piquet P, Schlichter R. Lability of the pacemaker activity in the rat rostro-ventrolateral medulla: effects of noradrenaline. Brain Research. 1998;796:1–12. doi: 10.1016/s0006-8993(98)00171-1. [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Rostroventrolateral medulla neurons preferentially project to target-specified sympathetic preganglionic neurons. Neuroscience. 1998;83:617–631. doi: 10.1016/s0306-4522(97)00355-2. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Postnatal development of catecholamine inputs to the paraventricular nucleus of the hypothalamus in rats. Journal of Comparative Neurology. 2001;438:411–422. doi: 10.1002/cne.1324. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Rostral ventrolateral medulla: selective projections to the thoracic autonomic cell column from the region containing C1 adrenergic neurons. Journal of Comparative Neurology. 1984a;228:168–185. doi: 10.1002/cne.902280204. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Meeley MP, Park DH, Joh TH, Reis DJ. A new group of neurons in hypothalamus containing phenylethanolamine N-methyltransferase (PNMT) but not tyrosine hydroxylase. Brain Research. 1984b;306:349–353. doi: 10.1016/0006-8993(84)90385-8. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. Journal of Neuroscience. 1984c;4:474–494. doi: 10.1523/JNEUROSCI.04-02-00474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. Journal of Comparative Neurology. 1997;387:524–536. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Sympathetic reflexes after depletion of bulbospinal catecholaminergic neurons with anti-DβH-saporin. American Journal of Physiology. 2000;279:R729–R742. doi: 10.1152/ajpregu.2000.279.2.R729. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Sved AF. The ventrolateral medulla and sympathetic regulation of arterial pressure. In: Llewellyn-Smith IJ, Verberne AJM, editors. Central Regulation of Autonomic Functions. New Yourk: Oxford University Press, Inc; 2011. pp. 78–97. [Google Scholar]
- Sesack SR, Miner LH, Omelchenko N. Preembedding immunoelectron microscopy: Applications for studies of the nervous system. In: Zaborszky L, et al., editors. Neuroanatomical Tract-Tracing Molecules, Neurons, and Systems. Singapore: Springer; 2006. pp. 6–71. [Google Scholar]
- Spyer KM. Centeral nervous mechansims contributing to cardiovascular control. Journal of Physiology. 1994;474:1–19. doi: 10.1113/jphysiol.1994.sp019997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta R, Akey PJ, Guyenet PG. Location and electrophysiological characterization of rostral medullary adrenergic neurons that contain neuropeptide Y mRNA in rat medulla. Journal of Comparative Neurology. 1999;415:482–500. doi: 10.1002/(sici)1096-9861(19991227)415:4<482::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Stornetta R, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/GLUT2 is expressed by both C1 adrenergic and nonaminergic presympathetic vasomotor neurons of the rat medulla. Journal of Comparative Neurology. 2002;444:207–220. doi: 10.1002/cne.10142. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Macon CJ, Nguyen TM, Coates MB, Guyenet PG. Cholinergic neurons in the mouse rostral ventrolateral medulla target sensory afferent areas. Brain Structure and Function. 2012 doi: 10.1007/s00429-012-0408-3. In Press, Published On-Line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Schreihofer AM, Pelaez NM, Sevigny CP, Guyenet PG. Preproenkephalin mRNA is expressed by C1 and non-C1 barosensitive bulbospinal neurons in the rostral ventrolateral medulla of the rat. Journal of Comparative Neurology. 2001;435:111–126. doi: 10.1002/cne.1196. [DOI] [PubMed] [Google Scholar]
- Sun MK, Guyenet PG. Medullospinal sympathoexcitatory neurons in normotensive and spontaneously hypertensive rats. American Journal of Physiology. 1986;250:R910–R917. doi: 10.1152/ajpregu.1986.250.5.R910. [DOI] [PubMed] [Google Scholar]
- Sun MK, Hackett JT, Guyenet PG. Sympathoexcitatory neurons of rostral ventrolateral medulla exhibit pacemaker properties in presence of a glutamate receptor antagonist. Brain Research. 1988a;438:23–40. doi: 10.1016/0006-8993(88)91320-0. [DOI] [PubMed] [Google Scholar]
- Sun MK, Young BS, Hackett JT, Guyenet PG. Reticulospinal pacemaker neurons of the rat rostral ventrolateral medulla with putative sympathoexcitatory function: an intracellular study in vitro. Brain Research. 1988b;442:229–239. doi: 10.1016/0006-8993(88)91508-9. [DOI] [PubMed] [Google Scholar]
- Sved AF, Ito S, Sved JC. Brainstem mechanisms of hypertension: Role of the rostral ventrolateral medulla. Current Hypertension Reports. 2003;5:262–268. doi: 10.1007/s11906-003-0030-0. [DOI] [PubMed] [Google Scholar]
- Sved AF, Mancini DL, Graham JC, Schreihofer AM, Hoffman GE. PNMT-containing neurons of the C1 cell group express c-fos in response to changes in baroreceptor input. American Journal of Physiology. 1994;266:R361–R367. doi: 10.1152/ajpregu.1994.266.2.R361. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Structure of the Rat Brain. Amsterdam: Elsevier; 1998. Brain Maps. [Google Scholar]
- Tiveron M-C, Hirsch M-R, Brunet J-F. The expression pattern of the transcription factor Phox2 delineates synaptic pathways of the autonomic nervous system. Journal of Neuroscience. 1996;16:7649–7660. doi: 10.1523/JNEUROSCI.16-23-07649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DC, Saper CB, Ruggiero DA, Reis DJ. Organization of central adrenergic pathways: I. Relationships of ventrolateral medullary projections to the hypothalamus and spinal cord. Journal of Comparative Neurology. 1987;259:591–603. doi: 10.1002/cne.902590408. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele E, Pieribone VA, Aston-Jones G. Diverse afferents converge on the nucleus paragigantocellularis in the rat ventrolateral medulla: retrograde and anterograde tracing studies. Journal of Comparative Neurology. 1989;290:561–584. doi: 10.1002/cne.902900410. [DOI] [PubMed] [Google Scholar]
- Watson RE, Wiegand ST, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]