Abstract
Neuroendocrine tumors (NETs) are rare neoplasms arising from cells of the neuroendocrine system in a multitude of anatomic locations, and representing a wide range of histologies. Compared with other malignancies from the same organ (eg, pancreatic NET vs pancreatic ductal adenocarcinoma), NETs are often indolent in both biology and disease progression. However, metastasis will develop in almost 40% of patients with NETs during the course of their disease, most commonly to the liver.1 In contrast to other malignancies, in a proportion of patients with neuroendocrine liver metastasis (NELM) disabling clinical symptoms can develop secondary to the production of specific biogenic amines and polypeptide hormones. Therefore, treatment of patients with NELM is focused on optimizing quality of life through reduction of such hormone-related symptoms, and improving survival in patients with disease amenable to liver-directed therapy, including hepatic resection, thermal ablation, and intra-arterial therapy (IAT).2-8 To date, hepatic resection remains the only potentially curative option for patients with NELM, with 5-year survival after hepatectomy ranging from 60% to 80% in recent series.6-11 Until recent reports of the use of somatostatin analogues in neuroendocrine tumors of mid-gut origin, and sunitinib and everolimus in patients with advanced pancreatic NETs (PNETs), there has been little success in the use of systemic therapy to treat patients with advanced NELM, with historic responses ranging from 15% to 20%.12-15 As these systemic agents are not curative, there is still considerable interest in the use of liver-directed therapy to increase patient survival and address hormonally related symptoms. In addition to surgery, IAT has emerged as an alternative liver-directed approach to treat patients with NELM. We review the management of patients with NELM with an emphasis on clarifying the relative roles of surgery, IAT, as well as emerging systemic therapeutic agents. To accomplish this, we performed an extensive literature search in PubMed using medical subject headings (ie, neuroendocrine, carcinoid, liver metastasis, hepatic metastases, hepatectomy, liver resection, transplantation, intra-arterial therapy, radiation therapy, chemotherapy) to identify relevant articles for inclusion.
Patient evaluation
Classification of neuroendocrine tumors
Neuroendocrine tumors encompass a diverse set of rare neoplasms arising most commonly throughout the gastrointestinal tract and bronchopulmonary tree. Pancreatic NETs originate in the endocrine tissues of the pancreas, and carcinoid tumors arise in neuroendocrine cells in the bronchopulmonary tree, small intestine, appendix, rectum, and thymus. These tumors can vary in degree of differentiation and hormonal secretion. Within the gastrointestinal tract, NETs most commonly arise in the small bowel (42%), rectum (27%), appendix (5%), and stomach (9%).1 The potential for metastatic disease is dependent on location and cell of origin. An analysis of Surveillance, Epidemiology, and End Results data in the 1990s suggested that NETs of pancreatic origin have the greatest potential for distant metastasis, and the lowest incidence was observed for bronchopulmonary and rectum sites.1
In 2000, under the auspices of the WHO, an NET classification system was proposed that had 3 distinct categories: well-differentiated tumors with benign or uncertain behavior, well-differentiated carcinoma with malignant characteristics, and poorly differentiated carcinoma.16-18 Since 2000, this classification system has been updated, with the most recent changes made in 2010. The 2010 WHO classification takes into account the anatomic location of origin, specific amine or hormonal production, mitotic activity, and the proliferative index as defined by Ki-67 (Table 1).19 Components of this version of the WHO system classification were partially incorporated into the 7th edition of the American Joint Committee on Cancer staging manual, as well as into the recent National Comprehensive Cancer Network practice guidelines.20,21 However, it is important to note that the 7th edition of the American Joint Committee on Cancer classification system has applied the TNM staging system of pancreatic adenocarcinoma to PNETs without considering mitotic count, Ki-67 staining, or hormonal production, which are essential components of the WHO 2010 classification schema.
Table 1.
World Health Organization Classification of Gastrointestinal and Pancreatic Neuroendocrine Tumors
| Behavior | Metastasis | Size, cm | Muscularis propria invasion | Differentiation | Angio-invasion | Ki-67, % | Hormonal index |
|---|---|---|---|---|---|---|---|
| WHO criteria, gastrointestinal | |||||||
| Benign | − | ≤1 | − | Well | − | <2 | − |
| Benign/low-grade malignant | − | 1–2 | − | Well | ± | <2 | − |
| Low-grade malignant | + | >2 | + | Well | + | 2–20 | + |
| High-grade malignant | + | Any | + | Poorly | + | >20 | − |
| WHO criteria, pancreas | |||||||
| Benign | − | ≤1 | − | Well | − | <2 | − |
| Benign/low-grade malignant | − | 1–2 | − | Well | ± | <2 | ± |
| Low-grade malignant | + | >4 | + | Well | + | 2–20 | ± |
| High-grade malignant | + | Any | + | Poorly | + | >20 | − |
Presentation
Patients with NELM are diverse in their clinical presentation. Overall, approximately two thirds of patients report symptoms that are attributable to their primary or hepatic metastasis.8 Depending on the hormonal functional status of the cancer, the patient might exhibit some of the hallmark syndromes associated with varied primary NET histologies (eg, carcinoid syndrome from carcinoid primaries, hypoglycemic episodes from insulinomas, necrolytic migratory erythema from glucagonomas, etc). In the largest study to date, of 753 patients with NELM undergoing surgical management (resection ± ablation) or IAT, Mayo and colleagues reported that between 25% and 50% of patients presented with hormonally related symptoms.8 The degree of symptoms is often directly related to the extent of hepatic disease burden and ultimately can lead to both a decreased quality of life and worse long-term survival.1,3-6 Aside from hormonal symptoms, patients with synchronous primary and hepatic disease can present with symptoms from the primary tumor, with abdominal cramping often being the initial symptom.7
Among patients with NELM, it is estimated that close to 50% of patients will present with synchronous liver metastasis, and in the other half of patients, metachronous liver disease will develop during the course of their life.2,7 Regardless of presentation, patients often seek and benefit from hepatic cytoreduction for relief of their hormonal or other symptoms.22 Sarmiento and colleagues reported that hepatic cytoreduction of 90% reduced endocrine-related symptoms in 90% of patients.6 Cytoreduction, in addition to alleviating symptoms, might also be associated with improved long-term survival, although the data are retrospective and need to be interpreted with caution.6,22,23 In addition, the degree of hepatic cytoreduction necessary to achieve symptomatic relief is not based on any prospective, randomized data, largely due to the rarity of patients with NELM eligible for surgical management.
Liver-directed therapy
Surgery: resection and ablation
Resection of the both the primary NET and the hepatic disease remains the mainstay of treatment for patients with NELM. Patients with untreated hepatic metastasis have a 5-year survival rate in the range of 20% to 40%.2,3,24 The relatively long 5-year survival rate among patients with unresected NET disease underscores the comparatively indolent biology of NELM compared with other malignancies. Surgical treatment of NELM does appear to confer a survival benefit. Frilling and colleagues described the following patterns of NELM distribution: isolated single lesion of any size (type I); a large focus of metastatic bulk with smaller surrounding lesions, always with involvement of both hemilivers (type II); and, widely disseminated metastatic spread with involvement of both hemilivers and essentially no normal liver parenchyma appreciable on preoperative imaging (type III).10 Only patients in the type I category and select type II patients are typically eligible for hepatic resection. In general, about 15% to 50% of patients with NELM might be a candidate for some type of surgical procedure.6,25
Sarmiento and colleagues from the Mayo clinic were one of the first groups to report their large experience with hepatic resection and cytoreductive therapy for patients with NELM.6 More than half of the 170 patients in their study underwent a major hepatic resection. Symptom control was achieved in 96% of patients who had presented with symptoms; however, 5-year symptom recurrence was 59%. Overall, 84% of patients had evidence of disease recurrence at 5 years, with a survival of 61% and 35% at 5 and 10 years, respectively. Based on these data, the authors advocate an “aggressive” surgical approach when all disease could be extirpated; the authors also noted that debulking of disease should be considered when 80% of the intrahepatic disease could be feasibly removed, especially among symptomatic patients who might potentially benefit from a reduction of intrahepatic tumor burden. As noted here, however, the association of cytoreduction with improvements in long-term survival remains controversial. More recently, in a separate study, Mayo and colleagues reported on 339 patients who underwent surgical management of NELM at 1 of 8 major hepatobiliary centers.7 In this study, most patients had a pancreatic (40%) or small bowel (25%) NET primary, and carcinoid was the most common NET histological subtype (53%). Median survival after surgery was 125 months with, overall 5-and 10-year survival rates of 74%, and 51%, respectively (Fig. 1). Of note, Glazer and colleagues reported very similar overall outcomes (5- and 10-year survival rates of 77% and 50%, respectively) based on data derived from a smaller cohort of patients treated at the University of Texas MD Anderson Cancer Center.26 In the Mayo et al study, patients with hormonally functional NETs who had an R0/R1 resection were noted to benefit the most from surgery, and factors such as synchronous disease (hazard ratio = 1.9), nonfunctional NET hormonal status (hazard ratio = 2.0), and extrahepatic disease (EHD) (hazard ratio = 3.0) were predictive of worse survival (all p < 0.05). Extrahepatic sites of disease most commonly included the lung and peritoneum. Although EHD has been shown to have a worse prognosis in several series, patients with limited, stable EHD can be considered for surgery, especially if the patient is symptomatic and cytoreduction would provide palliation of symptoms due to hormonal excess.
Figure 1.
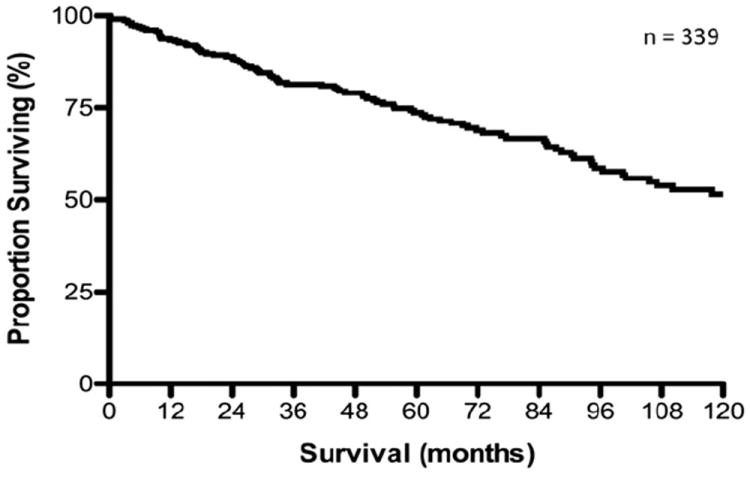
Overall survival from time of first liver-directed operation for patients with hepatic neuroendocrine metastasis. Median overall survival was 125.1 months, with 5- and 10-year survival of 74 and 51%, respectively. (With kind permission from Springer Science+Business Media: Annals of Surgical Oncology, Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis, vol 17, 2010, pages 3129–3136, Mayo SC, de Jong MC, Pulitano C, et al, Figure 1 [7].)
Although patients with symptomatic disease are often offered hepatic cytoreduction to help palliate their symptoms, the role of surgery for asymptomatic, hormonally/biochemically nonfunctional neuroendocrine liver metastases is more controversial. Although Sarmiento and others have reported that 80% to 90% debulking of disease in the setting of nonfunctional NELM was associated with improved survival, these data are retrospective and need to be interpreted with caution.6,22,23 As such, care of patients with asymptomatic disease in the setting of hormonally/biochemically nonfunctional tumors needs to be highly individualized. Although surgery in the setting of low-volume asymptomatic disease should be considered, surgery has a much more limited role for patients with high-volume disease who are probably best served by other therapeutic modalities, such as intra-arterial or systemic therapy.
The majority of patients with NELM will present with bilateral disease.7 As such, hepatic resection of NELM will frequently involve nonanatomic resections, with most patients undergoing multiple wedge resections to debulk multifocal, bilateral disease.7,26 Although many centers report an R0 resection of >90% for patients with resected colorectal metastasis, achieving an R0 resection for NELM appears to be significantly lower.27-29 In the series by Mayo and colleagues, only 53.7% of patients operated on with NELM had an R0 margin and almost one third had an R2 resection.7 The difference in pathologic margin status between patients with colorectal liver metastasis vs NELM is probably multifactorial. In light of data that debulking or cytoreduction of NELM can be instrumental in palliating patients with symptomatic disease and might be associated with a survival benefit when >80% to 90% of the disease is debulked, surgeons might be more willing to operate on NELM patients who have more extensive disease with a palliative or debulking intent.6,23
Because NELM is frequently multifocal and bilateral, resection is often combined with ablation to treat the entire intrahepatic tumor burden. In fact, ablation is used in up to one fifth of patients undergoing surgery for NELM as the indication.7 Ablation can be performed percutaneously, laparoscopically, or during laparotomy. Thermal ablation usually involves the use of either radio-frequency ablation (RFA) or microwave ablation energy devices. Presently, RFA is the most commonly used thermal ablative modality in the United States; as microwave ablation use continues to grow, it is currently used more in some Eastern centers.30 Assessing data on ablation vs resection can be problematic, and data on ablation of NELM is relatively scarce.31 In the Mayo and colleagues study, the authors did not find an increased risk of recurrence for patients who had an ablation vs hepatic resection alone.7 In a separate study, Mazzaglia and colleagues reported on 63 patients with NELM who underwent laparoscopic RFA.32 The 30-day perioperative morbidity was 5% and there were no deaths. Overall, at a median follow-up of 11 months, >90% of patients reported at least partial symptom relief and 70% of patients noted “complete” or “significant” symptomatic relief.32 Local recurrence after ablation of NELM probably is related to tumor size and number, as noted for other tumor types, such as colorectal liver metastasis and hepatocellular carcinoma.33-35 In Mazzaglia and colleagues’ study, the authors reported a 6.3% local recurrence with an associated median 3.9-year survival after the first RFA session.32 Unfortunately, surveillance imaging demonstrated progressive liver disease in 80% of patients.32 In reviewing the records of 16 patients who had >15 hepatic metastases treated with combined hepatectomy plus RFA, Elias and colleagues reported an overall survival of 84% at 3 years, with 50% recurrence/disease progression.36 The 5 patients reporting preoperative symptoms continued to be symptom-free at the time of last follow-up. Therefore, although ablation can be a helpful adjunct therapy to treat NELM with relatively low local recurrence, intrahepatic recurrence remains a problem.
Although liver-directed surgery for NELM is associated with prolonged survival, recurrent disease will develop in the majority of patients. In fact, recurrence is near universal, with reports of >90% of patients recurring by 5 years, most often intrahepatically.7 The role of repeat hepatectomy and ablation for recurrent disease remains controversial.7 In the study by Mayo and colleagues, 46 patients underwent repeat operation for recurrent NELM.7 At the time of the repeat operation, ablation was noted be used increasingly as both a stand-alone therapy and in combination with resection. Among patients undergoing a repeat surgery, median survival and 5-year survival rate were 83 months and 61%, respectively. In a separate study by Glazer and colleagues,26 80 of an initial 159 patients underwent a repeat operation for a hepatic recurrence. In this series, the authors reported a 5-year survival of >60% (Table 2). Collectively, although these data suggest that a subset of patients might benefit from a second operation, the selection of such patients remains ill-defined and should be based on multiple factors, including surgeon experience, patient symptoms, degree of functional hepatic reserve, and a thorough assessment of perioperative risk.
Table 2.
Summary of Recent Studies (Since 2003 with n ≥ 20) of Patients with Surgically Managed Neuroendocrine Liver Metastasis
| First author | Year | n | Combined modality (resection + RFA), % | Perioperative mortality, % | 5-Year survival, % | Recurrence at time of last follow-up, % |
|---|---|---|---|---|---|---|
| Elias73 | 2003 | 47 | 11 | 5 | 71 | Intrahepatic, 75 at 10 years |
| Osborne74 | 2006 | 61 | 18 | 0 | 60–80* | — |
| House24 | 2006 | 26 | 8 | 0 | 86 | 76 |
| Hibi75 | 2007 | 21 | 33 | 0 | 41–73* | — |
| Landry76 | 2008 | 23 | 57 | 0 | 75 | — |
| Chambers77 | 2008 | 30 | — | 0 | 74 | — |
| Ahmed78 | 2009 | 50 | — | 0 | 74 | — |
| Glazer26 | 2010 | 159 | 14 | 0 | 77 | — |
| Mayo7 | 2010 | 339 | 20 | <1 | 74 | 94 at 5 years; 99 at 10 years |
There was no distinction in these studies between palliative resections and resections undertaken with curative intent.
RFA, radiofrequency ablation.
Data from Elias and colleagues suggest a possible reason for the high incidence of intrahepatic recurrence.37 Specifically, Elias and colleagues demonstrated that modern preoperative cross-sectional imaging (eg, MRI, CT) can dramatically underestimate the true extent of NELM tumor burden within the liver by as much as 50%.37 In this study, the authors compared preoperative radiological findings with detailed pathological review of the surgical specimen to correlate the relative assessment of tumor burden. The authors noted that the amount of disease was underestimated by up to 50%, with many additional metastases <2 mm identified in the pathological specimen that were “missed” on preoperative imaging. These data suggest that when assessing many patients, surgeons might be underestimating the true burden of intrahepatic disease. In turn, complete extirpation of all visible disease might, in effect, just be a more thorough debulking of the liver rather than a true R0 resection. Given the high incidence of recurrence after surgery, some investigators have questioned the role of surgical management for NELM. These clinicians have suggested that IAT might be better suited as the primary therapy for NELM.38,39
Intra-arterial therapy
The intuitive appeal for IAT stems from the fact that NELM, unlike most hepatic metastasis, derive the majority of their blood supply from the hepatic artery and arterially enhance.40 IAT has been shown to be effective in retarding tumor progression as well as palliating symptoms related to NELM.40-43 Intra-arterial therapy can consist of transarterial chemoembolization (TACE), bland transarterial embolization, and chemoembolization with drug-eluting beads. Recently, there has been interest in combining TACE with drug-eluting beads. However, transarterial chemoembolization with drug-eluting beads has been associated with an increased risk of bilomas when used to treat NELM, especially among those patients with small, disseminated lesions.44 In turn, both Yttrium-90 (Y-90) resin-based (Sirspheres) and glass-based (Theraspheres) radioembolization have been used with increasing frequency to treat NELM.45,46 Most studies evaluating Y-90 radioembolization in NELM have been retrospective in design. Additionally, reports on the use of Y-90 for NELM often include other metastatic tumor types, making determinations of its efficacy for NELM difficult. One study by Kennedy and colleagues reviewed data from patients treated with Y-90 microsphere therapy for NELM at 10 institutions.47 The study included a total of 148 patients treated with 185 separate procedures. Median activity delivered was 1.14 gigabecquerel, with a median of 99% of the planned activity delivered. Fatigue (6.5%) was the most common side effect. Disease response was stable in 22.7%, partial in 60.5%, complete in 2.7%, and progressive in 4.9%. Median survival for all patients was 70 months, with no radiation-induced liver disease reported. In a separate study, Rhee and colleagues reported a multicenter open-label phase II study that examined the efficacy and safety of Y-90 radioembolization of patients with NELM.48 In this study, all patients underwent lobar radioembolization using glass or resin Y-90 radioembolic agents. A total of 42 patients treated with glass or resin microspheres were included. A significantly greater median radiation dose was delivered to each lobe using glass (right lobe 117 Gy; left lobe 108 Gy) than using resin (right 50.8 Gy; left 44.5 Gy) (p = 0.01). In assessing tumor response using the Response Evaluation Criteria in Solid Tumors criteria, 92% of glass and 94% of resin patients had a partial response or stable disease response at 6 months of follow-up. Six patients experienced grade 3/4 toxicities during the follow-up period. Median survival was similar with both modalities: 22 months (glass) vs 28 months (resin) (p = 0.82). A study by Bester and colleagues compared Y-90 (resin) vs supportive care in a cohort of 40 patients with metastatic NELM.49 The authors reported improved control of symptoms and improved survival with Y-90 treatment. Collectively, these studies suggest that radioembolization for NELM can improve both patient symptoms and overall survival with limited acute and chronic toxicity. Although not currently approved for use in the United States, the efficacy of peptide receptor radionuclide therapeutic agents, such as 90Y-DOTATOC and 177Lu-DOTATOC has recently been reported with favorable progression-free survival (PFS) and a low incidence of adverse side effects in patients with unresectable disease.50 Taken together, these data suggest that IAT, Y-90 radioembolization, and peptide receptor radionuclide therapeutic can play a role in the management of patients with NELM, especially patients with recurrent disease and those not fit for operative management.40-43
Identification of patients best managed with IAT vs surgery remains controversial. Mayo and colleagues reported on a large (n = 753) group of patients with NELM who were managed with either IAT or surgery.8,51 As expected, in this report, patients treated with IAT vs surgery had different clinicopathologic characteristics, making comparison of the 2 cohorts difficult.52 Specifically, patients managed with IAT had a higher burden of hepatic disease, were more likely to have extrahepatic disease, and more often had NET from an unknown primary, which are all factors associated with decreased survival.7,8 Using propensity score methods, the authors identified a subgroup of 118 patients (IAT, n =52 and surgery, n =66) who were matched based on having similar preoperative clinicopathological characteristics. In this cohort, the median overall survival was 38.9 months for IAT patients compared with 84.0 months for patients treated surgically (Fig. 2A). Additional analysis of this cohort revealed that patients with high-volume (>25% liver involvement) symptomatic disease appeared to benefit the most from surgical management of their disease (median survival: surgery, 87 months vs IAT, 51 months; p < 0.001) and patients with >25% liver tumor burden and asymptomatic disease did not derive a comparative benefit from surgery vs IAT (median survival: surgery, 16.7 months vs IAT, 18.5 months; p = 0.78) (Fig. 2B). The authors concluded that surgical management of NELM should be reserved for patients with a low systemic burden of disease and low-volume hepatic disease or for those patients with symptomatic high-volume disease. In contrast, patients with a high burden of intrahepatic disease, especially those with asymptomatic disease, are probably best served with locoregional IAT rather than surgical debulking.
Figure 2.
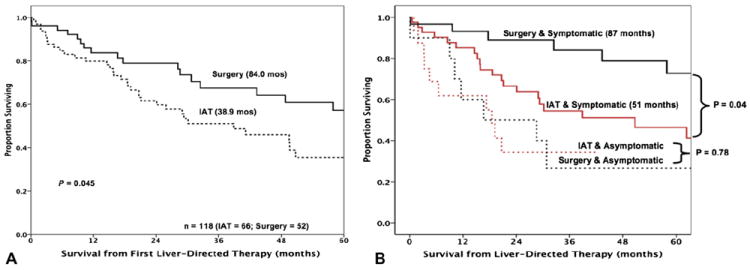
(A) Overall survival of propensity-matched patients stratified by receipt of surgery vs intra-arterial therapy (IAT) (p = 0.04). (B) Kaplan-Meier survival for propensity-matched patients with >25% hepatic tumor burden undergoing either IAT or hepatic resection of neuroendocrine liver metastasis (NELM). Patients with high-volume symptomatic disease benefited the most from surgical management (median survival: surgery, 87 months vs IAT, 51 months; p = 0.04). With kind permission from Springer Science+Business Media: Annals of Surgical Oncology, Surgery versus intra-arterial therapy for neuroendocrine liver metastasis: a multicenter international analysis, vol 18, 2011, pages 3657–3665, Mayo SC, de Jong MC, Bloomston M, et al, Figures 3C and 2 [8].
External beam radiation therapy
External beam radiation therapy historically has been limited by the low tolerance of the whole liver to radiation (eg, maximum dose 30 Gy). Improvements in radiation therapy techniques, such as intensity modulated radiation therapy and stereotactic body radiation therapy, now allow high doses of radiation therapy to be delivered to partial liver volumes.53 Hypofractionated high doses of radiation therapy (10 Gy in 5 fractions or 13 Gy in 3 fractions) have resulted in excellent local control/response (70% to 90%) in patients with primary hepatocellular carcinoma and metastasis from other sites.54-56 However, data evaluating intensity-modulated radiation therapy and stereotactic body radiation therapy in patients with NELM are currently limited. External beam radiation has been used more commonly for the palliation of patients with extrahepatic NELM. Contessa and colleagues reported on 36 patients with 49 treated sites, of which 23 patients had radiographic follow-up.57 The overall response to radiotherapy was 39% (13% complete response, 26% partial response, 56% stable disease and 4% progressive disease). Patients who received ≥49.6 Gy had less local progression compared with the cohort of patients who received ≤32 Gy. External beam radiation achieved palliation in 90% of patients, with either improvement or resolution of symptoms after radiotherapy.57
Liver transplantation
Orthotopic liver transplantation (OLT) for patients with NELM who have unresectable disease remains controversial and is performed at only a few centers, usually on a study protocol. Advocates for OLT note the relatively indolent nature of NETs, the propensity for liver only metastasis, and the high risk of intrahepatic recurrence after resection. To date, the largest series examining the role of OLT for patients with NELM included 85 patients. In this multicenter French study by Le Treut and colleagues, the authors reported an in-hospital post-transplantation mortality rate of 14% and an overall 5-year survival rate of 47%.58 Factors associated with a particularly poor prognosis after OLT included hepatomegaly and a primary NET arising from the duodenum or pancreas. Given the controversy and difficulty in selecting patients for OLT, Mazzaferro and colleagues have developed criteria analogous to the Milan criteria for hepatocellular carcinoma.59,60 The proposed inclusion criteria include confirmed carcinoid histology of a low-grade NET; primary NET must be resected and primary tumor must be in anatomic distribution that is drained by portal system (eg, rectal and lung NET excluded from consideration); ≤50% hepatic involvement by tumor metastasis; stable disease for at least 6 months during the pretransplantation period; and age 55 years or younger (Table 3). It remains to be seen whether OLT will assume a larger role in the managements of patients with NELM in the future, or whether systemic recurrences will doom this therapy to poor long-term outcomes. As of 2012, the National Comprehensive Cancer Network practice guidelines consider transplantation for NELM to be “investigational and not part of routine care at this time.”21
Table 3.
Milan Criteria for Indication to Liver Transplantation in Patients with Unresectable Neuroendocrine Liver Metastasis
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. Confirmed histology of carcinoid tumor (low-grade neuroendocrine tumors) with or without syndrome | 1. Small-cell carcinoma and high-grade neuroendocrine carcinomas (noncarcinoid tumors) |
| 2. Primary tumor drained by the portal system (pancreas and intermediate gut: from distal stomach to sigmoid colon) removed with a curative resection (pretransplantation removal of all extrahepatic tumor deposits) through surgical procedures different and separate from transplantation | 2. Other medical/surgical conditions contraindicating liver transplantation, including previous tumors |
| 3. Metastatic diffusion to liver parenchyma ≤50% | 3. Nongastrointestinal carcinoids or tumors not drained by the portal system |
| 4. Good response or stable disease for at least 6 months during the pretransplantation period | |
| 5. Age 55 years or younger |
Reprinted from Journal of Hepatology, vol 47, Mazzaferro B, Regalia E, Doci R, et al, Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? pages 460–466 [59], Copyright 2007, with permission from Elsevier.
Chemotherapy for neuroendocrine liver metastasis
Cytotoxic systemic therapy
Neuroendocrine tumors, as a whole, have traditionally exhibited a very poor response to conventional, cytotoxic chemotherapy. The exception has been poorly differentiated NETs with a high proliferative index (Ki-67 >20%), usually reflecting an aggressive tumor biology akin to that seen with small cell carcinoma. In such cases, even if the extent of NELM appears limited, cytotoxic chemotherapy should be considered the primary mode of treatment. Surgical therapy is usually not warranted for poorly differentiated NET with high Ki-67 because the potential for subclinical metastasis and an aggressive natural history is considerable. As such, locally directed therapies are unlikely to impact the overall course of the disease. Therefore, systemic chemotherapy—usually with a platinum-based regimen—is used. Although chemotherapy can induce a dramatic initial response, unfortunately such responses are not durable, progression is common, and the prognosis is grave.
For patients with low- to intermediate-grade NELM, the likelihood of response to cytotoxic chemotherapeutic agents is low. Streptozocin, an alkylating agent, was approved for the treatment of advanced NET 3 decades ago, but multiple studies have revealed response rates of only 10% to 20%, even when combined with other cytotoxic agents, and minimal survival benefit.61 Given its unfavorable toxicity profile, use of streptozocin has been limited in recent years. Numerous other agents have been considered for low- to intermediate-grade NELM, including doxorubicin, cisplatin, dacarbazine, and fluoropyrimidines, but none have gained traction.62 Temozolomide is an orally bioavailable alkylating agent, with a less intense side effect profile than streptozocin and, after a series of early phase clinical trials in the early part of this century, is now considered the backbone of systemic chemotherapy options for patients with advanced cases of NET.63 Temozolomide has been assessed in combination with numerous other agents, but responses remain disappointingly low. Most professional guidelines suggest that cytotoxic chemotherapy should only be contemplated in these patients after other therapeutic avenues have been exhausted and, ideally, in the context of a clinical trial, especially with the advent of the targeted therapies.
Targeted therapies
Although surgical resection and cytoreduction continue to be the primary treatments for patients with NELM amenable to an operative approach, several developments in targeted systemic therapy have recently emerged. Although these targeted agents rarely result in a reduction in tumor burden, targeted therapy has proven useful in slowing disease progression. During the last several years, 3 compounds have been approved for the treatment of advanced NET.
Mechanistically, somatostatin analogues act by binding the often overexpressed somatostatin receptors on neuroendocrine cells (particularly gastroenteropancreatic NETs) and ultimately limit hormonal release. Somatostatin analogues have long been used in the clinical setting to control symptoms caused by hormone excess in patients with carcinoid syndrome. However, the Placebo-Controlled Prospective Randomized Study on the Anti-proliferative Efficacy of Octreotide LAR in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID) study examined the anti-neoplastic effects of somatostatin analogues.64 Specifically, in the PROMID trial, 85 treatment-naïve patients with midgut carcinoid tumors without evidence of carcinoid syndrome were enrolled and randomly assigned to either placebo or monthly intramuscular octreotide LAR (Sandostatin LAR, a depot form of a somatostatin analogue). The investigators reported that the time to progression in the octreotide LAR group was 14.3 months vs 6 months in the placebo group (p = 0.000072) (Fig. 3A). After 6 months of treatment, there was stable disease in 66.7% of patients in the octreotide LAR group vs only 37.2% in the placebo group. These data provide sound evidence for the use of octreotide LAR therapy in patients with inoperable metastatic carcinoid tumors, even among those patients without symptomatic disease. The use of agents with greater target specificity than LAR has recently been reported for patients with disease refractory to LAR. One such agent, pasireotide (SOM230), is a multi-targeted somatostatin receptor analogue. Pasireotide has a high binding affinity to 4 of the 5 known somatostatin receptor subtypes, with a 39-fold greater affinity for sstr5 and a 30-fold and 5-fold greater affinity sstr1 and sstr3, respectively, than LAR.65 The use of pasireotide is currently under investigation in a phase II, open-label, multicenter study in patients with symptomatic carcinoid syndrome refractory to LAR.66
Figure 3.
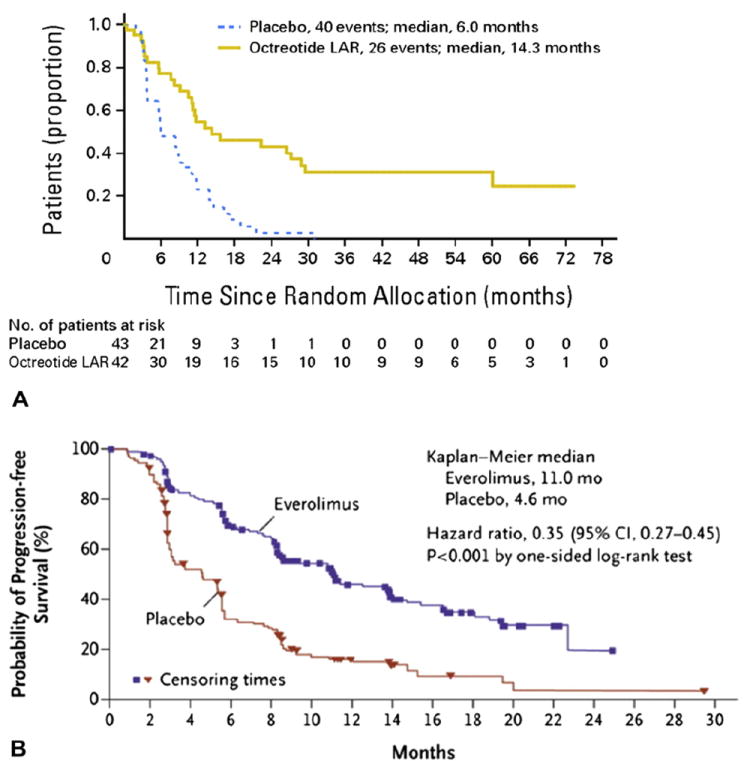
(A) Time to progression (TTP) in the Placebo-Controlled Prospective Randomized Study on the Antiproliferative Efficacy of Octreotide LAR in Patients with Metastatic Neuroendocrine Midgut Tumors trial for patients treated with octreotide LAR; the TTP in the octreotide LAR group was 14.3 months vs 6 months in the placebo group (p = 0.000072). Log-rank test stratified by functional activity: p = 0.000072; hazard ratio = 0.34 (95% CI, 0.20–0.59). (Reprinted with permission. © 2009 American Society of Clinical Oncology. All rights reserved. Rinke A, Müller HH, Schade-Brittinger C, et al; PROMID Study Group: Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol vol 27[28], 2009:4656–4663 [64].) (B) Progression-free survival (PFS) in the phase 3 RAD001 in Advanced Neuroendocrine Tumors (RADIANT-3) trial; patients in the everolimus group had a median PFS of 11.0 months compared with 4.6 months in the placebo group (p < 0.001). (From New England Journal of Medicine, Yao JC, Shah MH, Ito T; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group, Everolimus for advanced pancreatic neuroendocrine tumors, vol 364, pages 514–523. Copyright © 2011 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.)
Several other targeted agents have shown promise in the treatment of metastatic PNETs. Preclinical data suggest that inhibition of the mammalian target of rapamycin has a significant antiproliferative effect in poorly differentiated pancreatic neuroendocrine cell lines, which in turn has led to the clinical development of several mammalian target of rapamycin inhibitors.67 The phase 3 RAD001 in Advanced Neuroendocrine Tumors (RADIANT-3) trial enrolled 410 patients with advanced PNETs who had evidence of radiographic progression within the previous 12 months.14 Patients were randomly assigned either to treatment with the mammalian target of rapamycin inhibitor everolimus (RAD001) or to placebo, with a primary end point of PFS. Patients in the everolimus group had a median PFS of 11.0 months compared with 4.6 months in the placebo group (p < 0.001) (Fig. 3B).14 These data led to the approval of everolimus as a first-line treatment option for patients with advanced inoperable progressive PNET.
A number of other receptor tyrosine kinases, including vascular endothelial growth factor receptor and platelet-derived growth factor receptor, are known to be important in the proliferation of these tumors. As such, small molecule inhibitors of these kinases have undergone clinical assessment for the treatment of metastatic PNETs. In 2011, the FDA approved the multi-receptor tyrosine kinase inhibitor sunitinib (Sutent) after a phase III trial in patients with advanced, well-differentiated PNETs who had evidence of disease progression within the previous 12 months. In this randomized, double-blind, placebo-controlled trial, 171 patients were randomly assigned either to treatment with sunitinib or to placebo with PFS as the primary end point.12 At the first planned interim analysis, the median PFS was 11.4 months in the sunitinib group compared with 5.5 months in the placebo group (p < 0.001). The study was stopped and sunitinib is now approved for the treatment of patients with advanced PNETs.
Treatment with targeted agents in the adjuvant or perioperative setting seems to be the next logical step in eligible patients. Building on the success of sunitinib and everolimus, future therapeutic targets in NET include the complex angiogenic pathways and epidermal growth factor receptor–directed signaling, similar to many other tumor types.68 Agents exhibiting some promise in early-phase trials include imatinib, bevacizumab, and thalidomide, although much additional study is required.69-71 It will be intriguing to see if these or similar agents can be combined with the more traditional cytotoxic agents that have fallen out of favor in recent years to enhance responses in this disease. There is also interest in the systemic administration of receptor targeted therapy—radiolabeled somatostatin analogues allow delivery of Y-90 to cells expressing somatostatin receptors.72
CONCLUSIONS
Patients with NELM can achieve long-term survival with >50% survival rate at 10 years in patients whose disease is amenable to liver-directed therapies such as hepatic resection. Although intrahepatic disease progression/recurrence is near universal, appropriate liver-directed therapy can offer both durable symptom control and long-term survival in selected patients. Currently, hepatic resection should be the first line of therapy offered to patients with a good performance status who have localized disease and hormonally functional hepatic metastasis. Asymptomatic patients with a large hepatic disease burden (>25%) likely benefit the most from IAT. Those patients who have NELM diffusely throughout the hepatic parenchyma will not likely achieve any durable symptomatic or survival benefit from liver-directed therapies and might be more appropriate candidates for targeted chemotherapeutic agents, such as somatostatin inhibitors, sunitinib, and everolimus or rapamycin. Management of patients with NELM necessitates a multidisciplinary approach to tailor the treatment to both the unique aspects of the patient’s disease and their long-term goals. Future developments in predicting disease progression and refining targeted therapies exploiting the unique biology of NELM in combination with liver-directed therapies will ultimately lead to the greatest improvements in both patient survival and quality of life.
Abbreviations and Acronyms
- IAT
intra-arterial therapy
- NELM
neuroendocrine liver metastasis
- NET
neuroendocrine tumors
- OLT
orthotopic liver transplantation
- PFS
progression-free survival
- PNET
pancreatic neuroendocrine tumor
- RFA
radiofrequency ablation
- TACE
transarterial chemoembolization
- Y-90
Yttrium-90
Footnotes
Author Contributions
Study conception and design: Mayo, Pawlik
Acquisition of data: Mayo, Herman, Cosgrove, Bhagat, Kamel, Geschwind, Pawlik
Analysis and interpretation of data: Mayo, Herman, Cosgrove, Bhagat, Kamel, Geschwind, Pawlik
Drafting of manuscript: Mayo, Herman, Cosgrove, Bhagat, Kamel, Geschwind, Pawlik
Critical revision: Mayo, Herman, Cosgrove, Bhagat, Kamel, Geschwind, Pawlik
Disclosure Information: Nothing to disclose.
References
- 1.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432–445. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Hardacre JM, Uzar A, et al. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998;187:88–92. doi: 10.1016/s1072-7515(98)00099-4. discussion 92–93. [DOI] [PubMed] [Google Scholar]
- 4.Knox CD, Anderson CD, Lamps LW, et al. Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol. 2003;10:1171–1175. doi: 10.1245/aso.2003.04.533. [DOI] [PubMed] [Google Scholar]
- 5.Nave H, Mossinger E, Feist H, et al. Surgery as primary treatment in patients with liver metastases from carcinoid tumors: a retrospective, unicentric study over 13 years. Surgery. 2001;129:170–175. doi: 10.1067/msy.2001.110426. [DOI] [PubMed] [Google Scholar]
- 6.Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 7.Mayo SC, de Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol. 2010;17:3129–3136. doi: 10.1245/s10434-010-1154-5. [DOI] [PubMed] [Google Scholar]
- 8.Mayo SC, de Jong MC, Bloomston M, et al. Surgery versus intra-arterial therapy for neuroendocrine liver metastasis: a multicenter international analysis. Ann Surg Oncol. 2011;18:3657–3665. doi: 10.1245/s10434-011-1832-y. [DOI] [PubMed] [Google Scholar]
- 9.Norton JA, Warren RS, Kelly MG, et al. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134:1057–1063. doi: 10.1016/j.surg.2003.07.025. discussion 1063–1065. [DOI] [PubMed] [Google Scholar]
- 10.Frilling A, Li J, Malamutmann E, et al. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. Br J Surg. 2009;96:175–184. doi: 10.1002/bjs.6468. [DOI] [PubMed] [Google Scholar]
- 11.Scigliano S, Lebtahi R, Maire F, et al. Clinical and imaging follow-up after exhaustive liver resection of endocrine metastases: a 15-year monocentric experience. Endocr Relat Cancer. 2009;16:977–990. doi: 10.1677/ERC-08-0247. [DOI] [PubMed] [Google Scholar]
- 12.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 13.Engstrom PF, Lavin PT, Moertel CG, et al. Streptozocin plus fluorouracil versus doxorubicin therapy for metastatic carcinoid tumor. J Clin Oncol. 1984;2:1255–1259. doi: 10.1200/JCO.1984.2.11.1255. [DOI] [PubMed] [Google Scholar]
- 14.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Toole D, Hentic O, Corcos O, et al. Chemotherapy for gastro-enteropancreatic endocrine tumours. Neuroendocrinology. 2004;80(Suppl 1):79–84. doi: 10.1159/000080747. [DOI] [PubMed] [Google Scholar]
- 16.Rindi G, Capella C, Solcia E. Introduction to a revised clinicopathological classification of neuroendocrine tumors of the gastroenteropancreatic tract. Q J Nucl Med. 2000;44:13–21. [PubMed] [Google Scholar]
- 17.Rindi G, Kloppel G, Alhman H, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kloppel G, Rindi G, Anlauf M, et al. Site-specific biology and pathology of gastroenteropancreatic neuroendocrine tumors. Virchows Arch. 2007;451(Suppl 1):S9–S27. doi: 10.1007/s00428-007-0461-0. [DOI] [PubMed] [Google Scholar]
- 19.Klimstra DS, Arnold R, Capella C. Neuroendocrine neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. Lyon: IARC; 2010. pp. 322–326. [Google Scholar]
- 20.American Joint Commission on Cancer, American Cancer Society. 7. New York: Springer; 2010. [Google Scholar]
- 21. [April 2, 2012];The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines™) Neuroendocrine Tumors. (Version 1. 2012) Available at: www.NCCN.org.
- 22.McEntee GP, Nagorney DM, Kvols LK, et al. Cytoreductive hepatic surgery for neuroendocrine tumors. Surgery. 1990;108:1091–1096. [PubMed] [Google Scholar]
- 23.Que FG, Nagorney DM, Batts KP, et al. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg. 1995;169:36–42. doi: 10.1016/s0002-9610(99)80107-x. discussion 42–43. [DOI] [PubMed] [Google Scholar]
- 24.House MG, Cameron JL, Lillemoe KD, et al. Differences in survival for patients with resectable versus unresectable metastases from pancreatic islet cell cancer. J Gastrointest Surg. 2006;10:138–145. doi: 10.1016/j.gassur.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753–781. doi: 10.1016/j.bpg.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Glazer ES, Tseng JF, Al-Refaie W, et al. Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford) 2010;12:427–433. doi: 10.1111/j.1477-2574.2010.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Jong MC, Mayo SC, Pulitano C, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg. 2009;13:2141–2151. doi: 10.1007/s11605-009-1050-0. [DOI] [PubMed] [Google Scholar]
- 28.de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 29.Pawlik TM, Vauthey JN. Surgical margins during hepatic surgery for colorectal liver metastases: complete resection not millimeters defines outcome. Ann Surg Oncol. 2008;15:677–679. doi: 10.1245/s10434-007-9703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seki S, Sakaguchi H, Kadoya H, et al. Laparoscopic microwave coagulation therapy for hepatocellular carcinoma. Endoscopy. 2000;32:591–597. doi: 10.1055/s-2000-9014. [DOI] [PubMed] [Google Scholar]
- 31.Tsai S, Pawlik TM. Outcomes of ablation versus resection for colorectal liver metastases: are we comparing apples with oranges? Ann Surg Oncol. 2009;16:2422–2428. doi: 10.1245/s10434-009-0491-8. [DOI] [PubMed] [Google Scholar]
- 32.Mazzaglia PJ, Berber E, Milas M, et al. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery. 2007;142:10–19. doi: 10.1016/j.surg.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 33.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood TF, Rose DM, Chung M, et al. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol. 2000;7:593–600. doi: 10.1007/BF02725339. [DOI] [PubMed] [Google Scholar]
- 35.Bilchik AJ, Wood TF, Allegra DP. Radiofrequency ablation of unresectable hepatic malignancies: lessons learned. Oncologist. 2001;6:24–33. doi: 10.1634/theoncologist.6-1-24. [DOI] [PubMed] [Google Scholar]
- 36.Elias D, Goere D, Leroux G, et al. Combined liver surgery and RFA for patients with gastroenteropancreatic endocrine tumors presenting with more than 15 metastases to the liver. Eur J Surg Oncol. 2009;35:1092–1097. doi: 10.1016/j.ejso.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Elias D, Lefevre JH, Duvillard P, et al. Hepatic metastases from neuroendocrine tumors with a “thin slice” pathological examination: they are many more than you think. Ann Surg. 2010;251:307–310. doi: 10.1097/SLA.0b013e3181bdf8cf. [DOI] [PubMed] [Google Scholar]
- 38.Ho AS, Picus J, Darcy MD, et al. Long-term outcome after chemoembolization and embolization of hepatic metastatic lesions from neuroendocrine tumors. AJR Am J Roentgenol. 2007;188:1201–1207. doi: 10.2214/AJR.06.0933. [DOI] [PubMed] [Google Scholar]
- 39.Liapi E, Geschwind JF, Vossen JA, et al. Functional MRI evaluation of tumor response in patients with neuroendocrine hepatic metastasis treated with transcatheter arterial chemoembolization. AJR Am J Roentgenol. 2008;190:67–73. doi: 10.2214/ajr.07.2550. [DOI] [PubMed] [Google Scholar]
- 40.Gupta S, Johnson MM, Murthy R, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer. 2005;104:1590–1602. doi: 10.1002/cncr.21389. [DOI] [PubMed] [Google Scholar]
- 41.Eriksson BK, Larsson EG, Skogseid BM, et al. Liver embolizations of patients with malignant neuroendocrine gastrointestinal tumors. Cancer. 1998;83:2293–2301. [PubMed] [Google Scholar]
- 42.Gupta S, Yao JC, Ahrar K, et al. Hepatic artery embolization and chemoembolization for treatment of patients with metastatic carcinoid tumors: the MD Anderson experience. Cancer J. 2003;9:261–267. doi: 10.1097/00130404-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Roche A, Girish BV, de Baere T, et al. Trans-catheter arterial chemoembolization as first-line treatment for hepatic metastases from endocrine tumors. Eur Radiol. 2003;13:136–140. doi: 10.1007/s00330-002-1558-0. [DOI] [PubMed] [Google Scholar]
- 44.Guiu B, Deschamps F, Aho S, et al. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs drug-eluting beads. J Hepatol. 2012;56:609–617. doi: 10.1016/j.jhep.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Liapi E, Georgiades CC, Hong K, et al. Transcatheter arterial chemoembolization: current technique and future promise. Tech Vasc Interv Radiol. 2007;10:2–11. doi: 10.1053/j.tvir.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Saxena A, Chua TC, Bester L, et al. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg. 2010;251:910–916. doi: 10.1097/SLA.0b013e3181d3d24a. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy AS, Dezarn WA, McNeillie P, et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31:271–279. doi: 10.1097/COC.0b013e31815e4557. [DOI] [PubMed] [Google Scholar]
- 48.Rhee TK, Lewandowski RJ, Liu DM, et al. 90Y Radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg. 2008;247:1029–1035. doi: 10.1097/SLA.0b013e3181728a45. [DOI] [PubMed] [Google Scholar]
- 49.Bester L, Meteling B, Pocock N, et al. Radioembolization versus standard care of hepatic metastases: comparative retrospective cohort study of survival outcomes and adverse events in salvage patients. J Vasc Interv Radiol. 2012;23:96–105. doi: 10.1016/j.jvir.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 50.Pfeifer AK, Gregersen T, Gronbaek H, et al. Peptide receptor radionuclide therapy with Y-DOTATOC and (177)Lu-DOTATOC in advanced neuroendocrine tumors: results from a Danish cohort treated in Switzerland. Neuroendocrinology. 2011;93:189–196. doi: 10.1159/000324096. [DOI] [PubMed] [Google Scholar]
- 51.Mayo SC, de Jong MC, Pawlik TM. Surgical management and emerging therapies to prolong survival in metastatic neuroendocrine cancer. Ann Surg Oncol. 2011;18(Suppl 3):S220–S221. doi: 10.1245/s10434-010-1343-2. [DOI] [PubMed] [Google Scholar]
- 52.Gleisner AL, Choti MA, Assumpcao L, et al. Colorectal liver metastases: recurrence and survival following hepatic resection, radiofrequency ablation, and combined resection-radiofrequency ablation. Arch Surg. 2008;143:1204–1212. doi: 10.1001/archsurg.143.12.1204. [DOI] [PubMed] [Google Scholar]
- 53.Hoyer M, Swaminath A, Bydder S, et al. Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol Biol Phys. 2012;82:1047–1057. doi: 10.1016/j.ijrobp.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 54.Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486–493. doi: 10.1016/j.ijrobp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 55.Chang DT, Swaminath A, Kozak M, et al. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer. 2011;117:4060–4069. doi: 10.1002/cncr.25997. [DOI] [PubMed] [Google Scholar]
- 56.Stinauer MA, Diot Q, Westerly DC, et al. Fluorodeoxyglucose positron emission tomography response and normal tissue regeneration after stereotactic body radiotherapy to liver metastases. Int J Radiat Oncol Biol Phys. 2012;83:e613–e618. doi: 10.1016/j.ijrobp.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Contessa JN, Griffith KA, Wolff E, et al. Radiotherapy for pancreatic neuroendocrine tumors. Int J Radiat Oncol Biol Phys. 2009;75:1196–1200. doi: 10.1016/j.ijrobp.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 58.Le Treut YP, Grégoire E, Belghiti J, et al. Location of the primary tumor and liver size are predictors for survival after liver transplantation for endocrine metastases. An 85-case French multicentric series Liver Transplant. 2007;13:S 159. [Google Scholar]
- 59.Mazzaferro V, Pulvirenti A, Coppa J. Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? J Hepatol. 2007;47:460–466. doi: 10.1016/j.jhep.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 61.Moertel CG, Hanley JA. Combination chemotherapy trials in metastatic carcinoid tumor and the malignant carcinoid syndrome. Cancer Clin Trials. 1979;2:327–334. [PubMed] [Google Scholar]
- 62.Chan JA, Kulke MH. New treatment options for patients with advanced neuroendocrine tumors. Curr Treat Options Oncol. 2011;12:136–148. doi: 10.1007/s11864-011-0148-2. [DOI] [PubMed] [Google Scholar]
- 63.Ekeblad S, Sundin A, Janson ET, et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res. 2007;13:2986–2991. doi: 10.1158/1078-0432.CCR-06-2053. [DOI] [PubMed] [Google Scholar]
- 64.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 65.Schmid HA, Schoeffter P. Functional activity of the multiligand analog SOM230 at human recombinant somatostatin receptor subtypes supports its usefulness in neuroendocrine tumors. Neuroendocrinology. 2004;80(Suppl 1):47–50. doi: 10.1159/000080741. [DOI] [PubMed] [Google Scholar]
- 66.Kvols L, Oberg KE, O’Dorisio T, et al. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a Phase II study. Endocr Relat Cancer. 2012 Jul 17; doi: 10.1530/ERC-11-0367. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 67.Catena L, Bajetta E, Milione M, et al. Mammalian target of rapamycin expression in poorly differentiated endocrine carcinoma: clinical and therapeutic future challenges. Target Oncol. 2011;6:65–68. doi: 10.1007/s11523-011-0171-z. [DOI] [PubMed] [Google Scholar]
- 68.Terris B, Scoazec JY, Rubbia L, et al. Expression of vascular endothelial growth factor in digestive neuroendocrine tumours. Histopathology. 1998;32:133–138. doi: 10.1046/j.1365-2559.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 69.Yao JC, Zhang JX, Rashid A, et al. Clinical and in vitro studies of imatinib in advanced carcinoid tumors. Clin Cancer Res. 2007;13:234–240. doi: 10.1158/1078-0432.CCR-06-1618. [DOI] [PubMed] [Google Scholar]
- 70.Yao JC, Phan A, Hoff PM, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008;26:1316–1323. doi: 10.1200/JCO.2007.13.6374. [DOI] [PubMed] [Google Scholar]
- 71.Varker KA, Campbell J, Shah MH. Phase II study of thalidomide in patients with metastatic carcinoid and islet cell tumors. Cancer Chemother Pharmacol. 2008;61:661–668. doi: 10.1007/s00280-007-0521-9. [DOI] [PubMed] [Google Scholar]
- 72.Cwikla JB, Sankowski A, Seklecka N, et al. Efficacy of radionuclide treatment DOTATATE Y-90 in patients with progressive metastatic gastroenteropancreatic neuroendocrine carcinomas (GEP-NETs): a phase II study. Ann Oncol. 2010;21:787–794. doi: 10.1093/annonc/mdp372. [DOI] [PubMed] [Google Scholar]
- 73.Elias D, Lasser P, Ducreux M, et al. Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: a 15-year single center prospective study. Surgery. 2003;133:375–382. doi: 10.1067/msy.2003.114. [DOI] [PubMed] [Google Scholar]
- 74.Osborne DA, Zervos EE, Strosberg J, et al. Improved outcome with cytoreduction versus embolization for symptomatic hepatic metastases of carcinoid and neuroendocrine tumors. Ann Surg Oncol. 2006;13:572–581. doi: 10.1245/ASO.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 75.Hibi T, Sano T, Sakamoto Y, et al. Surgery for hepatic neuroendocrine tumors: a single institutional experience in Japan. Jpn J Clin Oncol. 2007;37:102–107. doi: 10.1093/jjco/hyl140. [DOI] [PubMed] [Google Scholar]
- 76.Landry CS, Scoggins CR, McMasters KM, et al. Management of hepatic metastasis of gastrointestinal carcinoid tumors. J Surg Oncol. 2008;97:253–258. doi: 10.1002/jso.20957. [DOI] [PubMed] [Google Scholar]
- 77.Chambers AJ, Pasieka JL, Dixon E, et al. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery. 2008;144:645–651. doi: 10.1016/j.surg.2008.06.008. discussion 651–653. [DOI] [PubMed] [Google Scholar]
- 78.Ahmed A, Turner G, King B, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer. 2009;16:885–894. doi: 10.1677/ERC-09-0042. [DOI] [PubMed] [Google Scholar]


