Abstract
Objective
To determine the association between asthma and proinflammatory conditions.
Participants and Methods
This population-based retrospective matched cohort study enrolled all asthmatic patients among Rochester, Minnesota, residents between January 1, 1964, and December 31, 1983. For each asthmatic patient, 2 age-and sex-matched nonasthmatic individuals were drawn from the same population. The asthmatic and nonasthmatic cohorts were followed forward in the Rochester Epidemiology Project diagnostic index for inflammatory bowel disease (IBD), rheumatoid arthritis (RA), diabetes mellitus (DM), and coronary heart disease (CHD) as outcome events. Data were fitted to Cox proportional hazards models.
Results
We identified 2392 asthmatic patients and 4784 nonasthmatic controls. Of the asthmatic patients, 1356 (57%) were male, and mean age at asthma onset was 15.1 years. Incidence rates of IBD, RA, DM, and CHD in nonasthmatic controls were 32.8, 175.9, 132.0, and 389.7 per 100,000 person-years, respectively; those for asthmatic patients were 41.4, 227.9, 282.6, and 563.7 per 100,000 person-years, respectively. Asthma was associated with increased risks of DM (hazard ratio, 2.11; 95% confidence interval, 1.43-3.13; P<.001) and CHD (hazard ratio, 1.47; 95% confidence interval, 1.05-2.06; P=.02) but not with increased risks of IBD or RA.
Conclusion
Although asthma is a helper T cell type 2–predominant condition, it may increase the risks of helper T cell type 1–polarized proinflammatory conditions, such as CHD and DM. Physicians who care for asthmatic patients need to address these unrecognized risks in asthmatic patients.
Abbreviations and Acronyms: CHD, coronary heart disease; CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; IBD, inflammatory bowel disease; ICD, International Classification of Diseases; RA, rheumatoid arthritis; REP, Rochester Epidemiology Project; TH, helper T cell
Asthma is the most common chronic illness in childhood and a major cause of morbidity in adults, affecting 4% to 17% of children and 7.3% to 10.1% of adults in the United States1,2 and, overall, nearly 30 million Americans and 300 million people globally.3 Asthma prevalence continues to increase in many parts of the world.4 For example, the Centers for Disease Control and Prevention reported that the asthma prevalence in persons of all ages in the United States increased from 7.3% in 2001 to 8.2% in 2009, a 12.3% increase in 8 years.5 The total incremental cost of asthma to society was estimated to be $56 billion,6 suggesting that asthma is a major medical and economic burden globally.
There have been numerous studies concerning the etiology of asthma, but the effect of asthma on the epidemiologic characteristics of other chronic diseases has been relatively underinvestigated. There have been studies that assessed the relationship between asthma and chronic conditions (ie, proinflammatory conditions), such as rheumatoid arthritis (RA),7-9 inflammatory bowel disease (IBD),9-12 diabetes mellitus (DM),8,9,13-16 and coronary heart disease (CHD).14 However, most of these studies were based on cross-sectional studies using International Classification of Diseases (ICD) codes for asthma and chronic diseases as outcomes. To our knowledge, no population-based cohort study has examined the effect of asthma using predetermined criteria on the incidence of these proinflammatory conditions. Considering the major burden and major effects of these proinflammatory conditions, the study results have important implications on clinical practice and public health.
We hypothesized that there may be an inverse relationship between asthma as a helper T cell (TH) type 2–polarized condition and proinflammatory diseases as TH1-predominant immune conditions given the reciprocal regulation between TH1 and TH2.17,18 To test this hypothesis, we conducted a population-based retrospective matched cohort study in Rochester, Minnesota, that determined the association of asthma with RA, IBD, DM, and CHD.
Participants and Methods
This study was designed as a population-based retrospective matched cohort study and was approved by the institutional review boards at Mayo Clinic and Olmsted Medical Center. We compared the incidence rates of proinflammatory conditions between Rochester residents with asthma (exposed group) and those without asthma (unexposed group) between January 1, 1964, and December 31, 1983.
Study Population and Setting
Rochester is centrally located in Olmsted County. During the study period, characteristics of the City of Rochester and Olmsted County populations were similar to those of the US white population except that a higher proportion of the working population is employed in the health care industry.19-21 Rochester is an excellent setting in which to conduct a population-based epidemiologic study because medical care is virtually self-contained within the community. In addition, when patients register at any health care provider in the community for the first time (eg, as newborns), they are asked whether they authorize using their medical records for research. Each patient who grants authorization (95%) for the use of their medical records for research is assigned a unique identifier under the auspices of the Rochester Epidemiology Project (REP),22 which has been continuously funded and maintained since 1960. All clinical diagnoses are electronically indexed, and information from every episode of care is contained in detailed patient-based medical records; essentially, all medical care settings and providers are linked. This unique longitudinal population-based resource has been the source of more than 2000 publications on the epidemiologic study of disease.23 Using REP resources, we previously demonstrated that incidence rates of asthma for this community are similar to those for other communities. For example, the annual incidence rate of asthma in Rochester was 238 cases per 100,000 persons, which is comparable with that in other communities, such as Tecumseh, Michigan (250 cases per 100,000 persons), during the study period.24
Exposure Ascertainment (Asthma)
The methods used to determine asthma status were previously reported.21,25 We enrolled a previously assembled cohort of all asthmatic patients as an exposed group (study cohort) among the Rochester residents between 1964 and 1983. Although the study period was not recent, it was a population-based asthma cohort based on ascertainment of asthma using predetermined criteria for asthma (not ICD codes or self-report). This unique asthma cohort also allowed us to examine more clearly the effect of asthma on the risk of proinflammatory conditions. This period was before the introduction and widespread use of anti-inflammatory therapeutics, which might alter the relationship between asthma and the risk of proinflammatory conditions. To assemble this asthmatic cohort, a previous study identified all individuals with a potential asthma diagnosis and symptoms such as wheezing using the REP medical diagnostic list. Then the medical records for all potential asthmatic patients were reviewed to confirm asthma using predetermined criteria (Table 1). Diagnostic categories have been linked across the many updates of the diagnostic indices, including revisions of the ICD or Hospital International Classification of Diseases Adapted and Berkson codes. Of the 18,000 potential patients with asthma, 2499 met the criteria for asthma delineated in Table 1. In this study, we used this previously assembled asthma cohort (1964-1983).
TABLE 1.
| 1. History of cough, dyspnea, or wheezing OR history of cough or dyspnea plus wheezing on examination |
| 2. Substantial variability in symptoms from time to time or periods of weeks or more when symptoms were absent |
| 3. Two or more of the following: |
| Sleep disturbance by nocturnal cough and wheeze |
| Nonsmoker (age ≥14 y) |
| Nasal polyps |
| Blood eosinophil count >300/μL |
| Positive wheal and flare skin test results OR elevated serum IgE level |
| History of hay fever or infantile eczema OR cough, dyspnea, and wheezing regularly on exposure to an antigen |
| Pulmonary function tests showing an FEV1 or FVC <70% of predicted and another with ≥20% improvement to an FEV1 >70% of predicted OR a methacholine challenge test showing a ≥20% decrease in FEV1 |
| Favorable clinical response to bronchodilator therapy |
| Patients were excluded from the study if any of the following conditions were present: |
| Pulmonary function tests that showed FEV1 to be consistently <50% of predicted or diminished diffusion capacity |
| Tracheobronchial foreign body on or about the incidence date |
| Hypogammaglobulinemia (IgG level <2.0 mg/mL) or another immunodeficiency disorder |
| Wheezing occurring only in response to anesthesia or medication administration |
| Bullous emphysema or pulmonary fibrosis on chest radiography |
| PiZZ α1-antitrypsin |
| Cystic fibrosis |
| Other major chest disease, such as juvenile kyphoscoliosis or bronchiectasis |
FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
Patients were considered to have definite asthma if a physician had made a diagnosis of asthma or if each of the 3 conditions were present, and they were considered to have probable asthma if only the first 2 conditions were present.
Excluded from the original study were (1) non-Rochester residents (to exclude referral patients from elsewhere), (2) those without research authorization for using medical records for research, and (3) individuals with any of the medical conditions listed in Table 1, which make ascertainment of asthma difficult.
Selection of Nonasthmatic Controls (Unexposed Group)
The REP resources were also used to generate a list of all Rochester residents without asthma (unexposed group) by excluding those who met the criteria for asthma (exposed group). Two nonasthmatic individuals as an unexposed group were matched to each corresponding asthmatic patient as an exposed group regarding sex and birthday (within 2 months for <18 years of age and within 1 year for ≥18 years of age) as the date criteria for asthma were first met by the case (ie, the patient's index date). Other criteria for nonasthmatic controls (unexposed group) included having been registered at Mayo Clinic as a resident of Rochester in the index year (±1 year) of their matched case. The index date for nonasthmatic controls was the closest clinic visit or hospitalization day to the index date of asthma for their matched case. Therefore, we ensured that asthmatic patients and nonasthmatic controls had a similar starting point of follow-up. The exclusion criteria for the unexposed group were the same as those for the exposed group.
Identification of Proinflammatory Conditions (Outcomes or Diseases)
Asthmatic patients (exposed group) and nonasthmatic controls (unexposed group or comparison cohort) who refused authorization for the use of medical records in research (typically 5%-6% in REP studies) were excluded from the study. We followed the exposed and nonexposed cohorts from the index date until the earliest of emigration, death, or the end of the study (December 31, 1983) to compare the cumulative incidence of the outcome disease of interest. We included only incident cases of proinflammatory conditions based on diagnostic codes by excluding the prevalent cases of proinflammatory conditions before the index date of asthma.
Ascertainment of IBD, DM, CHD, and RA as outcome events was based on the REP diagnostic index codes (ICD and Berkson codes) for individuals with IBD,26-30 DM,31-34 CHD,35-37 and RA38-40 from the medical record. Briefly, all medical index codes for DM that included a diagnosis of DM and DM-related complications (eg, diabetic retinopathy, nephropathy, gastroparesis, neuropathy, and ketoacidosis) were used. Similarly, all diagnostic index codes pertaining to angina pectoris, coronary disease, coronary atherosclerosis, myocardial infarction, and ischemic heart diseases were included for identification of CHD. All medical index codes for IBD including a diagnosis of IBD (eg, Crohn disease, ulcerative colitis, regional enteritis, regional jejunitis or ileitis, and IBD) were used. The REP computerized diagnostic index was searched for any diagnosis of RA (excluding degenerative arthritis or osteoarthritis and juvenile RA).
Statistical Analyses
The incidence rates and their corresponding 95% confidence intervals (CIs) of CHD, DM, RA, and IBD were calculated using the number of outcome events as the numerator and the total number of person-years of follow-up as the denominator. Since RA did not include juvenile RA and CHD is rare in children, we calculated the incidence rates of RA and CHD based on adult participants only. The incidence rate of each condition was compared between individuals with and those without asthma using nonasthmatic individuals as a reference group. We applied survival analysis using the Kaplan-Meier curve to compare survival free of outcome events between the asthmatic and nonasthmatic groups. Cox proportional hazards models were fit to calculate the hazard ratios (HRs) and their corresponding 95% CIs, adjusting for race/ethnicity. All the tests were 2-sided, with significance set at P<.05, and were performed using a commercially available software program (SAS, version 9.1; SAS Institute, Inc, Cary, NC).
Results
Study Participants
Of the original asthma study cohort (n=2499), 107 patients were excluded (72 owing to no research authorization for the present study and 35 owing to mismatching of clinic number). Demographic characteristics are summarized in Table 2. In this study, 2392 patients with asthma and 4784 controls without asthma were enrolled. Of the asthmatic patients, 1356 (57%) were male, and 1074 of 1099 patients with known race/ethnicity (98%) were white. The median and mean ages at asthma onset were 5 and 15.1 years, respectively. The median (and interquartile range) follow-up durations after the index date of asthma in asthmatic patients and nonasthmatic controls were 7.0 years (2.9-13.2 years) and 5.8 years (1.9-12.0 years), respectively (P=.001).
TABLE 2.
Demographic and Clinical Characteristics of the Study Participantsa
| Asthmatic group (N=2392) | Nonasthmatic group (N=4784) | |
|---|---|---|
| Age at index date (y)b | ||
| Mean ± SD | 15.1±20.5 | 15.1±20.5 |
| Median (IQR) | 5 (1-22) | 5 (1-22) |
| Age group at last follow-up or at the end of the study, No. (%) | ||
| Children (<18 y) | 1082 (45) | 2094 (44) |
| Adults (≥18 y) | 1310 (55) | 2690 (56) |
| Male sex, No. (%) | 1356 (57) | 2712 (57) |
| White race, No. (%)c | 1074 (98) | 1771 (91) |
IQR = interquartile range.
The closest clinic visit date of nonasthmatic controls (unexposed group) was matched to the index date of their corresponding asthmatic patients (exposed group).
The proportion of race/ethnicity was based on known race/ethnicity data for asthmatic patients (n=1099, 46%) and nonasthmatic controls (n=1948, 41%).
Asthma and Proinflammatory Conditions
The incidence rates of IBD, RA, DM, and CHD in the nonexposed group were 32.8, 175.9, 132.0, and 389.7 per 100,000 person-years, respectively, and those for the exposed group were 41.4, 227.9, 282.6, and 563.7 per 100,000 person-years, respectively. The main results are summarized in Tables 3 and 4 and in Figures 1 to 3. (Although median follow-up was 7.0 years for asthmatic patients and 5.8 years for nonasthmatic individuals, the data presented in Figures 1 to 3 show cumulative incidence rates throughout the study period of 20 years.) Asthma status was associated with increased risks of DM (unadjusted HR, 2.11; 95% CI, 1.43-3.13; P<.001) and CHD (unadjusted HR, 1.47; 95% CI, 1.05-2.06; P=.02), whereas individuals with asthma did not have significantly increased risks of IBD (unadjusted HR, 1.31; 95% CI, 0.53-3.25; P=.56) and RA (unadjusted HR, 1.30; 95% CI, 0.78-2.18; P=.31). Based on participants whose race/ethnicity was known, white race was associated with asthma (P<.001) (Table 2). However, after adjusting for race/ethnicity, there were no significant changes in the HRs for the association of asthma status with the risks of CHD and DM in multivariate Cox models (Table 4), and white patients had a lower risk of CHD compared with nonwhite patients (HR, 0.26; 95% CI, 0.17-0.40). The survival curves show that the incidence of CHD and DM in asthmatic patients continued to increase with increasing years of follow-up. Although there were trends toward a higher incidence of IBD and RA in asthmatic patients compared with nonasthmatic controls during the later follow-up period, there were no significant associations between asthma and the risk of RA or IBD.
TABLE 3.
| Outcome | Childrenc |
Adultsc |
||
|---|---|---|---|---|
| With asthma | Without asthma | With asthma | Without asthma | |
| CHD | NA | NA | 563.7 (431.2-724.1) | 389.7 (307.5-487.1) |
| DM | 60.0 (19.5-139.8) | 48.4 (19.5-99.6) | 454.7 (336.3-601.2) | 193.6 (137-265.7) |
| RA | NA | NA | 227.9 (147.4-336.4) | 175.9 (122.5-244.7) |
| IBD | 12.0 (0.3-66.6) | 13.8 (1.7-49.8) | 63.7 (25.6-131) | 45.1 (20.6-85.7) |
CHD = coronary heart disease; CI = confidence interval; DM = diabetes mellitus; IBD = inflammatory bowel disease; NA, not applicable; RA = rheumatoid arthritis.
The incidence rates and their corresponding 95% CIs were calculated per 100,000 person-years.
Stratification of age group was based on age at last follow-up or at the end of the study.
TABLE 4.
| Outcome | Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI)c | P value |
|---|---|---|---|---|
| DM | 2.11 (1.43-3.13) | <.001 | 2.12 (1.43-3.14) | <.001 |
| CHD | 1.47 (1.05-2.06) | .02 | 1.50 (1.07-2.10) | .02 |
| RA | 1.30 (0.78-2.18) | .31 | 1.30 (0.78-2.18) | .31 |
| IBD | 1.31 (0.53-3.25) | .56 | 1.30 (0.52-3.23) | .58 |
CHD = coronary heart disease; CI = confidence interval; DM = diabetes mellitus; HR = hazard ratio; IBD = inflammatory bowel disease; RA = rheumatoid arthritis.
The HRs and 95% CIs for the association between white race and risks of DM, CHD, RA, and IBD in the Cox models for adjustment were 0.85 (0.57-1.26), 0.26 (0.17-0.40), 0.93 (0.59-1.46), and 1.69 (0.68-4.22), respectively.
Adjusted HRs for the association between asthma and risks of proinflammatory conditions controlling for race/ethnicity (white vs nonwhite).
FIGURE 1.
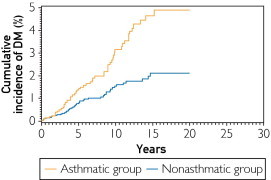
Cumulative incidence of diabetes mellitus (DM) in the asthmatic and nonasthmatic groups (P<.001 based on univariate Cox regression). The median (95% confidence interval) follow-up durations for the asthmatic and nonasthmatic groups were 7 (2.9-13.2) and 5.7 (1.9-12.0) years, respectively.
FIGURE 2.
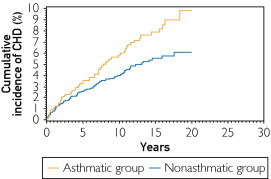
Cumulative incidence of coronary heart disease (CHD) in the asthmatic and nonasthmatic groups (P=.04 based on univariate Cox regression). The median (95% confidence interval) follow-up durations for the asthmatic and nonasthmatic groups were 7 (2.9-13.2) and 5.7 (1.9-12.0) years, respectively.
FIGURE 3.
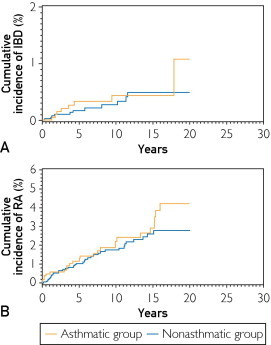
Cumulative incidence of inflammatory bowel disease (IBD) (A) and rheumatoid arthritis (RA) (B) in the asthmatic and nonasthmatic groups (no statistically significant differences). The median (95% confidence interval) follow-up durations for the asthmatic and nonasthmatic groups were 7 (2.9-13.2) and 5.7 (1.9-12.0) years, respectively.
Discussion
Contrary to the original hypothesis that asthma, a TH2-predominant condition, may have inverse relationships with proinflammatory conditions with TH1 immune milieu, these study results showed increased risks of certain proinflammatory conditions, such as CHD and DM. We observed significant associations between asthma as the explanatory variable and the risk of CHD (adjusted HR, 1.46) and DM (adjusted HR, 2.12) even after controlling for race/ethnicity. However, we did not observe similar associations between asthma and risks of RA and IBD, although there were trends toward a positive association. The associations between asthma and these conditions might be due to detection bias since comorbid conditions in asthmatic patients might be more likely to be detected by physicians than those in nonasthmatic individuals, who may not require medical care as often. However, we do not believe that detection bias entirely accounts for the findings because ascertainment of asthma in this study was not based on a physician diagnosis or self-report but rather on predetermined criteria for asthma. In addition, detection bias is more likely to occur when outcome diseases are subclinical and their detections depend on screening tests or evaluations by physicians prompted by exposure status. However, the outcome events in this study, such as DM and CHD, are unlikely to go undetected for a period of time. In addition, our previous work showed that the role of detection bias in epidemiologic research concerning the association of asthma with other disease outcomes is minimal or unlikely. For example, we showed similar health care use between asthmatic patients and nonasthmatic controls, suggesting that asthma status does not influence health care use or affect the likelihood of detecting health outcomes differentially between asthmatic patients and nonasthmatic controls in this study setting.41-44
In support of these study findings, previous studies have demonstrated an association between asthma and CHD14 and DM.8,14-16 Thus, the present study results provide an important basis for the potential association between asthma and proinflammatory conditions, although the present study results were not fully adjusted for all pertinent covariates or confounders.
When comparing the incidence rates of CHD and DM reported in previous studies conducted in the Rochester community (571-699 per 100,000 person-year36 and 240-256 per 100,000 person-years,33 respectively), the incidence rates of CHD and DM in the present study (390-564 per 100,000 person-years and 132-283 per 100,000 person-years, respectively) seem to be similar or slightly lower. The previous studies included older participants (ie, >30-35 years of age) than the present study, and the mean age of individuals in these previous studies were likely to be older than the present study patients. On the other hand, the incidence rates of RA and IBD in this study (176-228 per 100,000 person-years and 12-64 per 100,000 person-years, respectively) were slightly higher than the incidence estimates reported in previous studies (75.3 per 100,000 person-years for RA,40 9.4-10.1 per 100,000 person-years for ulcerative colitis, and 6.5-7.9 per 100,000 person-years for Crohn disease29). Because of a smaller number of patients with IBD, we calculated the overall incidence of IBD only, which makes it difficult to compare with the standardized incidence of ulcerative colitis and Crohn disease of previous studies. Alternatively, the present study was based on the Rochester residents (urban setting), which has been reported to have a higher incidence of ulcerative colitis than a rural setting, whereas the previous study was based on Olmsted County residents (urban and rural residents).30 Also, ascertainment of RA and IBD in previous studies was based on predetermined criteria for RA and IBD, whereas in the present study it was based on medical index search codes, which may inflate the incidence rate. However, this is likely to be subject to a nondifferential misclassification and is unlikely to affect the interpretation of the main results on the association of asthma with the risks of CHD and DM.
The lack of association between asthma and RA and IBD could be explained by statistical or potential immunologic reasons. The incidence rates of RA and IBD were lower than those of CHD and DM in adults, which may limit statistical power to detect the reported effect size.29,33,36,40 Immunologically, during the study period, inhaled corticosteroids were not widely available for the treatment of asthma, and systemic corticosteroids were used for the treatment of asthma exacerbations and for asthma control. Systemic corticosteroid treatment for asthma might mitigate or slow the progress of RA and IBD (ie, may mask clinical symptoms for RA and IBD) in a way that attenuates the association of asthma with RA and IBD since systemic corticosteroids were a treatment choice for both conditions.
For the potential mechanisms underlying the associations of asthma with CHD and DM, we postulate potential intrinsic and extrinsic pathways. As an intrinsic mechanism, inflammatory cytokines, such as interleukin 6 and 17, have been reported to be involved in the inflammatory process in asthma45-47 and proinflammatory conditions, including CHD46,48 and DM.49 As an extrinsic mechanism, acute exacerbations of asthma may cause hypoxia and tachyarrhythmia, triggering CHD symptoms. Thus, asthmatic patients with a predisposition to CHD may be more likely to reveal symptoms of CHD, leading to evaluations and detection of CHD. Similarly, corticosteroid treatment for asthma might cause steroid-induced hyperglycemia and is more likely to reveal those with immunogenetic predisposition to DM.
Overall, these epidemiologic data suggest that the traditional TH1 vs TH2 paradigm may not be a simple reciprocal inhibitory relationship in the context of development of chronic inflammatory diseases. Rather, asthma may share common underlying immunogenetic or environmental mechanisms with proinflammatory conditions. This conceptual understanding may facilitate clinical and basic research in a way that leads to improved patient care (early identification and better management of chronic diseases in asthmatic patients) and guidance for efforts to identify mechanisms underlying the association between asthma and proinflammatory conditions.
This study has the inherent limitations of a retrospective study. Although we adjusted the main results for age, sex, and ethnicity, we did not adjust for other potential covariates and confounders, such as body mass index, smoking, and other risk factors for CHD and DM. A future study examining the association between asthma and individual chronic conditions needs to consider these covariates and confounders. The present study participants were predominantly of white race, which may limit generalizability of the study findings. However, at the cost of generalizability (ie, external validity), internal validity can be enhanced by minimizing the confounding effect of race/ethnicity on the study results. The strengths of this study include a population-based matched cohort design that examined the incidence of proinflammatory conditions between asthmatic patients and nonasthmatic controls. In addition, we used a well-established epidemiologic database (ie, the REP) for proinflammatory conditions and applied predetermined criteria to determine asthma status. Also, the study setting has epidemiologic advantages, with a self-contained health care environment and unified medical records for research for all the residents of Rochester.
Conclusion
Asthma does not reduce the risk of proinflammatory conditions but increases the risk of CHD and DM. Physicians need to be aware of these study findings and address symptoms of CHD and DM in asthmatic patients during asthma follow-up visits. Also, given the considerable proportion of people affected by asthma, public health communities may need to carefully monitor the potential impact of asthma epidemiologic features on the epidemiologic mechanisms of the chronic diseases assessed in this study. Further studies that assess the association between asthma and individual chronic inflammatory conditions are needed to confirm these study findings.
Acknowledgment
We thank the staff of the Pediatric Asthma Epidemiology Research Unit who made this study possible. We also thank Elizabeth Krusemark, BA, for administrative assistance.
Footnotes
Grant Support: This work was supported by the Scholarly Clinician Award from the Mayo Foundation and was made possible by the Rochester Epidemiology Project (grant number R01-AG034676; Principal Investigator: Walter A. Rocca, MD, and Barbara P. Yawn, MD, MSc).
Supplemental Online Material
Author Interview Video
References
- 1.Lethbridge-Cejku M., Vickerie J. National Center for Health Statistics; Hyattsville, MD: 2005. Summary of Health Statistics for US Adults: National Health Interview Survey, 2003. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Forecasted state-specific estimates of self-reported asthma prevalence—United States, 1998. MMWR Morb Mortal Wkly Rep. 1998;47(47):1022–1025. [PubMed] [Google Scholar]
- 3.World Health Organization Fact sheet for asthma No. 307. http://www.who.int/mediacentre/factsheets/fs307/en/index.html Accessed October 26, 2006.
- 4.Anandan C., Nurmatov U., van Schayck O.C.P., Sheikh A. Is the prevalence of asthma declining? systematic review of epidemiological studies. Allergy. 2010;65(2):152–167. doi: 10.1111/j.1398-9995.2009.02244.x. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Vital signs: asthma prevalence, disease characteristics, and self-management education—United States, 2001-2009. MMWR Morb Mortal Wkly Rep. 2011;60(17):547–552. [PubMed] [Google Scholar]
- 6.Barnett S.B., Nurmagambetov T.A. Costs of asthma in the United States: 2002-2007. J Allergy Clin Immunol. 2011;127(1):145–152. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 7.de Roos A.J., Cooper G.S., Alavanja M.C., Sandler D.P. Personal and family medical history correlates of rheumatoid arthritis. Ann Epidemiol. 2008;18(6):433–439. doi: 10.1016/j.annepidem.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kero J., Gissler M., Hemminki E., Isolauri E. Could TH1 and TH2 diseases coexist? evaluation of asthma incidence in children with coeliac disease, type 1 diabetes, or rheumatoid arthritis: a register study. J Allergy Clin Immunol. 2001;108(5):781–783. doi: 10.1067/mai.2001.119557. [DOI] [PubMed] [Google Scholar]
- 9.Tirosh A., Mandel D., Mimouni F.B., Zimlichman E., Shochat T., Kochba I. Autoimmune diseases in asthma. Ann Intern Med. 2006;144(12):877–883. doi: 10.7326/0003-4819-144-12-200606200-00004. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein C.N., Wajda A., Blanchard J.F. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129(3):827–836. doi: 10.1053/j.gastro.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Fenta YA, Tello N, Jung JA, et al. Inflammatory bowel disease and asthma: a population-based, case-control study. Inflamm Bowel Dis. 16(11):1957-1962. [DOI] [PMC free article] [PubMed]
- 12.Weng X., Liu L., Barcellos L.F., Allison J.E., Herrinton L.J. Clustering of inflammatory bowel disease with immune mediated diseases among members of a northern California-managed care organization. Am J Gastroenterol. 2007;102(7):1429–1435. doi: 10.1111/j.1572-0241.2007.01215.x. [DOI] [PubMed] [Google Scholar]
- 13.EURODIAB Substudy 2 Study Group Decreased prevalence of atopic diseases in children with diabetes. J Pediatr. 2000;137(4):470–474. doi: 10.1067/mpd.2000.109109. [DOI] [PubMed] [Google Scholar]
- 14.Prosser R., Carleton B., Smith A. The comorbidity burden of the treated asthma patient population in British Columbia. Chronic Dis Can. 2010;30(2):46–55. [PubMed] [Google Scholar]
- 15.Adams R.J., Wilson D.H., Taylor A.W. Coexistent chronic conditions and asthma quality of life: a population-based study. Chest. 2006;129(2):285–291. doi: 10.1378/chest.129.2.285. [DOI] [PubMed] [Google Scholar]
- 16.Zhang T., Carleton B.C., Prosser R.J., Smith A.M. The added burden of comorbidity in patients with asthma. J Asthma. 2009;46(10):1021–1026. doi: 10.3109/02770900903350473. [DOI] [PubMed] [Google Scholar]
- 17.van Den Broek J., Bachmann M., Kohler G. IL-4 and IL-10 antagonize IL-12-mediated protection against acute vaccine in a virus infection with a limited role of IFN-γ and nitric oxide synthetase 2. J Immunol. 2000;164(1):371–378. doi: 10.4049/jimmunol.164.1.371. [DOI] [PubMed] [Google Scholar]
- 18.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18(6):263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 19.US Census Bureau . US Census Bureau; Washington, DC: 1983 and 1993. 1980 and 1990 Census of Population and Housing. [Google Scholar]
- 20.Katusic S.K., Colligan R.C., Barbaresi W.J., Schaid D.J., Jacobsen S.J. Potential influence of migration bias in birth cohort studies. Mayo Clin Proc. 1998;73(11):1053–1061. doi: 10.4065/73.11.1053. [DOI] [PubMed] [Google Scholar]
- 21.Yunginger J.W., Reed C.E., O'Connell E.J., Melton L.J., III, O'Fallon W.M., Silverstein M.D. A community-based study of the epidemiology of asthma: incidence rates, 1964-1983. Am Rev Respir Dis. 1992;146(4):888–894. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- 22.Kurland L.T., Molgaard C.A. The patient record in epidemiology. Sci Am. 1981;245(4):54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 23.Melton L. History of the Rochester Epidemiology Project. Mayo Clinic Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 24.Broder I., Higgins M.W., Mathews K.P., Keller J.B. Epidemiology of asthma and allergic rhinitis in a total community, Tecumseh, Michigan, IV: natural history. J Allergy Clin Immunol. 1974;54(2):100–110. doi: 10.1016/0091-6749(74)90038-4. [DOI] [PubMed] [Google Scholar]
- 25.Juhn Y.J., Kita H., Yawn B.P. Increased risk of serious pneumococcal disease in patients with asthma. J Allergy Clin Immunol. 2008;122(4):719–723. doi: 10.1016/j.jaci.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stonnington C.M., Phillips S.F., Melton L.J., III, Zinsmeister A.R. Chronic ulcerative colitis: incidence and prevalence in a community. Gut. 1987;28(4):402–409. doi: 10.1136/gut.28.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gollop J.H., Phillips S.F., Melton L.J., III, Zinsmeister A.R. Epidemiologic aspects of Crohn's disease: a population based study in Olmsted County, Minnesota, 1943-1982. Gut. 1988;29(1):49–56. doi: 10.1136/gut.29.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loftus E.V., Jr, Silverstein M.D., Sandborn W.J., Tremaine W.J., Harmsen W.S., Zinsmeister A.R. Crohn's disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology. 1998;114(6):1161–1168. doi: 10.1016/s0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- 29.Loftus C.G., Loftus E.V., Jr, Harmsen W.S. Update on the incidence and prevalence of Crohn's disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007;13(3):254–261. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 30.Loftus E.V., Jr, Silverstein M., Sandborn W., Tremaine W., Harmsen W., Zinsmeister A. Ulcerative colitis in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gut. 2000;46(3):336–343. doi: 10.1136/gut.46.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leibson C., Milton L.J., III, Palumbo P.J. Temporal trends in diabetes incidence and prevalence. Diabetes Care. 1997;20(3):460–462. doi: 10.2337/diacare.20.3.460b. [DOI] [PubMed] [Google Scholar]
- 32.Dinneen S.F., Maldonado D., III, Leibson C.L. Effects of changing diagnostic criteria on the risk of developing diabetes. Diabetes Care. 1998;21(9):1408–1413. doi: 10.2337/diacare.21.9.1408. [DOI] [PubMed] [Google Scholar]
- 33.Burke J.P., O'Brien P., Ransom J. Impact of case ascertainment on recent trends in diabetes incidence in Rochester, Minnesota. Am J Epidemiol. 2002;155(9):859–865. doi: 10.1093/aje/155.9.859. [DOI] [PubMed] [Google Scholar]
- 34.Thomas R.J., Palumbo P.J., Melton L.J., III Trends in the mortality burden associated with diabetes mellitus: a population-based study in Rochester, Minn, 1970-1994. Arch Intern Med. 2003;163(4):445–451. doi: 10.1001/archinte.163.4.445. [DOI] [PubMed] [Google Scholar]
- 35.Goraya T.Y., Jacobsen S.J., Kottke T.E., Frye R.L., Weston S.A., Roger V.L. Coronary heart disease death and sudden cardiac death: a 20-year population-based study. Am J Epidemiol. 2003;157(9):763–770. doi: 10.1093/aje/kwg057. [DOI] [PubMed] [Google Scholar]
- 36.Elveback L.R., Connolly D.C., Kurland L.T. Coronary heart disease in residents of Rochester, Minnesota, II: mortality, incidence, and survivorship, 1950-1975. Mayo Clin Proc. 1981;56(11):665–672. [PubMed] [Google Scholar]
- 37.Roger V.L., Jacobsen S.J., Weston S.A., Bailey K.R., Kottke T.E., Frye R.L. Trends in heart disease deaths in Olmsted County, Minnesota, 1979-1994. Mayo Clin Proc. 1999;74(7):651–657. doi: 10.4065/74.7.651. [DOI] [PubMed] [Google Scholar]
- 38.Doran M.F., Pond G.R., Crowson C.S., O'Fallon W.M., Gabriel S.E. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002;46(3):625–631. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 39.Arnett F.C., Edworthy S.M., Bloch D.A. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 40.Gabriel S.E., Crowson C.S., O'Fallon W.M. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955-1985. Arthritis Rheum. 1999;42(3):415–420. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 41.Juhn Y.J., Johnson S.K., Hashikawa A.H. The potential biases in studying the relationship between asthma and microbial infection. J Asthma. 2007;44(10):827–832. doi: 10.1080/02770900701743804. [DOI] [PubMed] [Google Scholar]
- 42.Frey D., Jacobson R., Poland G., Li X., Juhn Y. Assessment of the association between pediatric asthma and Streptococcus pyogenes upper respiratory infection. Allergy Asthma Proc. 2009;30(5):540–545. doi: 10.2500/aap.2009.30.3268. [DOI] [PubMed] [Google Scholar]
- 43.Lynch B.A., Fenta Y., Jacobson R., Li X., Juhn Y.J. Impact of delay in asthma diagnosis on chest x-ray and antibiotic utilization by clinicians. J Asthma. 2012;49(1):23–28. doi: 10.3109/02770903.2011.637596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lynch B.A., Van Norman C.A., Jacobson R.A., Weaver A.L., Juhn Y.J. Impact of delay in asthma diagnosis on health care service utilization. Allergy Asthma Proc. 2010;31(4):48–52. doi: 10.2500/aap.2010.31.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong C.K., Ho C.Y., Ko F.W. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-γ, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001;125(2):177–183. doi: 10.1046/j.1365-2249.2001.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu T.L., Chang P.Y., Tsao K.C., Sun C.F., Wu L.L., Wu J.T. A panel of multiple markers associated with chronic systemic inflammation and the risk of atherogenesis is detectable in asthma and chronic obstructive pulmonary disease. J Clin Lab Anal. 2007;21(6):367–371. doi: 10.1002/jcla.20197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Y., Yang J., Gao Y.D., Guo W. Th17 immunity in patients with allergic asthma. Int Arch Allergy Immunol. 2010;151(4):297–307. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]
- 48.Patterson C.C., Smith A.E., Yarnell J.W., Rumley A., Ben-Shlomo Y., Lowe G.D. The associations of interleukin-6 (IL-6) and downstream inflammatory markers with risk of cardiovascular disease: the Caerphilly Study. Atherosclerosis. 2010;209(2):551–557. doi: 10.1016/j.atherosclerosis.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 49.Pradhan A.D., Manson J.E., Rifai N., Buring J.E., Ridker P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview Video


