Abstract
Human herpesviruses (HHVs) have frequently been suspected as etiologic agents or cofactors in cutaneous disease. However, clearly established associations are rare. Investigations into an etiologic association between HHVs and cutaneous disease are complicated by the ubiquity and nearly universal prevalence of some herpesviruses. This article summarizes the associations between cutaneous disease and HHV-6, HHV-7, and HHV-8. In addition to a personal library of references, the PubMed database of biomedical literature was searched using the following Medical Subject Heading terms: HHV-6, HHV-7, and HHV-8, each in conjunction with cutaneous manifestations, virology, epidemiology, dermatopathology, and therapeutics, between 1998 and March 2011. Free-text searches with known or suspected disease associations were added for broader coverage. The results have been summarized to provide a practical review for the physician likely to encounter cutaneous diseases.
Abbreviations and Acronyms: CMV, Cytomegalovirus; DIHS, drug-induced hypersensitivity syndrome; DRESS, drug reaction with eosinophilia and systemic symptoms; HHV, human herpesvirus; KS, Kaposi sarcoma
Three recently discovered members of the human herpesvirus (HHV) family are HHV-6, HHV-7, and HHV-8. The closely homologous HHV-6 and HHV-7 are ubiquitous, with nearly universal prevalence in persons older than 6 years. Human herpesvirus 8 partly shares the predilection of its 2 siblings for latency in lymphoid tissues but has a more variable demographic distribution. All 3 HHVs are actively investigated for their role in multiple pathologic abnormalities and have important cutaneous manifestations. Dermatologists typically encounter HHV-6 and HHV-7 in classic roseola infantum, whereas HHV-8 is also known as Kaposi sarcoma (KS)–associated herpesvirus. However, new disease associations, novel diagnostic evaluations, and developments in antiviral therapies herald the possibility of new advances in the diagnosis and treatment of HHV-6, HHV-7, and HHV-8. In this article, we provide a broad review of HHV-6, HHV-7, and HHV-8 from a dermatologic perspective. In addition to a personal library of references, the PubMed database was searched using the following Medical Subject Heading terms: HHV-6, HHV-7, and HHV 8, each in conjunction with cutaneous manifestations, virology, epidemiology, dermatopathology, and therapeutics. Free-text searches with known or suspected disease associations were added for broader coverage. Coverage dates were limited by online available materials, and preference was given to newer studies. The results have been summarized to provide a practical review for the physician likely to encounter cutaneous diseases.
Virologic Classification
HHV-6 and HHV-7 and Their Epidemiologic Features
Classification of the herpesviruses includes alpha herpesviruses (HHV-1 and HHV-2), which are fast growers; beta herpesviruses, which are slow growers and include the genus Roseolovirus (HHV-6 and HHV-7) and the genus Cytomegalovirus (CMV); and gamma herpesviruses, which include Epstein-Barr virus in the genus Lymphocryptovirus. This discussion is restricted to HHV-6, HHV-7, and HHV-8. Human herpesvirus 6 has 2 variants, A and B, although new evidence suggests that these subtypes are distinct viruses, each with its own strains.1 Human herpesvirus 6A has been found more commonly in skin biopsy specimens from immunocompromised patients and is considered a possible cofactor in AIDS progression.2,3 In comparison, HHV-6B is thought to be the primary etiologic agent in roseola.
The cellular receptor for HHV-6 is CD46, which is expressed on all nucleated cells. It accounts for the wider tropism of HHV-6 compared with that of HHV-7, which uses CD4, the marker of a T-lymphocyte subclass.4 An anatomical mapping study of 8 autopsies (without known evidence of HHV infection) identified HHV-6 and HHV-7 in all cases, particularly in the salivary glands. An estimated 80% to 90% of the population sheds HHV-6 and HHV-7 intermittently in saliva.5 Human herpesvirus 6 can also integrate into the human chromosome as an ingenious means of achieving latency and vertical transmission. A prospective study of 5638 children showed that congenital HHV-6 infection occurred in approximately 1% of births.6 A recent chromosomal analysis confirmed that HHV-6 can be present in every cell of congenitally infected children.7 Such congenital presence of the virus is a confounding factor in epidemiologic and diagnostic studies. Clinically, the mode of transmission for HHV-6 and HHV-7 is probably less relevant because their ubiquity and nearly universal childhood serum positivity suggest that exposure prevention is unlikely to be an option for controlling the spread of HHV-6 and HHV-7.
Similar to HHV-8, HHV-6 and HHV-7 may also have oncogenic properties. Although no cutaneous malignancy has been clearly linked to any beta Herpesviridae, neoplastic transformation of human epidermal keratinocytes has been shown in vitro.8 The integration of HHV-6 into telomeres with subsequent viral-induced telomere elongation could account for part of its oncogenic properties and part of its ability to evade the human immune system.9
HHV-8 and Its Epidemiologic Features
Human herpesvirus 8 is a member of the gamma Herpesviridae subfamily and a member of the Rhadinovirus genus. It has multiple distinct subtypes and, similar to HHV-6 and HHV-7, a predilection for lymphoid tissue. Longitudinal detection difficulties preclude establishment of HHV-8 prevalence, which is thought to approximately mirror that of KS, with relatively low rates in the United States, except where KS is endemic and high rates exist.10 However, the prevalence of HHV-8 does not completely correlate with KS prevalence because there are subpopulations with high HHV-8 seropositivity and nearly unknown KS.11 In the United States, the seroprevalence of HHV-8 is usually estimated to be less than 10%, whereas estimates of the prevalence of KS usually fall below 0.1%.12
Human herpesvirus 8 has been found in seminal fluid, nasal secretions, and saliva, but its mode of transmission remains enigmatic.13 Seropositivity is unusual in infancy,14 which argues against widespread vertical transmission. Kaposi sarcoma is more common in homosexual than heterosexual persons with AIDS.15 The high prevalence in prepubertal children in endemic areas suggests that sexual transmission is unlikely to be the sole mode of infection. A study of HHV-8–seropositive men who had sex with men and no clinical evidence of KS showed that exposure to infectious saliva is a risk factor for HHV-8 acquisition.16 However, this association alone does not explain the prevalence of disparities between homosexual and heterosexual populations. Other likely routes of transmission are the transplant of infected organs,17 transfusion of unprocessed blood,18 and shared use of injection needles.19
Disease Associations
Cutaneous Disease Caused by HHV-6 and HHV-7
The identified disease association of HHV-6 and HHV-7 is roseola infantum, also known as exanthem subitum,20 which develops in only a few infected children. On the basis of isolation of the virus and seroconversion in blood samples from 4 patients in the febrile phase of the illness, Yamanishi et al21 proposed a causal association in 1988. One year later, a report from a prospective study of 38 children confirmed HHV-6 viremia in 100% of patients with roseola infantum in the early stages of the disease.22 More recently, roseola and the cutaneous eruptions associated with primary HHV-6 infection have been connected to a specific type of viral encephalopathy. On the basis of a series of 10 patients, a Japanese group proposed a new subtype of encephalitis called human herpesvirus 6 encephalopathy with cluster of convulsions during eruptive stage.23 In 2009, a nationwide survey in Japan determined that “exanthem subitum–associated encephalitis” had an unexpectedly poor prognosis.24 Human HHV-6 DNA was detected in only 53.8% of the patients. Consequently, HHV-6 encephalopathy and exanthem subitum encephalitis are probably distinct, although overlapping, entities.
A more debated association of the 2 viruses is pityriasis rosea (Figure 1).25 Studies linking HHV-6 and HHV-7 with pityriasis have involved tissue, whole blood, and serum analysis. Temporal case clustering from different world regions using regression analysis supported a pattern of seasonal cases compatible with an infectious etiology.26 Although some studies showed a clear association with HHV-6 and HHV-7,27,28 others found HHV-6 and HHV-7 to occur more commonly in controls than in persons with pityriasis.29 However, not all studies can be taken at face value. Given the nearly universal prevalence of HHV-6 and HHV-7, checking for viral DNA alone, without testing for an altered antibody response to show active infection, is insufficient to establish or disprove an association. The medical literature associating pityriasis rosea with HHV-6 and HHV-7 was reviewed by the team of Drago, Broccolo, and Rebora in 200930 and then again in 201031 to outline the existing controversies. Although an association seems likely, the investigators concluded that cutaneous signs of the disease are more likely a reactive response than an effect of direct infection of skin cells.
FIGURE 1.
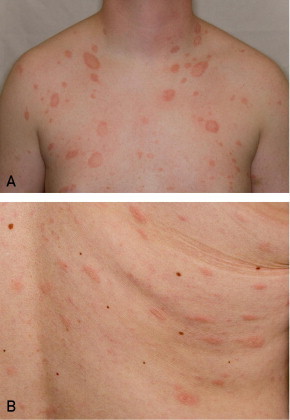
Pityriasis rosea. A, Classic oval-shaped, scaly patches. B, Eruption along Langer lines.
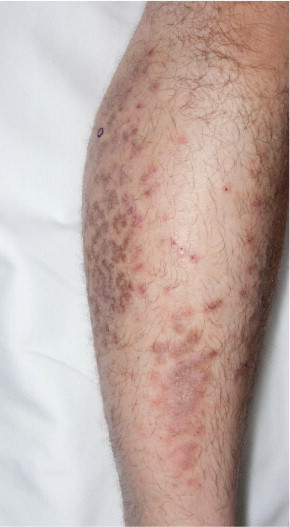
Human herpesvirus 7, but not HHV-6, is currently also a candidate for the etiology of lichen planus (Figure 2). In skin biopsy specimens from 33 patients, cells infected by HHV-7 were identified more frequently in lichen planus lesions than in skin without lesions or in psoriatic or healthy skin.32 Moreover, clinical remission after treatment was associated with a decrease in HHV-7 viral DNA.33 In addition, multiple case reports link HHV-6 and HHV-7 to other cutaneous disorders, including HHV-6 in Stevens-Johnson syndrome,34 thrombocytopenic purpura,35 purpura fulminans (Figure 3),36 papular-purpuric gloves and socks syndrome,37,38 and Gianotti-Crosti syndrome (Figure 4).39 The latter was investigated in a Chinese prospective study of 10 patients and 10 age-matched controls.26 Although HHV-6 DNA was detected in both groups, active HHV-6 infection was found in only 2 of the 10 patients. Both were younger than 1 year and, thus, in the age bracket most commonly affected by primary HHV-6 infection.
FIGURE 2.
Lichen planus. Purple polygonal papules.
FIGURE 3.
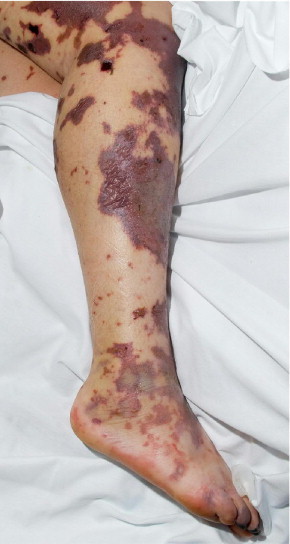
Purpura fulminans. Cutaneous hemorrhage and necrosis.
FIGURE 4.
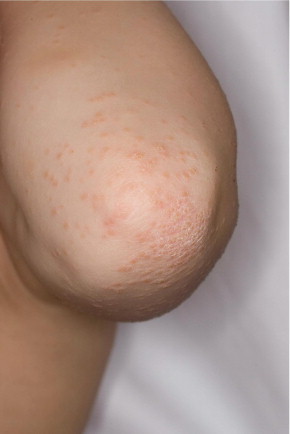
Gianotti-Crosti syndrome. Monomorphous pink papulovesicles on an extensor surface.
Human herpesvirus 6 and HHV-7 may also contribute to other disease processes with dermatologic implications, such as drug reactions and transplant complications. Herpesviridae are strong candidates in the quest to better understand the pathogenesis of drug-induced hypersensitivity syndrome (DIHS), particularly drug reaction with eosinophilia and systemic symptoms (DRESS).40 Data from 40 patients with DRESS suggest that it is a consequence of activated immune cells directed against herpesvirus antigens.41 Seventy-six percent of these patients showed reactivation of Epstein-Barr virus, HHV-6, or HHV-7. This reactivation, in turn, led to oligoclonal proliferation of activated CD8+ T lymphocytes directed against viral antigens and to the inappropriate attack of host visceral and cutaneous tissue. Thus, the viral involvement in DRESS may result partly from drug-induced HHV reactivation.42 In addition, the pathogenesis can be enhanced by the combined modulation of inflammatory cytokines produced by the interplay of the suspected drug and the virus.43 Traditionally, clinically significant reactivation of HHV-6 has been detected only after DIHS onset. However, a recent case report directly linked the pathogenesis of DIHS with HHV-6 when HHV-6 viremia was detected 3 days after onset of the cutaneous eruption.44 One piece of this puzzle may be found in a study of 10 patients with DIHS who were compared with 3 healthy individuals and 5 patients with other drug allergies.45 The emergence of circulating monomyeloid precursors, which are reservoirs for latent HHV-6, was found to be predictive of the HHV-6 reactivation. Drug-induced hypersensitivity syndrome has also been linked to reactivation of Epstein-Barr virus, CMV, and HHV-7, but the association with HHV-6 has been the most consistent. Indeed, that association has been strong enough for a Japanese consensus group to include HHV-6 reactivation in its DIHS diagnostic criteria.46
Posttransplant reactivation or donor transmission of HHV-6 and HHV-7 has been associated with multiple pathologic abnormalities, including cutaneous manifestations of graft-vs-host disease.47 In a prospective study of 15 patients who underwent allogeneic stem cell transplant, cutaneous graft-vs-host disease developed in 10.48 Of these 10 patients, 8 had new evidence of HHV-6 DNA levels that correlated with their cutaneous manifestations. In addition, HHV-6 infection has been said to facilitate superinfections in transplant recipients, which may account for some skin abnormalities in this classically immunocompromised group.49 However, establishing etiology rather than association is difficult because multiple pathogens are reactivated in the immunocompromised host and because HHV-6 and HHV-7 are ubiquitous in the general population. Viral-to-viral transactivation further obscures the picture. For example, HHV-6 and HHV-7 may cause or aggravate CMV infection in renal transplant patients.50,51
In a cross-sectional study of 58 patients and 38 controls that broadened the range to other states of an altered immune system, HHV-6 viremia was more common in patients with autoimmune connective tissue diseases.52 Whether HHV-6 is a predisposing factor or a more potent pathogen in a susceptible host, this association could be of importance in patients with cutaneous autoimmune diseases and in chronically immunosuppressed patients. Indeed, a particularly strong association was found between HHV-6 reactivation and scleroderma. Conversely, in a recent case report of fatal disseminated HHV-6 infection, the immunocompromised patient initially presented with a macular eruption.53 Thus, the viral involvement in some autoimmune diseases might parallel the role of viruses in DIHS. An immune system altered by drugs or diseases could favor viral reactivation.52 In turn, the viral-induced or viral-responsive proinflammatory drive could account for the cutaneous eruptions in each case by a pathway converging at an altered cytokine milieu (Table).
TABLE.
Cutaneous Disease Associations of HHV-6, HHV-7, and HHV-8
| HHV-6 |
| Roseola infantum |
| HHV-6 encephalopathy (with cluster of convulsions in eruptive stage) |
| Pityriasis rosea |
| Lichen planus |
| Scleroderma |
| Gianotti-Crosti syndrome |
| DIHS/DRESS |
| Stevens-Johnson syndrome |
| Gloves and socks syndrome |
| Thrombocytopenic purpura/purpura fulminans |
| Graft-vs-host disease |
| HHV-7 |
| Roseola infantum |
| Pityriasis rosea |
| Lichen planus |
| DIHS/DRESS |
| Graft-vs-host disease |
| HHV-8 |
| Kaposi sarcoma |
| Exanthem with primary infection |
DIHS = drug-induced hypersensitivity syndrome; DRESS = drug reaction with eosinophilia and systemic symptoms; HHV = human herpesvirus.
Cutaneous Disease Caused by HHV-8
During the early days of the AIDS pandemic, HHV-8 was identified in patients with KS. Human herpesvirus 8 has also been found in classic, endemic, and transplant-related forms of the disease.54 After the discovery of HHV-8 by representational difference analysis, numerous case reports and seroepidemiologic studies consistently documented findings of HHV-8 in KS lesions.55 In addition, patients with primary HHV-8 infection may present with cutaneous findings (Table), as evidenced in a cohort study of 86 immunocompetent children; 6 of these patients were suspected of having primary HHV-8, and all but 1 had a maculopapular exanthem.56 Established noncutaneous associations, multicentric Castleman disease, hemophagocytic lymphohistiocytosis, and primary effusion lymphoma can also have varying skin manifestations, although diagnosis and management belong more properly in the realm of hematology.
Other associations have been suggested but not proved. Human herpesvirus 6, HHV-7, and HHV-8 have all been candidates for the pathogenesis of parapsoriasis and cutaneous T-cell lymphoma. However, recent studies have not identified the viral DNA in lesional skin.57-59 As with HHV-6 and HHV-7, the spectrum of HHV-8–associated pathologic abnormality has not been completely described, and the identification of HHV-8 in multiple epithelial cell lineages suggests considerable need for research.
Presentation
Clinical Features of Cutaneous Diseases Associated With HHV-6 and HHV-7
Diagnosis of viral exanthems is often easier said than done. In a Brazilian study of 223 children, 43.5% had evidence of primary HHV-6 infection.60 However, only 21% with HHV-6 had a typical roseola-like illness, whereas 73% and 46% met the clinical criteria for measles and rubella, respectively. The diagnosis assigned most commonly to children with serologically proven primary HHV-6 infection was “unknown.” Future proposed clinical criteria for such diagnoses would be important not only for epidemiologic studies but also for optimal patient management in clinical practice.
The typical patient with roseola infantum is a child, usually younger than 2 years, with a high fever lasting 3 days and possibly involving febrile seizures.61 Human herpesvirus 6 and HHV-7 are considered the most common causes of infantile seizures (with and without roseola). As the child defervesces, a nonpruritic morbilliform exanthem develops, usually starting on the trunk and spreading centrifugally. Small, pink macules or papules may be surrounded by a light halo and are usually not painful and do not blanch or blister. Characteristic signs of viral exanthem are periorbital edema and erythematous papules on the soft palate and uvula. In immunocompetent patients, the disease is mild and self-limiting.
Pityriasis rosea classically begins with a single rose-colored, scaling, “herald” patch, although noncutaneous prodromal syndromes are not uncommon.62 Within 1 to 3 weeks, a generalized eruption of similar but smaller oval-shaped patches, papules, or plaques occurs along Langer lines, primarily on the trunk but also spreading to the limbs and, more rarely, the face. Unlike syphilis, pityriasis does not affect the palms and soles. Oral involvement may occur with punctate hemorrhages, ulcers, bullae, or erythematous plaques. Scaling appears on the inside of an erythematous circlet, which can help distinguish pityriasis from tinea infection. Varying degrees of pruritus are common but not universal. Multiple variations have been described, including vesicular, purpuric, and edematous.30 Atypical morphologic features, sites, and symptoms can cause confusion, likely leading to underdiagnosis by physicians other than dermatologists. Similar to roseola, pityriasis is usually benign and typically resolves within 6 weeks, but symptoms can cause severe distress and residual hypopigmentation or hyperpigmentation.
Lichen planus is an inflammatory eruption that typically causes purple, polygonal, pruritic papules. The lesions, with characteristically fine reticular scaling, are most commonly found on the flexor surfaces of the upper extremities, particularly the wrists, on the genitals and in the oral mucosa. However, they may affect all mucosal surfaces and the entire gastrointestinal tract. Ungual findings, such as nail plate thinning and dyspigmentation, are not uncommon. Lichen planus has a confusing array of variations, ranging from hypertrophic to atrophic, linear to annular, and vesicular to ulcerative. Involvement of hair-bearing skin of the scalp and axillae is well known. With analogous variability, lesions tend to resolve in a few weeks to a few years.
Drug-induced hypersensitivity syndrome commonly manifests on the skin. It typically develops 2 to 6 weeks after initiation of the precipitating medication.41 However, even after discontinuation of the offending agent, cutaneous eruptions may flare or relapse. Localized macules, papules, and pustules can progress to generalized erythroderma. Pruritus is common, although not universal, and diffuse edema may occur. Lesions may mimic erythema multiforme, Stevens-Johnson syndrome, or toxic epidermal necrolysis, although these entities are clinically distinct. DRESS is a multisystem disorder that usually begins several weeks after initiation of a new medication. Cutaneous manifestations typically involve a papular, pustular, bullous, or morbilliform exanthem that begins with facial and acral edema. Lesions can involve the mucous membranes and can evolve into a life-threatening exfoliative dermatitis. Medications commonly associated with DRESS include anticonvulsants, some antihypertensives, sulfonamides, allopurinol, and minocycline.63
Superficially, the cutaneous disease associations of HHV-6 and HHV-7 seem to differ markedly. However, the features of a viral exanthem or, in the case of lichen planus, localized eruptions, can be found in each of them, even in DIHS and DRESS, which, similar to primary childhood infection with HHV-6 or HHV-7, can mimic mononucleosis. Similarly, even the typical roseola eruption is often mistaken for another viral exanthem or a drug reaction.60
Clinical Features of KS
The typical patient with classic KS is a middle-aged man of Mediterranean or eastern European descent who presents with multiple discrete red to blue-purple or black patches on the lower extremities. The lesions can initially appear as bruises. Early macules or patches coalesce into characteristic plaques and nodules. However, in the early form of the lesion, the differential diagnosis is wider and includes various types of angiomas (primary or reactive [eg, bacillary angiomatosis]), acroangiodermatitis, and melanocytic lesions. In its classic form, KS can be considered a benign neoplasm because affected persons tend to die of other causes.64
The presentation of patients with endemic KS resembles that of classic KS, with the typical patient being male and middle aged, but patients with endemic KS are typically from the Middle East or Africa.65 However, endemic KS has a wider age range and more commonly involves organs other than the skin. With increasing or stagnant rates of infection with human immunodeficiency virus, AIDS-associated KS has become the dominant form even in many endemic regions.
The incidence of transplant-associated KS is several hundred times greater than that of KS in the general population of the United States. The cutaneous presentation resembles that of classic KS, but the site of manifestation is much more variable. However, as is the case for AIDS-associated KS, the prognosis for patients with transplant-associated KS is also much poorer than that for patients with classic KS.
The typical patient with AIDS-associated KS is a homosexual man with clinically significant immunosuppression due to infection with the human immunodeficiency virus. The presentation of AIDS-associated KS involves more atypical lesions that, early on, often resemble subtle bruises or pyogenic granulomas.54 The lesions later develop into more classic pigmented nodules and plaques. Oral and mucosal involvement is more common in AIDS-associated KS than in classic KS. AIDS-associated KS carries an unfavorable prognosis, with substantial mortality and morbidity. Patients die of opportunistic infections or internal complications (eg, gastric or pulmonary hemorrhage).
The typical presentation has several variations. Cutaneous manifestations can occur anywhere and in different patterns. An interesting variant is exanthematic KS, which, similar to pityriasis rosea, can present in a fir tree–shaped distribution.66 Mucosal surfaces may be involved and may represent the initial site of presentation. Skin breakdown can lead to ulcers and fungating masses. Local inflammation and edema may occur. The growth rate and aggression of the tumor often correlate with the degree of the host's immunosuppression. Other less common subtypes of KS include exophytic growths, cavernous tumors, telangiectatic or ecchymotic sarcomas, lymphangioma like eruptions, and multifocal friable tumor aggregations.67 Different clinical staging systems are available, all of which tend to rely on the extent of dissemination and immunosuppression (Figure 5).
FIGURE 5.
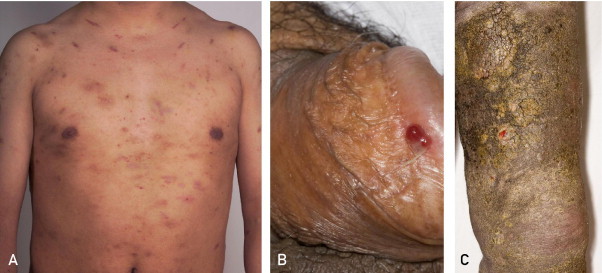
Kaposi sarcoma. A, Multiple discrete red-purple patches and plaques. B, Ulcerative plaque on the glans penis. C, Verruciform patches.
Viral Diagnostic Testing
In clinical practice, the diagnosis and management of HHV-6 and HHV-7 by cutaneous manifestations usually depend on clinical acumen rather than viral detection. Each disease might also have unique nonviral features. However, discerning the presence of HHV-6 and HHV-7, if not necessarily their contribution to skin abnormalities, has become increasingly sophisticated and may play an increasing role in the future, as evidenced by the Japanese consensus group diagnostic criteria for DIHS.46 Similarly, the presence of HHV-8 is helpful in diagnosing KS, particularly when there are early lesions that are easily confused with bruises or other vascular tumors.
Viral diagnostic techniques can be divided broadly into DNA/RNA detection, antigen assays, and antibody assays. Generally, the probability of viral detection can be amplified by culture, but the procedure is typically too time-consuming to have clinical utility. Skin biopsy specimens are tested by antigen assays, polymerase chain reaction, or, if available, electron microscopy. When applied to tissue, polymerase chain reaction, particularly real-time techniques, is highly sensitive and facilitates discrimination even of viral subtypes such as HHV-6A and HHV-6B. However, on the one hand, viral DNA may still be below the detection limits in biopsied tissues, and, on the other hand, the mere detection of viral nucleic acid does not prove its contribution to pathologic abnormalities. When applied to serum, nucleic acid amplification techniques tend to be less sensitive than do HHV-specific antibodies and may result in false-positives from contamination, particularly in persons with congenital HHV.9 Multiple samples are needed to demonstrate seroconversion, which is traditionally an indispensable step in proving the active pathologic role of the virus. Similar to nucleic acid amplification, antigen detection can be useful for testing biopsied tissue. Conversely, latency-associated nuclear antigen can detect HHV-8 in tissue and blood samples. Plasma or serum antibody titers can be measured by conventional indirect immunofluorescent assays or enzyme-linked immunosorbent assays. However, the antigenic similarity between Herpesviridae creates the problem of cross-reactivity. More important, in a population with naturally high seropositivity, many serologic assays cannot distinguish between active and latent infection.30 Testing for avidity helps reduce this limitation but is more labor intensive. In addition, persistent viremia may not correlate with persistent antibody titers, and seronegativity has been shown to sometimes follow seropositivity.68,69 Although this can be a concern in epidemiologic studies, it is particularly problematic in everyday clinical practice. Dermatologists must, therefore, be especially careful when using serum-derived data.
Clinically, awareness of available detection mechanisms may be increasingly important in immunocompromised patients, in whom the stakes are higher and antiviral therapy might be crucial. As our understanding of viral pathogenesis improves, viral diagnosis and targeted therapy may also become increasingly commonplace. The current variation in and disagreement about disease associations of the Herpesviridae is partly due to the variability of diagnostic techniques. Therefore, enhancement and standardization of detection and diagnostic techniques will be important in clarifying the pathogenesis of HHV-associated diseases.
Antiviral Treatments
Better understanding of their pathogenesis may lead to better targeting of HHV and HHV-associated diseases. Tailored therapies for HHV-6, HHV-7, and HHV-8 are still in their infancy. There are currently no approved treatments for HHV-6 and HHV-7 infections. From a practical perspective, immunocompetent persons with uncomplicated skin manifestations associated with HHV-6 and HHV-7, particularly roseola infantum and pityriasis rosea, need only symptomatic and supportive management. In addition, there are separate, nonantiviral treatment options, such as corticosteroids for DRESS. However, since HHV-6 and HHV-7 can cause havoc in the immunocompromised patient, and since their disease spectrum seems far from exhausted, new antiviral therapies should gain in clinical importance. A study of 87 patients with pityriasis rosea showed that acyclovir may be effective, especially in the first week after onset.70 By day 14 of treatment, 78.6% of patients receiving acyclovir had fully cleared cutaneous lesions compared with 4.4% receiving placebo. The study was neither double blinded nor randomized, and the authors pointed out that hitherto acyclovir has shown little activity against HHV-7. In vitro and isolated in vivo studies suggest that anti-CMV drugs, such as ganciclovir and valganciclovir, cidofovir, and foscarnet, have activity against HHV-6 and HHV-7, with the latter being more sensitive to cidofovir or foscarnet than to ganciclovir.71,72 By analogy, artesunate and other drugs with activity against CMV might be useful.
Several experimental drugs, such as cyclopropavir, also have promising potential against HHV-6; these drugs were recently reviewed by Flamand et al.9 Similarly, cyclotriazadisulfonamide73 and 9-R-2-phosphonomethoxypropyl adenine74 have shown promise against HHV-7. However, broad-spectrum antivirals carry considerable toxicity risk and are, therefore, still unsuitable for large-scale prophylactic or therapeutic trials.
Anti–HHV-8 therapies have been intimately linked to KS treatment, although some anecdotal evidence also exists for multicentric Castleman disease. Ganciclovir and foscarnet have been associated with reduced development of KS, whereas acyclovir has not been shown to be effective.75,76 On the basis of a study of 2 patients, cidofovir therapy was found to result in a reduction in HHV-8 viral load.77 However, a trial of cidofovir treatment in 7 patients did not reproduce these results.78 Most such studies have been small; the challenges involved in the diagnosis of HHV-8–associated disease also impede interpretation of treatment results. The proliferative, angiogenic, and inflammatory elements of KS seem to depend on lytic replication and latency of HHV-8.79 Thus, the effectiveness of antiviral therapy will depend partly on the level of HHV-8 production and on the life span of latently infected cells, which would determine treatment duration. Although the scope of HHV-8 infections is still being investigated, nonviral treatment modalities, including surgical, cytotoxic, and immunomodulating therapies, are likely to remain at the forefront of clinical practice. However, effective antivirals could lead to better therapeutic outcomes if combined with these other treatment modalities.80
Conclusion
Investigation of the 3 most recently identified HHV viruses is fascinating for several reasons. First, although the currently available data mostly allow disease association rather than causation analyses, it is likely that these 3 viral siblings serve as provoking, contributing, or complicating factors in multiple cutaneous disease presentations. Second, HHV-6, HHV-7, and HHV-8 have ingeniously incorporated key host DNA fragments that allow them to adapt more easily, to escape immunodetection, and to manipulate the cellular machinery to create an environment conducive to viral survival. Gene studies of these viruses will improve our understanding not only of the mechanisms by which HHV escapes immunity but also of virology as a whole and of cellular dynamics. Third, whereas HHV-8 is considered an oncogenic virus, HHV-6 and HHV-7 might also harbor oncogenic potential. The lymphoid cell tropism of HHV-6, HHV-7, and HHV-8 could make them recruits in the fight against neoplastic and immune diseases. For the practicing dermatologist, this means being on the lookout for new disease associations, new diagnostic techniques and treatments, and, not least of all, new answers to the probing questions of modern patients about why a particular skin abnormality developed.
Article Highlights.
-
■
Human herpesvirus (HHV) 6 and HHV-7 are ubiquitous and have been associated with roseola infantum, pityriasis rosea, lichen planus, hypersensitivity reactions, graft-vs-host disease, and multiple other cutaneous manifestations.
-
■
HHV-8 causes Kaposi sarcoma, the most common AIDS-associated malignancy; as a primary infection, HHV-8 may manifest as a cutaneous eruption.
-
■
Ongoing research suggests an expanding disease spectrum for HHV-6, HHV-7, and HHV-8.
-
■
All 3 variants can cause clinically significant morbidity in immunocompromised persons.
-
■
Novel diagnostic techniques will lead to better viral detection, and targeted antiviral therapy could lead to improved therapeutic outcomes in the future.
Supplemental Online Material
Author Interview Video
References
- 1.Achour A., Malet I., Le Gal F. Variability of gB and gH genes of human herpesvirus-6 among clinical specimens. J Med Virol. 2008;80(7):1211–1221. doi: 10.1002/jmv.21205. [DOI] [PubMed] [Google Scholar]
- 2.Iuliano R., Trovato R., Lico S. Human herpesvirus-6 reactivation in a longitudinal study of two HIV-1 infected patients. J Med Virol. 1997;51(4):259–264. [PubMed] [Google Scholar]
- 3.Chen H., Pesce A.M., Carbonari M. Absence of antibodies to human herpesvirus-6 in patients with slowly-progressive human immunodeficiency virus type 1 infection. Eur J Epidemiol. 1992;8(2):217–221. doi: 10.1007/BF00144803. [DOI] [PubMed] [Google Scholar]
- 4.Mori Y., Yamanishi K. HHV-6A, 6B, and 7: pathogenesis, host response, and clinical disease. In: Arvin A., Campadelli-Fiume G., Mocarski E., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge, NY: 2007. pp. 833–842. [Google Scholar]
- 5.Di Luca D., Mirandola P., Ravaioli T. Human herpesviruses 6 and 7 in salivary glands and shedding in saliva of healthy and human immunodeficiency virus positive individuals. J Med Virol. 1995;45(4):462–468. doi: 10.1002/jmv.1890450418. [DOI] [PubMed] [Google Scholar]
- 6.Hall C.B., Caserta M.T., Schnabel K.C. Congenital infections with human herpesvirus 6 (HHV6) and human herpesvirus 7 (HHV7) J Pediatr. 2004;145(4):472–477. doi: 10.1016/j.jpeds.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Arbuckle J.H., Medveczky M.M., Luka J. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro. Proc Natl Acad Sci U S A. 2010;107(12):5563–5568. doi: 10.1073/pnas.0913586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razzaque A., Williams O., Wang J., Rhim J.S. Neoplastic transformation of immortalized human epidermal keratinocytes by two HHV-6 DNA clones. Virology. 1993;195(1):113–120. doi: 10.1006/viro.1993.1351. [DOI] [PubMed] [Google Scholar]
- 9.Flamand L., Komaroff A.L., Arbuckle J.H., Medveczky P.G., Ablashi D.V. Review, part 1: human herpesvirus-6-basic biology, diagnostic testing, and antiviral efficacy. J Med Virol. 2010;82(9):1560–1568. doi: 10.1002/jmv.21839. [DOI] [PubMed] [Google Scholar]
- 10.Mesri E.A., Cesarman E., Boshoff C. Kaposi's sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10(10):707–719. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biggar R.J., Whitby D., Marshall V., Linhares A.C., Black F. Human herpesvirus 8 in Brazilian Amerindians: a hyperendemic population with a new subtype. J Infect Dis. 2000;181(5):1562–1568. doi: 10.1086/315456. [DOI] [PubMed] [Google Scholar]
- 12.Prober C.G. Human herpesvirus 6. Adv Exp Med Biol. 2011;697:87–90. doi: 10.1007/978-1-4419-7185-2_7. [DOI] [PubMed] [Google Scholar]
- 13.Dukers N.H., Renwick N., Prins M. Risk factors for human herpesvirus 8 seropositivity and seroconversion in a cohort of homosexual men. Am J Epidemiol. 2000;151(3):213–224. doi: 10.1093/oxfordjournals.aje.a010195. [DOI] [PubMed] [Google Scholar]
- 14.Plancoulaine S., Abel L., van Beveren M. Human herpesvirus 8 transmission from mother to child and between siblings in an endemic population. Lancet. 2000;356(9235):1062–1065. doi: 10.1016/S0140-6736(00)02729-X. [DOI] [PubMed] [Google Scholar]
- 15.Johnston C., Orem J., Okuku F. Impact of HIV infection and Kaposi sarcoma on human herpesvirus-8 mucosal replication and dissemination in Uganda. PLoS One. 2009;4(1):e4222. doi: 10.1371/journal.pone.0004222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pauk J., Huang M.L., Brodie S.J. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med. 2000;343(19):1369–1377. doi: 10.1056/NEJM200011093431904. [DOI] [PubMed] [Google Scholar]
- 17.Barozzi P., Luppi M., Facchetti F. Post-transplant Kaposi sarcoma originates from the seeding of donor-derived progenitors [published correction appears in Nat Med. 2003;9(7):975] Nat Med. 2003;9(5):554–561. doi: 10.1038/nm862. [DOI] [PubMed] [Google Scholar]
- 18.Hladik W., Dollard S.C., Mermin J. Transmission of human herpesvirus 8 by blood transfusion. N Engl J Med. 2006;355(13):1331–1338. doi: 10.1056/NEJMoa055009. [DOI] [PubMed] [Google Scholar]
- 19.Cannon M.J., Dollard S.C., Smith D.K., HIV Epidemiology Research Study Group Blood-borne and sexual transmission of human herpesvirus 8 in women with or at risk for human immunodeficiency virus infection. N Engl J Med. 2001;344(9):637–643. doi: 10.1056/NEJM200103013440904. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K., Kondo T., Torigoe S., Okada S., Mukai T., Yamanishi K. Human herpesvirus 7: another causal agent for roseola (exanthem subitum) J Pediatr. 1994;125(1):1–5. doi: 10.1016/s0022-3476(94)70113-x. [DOI] [PubMed] [Google Scholar]
- 21.Yamanishi K., Okuno T., Shiraki K. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 22.Asano Y., Yoshikawa T., Suga S. Viremia and neutralizing antibody response in infants with exanthem subitum. J Pediatr. 1989;114(4, pt 1):535–539. doi: 10.1016/s0022-3476(89)80689-4. [DOI] [PubMed] [Google Scholar]
- 23.Nagasawa T., Kimura I., Abe Y., Oka A. HHV-6 encephalopathy with cluster of convulsions during eruptive stage. Pediatr Neurol. 2007;36(1):61–63. doi: 10.1016/j.pediatrneurol.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 24.Yoshikawa T., Ohashi M., Miyake F. Exanthem subitum-associated encephalitis: nationwide survey in Japan. Pediatr Neurol. 2009;41(5):353–358. doi: 10.1016/j.pediatrneurol.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Broccolo F., Drago F., Careddu A.M. Additional evidence that pityriasis rosea is associated with reactivation of human herpesvirus-6 and -7. J Invest Dermatol. 2005;124(6):1234–1240. doi: 10.1111/j.0022-202X.2005.23719.x. [DOI] [PubMed] [Google Scholar]
- 26.Chuh A.A., Chan H.H., Chiu S.S., Ng H.Y., Peiris J.S. A prospective case control study of the association of Gianotti-Crosti syndrome with human herpesvirus 6 and human herpesvirus 7 infections. Pediatr Dermatol. 2002;19(6):492–497. doi: 10.1046/j.1525-1470.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- 27.Drago F., Ranieri E., Malaguti F., Battifoglio M.L., Losi E., Rebora A. Human herpesvirus 7 in patients with pityriasis rosea: electron microscopy investigations and polymerase chain reaction in mononuclear cells, plasma and skin. Dermatology. 1997;195(4):374–378. doi: 10.1159/000245991. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T., Kawamura T., Jacob S.E. Pityriasis rosea is associated with systemic active infection with both human herpesvirus-7 and human herpesvirus-6. J Invest Dermatol. 2002;119(4):793–797. doi: 10.1046/j.1523-1747.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- 29.Kosuge H., Tanaka-Taya K., Miyoshi H. Epidemiological study of human herpesvirus-6 and human herpesvirus-7 in pityriasis rosea. Br J Dermatol. 2000;143(4):795–798. doi: 10.1046/j.1365-2133.2000.03778.x. [DOI] [PubMed] [Google Scholar]
- 30.Drago F., Broccolo F., Rebora A. Pityriasis rosea: an update with a critical appraisal of its possible herpesviral etiology. J Am Acad Dermatol. 2009;61(2):303–318. doi: 10.1016/j.jaad.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 31.Rebora A., Drago F., Broccolo F. Pityriasis rosea and herpesviruses: facts and controversies. Clin Dermatol. 2010;28(5):497–501. doi: 10.1016/j.clindermatol.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 32.De Vries H.J., van Marle J., Teunissen M.B. Lichen planus is associated with human herpesvirus type 7 replication and infiltration of plasmacytoid dendritic cells. Br J Dermatol. 2006;154(2):361–364. doi: 10.1111/j.1365-2133.2005.06999.x. [DOI] [PubMed] [Google Scholar]
- 33.De Vries H.J., Teunissen M.B., Zorgdrager F., Picavet D., Cornelissen M. Lichen planus remission is associated with a decrease of human herpes virus type 7 protein expression in plasmacytoid dendritic cells. Arch Dermatol Res. 2007;299(4):213–219. doi: 10.1007/s00403-007-0750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peppercorn A.F., Miller M.B., Fitzgerald D., Weber D.J., Groben P.A., Cairns B.A. High-level human herpesvirus-6 viremia associated with onset of Stevens-Johnson syndrome: report of two cases. J Burn Care Res. 2010;31(2):365–368. doi: 10.1097/BCR.0b013e3181d0f48b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saijo M., Saijo H., Yamamoto M. Thrombocytopenic purpura associated with primary human herpesvirus 6 infection. Pediatr Infect Dis J. 1995;14(5):405. doi: 10.1097/00006454-199505000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Boccara O., Lesage F., Regnault V. Nonbacterial purpura fulminans and severe autoimmune acquired protein S deficiency associated with human herpesvirus-6 active replication. Br J Dermatol. 2009;161(1):181–183. doi: 10.1111/j.1365-2133.2009.09264.x. [DOI] [PubMed] [Google Scholar]
- 37.Ruzicka T., Kalka K., Diercks K., Schuppe H.C. Papular-purpuric ”gloves and socks” syndrome associated with human herpesvirus 6 infection. Arch Dermatol. 1998;134(2):242–244. doi: 10.1001/archderm.134.2.242. [DOI] [PubMed] [Google Scholar]
- 38.Fretzayas A., Douros K., Moustaki M., Nicolaidou P. Papular-purpuric gloves and socks syndrome in children and adolescents. Pediatr Infect Dis J. 2009;28(3):250–252. doi: 10.1097/INF.0b013e31818cb289. [DOI] [PubMed] [Google Scholar]
- 39.Yasumoto S., Tsujita J., Imayama S., Hori Y. Case report: Gianotti-Crosti syndrome associated with human herpesvirus-6 infection. J Dermatol. 1996;23(7):499–501. [PubMed] [Google Scholar]
- 40.Ichiche M., Kiesch N., De Bels D. DRESS syndrome associated with HHV-6 reactivation. Eur J Intern Med. 2003;14(8):498–500. doi: 10.1016/j.ejim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Picard D., Janela B., Descamps V. Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Sci Transl Med. 2010;2(46):46ra62. doi: 10.1126/scitranslmed.3001116. [DOI] [PubMed] [Google Scholar]
- 42.Walsh S.A., Creamer D. Drug reaction with eosinophilia and systemic symptoms (DRESS): a clinical update and review of current thinking. Clin Exp Dermatol. 2011;36(1):6–11. doi: 10.1111/j.1365-2230.2010.03967.x. [DOI] [PubMed] [Google Scholar]
- 43.Ablashi D.V., Devin C.L., Yoshikawa T. Review, part 3: human herpesvirus-6 in multiple non-neurological diseases. J Med Virol. 2010;82(11):1903–1910. doi: 10.1002/jmv.21860. [DOI] [PubMed] [Google Scholar]
- 44.Hubiche T., Milpied B., Cazeau C., Taieb A., Leaute-Labreze C. Association of immunologically confirmed delayed drug reaction and human herpesvirus 6 viremia in a pediatric case of drug-induced hypersensitivity syndrome. Dermatology. 2011;222(2):140–141. doi: 10.1159/000324506. [DOI] [PubMed] [Google Scholar]
- 45.Hashizume H., Aoshima M., Ito T., Seo N., Takigawa M., Yagi H. Emergence of circulating monomyeloid precursors predicts reactivation of human herpesvirus-6 in drug-induced hypersensitivity syndrome. Br J Dermatol. 2009;161(2):486–488. doi: 10.1111/j.1365-2133.2009.09280.x. [DOI] [PubMed] [Google Scholar]
- 46.Shiohara T., Iijima M., Ikezawa Z., Hashimoto K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol. 2007;156(5):1083–1084. doi: 10.1111/j.1365-2133.2007.07807.x. [DOI] [PubMed] [Google Scholar]
- 47.de Pagter P.J., Schuurman R., Meijer E., van Baarle D., Sanders E.A., Boelens J.J. Human herpesvirus type 6 reactivation after haematopoietic stem cell transplantation. J Clin Virol. 2008;43(4):361–366. doi: 10.1016/j.jcv.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Kitamura K., Asada H., Iida H. Relationship among human herpesvirus 6 reactivation, serum interleukin 10 levels, and rash/graft-versus-host disease after allogeneic stem cell transplantation. J Am Acad Dermatol. 2008;58(5):802–809. doi: 10.1016/j.jaad.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Ljungman P., Singh N. Human herpesvirus-6 infection in solid organ and stem cell transplant recipients. J Clin Virol. 2006;37(suppl 1):S87–S91. doi: 10.1016/S1386-6532(06)70018-X. [DOI] [PubMed] [Google Scholar]
- 50.Ratnamohan V.M., Chapman J., Howse H. Cytomegalovirus and human herpesvirus 6 both cause viral disease after renal transplantation. Transplantation. 1998;66(7):877–882. doi: 10.1097/00007890-199810150-00011. [DOI] [PubMed] [Google Scholar]
- 51.Tong C.Y., Bakran A., Williams H., Cheung C.Y., Peiris J.S. Association of human herpesvirus 7 with cytomegalovirus disease in renal transplant recipients. Transplantation. 2000;70(1):213–216. [PubMed] [Google Scholar]
- 52.Broccolo F., Drago F., Paolino S. Reactivation of human herpesvirus 6 (HHV-6) infection in patients with connective tissue diseases. J Clin Virol. 2009;46(1):43–46. doi: 10.1016/j.jcv.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Galan A., McNiff J.M., Choi J.N., Lazova R. Fatal HHV6 infection in an immunocompromised patient presenting with skin involvement. J Cutan Pathol. 2010;37(2):277–281. doi: 10.1111/j.1600-0560.2009.01291.x. [DOI] [PubMed] [Google Scholar]
- 54.Antman K., Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342(14):1027–1038. doi: 10.1056/NEJM200004063421407. [DOI] [PubMed] [Google Scholar]
- 55.Tuttleton Arron S., Jennings L., Nindl I., Viral Working Group of the International Transplant Skin Cancer Collaborative (ITSCC) & Skin Care in Organ Transplant Patients, Europe (SCOPE) Viral oncogenesis and its role in nonmelanoma skin cancer. Br J Dermatol. 2011;164(6):1201–1213. doi: 10.1111/j.1365-2133.2011.10322.x. [DOI] [PubMed] [Google Scholar]
- 56.Andreoni M., Sarmati L., Nicastri E. Primary human herpesvirus 8 infection in immunocompetent children. JAMA. 2002;287(10):1295–1300. doi: 10.1001/jama.287.10.1295. [DOI] [PubMed] [Google Scholar]
- 57.Quereux G., Andre-Garnier E., Knol A.C., Imbert-Marcille B.M., Dreno B. Evaluation of the role of human herpes virus 6 and 8 in parapsoriasis. Exp Dermatol. 2009;18(4):357–361. doi: 10.1111/j.1600-0625.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- 58.Drago F., Broccolo F., Rebora A. Human herpesvirus 7 and cutaneous T-cell lymphomas. Br J Dermatol. 2009;161(1):199. doi: 10.1111/j.1365-2133.2009.09185.x. [DOI] [PubMed] [Google Scholar]
- 59.Amitay-Laish I., Sarid R., Ben-Amitai D. Human herpesvirus 8 is not detectable in lesions of large plaque parapsoriasis, and in early-stage sporadic, familial, and juvenile cases of mycosis fungoides. J Am Acad Dermatol. 2012;66(1):46–50. doi: 10.1016/j.jaad.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 60.Vianna R.A., de Oliveira S.A., Camacho L.A. Role of human herpesvirus 6 infection in young Brazilian children with rash illnesses. Pediatr Infect Dis J. 2008;27(6):533–537. doi: 10.1097/INF.0b013e3181673c50. [DOI] [PubMed] [Google Scholar]
- 61.Dockrell D.H., Smith T.F., Paya C.V. Human herpesvirus 6. Mayo Clin Proc. 1999;74(2):163–170. doi: 10.4065/74.2.163. [DOI] [PubMed] [Google Scholar]
- 62.Bjornberg A., Hellgren L. Pityriasis rosea: a statistical, clinical, and laboratory investigation of 826 patients and matched healthy controls. Acta Derm Venereol Suppl (Stockh) 1962;42(suppl 50):1–68. doi: 10.2340/0001555542168. [DOI] [PubMed] [Google Scholar]
- 63.Tohyama M., Hashimoto K. New aspects of drug-induced hypersensitivity syndrome. J Dermatol. 2011;38(3):222–228. doi: 10.1111/j.1346-8138.2010.01176.x. [DOI] [PubMed] [Google Scholar]
- 64.Schwartz R.A., Micali G., Nasca M.R., Scuderi L. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59(2):179–206. doi: 10.1016/j.jaad.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Geraminejad P., Memar O., Aronson I., Rady P.L., Hengge U., Tyring S.K. Kaposi's sarcoma and other manifestations of human herpesvirus 8. J Am Acad Dermatol. 2002;47(5):641–655. doi: 10.1067/mjd.2002.128383. [DOI] [PubMed] [Google Scholar]
- 66.Wollenberg A., Eames T. Skin diseases following a Christmas tree pattern. Clin Dermatol. 2011;29(2):189–194. doi: 10.1016/j.clindermatol.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 67.Rappersberger K., Stingl G., Wolff K. Kaposi's sarcoma. In: Freedberg I.M., Eisen A.Z., Wolff K., Austen K.F., Goldsmith L.A., Katz S.I., editors. Vol 1. McGraw-Hill; New York, NY: 2003. pp. 1020–1026. (Fitzpatrick's Dermatology in General Medicine). [Google Scholar]
- 68.Wang Q.J., Jenkins F.J., Jacobson L.P. Primary human herpesvirus 8 infection generates a broadly specific CD8(+) T-cell response to viral lytic cycle proteins [published correction appears in Blood. 2002;99(10):3499] Blood. 2001;97(8):2366–2373. doi: 10.1182/blood.v97.8.2366. [DOI] [PubMed] [Google Scholar]
- 69.Quinlivan E.B., Wang R.X., Stewart P.W., Swiss HIV Cohort Study Longitudinal sero-reactivity to human herpesvirus 8 (KSHV) in the Swiss HIV Cohort 4.7 years before KS. J Med Virol. 2001;64(2):157–166. doi: 10.1002/jmv.1031. [DOI] [PubMed] [Google Scholar]
- 70.Drago F., Vecchio F., Rebora A. Use of high-dose acyclovir in pityriasis rosea. J Am Acad Dermatol. 2006;54(1):82–85. doi: 10.1016/j.jaad.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 71.Yoshida M., Yamada M., Tsukazaki T. Comparison of antiviral compounds against human herpesvirus 6 and 7. Antiviral Res. 1998;40(1-2):73–84. doi: 10.1016/s0166-3542(98)00049-7. [DOI] [PubMed] [Google Scholar]
- 72.De Bolle L., Naesens L., De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18(1):217–245. doi: 10.1128/CMR.18.1.217-245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vermeire K., Zhang Y., Princen K. CADA inhibits human immunodeficiency virus and human herpesvirus 7 replication by down-modulation of the cellular CD4 receptor. Virology. 2002;302(2):342–353. doi: 10.1006/viro.2002.1624. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y., Schols D., De Clercq E. Selective activity of various antiviral compounds against HHV-7 infection. Antiviral Res. 1999;43(1):23–35. doi: 10.1016/s0166-3542(99)00031-5. [DOI] [PubMed] [Google Scholar]
- 75.Glesby M.J., Hoover D.R., Weng S. Use of antiherpes drugs and the risk of Kaposi's sarcoma: data from the Multicenter AIDS Cohort Study. J Infect Dis. 1996;173(6):1477–1480. doi: 10.1093/infdis/173.6.1477. [DOI] [PubMed] [Google Scholar]
- 76.Ioannidis J.P., Collier A.C., Cooper D.A. Clinical efficacy of high-dose acyclovir in patients with human immunodeficiency virus infection: a meta-analysis of randomized individual patient data. J Infect Dis. 1998;178(2):349–359. doi: 10.1086/515621. [DOI] [PubMed] [Google Scholar]
- 77.Mazzi R., Parisi S.G., Sarmati L. Efficacy of cidofovir on human herpesvirus 8 viraemia and Kaposi's sarcoma progression in two patients with AIDS. AIDS. 2001;15(15):2061–2062. doi: 10.1097/00002030-200110190-00026. [DOI] [PubMed] [Google Scholar]
- 78.Little R.F., Merced-Galindez F., Staskus K. A pilot study of cidofovir in patients with Kaposi sarcoma. J Infect Dis. 2003;187(1):149–153. doi: 10.1086/346159. [DOI] [PubMed] [Google Scholar]
- 79.Ganem D. KSHV-induced oncogenesis. In: Arvin A., Campadelli-Fiume G., Mocarski E., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge, NY: 2007. pp. 1007–1028. [PubMed] [Google Scholar]
- 80.Corbellino M., Bestetti G., Scalamogna C. Long-term remission of Kaposi sarcoma-associated herpesvirus-related multicentric Castleman disease with anti-CD20 monoclonal antibody therapy. Blood. 2001;98(12):3473–3475. doi: 10.1182/blood.v98.12.3473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview Video


