Abstract
Brain metastases are a major cause of morbidity and mortality in patients with advanced melanoma. With the development of targeted agents for the treatment of metastatic melanoma, a great deal of interest has focused on whether selective BRAF inhibitors may play a role in the treatment of brain metastases in lieu of or in addition to surgery and/or radiation therapy. However, relatively little is known about the intracranial effectiveness of vemurafenib, the only US Food and Drug Administration–approved selective BRAF V600E inhibitor, because patients with brain metastases have historically been excluded from vemurafenib clinical trials. We describe 3 patients with BRAF V600E mutation metastatic melanoma in whom treatment with vemurafenib resulted in prompt extracranial disease response but progression of metastatic disease in the brain. Further, we discuss possible mechanisms responsible for the suboptimal central nervous system response observed in these patients and alternative therapies for patients with melanoma metastatic to the brain.
Abbreviations and Acronyms: BBB, blood-brain barrier; BRAFi, BRAF inhibitor; CNS, central nervous system; FDA, Food and Drug Administration; MRI, magnetic resonance imaging; WBRT, whole-brain radiation therapy
Brain metastases are a major cause of morbidity and mortality in patients with advanced melanoma. With the development of targeted agents for the treatment of metastatic melanoma, a great deal of interest has focused on whether selective BRAF inhibitors may play a role in the treatment of brain metastases in lieu of or in addition to surgery and/or radiation therapy. In this report, we describe 3 patients with BRAF V600E mutation metastatic melanoma in whom treatment with vemurafenib, the only US Food and Drug Administration (FDA)–approved selective BRAF V600E inhibitor, resulted in prompt extracranial disease response but progression of metastatic disease in the brain.
Case 1
A 26-year-old man presented with back and abdominal pain, shortness of breath, fatigue, hypercalcemia, and acute renal insufficiency. Imaging studies revealed numerous solid masses suggestive of widespread metastatic malignancy, including extensive involvement of the vertebral column. Brain magnetic resonance imaging (MRI) revealed no intracranial disease. The patient was subsequently diagnosed as having metastatic melanoma with an unknown primary skin malignancy, stage M1c, BRAF V600E mutation. Palliative treatment with external beam radiation to the spine and high-dose corticosteroid therapy was initiated. Given rapid radiologic and symptomatic disease progression (Figure 1, A), vemurafenib at 960 mg twice daily was administered concurrently with radiation therapy. Treatment was well tolerated, with the exception of development of grade 1 arthralgia and a grade 2 maculopapular rash. After initiation of systemic therapy, the patient's presenting clinical symptoms improved in less than 2 weeks, and restaging evaluation with computed tomography at 1 month revealed a considerable decrease in the size of the previously noted metastatic lesions (Figure 1, B). Over the next 1 to 2 weeks, however, the patient experienced new-onset headaches, nausea, drowsiness, and memory problems. A repeated brain MRI revealed interval development of innumerable punctate foci of enhancement throughout both cerebral hemispheres, the basal ganglia, and the cerebellum (with the largest lesion measuring approximately 5 mm), highly suggestive of interval development of central nervous system (CNS) metastatic disease (Figure 1, C), as well as diffuse leptomeningeal contrast enhancement suggestive of leptomeningeal carcinomatosis (Figure 1, D). Whole-brain radiation therapy (WBRT) was initiated; however, the patient's clinical condition deteriorated rapidly, and he died 2 weeks later.
FIGURE 1.
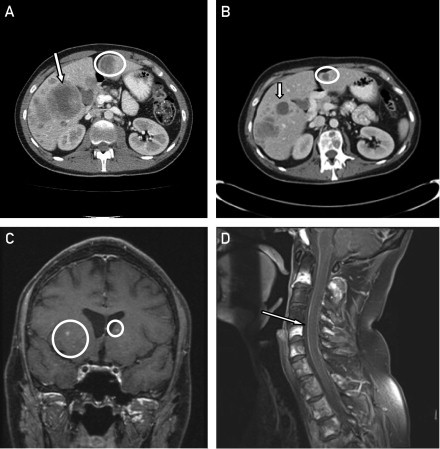
Computed tomographic scans demonstrating liver metastases (arrow and circle) before initiation of treatment with vemurafenib (A) and after 1 month of treatment (B). Magnetic resonance images showing brain (C, circles) and leptomeningeal (D, arrow) metastases after starting treatment with vemurafenib.
Case 2
A 42-year-old woman with a history of stage II cutaneous melanoma of the left preauricular area underwent cholecystectomy for presumed gallstone disease 2 years after the initial diagnosis. Pathologic examination showed a mural mass in the gallbladder and a single pericolic lymph node that were positive for metastatic malignant melanoma. Postoperatively, staging studies revealed no evidence of residual disease, and adjuvant immunotherapy with granulocyte-macrophage colony-stimulating factor was initiated. Two months later, however, disease recurred in the liver and gallbladder fossa, and she was treated intermittently with systemic chemotherapy involving a combination of paclitaxel, carboplatin, and bevacizumab, followed by temozolomide-bevacizumab and hepatic chemoembolization. More than 3 years after the initial diagnosis of metastatic disease, a routine brain MRI revealed 3 new lesions located in the left frontal lobe, left caudate head, and fornix (size range, 2-5 mm) that suggested brain metastasis. The patient underwent gamma knife radiosurgery to the brain lesions and was subsequently given ipilimumab for systemic disease progression. Unfortunately, within 2 months of initiating therapy, she had symptomatic and radiologic progression both systemically (Figure 2, C and D) and in the CNS, with new lesions in the cerebellum, right temporal lobe, and right frontal lobe (Figure 2, A), for which she was again treated with gamma knife radiosurgery. The patient's tumor was subsequently found to be positive for the BRAF V600E mutation, and vemurafenib, 960 mg twice daily, was administered on a compassionate-care basis. Approximately 2 months after initiation of therapy, she was found to have CNS disease progression (Figure 2, B), despite good systemic control (Figure. 2, E). The patient subsequently underwent WBRT while continuing vemurafenib therapy. She tolerated concurrent therapy well with no additional adverse effects (she had grade 1 nausea and diarrhea on single-agent vemurafenib). However, 3 months later, the patient was found to have substantial systemic disease progression, and vemurafenib therapy was discontinued.
FIGURE 2.
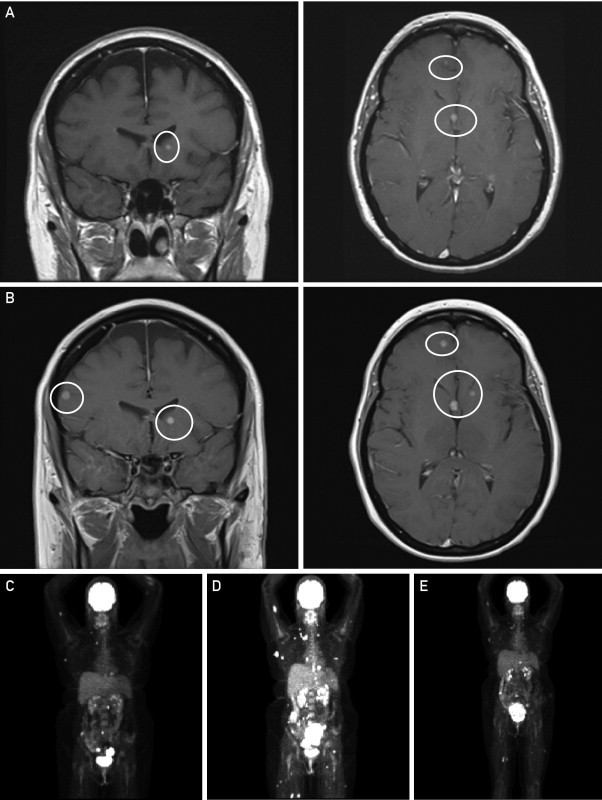
Magnetic resonance images of the brain demonstrating brain metastases (circles) before initiation of treatment with vemurafenib (A) and after 2 months of treatment (B). Positron emission tomographic images before initiation of ipilimumab therapy (C), after 2 cycles of ipilimumab showing a marked increase in tumor volume (D), and after 2 cycles of vemurafenib (E).
Case 3
A 62-year-old woman was diagnosed with stage IIIC cutaneous melanoma of the lower back, for which she underwent wide local excision and inguinal lymphadenectomy. Positron emission tomography performed at diagnosis revealed bilateral millimetric pulmonary nodules. Repeated imaging studies 6 weeks later showed progression of the lung lesions. Contrast-enhanced MRI of the brain demonstrated 6 enhancing lesions in the cerebral and cerebellar cortex, highly suggestive of CNS metastatic disease, with the largest lesion measuring 8 mm. Molecular analysis of the tumor confirmed the presence of BRAF V600E mutation. The patient underwent gamma knife radiosurgery to all 6 brain lesions, followed by vemurafenib therapy, 960 mg twice daily. Interval brain MRI at 4 weeks showed overall improvement in the size of the treated lesions and no new metastatic sites. Approximately 6 weeks after initiating vemurafenib, however, a grade 2 papular rash developed, as well as grade 3 elevation of liver enzymes, and therapy was temporarily discontinued. Restaging studies 8 weeks after initiation of therapy revealed interval response of the lung lesions (Figure 3, A), as well as improvement in the lactate dehydrogenase level. However, brain MRI showed numerous new enhancing lesions scattered throughout the cerebral and cerebellar hemispheres (Figure 3, B), indicating interval progression of CNS metastatic disease, and the patient subsequently received WBRT.
FIGURE 3.
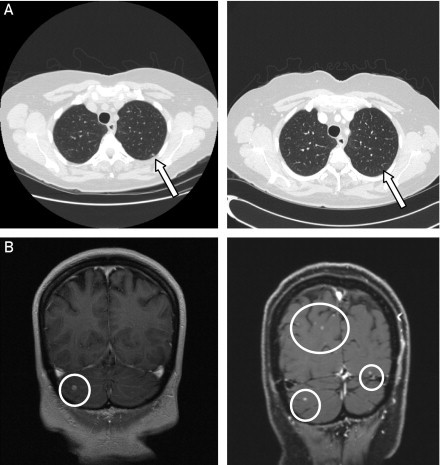
A, Computed tomographic scans of the chest at diagnosis (left) and 8 weeks after initiation of vemurafenib therapy (right) demonstrating interval response of metastatic lung lesions with almost complete disappearance of some nodules (arrows). B, Magnetic resonance images of the brain at diagnosis (left) and 8 weeks after initiating vemurafenib therapy (right) demonstrating numerous new enhancing lesions (circles) scattered throughout the cerebral and cerebellar hemispheres (note that only the cerebellar lesion treated with gamma knife radiation therapy [circle, left] showed continued improvement).
Discussion
Brain metastases are a major cause of morbidity and mortality in advanced melanoma, reflecting the propensity of this malignancy to involve the CNS.1 After cancers of the lung and breast, melanoma is the third most common cause of CNS metastases. In patients with newly diagnosed stage IV disease, brain metastases are present in 20% of cases,2 and among patients with documented brain involvement, these lesions contribute to death in up to 95%.3 In general, the median survival from the time of diagnosis of cerebral metastases is less than 6 months4; however, selected patients (ie, those with good performance status and limited extracranial and/or limited CNS disease) may respond to aggressive treatment and have improved clinical outcomes.3 Traditional treatment approaches for melanoma brain metastases have included surgical resection for patients with a single or limited number of lesions, stereotactic radiosurgery, or WBRT (with or without surgery/stereotactic radiosurgery). With the development of targeted agents for treatment of metastatic melanoma, a great deal of interest has focused on whether these drugs may play a role in the treatment of brain metastases in lieu of or in addition to surgery and/or radiation therapy.
Somatic point mutations in the BRAF gene, resulting in constitutive activation of the serine-threonine protein kinase BRAF and the RAS-RAF-MEK-ERK signaling pathway, are present in 40% to 60% of melanoma cases.5 The substitution of glutamic acid for valine at codon 600 (V600E) is the most prevalent BRAF mutation in invasive melanoma, found in approximately 80% of cases.6,7 In the metastatic setting, the presence of BRAF mutation seems to be associated with inferior patient survival in the absence of targeted therapy.6 Inhibitors of BRAF (BRAFi) have demonstrated dramatic and early antitumor activity in clinical trials in patients with advanced melanoma whose tumors harbor the V600E mutation. Based on the results of a randomized phase III clinical trial showing significant improvement in both progression-free and overall survival rates, as well as objective responses when compared with dacarbazine,8 vemurafenib was approved by the FDA in August 2011 for use in patients with advanced melanoma whose tumors express the V600E mutation. Most vemurafenib clinical studies, however, excluded patients with untreated CNS disease, and relatively little is known about the effectiveness of this agent in patients with melanoma brain metastases. If the premise is that melanoma cells do not change their BRAF mutation status on forming brain metastases,9,10 then BRAFi could represent an important therapeutic approach for these patients. A clinical trial evaluating the efficacy and safety of vemurafenib in patients with metastatic melanoma and treated or untreated brain lesions is currently under way (clinicaltrials.gov Identifier: NTC01378975). Nevertheless, until the results of this trial become available, most of our knowledge regarding the use of vemurafenib in this patient population is currently limited to a few published case reports11,12 that suggest activity of this agent in patients who received prior temozolomide and/or WBRT.
Herein, we describe our experience with 3 patients with BRAF V600E mutation metastatic melanoma in whom progression of CNS metastatic disease during treatment with vemurafenib was observed despite notable extra-cranial disease response. Similar to findings in our previously reported case,11 2 patients in the current series had previously been diagnosed with metastatic brain disease and were treated with gamma knife radiosurgery, while brain metastases developed de novo in 1 patient after initiation of vemurafenib therapy. Early progression of disease in the brain in patients in our series despite marked peripheral and lactate dehydrogenase response raises the question of whether this reflects true resistance to therapy (such as development of restricted resistant subclones or clones harboring wild-type BRAF) vs restriction of vemurafenib distribution in the brain due to an impermeable blood-brain barrier (BBB) or active drug efflux.13 Recently published preclinical data suggest that restriction of vemurafenib brain distribution by efflux transporters such as P-glycoprotein and breast cancer resistance protein may play a major role in limiting the delivery of this agent to brain metastases.13,14 In animal studies, differences in temporal expression and developmental changes in P-glycoprotein function have been implicated in the observed differences in drug responses with BBB maturation. Data in humans are limited, although increased BBB penetration in children and young adults is frequently cited; this could have perhaps contributed, among other factors, to the observed response to vemurafenib in our previously reported case.11 It is also possible that other factors might influence differential penetration of this agent, modulated by changes in the BBB function induced by different pathologic conditions such as partial disruption of the barrier in patients with preexisting brain metastases, hypoxic or ischemic insults, inflammation with release of proinflammatory mediators (ie, cytokines, reactive oxygen species, eicosanoids) inducing endothelial up-regulation of surface adhesion molecules (platelet endothelial cell adhesion molecule 1, E-selectin, intercellular adhesion molecule 1) and increases in vascular permeability,14 or use of corticosteroid treatment that may limit drug penetration.
Another specific BRAFi, dabrafenib (GSK2118436), has also shown activity in patients with metastatic melanoma in early clinical studies,15 and a phase III trial demonstrating a significant improvement in progression-free survival and overall response rate over dacarbazine was recently reported.16 This agent had also shown activity in patients with previously untreated asymptomatic brain metastases, with 9 of 10 patients achieving objective responses in a phase I/II study.17 These favorable results have recently been confirmed in a large international study of targeted systemic therapy in 172 patients with BRAF V600E/K mutation melanoma and previously treated or untreated brain metastases (BREAK-MB phase II study), in whom dabrafenib showed high clinical activity for both intracranial and extracranial disease, with an overall disease control rate of approximately 80%.18 Interestingly, dabrafenib seems to have been synthesized specifically to prevent BBB penetration as a way of limiting toxic neurologic adverse effects.17 It is therefore unclear whether disruption of the barrier by preexisting brain metastases or other mechanisms operating differently from vemurafenib may be responsible for the increased CNS penetration of this drug. Although dabrafenib is not yet FDA approved for the treatment of V600E/K melanoma, its unprecedented activity in patients with brain metastases makes it a desirable treatment option in this previously underrepresented patient population.
Due to the limited number of cases presented herein and the anecdotal nature of the evidence provided in these cases, we cannot draw definitive conclusions regarding vemurafenib effectiveness in treating brain metastases. Based on our observations with these 3 cases, we suggest that a high index of suspicion should be maintained and that patients should be closely monitored for signs of intracranial progression despite peripheral disease response to BRAF-targeted therapies. Nevertheless, these agents seem to be well tolerated when administered concurrently with radiation therapy. Both BRAFi and radiation therapy can be associated with adverse skin reactions, and although a recent case study reported the development of a severe maculopapular rash in 4 of 13 patients treated with vemurafenib after ipilimumab,19 we have noted only moderate skin reactions that did not necessitate treatment interruption in 1 of the 2 patients treated with concurrent therapy.
The increased understanding of melanoma biology and the development of molecularly targeted agents have resulted in important scientific and clinical breakthroughs for the care of patients with melanoma. However, challenges still remain, and it is imperative to conduct clinical trials that include patients with metastatic brain disease to assess new drugs in this patient population.
Acknowledgment
Ms Rochet and Dr Dronca contributed equally to this work.
Supplemental Online Material
Author Interview Video
References
- 1.Johnson J.D., Young B. Demographics of brain metastasis. Neurosurg Clin N Am. 1996;7(3):337–344. [PubMed] [Google Scholar]
- 2.Davies M.A., Liu P., McIntyre S. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117(8):1687–1696. doi: 10.1002/cncr.25634. [DOI] [PubMed] [Google Scholar]
- 3.Sampson J.H., Carter J.H., Jr, Friedman A.H., Seigler H.F. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88(1):11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 4.Fife K.M., Colman M.H., Stevens G.N. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22(7):1293–1300. doi: 10.1200/JCO.2004.08.140. [DOI] [PubMed] [Google Scholar]
- 5.Davies H., Bignell G.R., Cox C. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 6.Long G.V., Menzies A.M., Nagrial A.M. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29(10):1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 7.Spittle C., Ward M.R., Nathanson K.L. Application of a BRAF pyrosequencing assay for mutation detection and copy number analysis in malignant melanoma. J Mol Diagn. 2007;9(4):464–471. doi: 10.2353/jmoldx.2007.060191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman P.B., Hauschild A., Robert C., BRIM-3 Study Group Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capper D., Berghoff A.S., Magerle M. Immunohistochemical testing of BRAF V600E status in 1,120 tumor tissue samples of patients with brain metastases. Acta Neuropathol. 2012;123(2):223–233. doi: 10.1007/s00401-011-0887-y. [DOI] [PubMed] [Google Scholar]
- 10.Colombino M., Capone M., Maio M., Italian Melanoma Intergroup (IMI) Mutation frequency in BRAF and NRAS genes among primary tumors and different types of metastasis from melanoma patients [abstract 8574] J Clin Oncol (Meeting Abstracts) 2011;29(15, suppl):8574. [Google Scholar]
- 11.Rochet N.M., Kottschade L.A., Markovic S.N. Vemurafenib for melanoma metastases to the brain. N Engl J Med. 2011;365(25):2439–2441. doi: 10.1056/NEJMc1111672. [DOI] [PubMed] [Google Scholar]
- 12.Dummer R., Rinderknecht J., Goldinguer S.M. An open-label pilot study of vemurafenib in previously treated metastatic melanoma patients with brain metastases [abstract 8548] J Clin Oncol (Meeting Abstracts) 2011;29(15, suppl):8548. [Google Scholar]
- 13.Mittapalli R.K., Vaidhyanathan S., Sane R., Elmquist W.F. Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on the brain distribution of a novel BRAF inhibitor: vemurafenib (PLX4032) J Pharmacol Exp Ther. 2012;342(1):33–40. doi: 10.1124/jpet.112.192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber J.D., Egleton R.D., Davis T.P. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24(12):719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- 15.Kefford R., Arkenau H., Brown M.P. Phase I/II study of GSK2118436, a selective inhibitor of oncogenic mutant BRAF kinase, in patients with metastatic melanoma and other solid tumors [abstract 8503] J Clin Oncol (Meeting Abstracts) 2010;28(15, suppl):8503. [Google Scholar]
- 16.Hauschild A., Grob J.J., Demidov L.V. Phase III, randomized, open-label, multicenter trial (BREAK-3) comparing the BRAF kinase inhibitor dabrafenib (GSK2118436) with dacarbazine (DTIC) in patients with BRAFV600E-mutated melanoma [abstract LBA8500] J Clin Oncol. 2012;30(15, suppl):LBA8500. [Google Scholar]
- 17.Falchook G.S., Long G.V., Kurzrock R. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379(9829):1893–1901. doi: 10.1016/S0140-6736(12)60398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkwood J.M., Long G.V., Trefzer U. BREAK-MB: a phase II study assessing overall intracranial response rate (OIRR) to dabrafenib (GSK2118436) in patients (pts) with BRAF V600E/k mutation-positive melanoma with brain metastases (mets) J Clin Oncol. 2012;30(15, suppl):8501. [Google Scholar]
- 19.Harding J.J., Pulitzer M., Chapman P.B. Vemurafenib sensitivity skin reaction after ipilimumab. N Engl J Med. 2012;366(9):866–868. doi: 10.1056/NEJMc1114329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview Video


