Abstract
Objective
To promote wider recognition and further understanding of cannabinoid hyperemesis (CH).
Patients and Methods
We constructed a case series, the largest to date, of patients diagnosed with CH at our institution. Inclusion criteria were determined by reviewing all PubMed indexed journals with case reports and case series on CH. The institution's electronic medical record was searched from January 1, 2005, through June 15, 2010. Patients were included if there was a history of recurrent vomiting with no other explanation for symptoms and if cannabis use preceded symptom onset. Of 1571 patients identified, 98 patients (6%) met inclusion criteria.
Results
All 98 patients were younger than 50 years of age. Among the 37 patients in whom duration of cannabis use was available, most (25 [68%]) reported using cannabis for more than 2 years before symptom onset, and 71 of 75 patients (95%) in whom frequency of use was available used cannabis more than once weekly. Eighty-four patients (86%) reported abdominal pain. The effect of hot water bathing was documented in 57 patients (58%), and 52 (91%) of these patients reported relief of symptoms with hot showers or baths. Follow-up was available in only 10 patients (10%). Of those 10, 7 (70%) stopped using cannabis and 6 of these 7 (86%) noted complete resolution of their symptoms.
Conclusion
Cannabinoid hyperemesis should be considered in younger patients with long-term cannabis use and recurrent nausea, vomiting, and abdominal pain. On the basis of our findings in this large series of patients, we propose major and supportive criteria for the diagnosis of CH.
Cannabis is the most widely used illicit drug in the United States and in the world.1 The 2008 World Health Organization World Mental Health Surveys estimated that the cumulative, lifetime prevalence of cannabis use in the US population is 42% to 46%.2 While cannabis is well known for its antiemetic effects, more recently, long-term cannabis use has been associated with cyclic episodes of nausea, vomiting, and abdominal pain. In 2004, Allen et al3 coined the term cannabinoid hyperemesis (CH) after describing 9 patients with a cyclic vomiting illness that began in the setting of long-term cannabis use and resolved after cessation of the drug. Since 2004, 13 case reports and 3 small case series, the largest of which consisted of 9 patients, have been published in further support of CH.4-10 In 2009, Sontineni et al7 described important clinical features of CH, which included long-term cannabis use, cyclic vomiting, colicky abdominal pain, compulsive use of hot showers, and improvement of symptoms with cannabis cessation.
Given the prevalence of cannabis use worldwide, the very recent recognition of CH, and the paucity of CH literature, it is likely that this disease is underrecognized and underdiagnosed. Lack of awareness of the disease may lead to invasive and costly diagnostic tests, as well as patient and physician frustration. To promote wider recognition and further understanding of this condition, we conducted a case series, the largest to date, of patients diagnosed with CH at our institution.
Patients and Methods
Inclusion criteria were determined by reviewing all PubMed indexed journals with case reports and case series on CH. On the basis of the results of that review, patients were included if they had (1) long-term marijuana use before the start of symptoms, (2) a history of recurrent vomiting, and (3) the absence of a major illness that could explain the symptoms. Using institutional software, the electronic medical record was searched from January 1, 2005, through June 15, 2010, for the terms listed in Table 1, and 101 unique patients were identified. Two of the investigators (J.H.S., D.A.S.) independently reviewed each patient record to determine study eligibility, with 97% concordance between the reviewers on assignment of eligibility. For cases in which there was no consensus, a gastroenterologist (A.S.O.) determined eligibility. Fifty-five patients met the inclusion criteria. To ensure that all eligible patients were captured, a second search was performed of all gastroenterology notes from January 1, 2005, through June 15, 2010, with the following search terms: cannabinoid OR cannabis OR marijuana AND vomiting OR emesis OR hyperemesis. From this second search, 1470 patients were identified, for a total of 1571 potentially eligible patients. Two of the investigators (J.H.S., M.L.H.) evenly divided the newly identified patient records, screened their portions independently, and met to discuss unclear cases. As a result of the second search, 43 additional patients met inclusion criteria, bringing the total to 98 patients. The medical records of these 98 patients were then reviewed by one author (D.A.S.) who abstracted demographic and clinical information.
TABLE 1.
Terms Used in Initial Search of Electronic Medical Records to Identify Patients With Cannabinoid Hyperemesis
| Cannabinoid hyperemesis syndrome |
| Cannabinoid hyperemesis |
| Hyperemesis secondary to cannabis use |
| Cannabis hyperemesis syndrome |
| Cyclic vomiting secondary to chronic cannabis abuse |
| Cyclic vomiting secondary to chronic marijuana abuse |
| Hyperemesis secondary to marijuana abuse |
| Recurrent vomiting secondary to cannabis abuse |
| Recurrent vomiting secondary to marijuana abuse |
| Cannabinoid hyperemesis |
| Cannabinoid vomiting |
| Cannabinoid emesis |
| Cannabis hyperemesis |
| Cannabis vomiting |
| Cannabis emesis |
| Marijuana hyperemesis |
| Marijuana vomiting |
| Marijuana emesis |
Results
The demographic characteristics of the study population are shown in Table 2. Sixty-six patients (67%) were males, the mean ± SD age at evaluation was 32.3±9.9 years, and the mean ± SD age at symptom onset was 25.3±8.9 years (range, 14-48 years). Sixty patients (65%) had a body mass index (calculated as the weight in kilograms divided by the height in meters squared) of 25 or less, and only 11 (12%) were obese, defined as a body mass index of greater than 30. Forty-eight patients (49%) used tobacco, but only 10 (10%) reported weekly use of alcohol.
TABLE 2.
Characteristics of 98 Patients With Cannabinoid Hyperemesis
| Characteristic | Value |
|---|---|
| Age (y), mean ± SD | 32.3±9.89 |
| Age distribution (y) | |
| 20-30 | 51 (52) |
| 31-40 | 26 (27) |
| 41-50 | 15 (15) |
| ≥51 | 6 (6) |
| Gender | |
| Male | 66 (67) |
| Female | 32 (33) |
| Ethnicity | |
| Caucasian | 78 (80) |
| Hispanic | 3 (3) |
| African American | 5 (5) |
| Other | 12 (12) |
| State of residence | |
| Minnesota | 33 (34) |
| Other | 65 (66) |
| BMI (n=93) | |
| ≤20 | 25 (27) |
| 21-25 | 35 (37) |
| 26-30 | 22 (24) |
| ≥31 | 11 (12) |
| Nicotine use | |
| Yes | 48 (49) |
| No | 50 (51) |
| Alcohol use | |
| Yes | 10 (10) |
| No | 76 (78) |
| Unknown | 12 (12) |
| Employment status | |
| Employed | 62 (63) |
| Unemployed | 36 (37) |
Data are presented as No. (percentage) of patients unless indicated otherwise. BMI = body mass index.
The duration and frequency of cannabis use before symptom onset are shown in the Figure. This information was available for 37 (38%) and 75 (77%) of the patients, respectively. Twenty-five (68%) of 37 patients consumed cannabis products for more than 2 years before symptoms occurred. Forty-four patients (59%) used cannabis on a daily basis, while 71 patients (95%) used it more than once a week.
FIGURE.
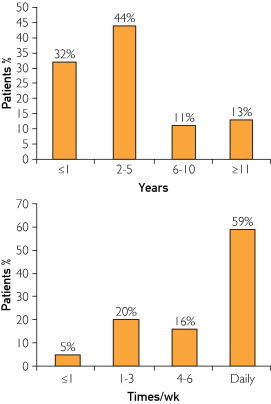
Duration of cannabis use before onset of symptoms (top) and frequency of cannabis use per week (bottom).
All patients had symptoms of nausea and vomiting, as noted in Table 3. In 53 patients (71%), the symptoms were present in the morning, while only 16 (21%) had symptoms associated with meals. Eighty-four patients (86%) had abdominal pain associated with nausea and vomiting. Among the 75 patients for whom location of pain was recorded, 46 (61%) described epigastric pain, while 17 (23%) reported a periumbilical location; the location of pain was not documented in 13 (15%) of the 84 patients who reported abdominal pain. The description of the pain varied and was described as burning, crampy, or sharp. Of 95 patients with bowel habits recorded, 64 (67%) reported normal bowel habits, while 22 patients (23%) reported diarrhea. Fifty-seven patients (58%) had documentation of the effects of hot water bathing on their symptoms; of these, 52 (91%) reported relief of their symptoms with hot showers or baths. There was no comment on the effect of bathing in hot water in 41 patients (42%), and only 5 patients (5%) specifically denied any relief with hot water bathing. Associated symptoms were reported by some patients and included diaphoresis in 20 (20%), bloating in 6 (6%), and flushing in 5 (5%). Eighty-one patients (83%) reported weight loss, with a mean loss of 14.2 kg (median, 12 kg).
TABLE 3.
Clinical Manifestations of Cannabinoid Hyperemesis in 98 Patients
| Symptom | No. (%) of patients |
|---|---|
| Nausea | 98 (100) |
| Emesis | 98 (100) |
| Time of symptoms (n=75) | |
| Morning | 53 (71) |
| Postprandial | 16 (21) |
| During defecation | 6 (8) |
| Abdominal pain | 84 (86) |
| Location of pain (n=75) | |
| Epigastric | 46 (61) |
| Periumbilical | 17 (23) |
| Diffuse | 4 (5) |
| Other | 8 (11) |
| Description of pain (n=48) | |
| Burning | 13 (27) |
| Crampy | 14 (29) |
| Sharp | 11 (23) |
| Other | 10 (21) |
| Bowel habits (n=95) | |
| Diarrhea | 22 (23) |
| Constipation | 7 (7) |
| Both | 2 (2) |
| Normal | 64 (67) |
| Relief with hot showers (n=57) | |
| Yes | 52 (91) |
| No | 5 (9) |
| Other symptoms | |
| Diaphoresis | 20 (20) |
| Bloating | 6 (6) |
| Flushing | 5 (5) |
| Chills | 2 (2) |
Diagnostic studies were performed in most patients and yielded negative results for alternative diagnoses; these studies included complete blood cell count, glucose level, liver biochemistries, pancreatic enzyme level, abdominal computed tomography, upper endoscopy, and colonoscopy. Sixty-one patients (62%) had gastric scintigraphy performed, with normal gastric emptying documented in 28 (46%), delayed emptying in 18 (30%), and rapid emptying in 15 (25%).
Follow-up was available in only 10 patients (10%). Three (30%) of these patients did not abstain from cannabis use and continued to have symptoms. Six patients (60%) stopped using cannabis and noted complete resolution of their symptoms. The time to improvement varied from 1 to 3 months. After not using cannabis for only 1 month, 1 patient (10%) experienced no symptomatic improvement, and no further follow-up was documented.
Discussion
Current knowledge of CH is based on several case reports and small case series. In fact, diagnostic clinical criteria originate from a review of only 13 cases of CH.7 Our case series, the largest to date, confirms the essential and major criteria previously proposed by Sontineni et al7 but additionally expands the major criteria and modifies the previously proposed “characteristics suggestive of the diagnosis.” Firstly, long-term cannabis use is essential for the diagnosis. The major features include (1) severe cyclic nausea and vomiting, (2) resolution with cannabis cessation, (3) relief of symptoms with hot showers or baths, (4) abdominal pain, and (5) weekly use of cannabis. Supportive features include (1) age younger than 50 years, (2) weight loss of greater than 5 kg, (3) morning predominance of symptoms, (4) normal bowel habits, and (5) negative findings on diagnostic evaluation. Lastly, our case series expands the general knowledge of CH.
Our data support the previously proposed essential criterion for a diagnosis of CH: long-term cannabis use. The duration of cannabis use before onset of symptoms varied greatly in our study (ranging from 4 months to 27 years), but the majority of our patients developed symptoms within 1 to 5 years from the onset of cannabis use. Because 32% of our patients reported cannabis use for less than 1 year, we believe that years of cannabis use are not essential for the diagnosis and that CH should even be considered in patients who report nausea and vomiting after using cannabis for less than 1 year. With the exception of the case series by Soriano-Co et al,8 which suggested that CH is preceded by many years of cannabis use, prior case reports and case series describe a distribution similar to our findings.3-7,9,10
Our data corroborate the previously proposed major criteria of severe, cyclic nausea and vomiting, with the majority of our study's patients (70%) reporting more than 7 episodes per year. Moreover, we propose several new major diagnostic criteria for CH as shown in Table 4. Patients in our case series described frequent hot water bathing during acute attacks, which is consistent with prior reports in the literature.3-10 Given the fact that 91% of our patients reported that hot water relieved symptoms of nausea and vomiting and because no other known vomiting syndromes share this phenomenon, we propose this behavior as a new major criterion. In addition, our data demonstrate that patients most commonly experience epigastric or periumbilical abdominal pain, which are locations previously described in pediatric patients with cyclic vomiting syndrome (CVS).11,12 Previously, colicky abdominal pain was deemed a symptom suggestive of CH; however, our data did not demonstrate a predominant pain type (Table 3). Lastly, the majority of patients reported smoking cannabis daily, but several patients reported less than once-weekly use, a finding consistent with prior reports.4-10 Thus, we have added weekly use of cannabis to the major diagnostic criteria.
TABLE 4.
Proposed Clinical Criteria for Cannabinoid Hyperemesis
| Essential for diagnosis |
| Long-term cannabis use |
| Major features |
| Severe cyclic nausea and vomiting |
| Resolution with cannabis cessation |
| Relief of symptoms with hot showers or baths |
| Abdominal pain, epigastric or periumbilical |
| Weekly use of marijuana |
| Supportive features |
| Age less than 50 y |
| Weight loss of >5 kg |
| Morning predominance of symptoms |
| Normal bowel habits |
| Negative laboratory, radiographic, and endoscopic test results |
Several supportive criteria were evident in our case series. The first is age younger than 50 years, a phenomenon consistent with the demographic group that habitually uses cannabis.13 We believe the second supportive criterion should be weight loss because of the notable weight loss reported by our patients; however, this finding may become less common as awareness of this disease grows and patients are diagnosed earlier. The third supportive criterion, a morning predominance of symptoms, is another symptom previously described in the literature for pediatric CVS but not for CH.11,14 The next supportive criterion of normal bowel habits is based on the fact that nearly two-thirds of our patients reported this feature; if patients present with a predominance of either diarrhea or constipation, CH may still be present, but additional diagnoses and testing may need to be considered. The final proposed supportive criterion of CH is failure to identify another cause of recurrent vomiting based on any testing that is performed; however, if CH is suspected before testing, we recommend that case-specific, judicious testing be performed.
In addition to modifying existing criteria, our case series elucidates other aspects of CH. Several patients reported autonomic symptoms such as flushing and diaphoresis; these symptoms reportedly persisted for hours to days. The majority of patients experienced recurrent symptoms at intervals of less than 2 months. The severity of CH symptoms varied, and many patients required frequent hospitalizations for hydration and intravenous antiemetics.
The pathophysiology of CH is not well understood. The antiemetic effects of cannabis and its derivatives have been recognized for some time.15 Cannabinoids act mainly through 2 receptors, CB1 and CB2, which are mainly located in the central nervous system, on the dorsal ganglia, hypothalamus, hippocampus, and cerebellum16,17; they are also found on the peripheral enteric nerves and on the presynaptic ganglia of the parasympathetic system.18,19 These receptors mediate the effects of cannabinoids by reducing the release of anterior pituitary hormones (prolactin, gonadotropin, growth hormone) and increasing corticotropin secretions.20 Disturbances of the hypothalamic-pituitary-adrenal axis and the presence of autonomic instability have been described as the framework for symptoms in those with CVS.21 Taché21 also characterized the increased secretion and activation of corticotropin-releasing factor in the development of CVS. Therefore, we propose that the central effects of long-term cannabis use on the hypothalamic-pituitary-adrenal axis might play a major role in the development of CH. CB1 receptors located in the preoptic area have also been reported to be involved in the hypothermic effects of cannabinoids.22,23 The impairment of the physiologic thermoregulation provoked by cannabis use might account for the relief of symptoms with compulsive hot bathing that is seen in most patients with CH. Experimental studies, likely involving murine models, would be necessary to confirm these hypotheses. The peripheral CB1 receptors of the enteric nerves have also been implicated in slowing gastrointestinal transit.18 Previously, it was suggested that decreased gastric emptying induced by cannabis use might be responsible for the recurrent emesis seen with CH. However, the majority of our patients had either normal or increased gastric transit. Also, the patients did not have features suggestive of delayed gastric emptying, such as early satiety, bloating, or postprandial predominance of symptoms.24
This case series was performed at a single tertiary care center, which might limit the generalizability of our findings; however, 66% of our patients were from out-of-state, with 28 states and 1 other country (Canada) being represented. In addition, because of the nature of being a tertiary care center, long-term follow-up was considerably limited. Follow-up was available in only 10 patients (10%). Of those 10, 7 (70%) stopped using cannabis and 6 of these 7 (86%) noted complete resolution of their symptoms. The only patient who did not notice any improvement stopped consuming marijuana for only 1 month. The half-life of cannabinoids varies greatly, with a long terminal elimination time, especially in long-term daily consumers.25 Therefore, prolonged abstinence should be recommended.
Given the retrospective nature of our study, patient recall bias may exist, and some clinical details were not available for all patients, including potential predisposing factors. The majority of marijuana users do not develop this syndrome, and further studies are needed to identify risk factors. The major strength of our study, however, is the large number of cases, representing the largest case series of CH to date.
Conclusion
Cannabinoid hyperemesis should be considered in younger patients with long-term cannabis use and recurrent nausea, vomiting, and abdominal pain. The timing, location, and characteristics of symptoms can be helpful in determining the diagnosis of CH, and patients should be asked about the relief of symptoms with hot water bathing. Cessation of cannabis use should result in improvement of clinical symptoms. Studies with higher rates of follow-up are needed, and validation of the proposed diagnostic criteria is required.
Acknowledgments
We thank Donna K. Lawson, LPN, (Mayo Clinic, Rochester, MN) for assistance with data collection and Felicity T. Enders, PhD, (Mayo Clinic, Rochester, MN) for assistance with data analysis.
Footnotes
For editorial comment, seepage 107
Supplemental Online Material
Author Interview Video
References
- 1.Leggett T., United Nations Office on Drugs and Crime A review of the world cannabis situation. Bull Narc. 2006;58(1-2):1–155. [PubMed] [Google Scholar]
- 2.Degenhardt L., Chiu W.T., Sampson N. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5(7):e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen J.H., de Moore G.M., Heddle R., Twartz J.C. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut. 2004;53(11):1566–1570. doi: 10.1136/gut.2003.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang Y.H., Windish D.M. Cannabinoid hyperemesis relieved by compulsive bathing. Mayo Clin Proc. 2009;84(1):76–78. doi: 10.4065/84.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chepyala P., Olden K.W. Cyclic vomiting and compulsive bathing with chronic cannabis abuse. Clin Gastroenterol Hepatol. 2008;6(6):710–712. doi: 10.1016/j.cgh.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Donnino M.W., Cocchi M.N., Miller J., Fisher J. Cannabinoid hyperemesis: a case series. J Emerg Med. 2011;40(4):e63–e66. doi: 10.1016/j.jemermed.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 7.Sontineni S.P., Chaudhary S., Sontineni V., Lanspa S.J. Cannabinoid hyperemesis syndrome: clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World J Gastroenterol. 2009;15(10):1264–1266. doi: 10.3748/wjg.15.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soriano-Co M., Batke M., Cappell M.S. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: a report of eight cases in the United States. Dig Dis Sci. 2010;55(11):3113–3119. doi: 10.1007/s10620-010-1131-7. [DOI] [PubMed] [Google Scholar]
- 9.Wallace D., Martin A.L., Park B. Cannabinoid hyperemesis: marijuana puts patients in hot water. Australas Psychiatry. 2007;15(2):156–158. doi: 10.1080/10398560701196778. [DOI] [PubMed] [Google Scholar]
- 10.Watts M. Cannabinoid hyperemesis presenting to a New Zealand hospital. N Z Med J. 2009;122(1290):116–118. [PubMed] [Google Scholar]
- 11.Catto-Smith A.G., Ranuh R. Abdominal migraine and cyclical vomiting. Semin Pediatr Surg. 2003;12(4):254–258. doi: 10.1053/j.sempedsurg.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Li B.U., Fleisher D.R. Cyclic vomiting syndrome: features to be explained by a pathophysiologic model. Dig Dis Sci. 1999;44(8, suppl):13S–18S. [PubMed] [Google Scholar]
- 13.Degenhardt L., Chiu W.T., Sampson N., Kessler R.C., Anthony J.C. Epidemiological patterns of extra-medical drug use in the United States: evidence from the National Comorbidity Survey Replication, 2001-2003. Drug Alcohol Depend. 2007;90(2-3):210–223. doi: 10.1016/j.drugalcdep.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleisher D.R., Matar M. The cyclic vomiting syndrome: a report of 71 cases and literature review. J Pediatr Gastroenterol Nutr. 1993;17(4):361–369. [PubMed] [Google Scholar]
- 15.Walsh D., Nelson K.A., Mahmoud F.A. Established and potential therapeutic applications of cannabinoids in oncology. Support Care Cancer. 2003;11(3):137–143. doi: 10.1007/s00520-002-0387-7. [DOI] [PubMed] [Google Scholar]
- 16.Croxford J.L. Therapeutic potential of cannabinoids in CNS disease. CNS Drugs. 2003;17(3):179–202. doi: 10.2165/00023210-200317030-00004. [DOI] [PubMed] [Google Scholar]
- 17.González S., Cebeira M., Fernández-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav. 2005;81(2):300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Izzo A.A., Sharkey K.A. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther. 2010;126(1):21–38. doi: 10.1016/j.pharmthera.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Storr M.A., Sharkey K.A. The endocannabinoid system and gut-brain signalling. Curr Opin Pharmacol. 2007;7(6):575–582. doi: 10.1016/j.coph.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Wenger T., Moldrich G. The role of endocannabinoids in the hypothalamic regulation of visceral function. Prostaglandins Leukot Essent Fatty Acids. 2002;66(2-3):301–307. doi: 10.1054/plef.2001.0353. [DOI] [PubMed] [Google Scholar]
- 21.Taché Y. Cyclic vomiting syndrome: the corticotropin-releasing-factor hypothesis. Dig Dis Sci. 1999;44(8, suppl):79S–86S. [PubMed] [Google Scholar]
- 22.Hayakawa K., Mishima K., Nozako M. Delta9-tetrahydrocannabinol (Delta9-THC) prevents cerebral infarction via hypothalamic-independent hypothermia. Life Sci. 2007;80(16):1466–1471. doi: 10.1016/j.lfs.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Sim-Selley L.J. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15(2):91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- 24.Hasler W.L. Gastroparesis: symptoms, evaluation, and treatment. Gastroenterol Clin North Am. 2007;36(3):619–647. doi: 10.1016/j.gtc.2007.07.004. ix. [DOI] [PubMed] [Google Scholar]
- 25.Lowe R.H., Abraham T.T., Darwin W.D., Herning R., Cadet J.L., Huestis M.A. Extended urinary Delta9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug Alcohol Depend. 2009;105(1-2):24–32. doi: 10.1016/j.drugalcdep.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author Interview Video


