Abstract
Objective
To study the effectiveness and safety of vancomycin compared with that of other antibiotics for the treatment of gram-positive infections.
Methods
Major electronic databases were searched. Data from published randomized controlled trials (January 1, 1950, to September 15, 2011) were pooled using a meta-analytic method.
Results
Fifty-three trials comparing vancomycin with linezolid, daptomycin, quinupristin-dalfopristin, tigecycline, ceftaroline, ceftobiprole, telavancin, teicoplanin, iclaprim, and dalbavancin were included in the meta-analysis. Individual antibiotics were as effective as vancomycin, except for linezolid, which was more effective than vancomycin for the treatment of skin and soft tissue infections (odds ratio [OR], 1.61; 95% confidence interval [CI], 1.07-2.43). Comparators were as effective as vancomycin in the intent-to-treat population (OR, 1.08; 95% CI, 0.98-1.18) but were more effective in the clinically evaluable population (OR, 1.14; 95% CI, 1.02-1.27) when all infections were pooled. When available data from all trials were pooled, no differences were noted when patients with febrile neutropenia (OR, 1.07; 95% CI, 0.82-1.39), pneumonia (OR, 1.10; 95% CI, 0.87-1.37), bacteremia (OR, 1.05; 95% CI, 0.76-1.45), and skin and soft tissue infections (OR, 1.11; 95% CI, 0.89-1.39) were studied. Comparators were more effective in open-label (OR, 1.28; 95% CI, 1.08-1.50) but not in double-blind trials (OR, 1.04; 95% CI, 0.90-1.20). Total adverse events attributed to studied antibiotics (OR, 1.07; 95% CI, 0.90-1.28) and patients withdrawn from trials (OR, 0.86; 95% CI, 0.68-1.09) were similar in the compared groups. Mortality was not different between vancomycin and comparator antibiotics when all trials were included in the analysis (OR, 1.09; 95% CI, 0.96-1.23). Comparators were associated with higher mortality in open-label (OR, 1.27; 95% CI, 1.05-1.54) but not double-blind trials (OR, 0.96; 95% CI, 0.80-1.14).
Conclusion
On the basis mainly of data from open-label trials, vancomycin is a treatment choice that is as effective as other available antibiotics for patients with gram-positive infections. Study design seems to make a major contribution to the outcome.
Abbreviations and Acronyms: CE, clinically evaluable; CI, confidence interval; ITT, intention to treat; OR, odds ratio; ME, microbiologically evaluable; MIC, minimal inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus
Since its discovery in the 1950s, vancomycin has been a widely used antibiotic for the treatment of infections due to resistant gram-positive cocci. In addition, its use has increased throughout the years, especially in some settings,1 mainly because of the emergence of β-lactam–resistant staphylococci and enterococci. Although vancomycin has proved effective for the treatment of patients with gram-positive infections, several studies have emphasized some important disadvantages, including nephrotoxicity and other adverse reactions,2-4 the need for continuous monitoring of its trough serum levels,5 relatively poor pharmacokinetic properties (especially in the lungs),6,7 data pointing toward reduced effectiveness when the minimal inhibitory concentration (MIC) of Staphylococcus aureus was higher than 1 μg/mL,8-11 and vancomycin resistance or increasing MICs.12 However, vancomycin continues to be the treatment of choice for serious resistant gram-positive infections (including bacteremia, pneumonia, endocarditis, and complicated skin and soft tissue infections [SSTIs]) in the published guidelines of medical societies.13-16
During the past 20 years, several antibiotics with similar spectra to that of vancomycin and effectiveness against resistant gram-positive cocci have become available. Some of them belong to the glycopeptide (teicoplanin) or a similar (telavancin, oritavancin, or dalbavancin) class, whereas others belong to other classes (linezolid, daptomycin, quinupristin-dalfopristin, iclaprim, tigecycline, ceftobiprole, or ceftaroline). In addition, some of them exhibit an entirely different mechanism of action that gave hope for decreasing drug resistance or cross-resistance. Among them, linezolid and teicoplanin are the most extensively studied antibiotics. However, linezolid has a high acquisition cost and is primarily bacteriostatic, whereas teicoplanin was never approved for use in the United States.
Several randomized controlled trials have compared vancomycin with other antibiotics. Several meta-analyses comparing vancomycin with linezolid and teicoplanin have also been published,17-19 but since then, additional data have become available. We sought to summarize and study systematically the effectiveness and safety of vancomycin compared with other approved or investigational antibiotics using a meta-analytic method.
Methods
Data Sources
A search of PubMed (January 1, 1950, to September 15, 2011), Current Contents, Embase, Scopus, Cochrane Central Register of Trials, clinicaltrials.gov, and clinicalstudyresults.org was performed to identify relevant trials for the meta-analysis. Search terms included vancomycin, teicoplanin, glycopeptides, linezolid, telavancin, dalbavancin, oritavancin, ceftaroline, ceftobiprole, daptomycin, quinupristin-dalfopristin, tigecycline, iclaprim, methicillin-resistant, β-lactams, skin and soft tissue, pneumonia, bacteremia, gram-positive cocci, S aureus, MRSA, and enterococcus. References from relevant articles, including review articles, were also examined. Trials reported as abstracts in conferences were searched but not included in the meta-analysis.20
Study Selection
Two reviewers (K.Z.V. and N.R.) independently searched the literature and examined relevant trials for further assessment of data on effectiveness and toxicity. A study was considered eligible if it was a randomized controlled clinical trial, if it studied the role of vancomycin compared with another antibiotic in the treatment of gram-positive infections, and if it assessed the effectiveness, toxicity, or mortality of both therapeutic regimens. Hospital admission of patients was not required for eligibility. No language restriction was set. Trials with masked and unmasked design were included.
Experimental trials and trials that focused on pharmacokinetic or pharmacodynamic variables were excluded. Trials that compared vancomycin with older antibiotics that may still be partially effective for resistant gram-positive infections (eg, clindamycin and trimethoprim-sulfamethoxazole) or β-lactams (usually not effective against S aureus and mainly methicillin-resistant S aureus [MRSA]) were excluded. Finally, trials comparing vancomycin with metronidazole or other antibiotics for Clostridium difficile–associated diarrhea or vancomycin with other antibiotics for peritonitis in patients undergoing continuous ambulatory peritoneal dialysis were also excluded. Additional antimicrobial agents (those with effectiveness against gram-negative rods that could be involved in polymicrobial infections) could be used in the trials.
Data Extraction
Data regarding clinical setting, patient population (intention to treat [ITT], clinically evaluable [CE] and microbiologically evaluable [ME] populations), number of patients, antimicrobial agents and doses used, clinical and microbiological outcomes, toxicity, and mortality were extracted. The ITT population comprised patients who received at least 1 dose of the medications studied in the individual trials. The CE population comprised patients who fulfilled all inclusion and exclusion criteria in the individual trials, who had complete follow-up, and for whom data on treatment outcomes were available but not indeterminate. The ME population was a subset of the CE population who also had microbiologically documented infections.
Two reviewers (M.N.M. and N.R.) independently extracted the relevant data. Any disagreement was resolved by consensus in meetings with all investigators. A quality review of each trial was performed to include details of randomization, generation of random numbers, details of the double-blinding procedure, information on withdrawals, and allocation concealment. One point was awarded for the specification of each criterion, with a maximum score of 5. High-quality trials scored 3 or more points, whereas low-quality trials scored 2 or fewer points, according to a modified Jadad score.21
Definitions of Infections
Infections were defined according to the definitions used by the individual trials. Definitions of infections did not differ substantially in the trials included in the meta-analysis. Both complicated (defined as needing surgical intervention or involving deeper tissues [eg, fascia, muscle, or bone] or presenting in patients with comorbidities such as diabetes mellitus, human immunodeficiency virus coinfection, or other immunocompromised status) and uncomplicated SSTIs could be included in the meta-analysis. At least one of the following symptoms of infection needed to have been present: drainage or discharge, erythema, fluctuance, heat or localized warmth, pain or tenderness to palpation, swelling, or induration.
For bacteremia, culture of at least 1 blood sample needed to yield gram-positive cocci (at least 2 for coagulase-negative staphylococci), and the clinical profile had to be compatible with a diagnosis of bacteremia with one of the following findings: fever, chills, leukocytosis with prominent left shift, changes in vital signs, signs of septic shock (decreased peripheral perfusion or hypotension), and petechiae or purpura.
For a diagnosis of pneumonia, the baseline chest radiograph needed to show new or progressive infiltrates, consolidation with or without effusion, and 2 of the following signs and symptoms: cough, new or worsened purulent sputum production, rales and/or signs of pulmonary consolidation, dyspnea, tachypnea, and/or hypoxemia. In addition, at least 2 of the following findings were necessary: fever (temperature ≥38°C taken orally), respiratory rate of 30/min, systolic hypotension (<90 mm Hg), heart rate of at least 120/min, altered mental status, requirement for mechanical ventilation, elevated total peripheral leukocyte count of at least 10 × 109/L (to convert to /μL, divide by 0.001) with at least 15% immature neutrophils (band forms), or leukopenia (leucocyte count <4.5 × 109/L). For a diagnosis of nosocomial pneumonia, the symptoms or radiologic findings should have started more than 48 hours after hospital admission.
Analyzed Outcomes
Treatment success (cure, defined as resolution of all symptoms and signs of infections, or improvement, defined as resolution of ≥2 of the baseline symptoms or signs of infections in the ITT and CE populations), all-cause mortality in the ITT population, and adverse effects probably or possibly related to study regimens were used as primary outcome measures for this meta-analysis. We did not devise a composite index based on the 3 primary outcome measures of our meta-analysis as the main outcome of the analysis. Treatment success was assessed in all patients and separately in patients with febrile neutropenia, SSTIs, bacteremia, and pneumonia. The effectiveness of the empirical regimen was estimated at the test-of-cure visit. The CE patients in the individual trials who had an indeterminate clinical outcome (ie, their condition could not be assessed as improvement or failure or follow-up data were inadequate) at the test-of-cure visit were deemed unassessed for the analysis of treatment success. All-cause mortality was analyzed based on the reported data for mortality during the study period (eg, during treatment and follow-up period). Treatment duration (ITT population, intravenous and oral administration of study regimens combined) and microbiological assessment (especially S aureus, methicillin-resistant and methicillin-susceptible strains) were secondary outcomes.
Statistical Analyses
Statistical analyses were performed with Review Manager (RevMan), version 5.0 (Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark, 2008). The heterogeneity among the trials was assessed by using a χ2 test (P<.10 was defined to indicate significant heterogeneity) or I2 (>40%). Publication bias was assessed using the Egger test by the funnel plot method, with P<.05 indicating potential bias.22 Pooled odds ratios (ORs) and 95% confidence intervals (CIs) for all primary and secondary outcomes (including the ITT, CE, and ME populations) were calculated by the Mantel-Haenszel fixed-effect model or the DerSimonian-Laird random-effects model.23,24 For all analyses, results from the fixed-effect model are presented only when there was no heterogeneity among the trials; otherwise, results from the random-effects model are presented.
Results
Figure 1 shows the selection process applied to identify the pool of trials used in the final analysis. We identified 2512 published reports that dealt with patients with infections caused by gram-positive cocci treated with vancomycin. Eighty-four reports were selected for further investigation. Thirty-one reports of trials were excluded from the meta-analysis for the reasons depicted in Figure 1. Thus, 53 trials that enrolled 17,420 patients were included in the meta-analysis.25-76
FIGURE 1.
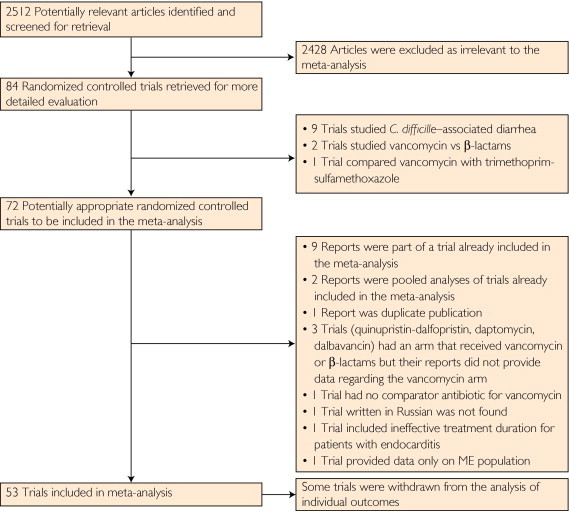
The selection process applied to identify the pool of trials used in the final analysis. ME = microbiologically evaluable.
The main characteristics of the analyzed trials are given in the supplemental online table (available at www.mayoclinicproceedings.org). The mean quality score of the included trials was 2.7, whereas the median was 2 (range, 1-5). The quality of 26 trials (49%) was high (score ≥3). All enrolled patients had a presumed or definitive infection caused by gram-positive cocci. Publication bias was not detected. Although for some trials the dropout rate was high, no significant differences were found among the treatment groups in the individual trials. The demographic characteristics of patients varied among the studies.
Vancomycin was compared with linezolid in 14 trials,25-38 ceftaroline in 3,39-41 ceftobiprole in 2,42,43 tigecycline in 3,44-46 quinupristin-dalfopristin in 2,47,48 dalbavancin in 1,49 telavancin in 4,50-53 teicoplanin in 20,54-73 daptomycin in 3,74-76 and iclaprim in 1.77 In trials studying febrile neutropenic patients, vancomycin and comparator antibiotics were included in the first-line empirical regimen in 7 trials,32,54,61,62,64,67,69 in 2 trials they were administered after treatment failure of the initial regimen (usually a β-lactam with or without an aminoglycoside) was encountered,59,71 and in the remaining 3 randomization was performed after the isolation of gram-positive cocci.55,57,73 Finally, 1 trial did not report the time of randomization.66
Patients with concomitant gram-negative or mixed infections were treated with appropriate regimens (mainly aztreonam or other β-lactams and aminoglycosides). The administration of study antibiotics to patients before enrollment was not allowed except for trials studying neutropenic patients who could have received prophylactic antibiotics. Patients were excluded from the effectiveness analysis in each trial if they received any antibiotic effective against gram-positive infections in the previous 24 to 48 hours, unless the isolated cocci were resistant to these antibiotics.
Linezolid
Linezolid was more effective than vancomycin in the ITT population (OR, 1.20; 95% CI, 1.01-1.43) and in its subset of patients with SSTIs (OR, 1.56; 95% CI, 1.01-2.40); on the other hand, no difference was found in the ITT patients with pneumonia (OR, 1.02; 95% CI, 0.78-1.35). Linezolid was also more effective than vancomycin in the CE population (OR, 1.39; 95% CI, 1.07-1.80) and in its subset of patients with SSTIs (OR, 1.61; 95% CI, 1.07-2.43). No difference was seen in patients with pneumonia (OR, 1.13; 95% CI, 0.83-1.54), bacteremia (OR, 0.88; 95% CI, 0.49-1.57), and neutropenic fever (1 trial). Treatment success with linezolid was higher in ME patients (OR, 1.36; 95% CI, 1.09-1.71) and in those with S aureus infections (OR, 1.70; 95% CI, 1.33-2.16) but not in patients with MRSA (OR, 1.80; 95% CI, 0.96-2.33; P=.08). Mortality was similar in patients receiving linezolid or vancomycin (OR, 1.08; 95% CI, 0.90-1.30). Treatment-related adverse events were similar between linezolid and vancomycin patients (OR, 1.08; 95% CI, 0.83-1.39), but linezolid was associated with fewer withdrawals due to adverse events (OR, 0.57; 95% CI, 0.34-0.96). Linezolid was associated with fewer episodes of nephrotoxicity (OR, 0.38; 95% CI, 0.19-0.79) but more episodes of thrombocytopenia (OR, 9.94; 95% CI, 3.97-24.87).
Teicoplanin
Teicoplanin was compared with vancomycin mainly in neutropenic patients with fever. No difference was noticed in the ITT (OR, 1.05; 95% CI, 0.60-1.84), CE (OR, 1.01; 95% CI, 0.79-1.29), and ME (OR, 0.74; 95% CI, 0.49-1.12) populations. No difference was noticed in patients with febrile neutropenia (OR, 1.02; 95% CI, 0.77-1.35), bacteremia (OR, 0.63; 95% CI, 0.35-1.17), and SSTIs (OR, 0.84; 95% CI, 0.25-2.81). Teicoplanin was as effective as vancomycin in patients with S aureus (OR, 1.04; 95% CI, 0.50-2.19) and MRSA infections (OR, 1.41; 95% CI, 0.39-5.11). Mortality was similar between teicoplanin and vancomycin users (OR, 0.93; 95% CI, 0.66-1.32). Teicoplanin was associated with fewer treatment-related adverse events (OR, 0.33; 95% CI, 0.19-0.55), especially nephrotoxicity (OR, 0.35; 95% CI, 0.21-0.58). However, withdrawals due to adverse events were similar in the compared groups (OR, 0.63; 95% CI, 0.32-1.25).
Telavancin
In the whole ITT population, telavancin was as effective as vancomycin (OR, 1.03; 95% CI, 0.90-1.19) as well as in the subsets of patients with pneumonia (OR, 0.97; 95% CI, 0.79-1.19) and SSTIs (OR, 1.09; 95% CI, 0.90-1.33). No difference among the compared antibiotics was noticed in the CE (OR, 1.11; 95% CI, 0.87-1.40) and ME (OR, 1.15; 95% CI, 0.77-1.72) populations or in the subsets of patients with pneumonia (OR, 1.12; 95% CI, 0.76-1.67), SSTIs (OR, 1.10; 95% CI, 0.82-1.48), S aureus infections (OR, 1.28; 95% CI, 0.97-1.69), and MRSA infections (OR, 1.31; 95% CI, 0.92-1.87). Mortality (OR, 1.09; 95% CI, 0.85-1.39) and treatment-related adverse events (OR, 1.30; 95% CI, 0.84-2.02) were similar between the telavancin and vancomycin groups. More episodes of nephrotoxicity (OR, 1.66; 95% CI, 1.22-2.26) and more patients withdrawn from trials because of adverse events (OR, 1.48; 95% CI, 1.14-1.93) were reported with telavancin treatment.
Daptomycin and Quinupristin-Dalfopristin
Few data were available regarding the comparative effectiveness between daptomycin and quinupristin-dalfopristin with vancomycin. Daptomycin was as effective as vancomycin in all patient populations (ITT patients: OR, 1.05; 95% CI, 0.58-1.91; CE patients: OR, 0.95; 95% CI, 0.22-3.95; ME patients: OR, 1.05; 95% CI, 0.38-2.86; patients with SSTIs: OR, 0.58; 95% CI, 0.06-6.10; and patients with MRSA infections: OR, 0.90; 95% CI, 0.23-3.52). No difference was reported in mortality (OR, 1.59; 95% CI, 0.58-4.38) and withdrawals due to adverse events (OR, 0.36; 95% CI, 0.10-1.33). More treatment-related adverse events were observed in the daptomycin group (OR, 2.50; 95% CI, 1.10-5.71).
Similarly, no difference was seen in the effectiveness of quinupristin-dalfopristin compared with vancomycin in all patients populations (ITT patients: OR, 0.98; 95% CI, 0.63-1.52; CE patients: OR, 0.94; 95% CI, 0.52-1.68; ME patients: OR, 0.94; 95% CI, 0.52-1.68; and patients with bacteremia: OR, 2.92; 95% CI, 0.70-12.17). No difference was seen in mortality (OR, 1.25; 95% CI, 0.74-2.10) and withdrawal due to adverse events (OR, 1.11; 95% CI, 0.31-3.94), but quinupristin-dalfopristin was associated with more treatment-related adverse events (OR, 3.56; 95% CI, 2.15-5.90).
Ceftaroline
Ceftaroline and vancomycin were compared only in patients with SSTIs. No difference was observed between the 2 antibiotics in the ITT (OR, 1.06; 95% CI, 0.79-1.42), CE (OR, 0.92; 95% CI, 0.61-1.39), and ME (OR, 0.85; 95% CI, 0.51-1.41) populations. Patients with secondary bacteremia also had similar outcomes (OR, 0.22; 95% CI, 0.02-2.06). Treatment success was similar in patients with MRSA infections (OR, 0.77; 95% CI, 0.30-1.99). Only 3 deaths were recorded in the ceftaroline-treated patients compared with none in vancomycin-treated patients (OR, 6.98; 95% CI, 0.36-135.63). No difference was noticed between ceftaroline and vancomycin regarding treatment-related adverse events (OR, 0.86; 95% CI, 0.67-1.10) and withdrawals due to adverse events (OR, 0.63; 95% CI, 0.37-1.09).
Ceftobiprole
Ceftobiprole and vancomycin were also compared only in patients with SSTIs. No difference between ceftobiprole and vancomycin was seen in the ITT (OR, 1.04; 95% CI, 0.81-1.34), CE (OR, 1.08; 95% CI, 0.68-1.72), and ME (OR, 1.17; 95% CI, 0.71-1.94) populations. No difference was noticed in patients with MRSA (OR, 1.32; 95% CI, 0.56-3.11). No difference was also noted in mortality (OR, 0.52; 95% CI, 0.12-2.28), treatment-related adverse events (OR, 1.01; 95% CI, 0.83-1.24), and withdrawals due to adverse events (OR, 0.89; 95% CI, 0.55-1.45).
Tigecycline
No data on tigecycline were available regarding the ITT population. Tigecycline was as effective as vancomycin in the CE population (OR, 0.82; 95% CI, 0.55-1.21) and the subsets of patients with SSTIs (OR, 0.79; 95% CI, 0.53-1.17) and bacteremia (OR, 0.90; 95% CI, 0.24-3.45). No difference between tigecycline and vancomycin was noticed in the ME population (OR, 0.74; 95% CI, 0.34-1.62) and in patients with MRSA infections (1 trial). No difference between tigecycline and vancomycin was seen in mortality (OR, 2.12; 95% CI, 0.65-6.92) and withdrawals due to adverse events (OR, 0.91; 95% CI, 0.42-2.00); however, treatment-related adverse events were observed more frequently with tigecycline (OR, 1.40; 95% CI, 1.09-1.79).
Dalbavancin and Iclaprim
Few data regarding dalbavancin and iclaprim were available (1 trial each), and no further analysis could be performed. No differences were observed in these trials between dalbavancin or iclaprim with vancomycin.
Overall Assessment
Mortality was not different between comparator antibiotics and vancomycin when all trials were included in the analysis (OR, 1.09; 95% CI, 0.96-1.23; Figure 2). Figure 3 shows that mortality was also comparable between the 2 groups in double-blind trials (OR, 0.96; 95% CI, 0.80-1.14). However, in open-label trials, mortality was higher in the comparator antibiotics (OR, 1.27; 95% CI, 1.05-1.54), a figure that should be mainly attributed in 1 trial that compared linezolid with vancomycin for the treatment of patients with catheter-related bloodstream infections and SSTIs and included in the ITT mortality analysis patients with gram-negative infections.25 If this trial was excluded from the analysis, mortality was not different among patients receiving vancomycin or comparator antibiotics (OR, 1.23; 95% CI, 0.98-1.53). Mortality regarding patients infected with MRSA was reported in 6 randomized controlled trials only and was similar among the compared groups (OR, 1.16; 95% CI, 0.66-2.03). Data regarding mortality of patients with MRSA pneumonia were available in 2 trials only. In the analysis that included trials mainly studying patients with febrile neutropenia, pneumonia, and bacteremia, mortality was not statistically different between vancomycin and comparator antibiotics (OR, 0.86; 95% CI, 0.70-1.04). This analysis included only trials studying linezolid (5 trials), teicoplanin (12 trials), daptomycin (1 trial), and quinupristin-dalfopristin (2 trials).
FIGURE 2.

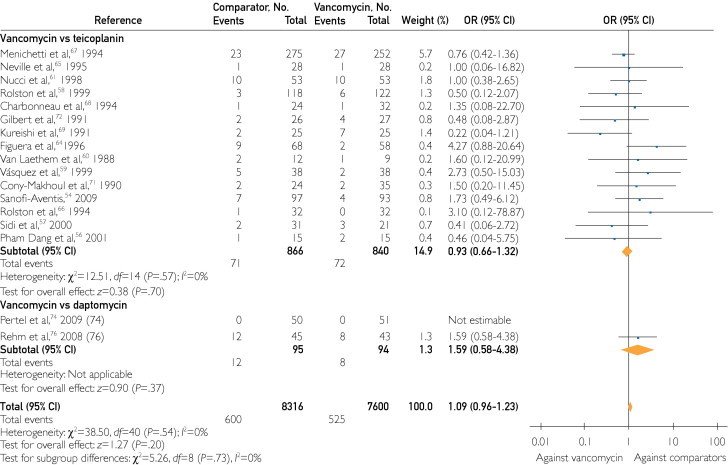
Odds ratios (ORs) of all-cause mortality for individual antibiotic comparison with vancomycin in the intent-to-treat population. Vertical line = the no difference point between the 2 regimens; square = OR; diamond = pooled OR for all randomized controlled trials; horizontal line = 95% confidence interval (CI). aThese numbers correspond to 2 different randomized controlled trials that were published as one in Clinical Infectious Diseases.50
FIGURE 3.

Odds ratios (ORs) of all-cause mortality for the intent-to-treat population in double-blind and open-label trials. Vertical line = the no difference point between the 2 regimens; square = OR; diamond = pooled OR for all randomized controlled trials; horizontal line = 95% confidence interval (CI). aThese numbers correspond to 2 different randomized controlled trials that were published as one in Clinical Infectious Diseases.50
When all trials that provided data on effectiveness in the ITT population were included in the analysis, comparator antibiotics were not more effective than vancomycin (OR, 1.08; 95% CI, 0.98-1.18). Similar effectiveness was found between compared antibiotics for ITT patients with pneumonia (OR, 0.98; 95% CI, 0.84-1.15), bacteremia (OR, 1.31; 95% CI, 0.80-2.14), and SSTIs (OR, 1.09; 95% CI, 0.96-1.24). No difference was found in the analysis of double-blind trials (OR, 1.04; 95% CI, 0.94-1.15), but comparators were more effective than vancomycin in open-label trials (OR, 1.22; 95% CI, 1.01-1.47).
When all trials that provided the relevant data were included in the analysis of effectiveness in the CE population, comparators were more effective than vancomycin (OR, 1.14; 95% CI, 1.02-1.27). Figure 4 shows that in double-blind trials no difference among the compared groups was seen (OR, 1.04; 95% CI, 0.90-1.20), whereas in open-label trials comparators were more effective (OR, 1.28; 95% CI, 1.08-1.50).
FIGURE 4.
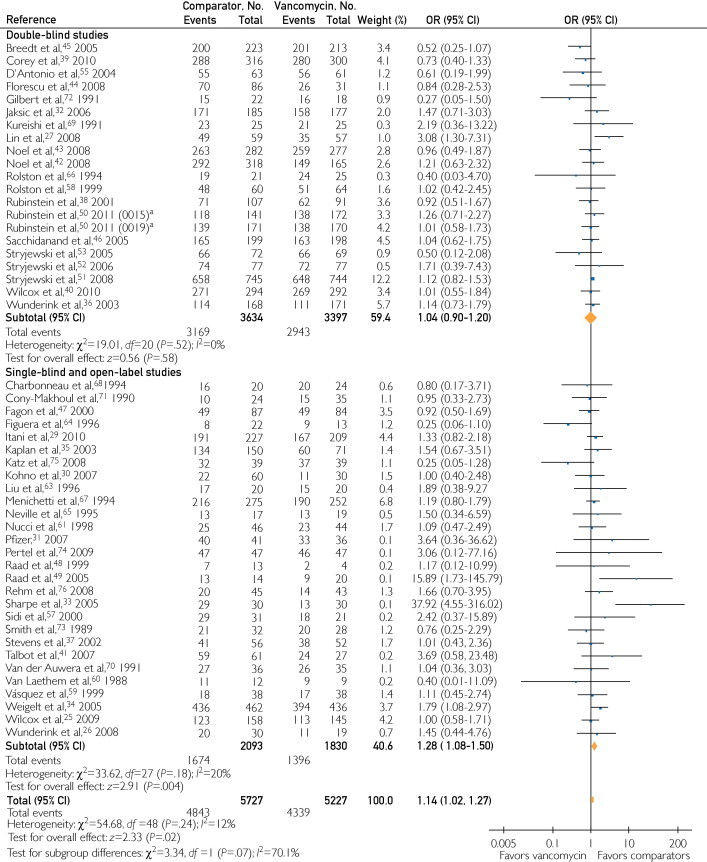
Odds ratios (ORs) of treatment success for the clinically evaluable patients in double-blind and open-label trials. Vertical line = the no difference point between the 2 regimens; square = OR; diamond = pooled OR for all randomized controlled trials; horizontal lines = 95% confidence interval (CI). aThese numbers correspond to 2 different randomized controlled trials that were published as one in Clinical Infectious Diseases.50
Vancomycin was as effective as comparator antibiotics in patients with febrile neutropenia (OR, 1.07; 95% CI, 0.82-1.39), pneumonia (OR, 1.09; 95% CI, 0.87-1.37), bacteremia (OR, 1.05; 95% CI, 0.76-1.45), and SSTIs (OR, 1.11; 95% CI, 0.89-1.37). In the ME population, no difference between the compared groups was noted (OR, 1.10; 95% CI, 0.95-1.26). Comparators were overall more effective than vancomycin in patients with confirmed S aureus (OR, 1.34; 95% CI, 1.14-1.56) and MRSA infections (OR, 1.35; 95% CI, 1.11-1.63). For MRSA infections, comparators were not more effective than vancomycin in double-blind (OR, 1.23; 95% CI, 0.93-1.64) and open-label trials (OR, 1.30; 95% CI, 0.88-1.91).
Adverse events attributed to the studied antibiotics were similar between the compared groups (OR, 1.07; 95% CI, 0.90-1.28). Adverse events were comparable between vancomycin users and users of other antibiotics in double-blind (OR, 1.00; 95% CI, 0.83-1.21) and open-label (OR, 1.19; 95% CI, 0.84-1.67) trials. Comparators were not associated with fewer adverse events that led to withdrawal of treatment (OR, 0.86; 95% CI, 0.68-1.09). Withdrawals due to adverse events were comparable between vancomycin and other antibiotics in double-blind (OR, 1.05; 95% CI, 0.88-1.26) and open-label (OR, 0.64; 95% CI, 0.38-1.05) trials. Finally, similar episodes of recurrent infections were reported in the compared groups of antibiotics (OR, 1.14; 95% CI, 0.84-1.56).
Discussion
The results of this meta-analysis suggest that vancomycin is as effective as comparator antibiotics for the treatment of patients with febrile neutropenia, pneumonia, and bacteremia due to gram-positive cocci. Vancomycin should also be considered equally effective for the treatment of patients with gram-positive SSTIs; linezolid can be considered an exception, but physicians should be aware that data regarding the comparative effectiveness of linezolid and vancomycin rely mainly on open-label trials. Overall, comparators were associated with higher treatment success in the analyses of patients with S aureus and MRSA infections. Again, only linezolid was more effective than vancomycin for S aureus infections; we also noticed a trend toward higher effectiveness of telavancin (P=.09). On the other hand, no single antibiotic was significantly more effective than vancomycin for MRSA infections. However, we should acknowledge that all these effects were mainly seen in the analysis of open-label trials, whereas in double-blind trials no difference between vancomycin and comparators was found.
No difference in mortality was noticed in the analysis of all trials. However, comparators were associated with higher mortality in open-label trials, a finding in contrast to the higher effectiveness noticed in the comparator antibiotics arm. This finding can be explained by various assumptions. First, the difference in mortality was observed in the analysis of open-label trials, whereas mortality was similar between vancomycin and comparators in the analysis of double-blind and high-quality trials. It has been shown that open-label trials have a higher potential for bias.25,78 For example, misclassification may have occurred on the assessment of the outcomes by the investigators performing the trials included in the meta-analysis. Treatment success is probably a less objective outcome than mortality. Furthermore, early determination in the assessment of treatment success may have contributed to misclassification of outcome.79
Second, it is evident that the observed higher mortality rate should be attributed to an open-label trial that studied the comparative effectiveness of linezolid and vancomycin for the treatment of patients with presumed or definitive gram-positive SSTIs and catheter-related bacteremia.25 The removal of this trial from the analysis led to a result with no difference in mortality between comparators and vancomycin. Furthermore, in this trial linezolid was associated with a higher mortality rate when patients with infections due to both gram-positive and gram-negative bacteria were included in the analysis. When patients with proven gram-positive infections were analyzed alone, mortality rates were not different between linezolid and vancomycin. However, the mortality rate was higher in patients allocated to the linezolid arm with infections due to gram-negative bacteria or when no microorganism was isolated. The authors of this trial could not find out why this “unexpected” difference was seen, despite the fact that they proceeded to additional post hoc analyses. However, they pointed out than more than half of patients with gram-negative infections did not receive adequate treatment in both treatment groups.
Tigecycline, daptomycin, and quinupristin-dalfopristin were associated with more adverse effects than vancomycin; teicoplanin was associated with fewer adverse effects; and linezolid, telavancin, ceftobiprole, and ceftaroline were associated with similar rates of adverse events with vancomycin. Nephrotoxicity was mainly seen with vancomycin, whereas disturbances of the gastrointestinal tract were observed with other antibiotics. Telavancin was the only antibiotic that was associated with more episodes of nephrotoxicity than vancomycin. We did not study specific adverse events caused by each comparator antibiotic because older studies or reviews had shown that each one could be associated with one or more specific reactions; linezolid, for example, was associated with more episodes of thrombocytopenia.17,80,81 In addition, such an assessment was beyond the scope of the meta-analysis.
Of 53 trials included in the meta-analysis, only 9 (1115 CE patients) reported outcomes for nosocomial pneumonia, pointing out the paucity of data regarding this serious infection. Moreover, 7 of these trials compared vancomycin with linezolid for patients with pneumonia. A trial that was published after the meta-analysis was performed showed that linezolid was more effective than vancomycin for the treatment of patients with MRSA nosocomial pneumonia in both the CE (57.6% vs 46.6%, P=.042) and modified ITT (54.8% vs 44.9%, P=.049) populations at the end of study. However, no difference in mortality rate was noticed (15.7% vs 17%).82 The lack of published data regarding the effectiveness of teicoplanin, an antibiotic that has been available for more than 2 decades in the European market, compared with vancomycin for patients with pneumonia is remarkable.
Ceftobiprole has been studied in a large trial for nosocomial pneumonia but was not compared with vancomycin.83,84 Ceftobiprole was noninferior to ceftazidime plus linezolid for all patients with nosocomial pneumonia, but it was less effective than ceftazidime plus linezolid for patients with ventilator-associated pneumonia.83,84 Tigecycline was compared with imipenem-cilastatin for patients with nosocomial pneumonia. Tigecycline was as effective as imipenem-cilastatin in patients who did not require mechanical ventilation, but it was inferior to imipenem-cilastatin for patients receiving mechanical ventilation.85 In a subset of patients with confirmed MRSA pneumonia, tigecycline was less effective than imipenem-cilastatin plus vancomycin (12/27 vs 23/30 patients, P=.005).85
One trial comparing linezolid with vancomycin for patients with febrile neutropenia and 11 trials comparing teicoplanin with vancomycin have been published. In most of these trials antibiotics were administered as first-line empirical treatment. The currently available data regarding patients with febrile neutropenia indicate that initial coverage against resistant gram-positive cocci is associated with higher treatment success of the infections and sooner defervescence, but mortality remains unaffected.86,87 We are not aware of published trials comparing newer antibiotics in this patient population. A trial comparing ceftobiprole with cefepime-vancomycin (NCT00529282) was terminated, whereas 2 small trials comparing daptomycin with vancomycin (NCT00296049, NCT00335478) have been completed but data are not available. However, data from nonrandomized studies indicate that these newer antibiotics might be useful for the treatment of such patients.88-90
Limited data are also available for the effectiveness of individual comparator antibiotics for the treatment of bacteremic patients. It is noteworthy that 850 CE patients were studied in 19 trials that reported the outcomes of patients with bacteremia. Vancomycin was not less effective than comparators in this patient population.
On the other hand, the available evidence for patients with SSTIs is probably adequate, at least for linezolid, telavancin, ceftaroline, and ceftobiprole. The data point toward higher effectiveness of linezolid when compared with vancomycin. The main drawback is that the data come mainly from open-label trials. In addition, despite its higher acquisition cost, linezolid has been shown in several studies to be more cost-effective than vancomycin because of the availability of an oral formulation and the possibility of early hospital discharge.91-93 Therefore, linezolid can probably be considered a more attractive choice in hospitalized patients with SSTIs due to resistant cocci.94-96 No other antibiotic showed significantly higher effectiveness than vancomycin for patients with SSTIs.
The analysis of the ME population showed that vancomycin was less effective than comparator antibiotics for infections due to S aureus and MRSA. More specifically, linezolid was more effective for the treatment of S aureus infections, but no single antibiotic proved more effective than vancomycin for MRSA infections. Accumulating data demonstrate that vancomycin is less effective when the MIC of S aureus is higher than 1 μg/mL than when the MIC is 1 μg/mL or less.8-11 The lack of relevant data in these trials did not allow us to study whether the higher effectiveness of comparator antibiotics could be due to higher MICs. Several trials reported (without presenting quantitative data) that development of resistance was not observed during the trial period, thus rejecting the hypothesis that resistance may have been the reason for vancomycin's lower effectiveness. Finally, the lack of monitoring of vancomycin's trough serum levels in several trials might have influenced the outcomes in favor of comparator antibiotics; again, data strongly supporting this assumption were not available in the individual trials included in the meta-analysis.
The present meta-analysis has the usual limitations found in similar works.78,97 The authors of the included trials were not contacted to provide additional data when these were missing from the original report. Trials published during a period of 20 years (1989-2009) were included. Studies with higher or lower methodological quality, double-blind, or open-label with adequate or inadequate allocation concealment were evaluable for inclusion if they fulfilled the criterion of randomization. Trials studying the comparative effectiveness of vancomycin with that of several other antibiotics with approximately the same spectrum were included. A significant proportion of patients also received additional antibiotics for gram-negative bacteria (mainly aztreonam and aminoglycosides), which in some cases may have contributed to the effectiveness of the studied medications. Furthermore, a significant proportion of patients had presumed, not proven, gram-positive infections. Finally, trials presented as abstracts in conferences were not included in the meta-analysis.
Conclusion
This meta-analysis shows that vancomycin is at least as effective as other commercially available or forthcoming antibiotics for the treatment of patients with pneumonia, bacteremia, and febrile neutropenia due to resistant gram-positive bacteria. Nonetheless, the data showed that vancomycin is less effective than linezolid for the treatment of SSTIs in open-label trials. Although open-label trials showed that the mortality rate was lower in the vancomycin arm, this finding was not evident in patients with severe infections and febrile neutropenia or those enrolled in double-blind trials. It is therefore evident that the quality of each study should be taken into account before making decisions for treatment. The vast experience and knowledge on vancomycin anticipate that it will be among the leading choices for the treatment of patients with gram-positive infections for the years to come. However, it is reassuring and promising that alternative, equally effective or, on specific occasions, better, antibiotics are already available for this patient population. Furthermore, the availability of several treatment options enables the physicians to choose the appropriate antibiotic based on its effectiveness and safety for a given indication according to the characteristics of the individual patient.
Supplementary Online Material
References
- 1.Pallares R., Dick R., Wenzel R.P., Adams J.R., Nettleman M.D. Trends in antimicrobial utilization at a tertiary teaching hospital during a 15-year period (1978-1992) Infect Control Hosp Epidemiol. 1993;14(7):376–382. doi: 10.1086/646765. [DOI] [PubMed] [Google Scholar]
- 2.Lodise T.P., Lomaestro B., Graves J. Larger vancomycin doses (≥4 grams/day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52:1330–1336. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine D.P. Vancomycin: a history. Clin Infect Dis. 2006;42(suppl 1):S5–S12. doi: 10.1086/491709. [DOI] [PubMed] [Google Scholar]
- 4.Rybak M.J., Albrecht L.M., Boike S.C., Chandrasekar P.H. Nephrotoxicity of vancomycin alone and with an aminoglycoside. J Antimicrob Chemother. 1990;25(4):679–687. doi: 10.1093/jac/25.4.679. [DOI] [PubMed] [Google Scholar]
- 5.Rybak M., Lomaestro B., Rotschafer J.C. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 6.Georges H., Leroy O., Alfandari S. Pulmonary disposition of vancomycin in critically ill patients. Eur J Clin Microbiol Infect Dis. 1997;16(5):385–388. doi: 10.1007/BF01726369. [DOI] [PubMed] [Google Scholar]
- 7.Scheetz M.H., Wunderink R.G., Postelnick M.J., Noskin G.A. Potential impact of vancomycin pulmonary distribution on treatment outcomes in patients with methicillin-resistant Staphylococcus aureus pneumonia. Pharmacotherapy. 2006;26(4):539–550. doi: 10.1592/phco.26.4.539. [DOI] [PubMed] [Google Scholar]
- 8.Tenover F.C., Moellering R.C., Jr. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis. 2007;44(9):1208–1215. doi: 10.1086/513203. [DOI] [PubMed] [Google Scholar]
- 9.Sakoulas G., Moise-Broder P.A., Schentag J., Moellering R.C., Jr, Eliopoulos G.M. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42(6):2398–2402. doi: 10.1128/JCM.42.6.2398-2402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hidayat L.K., Hsu D.I., Quist R., Shriner K.A., Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 11.Charles P.G., Ward P.B., Johnson P.D., Howden B.P., Grayson M.L. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis. 2004;38(3):448–451. doi: 10.1086/381093. [DOI] [PubMed] [Google Scholar]
- 12.Kollef M.H. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin Infect Dis. 2007;45(suppl 3):S191–S195. doi: 10.1086/519470. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura R.A., Carabello B.A., Faxon D.P., American College of Cardiology/American Heart Association Task Force ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118(8):887–896. doi: 10.1161/CIRCULATIONAHA.108.190377. [DOI] [PubMed] [Google Scholar]
- 14.Mermel L.A., Allon M., Bouza E. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens D.L., Bisno A.L., Chambers H.F., Infectious Diseases Society of America Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41(10):1373–1406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society; Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 17.Falagas M.E., Siempos I.I., Vardakas K.Z. Linezolid versus glycopeptide or beta-lactam for treatment of gram-positive bacterial infections: meta-analysis of randomised controlled trials. Lancet Infect Dis. 2008;8(1):53–66. doi: 10.1016/S1473-3099(07)70312-2. [DOI] [PubMed] [Google Scholar]
- 18.Svetitsky S., Leibovici L., Paul M. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob Agents Chemother. 2009;53(10):4069–4079. doi: 10.1128/AAC.00341-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beibei L., Yun C., Mengli C., Nan B., Xuhong Y., Rui W. Linezolid versus vancomycin for the treatment of gram-positive bacterial infections: meta-analysis of randomised controlled trials. Int J Antimicrob Agents. 2010;35(1):3–12. doi: 10.1016/j.ijantimicag.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Rosmarakis E.S., Soteriades E.S., Vergidis P.I., Kasiakou S.K., Falagas M.E. From conference abstract to full paper: differences between data presented in conferences and journals. FASEB J. 2005;19(7):673–680. doi: 10.1096/fj.04-3140lfe. [DOI] [PubMed] [Google Scholar]
- 21.Moher D., Pham B., Jones A. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609–613. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- 22.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantel N., Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 24.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Wilcox M.H., Tack K.J., Bouza E. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis. 2009;48(2):203–212. doi: 10.1086/595686. [DOI] [PubMed] [Google Scholar]
- 26.Wunderink R.G., Mendelson M.H., Somero M.S. Early microbiological response to linezolid vs vancomycin in ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus. Chest. 2008;134(6):1200–1207. doi: 10.1378/chest.08-0011. [DOI] [PubMed] [Google Scholar]
- 27.Lin D.F., Zhang Y.Y., Wu J.F. Linezolid for the treatment of infections caused by Gram-positive pathogens in China. Int J Antimicrob Agents. 2008;32(3):241–249. doi: 10.1016/j.ijantimicag.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Pfizer: Linezolid vs vancomycin/cefazolin in the treatment of hemodialysis patients with catheter-related gram-positive bloodstream infections: A5951105. http://www.clinicalstudyresults.org/documents/company-study_2421_0.pdf Accessed January 13, 2010.
- 29.Pfizer Linezolid in the treatment of subjects with complicated skin and soft tissue infections proven to be due to methicillin-resistant staphylococcus aureus: A5951002. http://www.clinicalstudyresults.org/documents/company-study_4375_0.pdf Accessed January 13, 2010.
- 30.Kohno S., Yamaguchi K., Aikawa N. Linezolid versus vancomycin for the treatment of infections caused by methicillin-resistant Staphylococcus aureus in Japan. J Antimicrob Chemother. 2007;60(6):1361–1369. doi: 10.1093/jac/dkm369. [DOI] [PubMed] [Google Scholar]
- 31.Pfizer: Time to efficacy and onset of action of linezolid compared to vancomycin in skin and soft tissue infections: OXAA-0026-162. http://www.clinicalstudyresults.org/documents/company-study_1864_0.pdf Accessed January 13, 2010.
- 32.Jaksic B., Martinelli G., Perez-Oteyza J., Hartman C.S., Leonard L.B., Tack K.J. Efficacy and safety of linezolid compared with vancomycin in a randomized, double-blind study of febrile neutropenic patients with cancer. Clin Infect Dis. 2006;42(5):597–607. doi: 10.1086/500139. [DOI] [PubMed] [Google Scholar]
- 33.Sharpe J.N., Shively E.H., Polk H.C., Jr Clinical and economic outcomes of oral linezolid versus intravenous vancomycin in the treatment of MRSA-complicated, lower-extremity skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. Am J Surg. 2005;189(4):425–428. doi: 10.1016/j.amjsurg.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Weigelt J., Itani K., Stevens D., Lau W., Dryden M., Knirsch C., Linezolid CSSTI Study Group Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrob Agents Chemother. 2005;49(6):2260–2266. doi: 10.1128/AAC.49.6.2260-2266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan S.L., Afghani B., Lopez P. Linezolid for the treatment of methicillin-resistant Staphylococcus aureus infections in children. Pediatr Infect Dis J. 2003;22(9, suppl):S178–S185. doi: 10.1097/01.inf.0000087020.75886.93. [DOI] [PubMed] [Google Scholar]
- 36.Wunderink R.G., Cammarata S.K., Oliphant T.H., Kollef M.H., Linezolid Nosocomial Pneumonia Study Group Continuation of a randomized, double-blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia. Clin Ther. 2003;25(3):980–992. doi: 10.1016/s0149-2918(03)80118-2. [DOI] [PubMed] [Google Scholar]
- 37.Stevens D.L., Herr D., Lampiris H., Hunt J.L., Batts D.H., Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002;34(11):1481–1490. doi: 10.1086/340353. [DOI] [PubMed] [Google Scholar]
- 38.Rubinstein E., Cammarata S., Oliphant T., Wunderink R., Linezolid Nosocomial Pneumonia Study Group Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin Infect Dis. 2001;32(3):402–412. doi: 10.1086/318486. [DOI] [PubMed] [Google Scholar]
- 39.Corey G.R., Wilcox M.H., Talbot G.H., Thye D., Friedland D., Baculik T., CANVAS 1 investigators CANVAS 1: the first Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother. 2010;65(suppl 4):iv41–iv51. doi: 10.1093/jac/dkq254. [DOI] [PubMed] [Google Scholar]
- 40.Wilcox M.H., Corey G.R., Talbot G.H., Thye D., Friedland D., Baculik T., CANVAS 2 Investigators CANVAS 2: the second Phase III, randomized, double-blind study evaluating ceftaroline fosamil for the treatment of patients with complicated skin and skin structure infections. J Antimicrob Chemother. 2010;65(suppl 4)):iv53–iv65. doi: 10.1093/jac/dkq255. [DOI] [PubMed] [Google Scholar]
- 41.Talbot G.H., Thye D., Das A., Ge Y. Phase 2 study of ceftaroline versus standard therapy in treatment of complicated skin and skin structure infections. Antimicrob Agents Chemother. 2007;51(10):3612–3616. doi: 10.1128/AAC.00590-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noel G.J., Bush K., Bagchi P., Ianus J., Strauss R.S. A randomized, double-blind trial comparing ceftobiprole medocaril with vancomycin plus ceftazidime for the treatment of patients with complicated skin and skin-structure infections. Clin Infect Dis. 2008;46(5):647–655. doi: 10.1086/526527. [DOI] [PubMed] [Google Scholar]
- 43.Noel G.J., Strauss R.S., Amsler K., Heep M., Pypstra R., Solomkin J.S. Results of a double-blind, randomized trial of ceftobiprole treatment of complicated skin and skin structure infections caused by gram-positive bacteria. Antimicrob Agents Chemother. 2008;52(1):37–44. doi: 10.1128/AAC.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Florescu I., Beuran M., Dimov R., 307 Study Group Efficacy and safety of tigecycline compared with vancomycin or linezolid for treatment of serious infections with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci: a Phase 3, multicentre, double-blind, randomized study. J Antimicrob Chemother. 2008;62(suppl 1):i17–i28. doi: 10.1093/jac/dkn250. [DOI] [PubMed] [Google Scholar]
- 45.Breedt J., Teras J., Gardovskis J., Tigecycline 305 cSSSI Study Group Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam. Antimicrob Agents Chemother. 2005;49(11):4658–4666. doi: 10.1128/AAC.49.11.4658-4666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sacchidanand S., Penn R.L., Embil J.M. Efficacy and safety of tigecycline monotherapy compared with vancomycin plus aztreonam in patients with complicated skin and skin structure infections: results from a phase 3, randomized, double-blind trial. Int J Infect Dis. 2005;9(5):251–261. doi: 10.1016/j.ijid.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Fagon J., Patrick H., Haas D.W., Nosocomial Pneumonia Group Treatment of gram-positive nosocomial pneumonia: prospective randomized comparison of quinupristin/dalfopristin versus vancomycin. Am J Respir Crit Care Med. 2000;161(3, pt 1):753–762. doi: 10.1164/ajrccm.161.3.9904115. [DOI] [PubMed] [Google Scholar]
- 48.Raad I., Bompart F., Hachem R. Prospective, randomized dose-ranging open phase II pilot study of quinupristin/dalfopristin versus vancomycin in the treatment of catheter-related staphylococcal bacteremia. Eur J Clin Microbiol Infect Dis. 1999;18(3):199–202. doi: 10.1007/s100960050258. [DOI] [PubMed] [Google Scholar]
- 49.Raad I., Darouiche R., Vazquez J. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis. 2005;40(3):374–380. doi: 10.1086/427283. [DOI] [PubMed] [Google Scholar]
- 50.Rubinstein E., Lalani T., Corey G.R., ATTAIN Study Group Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin Infect Dis. 2011;52(1):31–40. doi: 10.1093/cid/ciq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stryjewski M.E., Graham D.R., Wilson S.E., Assessment of Telavancin in Complicated Skin and Skin-Structure Infections Study Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin Infect Dis. 2008;46(11):1683–1693. doi: 10.1086/587896. [DOI] [PubMed] [Google Scholar]
- 52.Stryjewski M.E., Chu V.H., O'Riordan W.D., FAST 2 Investigator Group Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob Agents Chemother. 2006;50(3):862–867. doi: 10.1128/AAC.50.3.862-867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stryjewski M.E., O'Riordan W.D., Lau W.K., FAST Investigator Group Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin Infect Dis. 2005;40(11):1601–1607. doi: 10.1086/429914. [DOI] [PubMed] [Google Scholar]
- 54.Sanofi-Aventis Comparison of teicoplanin and vancomycin in initial empirical antibiotic regimen for febrile neutropenic patients: 2008. Trial NCT00454272. http://www.clinicalstudyresults.org/documents/company-study_8797_0.pdf Accessed January 13, 2010.
- 55.D'Antonio D., Staniscia T., Piccolomini R. Addition of teicoplanin or vancomycin for the treatment of documented bacteremia due to gram-positive cocci in neutropenic patients with haematological malignancies: microbiological, clinical and economic evaluation. Chemotherapy. 2004;50(2):81–87. doi: 10.1159/000077807. [DOI] [PubMed] [Google Scholar]
- 56.Pham Dang C., Gouin F., Touchais S., Richard C., Potel G. The comparative costs of vancomycin treatment versus teicoplanin in osteoarticular infection caused by methicillin-resistant staphylococci [in French] Pathol Biol (Paris) 2001;49(7):587–596. doi: 10.1016/s0369-8114(01)00203-6. [DOI] [PubMed] [Google Scholar]
- 57.Sidi V., Roilides E., Bibashi E., Gompakis N., Tsakiri A., Koliouskas D. Comparison of efficacy and safety of teicoplanin and vancomycin in children with antineoplastic therapy-associated febrile neutropenia and gram-positive bacteremia. J Chemother. 2000;12(4):326–331. doi: 10.1179/joc.2000.12.4.326. [DOI] [PubMed] [Google Scholar]
- 58.Rolston K.V., Bodey G.P., Chow A.W. Prospective, double-blind, randomized trial of teicoplanin versus vancomycin for the therapy of vascular access-associated bacteremia caused by gram-positive pathogens. J Infect Chemother. 1999;5(4):208–212. doi: 10.1007/s101560050037. [DOI] [PubMed] [Google Scholar]
- 59.Vázquez L., Encinas M.P., Morín L.S. Randomized prospective study comparing cost-effectiveness of teicoplanin and vancomycin as second-line empiric therapy for infection in neutropenic patients. Haematologica. 1999;84(3):231–236. [PubMed] [Google Scholar]
- 60.Van Laethem Y., Hermans P., De Wit S., Goosens H., Clumeck N. Teicoplanin compared with vancomycin in methicillin-resistant Staphylococcus aureus infections: preliminary results. J Antimicrob Chemother. 1988;21(suppl A):81–87. doi: 10.1093/jac/21.suppl_a.81. [DOI] [PubMed] [Google Scholar]
- 61.Nucci M., Biasoli I., Braggio S. Ceftazidime plus amikacin plus teicoplanin or vancomycin in the empirical antibiotic therapy in febrile neutropenic cancer patients. Oncol Rep. 1998;5(5):1205–1209. doi: 10.3892/or.5.5.1205. [DOI] [PubMed] [Google Scholar]
- 62.Auperin A., Cappelli C., Benhamou E. Teicoplanin or vancomycin in febrile neutropenic children with cancer: a randomized study on cost effectiveness. Med Mal Infect. 1997;27:984–988. [Google Scholar]
- 63.Liu C.Y., Lee W.S., Fung C.P. Comparative study of teicoplanin vs vancomycin for the treatment of methicillin-resistant Staphylococcus aureus bacteraemia. Clin Drug Investig. 1996;12:80–87. doi: 10.2165/00044011-199612020-00003. [DOI] [PubMed] [Google Scholar]
- 64.Figuera A., Tomás J.F., Hernández L. Imipenem combined with teicoplanin or vancomycin in the initial empirical treatment of febrile neutropenia: analysis of the primary response and of a global sequential strategy in 126 episodes. Rev Clin Esp. 1996;196(8):515–522. [PubMed] [Google Scholar]
- 65.Neville L.O., Brumfitt W., Hamilton-Miller J.M.T., Hardingb I. Teicoplanin vs. vancomycin for the treatment of serious infections: a randomised trial. Int J Antimicrob Agents. 1995;5:187–193. doi: 10.1016/0924-8579(95)00002-p. [DOI] [PubMed] [Google Scholar]
- 66.Rolston K.V., Nguyen H., Amos G., Elting L., Fainstein V., Bodey G.P. A randomized double-blind trial of vancomycin versus teicoplanin for the treatment of gram-positive bacteremia in patients with cancer. J Infect Dis. 1994;169(2):350–355. doi: 10.1093/infdis/169.2.350. [DOI] [PubMed] [Google Scholar]
- 67.Menichetti F., Martino P., Bucaneve G., Gimema Infection Program Effects of teicoplanin and those of vancomycin in initial empirical antibiotic regimen for febrile, neutropenic patients with hematologic malignancies. Antimicrob Agents Chemother. 1994;38(9):2041–2046. doi: 10.1128/aac.38.9.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Charbonneau P., Harding I., Garaud J.J., Aubertin J., Brunet F., Domart Y. Teicoplanin: a well-tolerated and easily administered alternative to vancomycin for gram-positive infections in intensive care patients. Intensive Care Med. 1994;20(suppl 4):S35–S42. doi: 10.1007/BF01713981. [DOI] [PubMed] [Google Scholar]
- 69.Kureishi A., Jewesson P.J., Rubinger M. Double-blind comparison of teicoplanin versus vancomycin in febrile neutropenic patients receiving concomitant tobramycin and piperacillin: effect on cyclosporine A-associated nephrotoxicity. Antimicrob Agents Chemother. 1991;35(11):2246–2252. doi: 10.1128/aac.35.11.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van der Auwera P., Aoun M., Meunier F. Randomized study of vancomycin versus teicoplanin for the treatment of gram-positive bacterial infections in immunocompromised hosts. Antimicrob Agents Chemother. 1991;35(3):451–457. doi: 10.1128/aac.35.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cony-Makhoul P., Brossard G., Marit G., Pellegrin J.L., Texier-Maugein J., Reiffers J. A prospective study comparing vancomycin and teicoplanin as second-line empiric therapy for infection in neutropenic patients. Br J Haematol. 1990;76(suppl 2):35–40. doi: 10.1111/j.1365-2141.1990.tb07934.x. [DOI] [PubMed] [Google Scholar]
- 72.Gilbert D.N., Wood C.A., Kimbrough R.C., The Infectious Diseases Consortium of Oregon Failure of treatment with teicoplanin at 6 milligrams/kilogram/day in patients with Staphylococcus aureus intravascular infection. Antimicrob Agents Chemother. 1991;35(1):79–87. doi: 10.1128/aac.35.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith S.R., Cheesbrough J., Spearing R., Davies J.M. Randomized prospective study comparing vancomycin with teicoplanin in the treatment of infections associated with Hickman catheters. Antimicrob Agents Chemother. 1989;33(8):1193–1197. doi: 10.1128/aac.33.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pertel P.E., Eisenstein B.I., Link A.S. The efficacy and safety of daptomycin vs. vancomycin for the treatment of cellulitis and erysipelas. Int J Clin Pract. 2009;63(3):368–375. doi: 10.1111/j.1742-1241.2008.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katz D.E., Lindfield K.C., Steenbergen J.N. A pilot study of high-dose short duration daptomycin for the treatment of patients with complicated skin and skin structure infections caused by gram-positive bacteria. Int J Clin Pract. 2008;62(9):1455–1464. doi: 10.1111/j.1742-1241.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 76.Rehm S.J., Boucher H., Levine D. Daptomycin versus vancomycin plus gentamicin for treatment of bacteraemia and endocarditis due to Staphylococcus aureus: subset analysis of patients infected with methicillin-resistant isolates. J Antimicrob Chemother. 2008;62(6):1413–1421. doi: 10.1093/jac/dkn372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krievins D., Brandt R., Hawser S., Hadvary P., Islam K. Multicenter, randomized study of the efficacy and safety of intravenous iclaprim in complicated skin and skin structure infections. Antimicrob Agents Chemother. 2009;53(7):2834–2840. doi: 10.1128/AAC.01383-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leibovici L., Falagas M.E. Systematic reviews and meta-analyses in infectious diseases: how are they done and what are their strengths and limitations? Infect Dis Clin North Am. 2009;23(2):181–194. doi: 10.1016/j.idc.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Siempos I.I., Vardakas K.Z., Manta K.G., Falagas M.E. Carbapenems for the treatment of immunocompetent adult patients with nosocomial pneumonia. Eur Respir J. 2007;29(3):548–560. doi: 10.1183/09031936.00080206. [DOI] [PubMed] [Google Scholar]
- 80.Falagas M.E., Siempos I.I., Papagelopoulos P.J., Vardakas K.Z. Linezolid for the treatment of adults with bone and joint infections. Int J Antimicrob Agents. 2007;29(3):233–239. doi: 10.1016/j.ijantimicag.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 81.Falagas M.E., Manta K.G., Ntziora F., Vardakas K.Z. Linezolid for the treatment of patients with endocarditis: a systematic review of the published evidence. J Antimicrob Chemother. 2006;58(2):273–280. doi: 10.1093/jac/dkl219. [DOI] [PubMed] [Google Scholar]
- 82.Wunderink R.G., Niederman M.S., Kollef M.H. Linezolid in methicillin-resistant staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54(5):621–629. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 83.Johnson and Johnson Ceftobiprole versus linezolid plus ceftazidime for patients with nosocomial pneumonia. http://filehosting.pharmacm.com/DownloadService.ashx?client=CTR_JNJ_6051&studyid=635&filename=CR005032_CR005140_CSR.pdf Accessed September 7, 2011.
- 84.Neuner E.A., Ritchie D.J., Micek S.T. New antibiotics for healthcare-associated pneumonia. Semin Respir Crit Care Med. 2009;30(1):92–101. doi: 10.1055/s-0028-1119813. [DOI] [PubMed] [Google Scholar]
- 85.Freire A.T., Melnyk V., Kim M.J. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis. 2010;68(2):140–151. doi: 10.1016/j.diagmicrobio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 86.Vardakas K.Z., Samonis G., Chrysanthopoulou S.A., Bliziotis I.A., Falagas M.E. The role of glycopeptides as part of the empirical treatment in febrile neutropenic patients: a meta-analysis of randomized controlled trials. Lancet Infect Dis. 2005;5(7):431–439. doi: 10.1016/S1473-3099(05)70164-X. [DOI] [PubMed] [Google Scholar]
- 87.Paul M., Borok S., Fraser A., Vidal L., Cohen M., Leibovici L. Additional anti-Gram-positive antibiotic treatment for febrile neutropenic cancer patients. Cochrane Database Syst Rev. 2005;(3) doi: 10.1002/14651858.CD003914.pub2. CD003914. [DOI] [PubMed] [Google Scholar]
- 88.Chemaly R.F., Hanmod S.S., Jiang Y. Tigecycline use in cancer patients with serious infections: a report on 110 cases from a single institution. Medicine (Baltimore) 2009;88(4):211–220. doi: 10.1097/MD.0b013e3181af01fc. [DOI] [PubMed] [Google Scholar]
- 89.Bubalo J.S., Munar M.Y., Cherala G., Hayes-Lattin B., Maziarz R. Daptomycin pharmacokinetics in adult oncology patients with neutropenic fever. Antimicrob Agents Chemother. 2009;53(2):428–434. doi: 10.1128/AAC.00943-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poutsiaka D.D., Skiffington S., Miller K.B., Hadley S., Snydman D.R. Daptomycin in the treatment of vancomycin-resistant Enterococcus faecium bacteremia in neutropenic patients. J Infect. 2007;54(6):567–571. doi: 10.1016/j.jinf.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Itani K.M., Weigelt J., Li J.Z., Duttagupta S. Linezolid reduces length of stay and duration of intravenous treatment compared with vancomycin for complicated skin and soft tissue infections due to suspected or proven methicillin-resistant Staphylococcus aureus (MRSA) Int J Antimicrob Agents. 2005;26(6):442–448. doi: 10.1016/j.ijantimicag.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 92.Vinken A., Li Z., Balan D., Rittenhouse B., Wilike R., Nathwani D. Economic evaluation of linezolid, flucloxacillin and vancomycin in the empirical treatment of cellulitis in UK hospitals: a decision analytical model. J Hosp Infect. 2001;49(suppl A):S13–S24. doi: 10.1016/s0195-6701(01)90030-1. [DOI] [PubMed] [Google Scholar]
- 93.Li J.Z., Willke R.J., Rittenhouse B.E., Rybak M.J. Effect of linezolid versus vancomycin on length of hospital stay in patients with complicated skin and soft tissue infections caused by known or suspected methicillin-resistant staphylococci: results from a randomized clinical trial. Surg Infect (Larchmt) 2003;4(1):57–70. doi: 10.1089/109629603764655290. [DOI] [PubMed] [Google Scholar]
- 94.Vardakas K.Z., Kioumis I., Falagas M.E. Association of pharmacokinetic and pharmacodynamic aspects of linezolid with infection outcome. Curr Drug Metabol. 2009;10(1):2–12. doi: 10.2174/138920009787048446. [DOI] [PubMed] [Google Scholar]
- 95.Falagas M.E., Vardakas K.Z. Benefit-risk assessment of linezolid for serious gram-positive bacterial infections. Drug Saf. 2008;31(9):753–768. doi: 10.2165/00002018-200831090-00004. [DOI] [PubMed] [Google Scholar]
- 96.Vardakas K.Z., Ntziora F., Falagas M.E. Linezolid: effectiveness and safety for approved and off-label indications. Expert Opin Pharmacother. 2007;8(14):2381–2400. doi: 10.1517/14656566.8.14.2381. [DOI] [PubMed] [Google Scholar]
- 97.Falagas M.E., Vardakas K.Z., Samonis G. Decreasing the incidence and impact of infections in neutropenic patients: evidence from meta-analyses of randomized controlled trials. Curr Med Res Opin. 2008;24(1):215–235. doi: 10.1185/030079908x253816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


