Abstract
Optical switch probes undergo rapid and reversible transitions between two distinct states, one of which may fluoresce. This class of probe is used in various super-resolution imaging techniques and in the high-contrast imaging technique of optical lock-in detection (OLID) microscopy. Here, we introduce optimized optical switches for studies in living cells under standard conditions of cell culture. In particular, a highly fluorescent cyanine probe (Cy or Cy3) is directly or indirectly linked to naphthoxazine (NISO), a highly efficient optical switch that undergoes robust, 405/532 nm-driven transitions between a colourless spiro (SP) state and a colourful merocyanine (MC) state. The intensity of Cy fluorescence in these Cy/Cy3-NISO probes is reversibly modulated between a low and high value in SP and MC states, respectively, as a result of Förster resonance energy transfer. Cy/Cy3-NISO probes are targeted to specific proteins in living cells where defined waveforms of Cy3 fluorescence are generated by optical switching of the SP and MC states. Finally, we introduce a new imaging technique (called OLID-immunofluorescence microscopy) that combines optical modulation of Cy3 fluorescence from Cy3/NISO co-labelled antibodies within fixed cells and OLID analysis to significantly improve image contrast in samples having high background or rare antigens.
Keywords: optical switches, optical lock-in detection, high-contrast fluorescence imaging
1. Introduction
A grand challenge in cell biology is to understand the molecular mechanisms that underlie the interactions and activities of proteins involved in the regulation of complex cellular processes, including motility and signalling [1,2]. This level of understanding requires optical probes and associated imaging technologies that can generate quantitative and dynamic maps of the distribution, activities and interactions of key proteins within living cells [1,3]. In this light, considerable progress has been made in recent years on the development and application of new imaging modalities that allow for super-resolution (SR) and high-contrast imaging of specific proteins and structures within fixed and living cells [4–6]. Both SR and optical lock-in detection (OLID) imaging techniques achieve their impressive levels of performance by controlling the spatial distribution of the non-fluorescent and fluorescent states in the sample. In the case of stimulated emission depletion microscopy [4,7], spatial control of the ground and excited states of an ensemble population of probe molecules at the focus, or of the fluorescent and non-fluorescent states of an optical switch in the case of reversible saturable optical fluorescence transitions (RESOLFT) [8], is used to limit fluorescence emission to a sub-diffraction region. Optical control of the fluorescent state of an irreversible or reversible optical switch probe is the key to the SR-imaging techniques, including photo-activation localization microscopy (PALM), stochastic reconstruction optical microscopy (STORM) and synchronously amplified fluorescence image recovery (SAFIRe) [5,9–12]. In these techniques, single (PALM) or multiple (STORM, SAFIRe) cycles of optical switching are carried out on a sparse and immobile population of probe molecules. The performance of these techniques is ultimately limited by background signals in the sample, which can amount to 10 000 equivalents of fluorescein within a typical live fibroblast cell [13]. While specialized forms of fluorescence microscopy, such as total internal reflection [14], can easily image single molecules within living cells, they do so by severely restricting the field to surface associated membranes, and are of little utility when imaging within a cell or in tissue.
OLID microscopy can be used to isolate a specific component of a fluorescence signal that originates from the probe of interest against all other signals in the sample [6]. Key to the success of OLID is a new type of probe (or fluorescent optical switch) that undergoes multiple cycles of high fidelity, rapid and reversible transitions between two distinct states, of which only one fluoresces [6]. OLID microscopy differs significantly from conventional forms of fluorescence microscopy, represented by the design of an optical probe, and the way that fluorescence signals from optical switch probes are generated and analysed. In OLID, the optical switch is optically manipulated between the fluorescent and non-fluorescent states to generate a defined fluorescence intensity waveform that is isolated from the unmodulated background [15]. An effective strategy to generate these unique time-dependent fluorescence signals or intensity waveforms from the fluorescent probe is schematized in figure 1c [6,15]. A fluorescence signal in a biological preparation that has a defined intensity waveform is clearly unique and differs significantly from background signals; we have shown that these modulated fluorescence signals can be isolated from other sources in the preparation by using OLID [15].
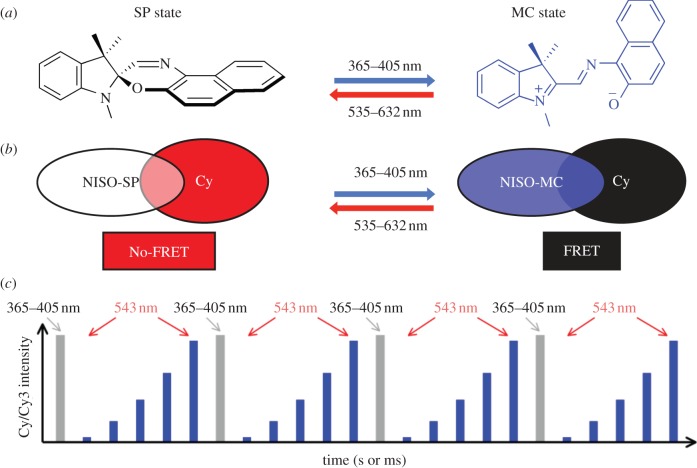
Figure 1.
(a) Generalized molecular structures of the spiro (SP) and merocyanine (MC) states of naphthoxazine (NISO) with details of the orthogonal, optically-triggered transitions between the two states. (b) Schematic of photochromic Förster resonance energy transfer (FRET) in the Cy-NISO probes. Optical switching between SP and MC states of NISO results in a time-varying change in FRET efficiency with concomitant modulation of the intensity of Cy fluorescence. (c) Optical modulation of the intensity of Cy fluorescence over multiple cycles of optical switching of the populations of the SP and MC states of NISO. The grey bars represent periods when the sample is exposed to 365/405 nm and the blue bars the periods the sample is exposed to 532–632 nm. The height of each bar represents the intensity of Cy fluorescence at specific times during the course of optical switching.
For most types of optical switch probe, the excited state of the coloured state decays either by transitioning to the colourless state (switching), or else by returning to the same ground state with emission of fluorescence, both with defined quantum yields [15,16]. Consequently, NitroBIPS, Dronpa and related photochromes are either good fluorophores but poor switches, or good switches but poor fluorophores [6,15]. An ideal probe, however, should exhibit high efficiencies for both fluorescence and optical switching. A fluorescent optical switch for OLID imaging should undergo excited-state transitions between the two states at wavelengths greater than 400 nm in order to reduce phototoxic effects. Finally, the optical switch should allow for true orthogonal optical control of the optical switching functions and recording of the fluorescence emission. These three ideal properties of an optical switch are represented in new types of photochromic Förster resonance energy transfer (FRET) probes [16] presented in this study. In particular, these probes are composed of a highly fluorescent cyanine donor and a highly efficient naphthoxazine (NISO) optical switch that are either linked in the same molecule or covalently attached to a single protein or sensor. FRET-based optical switch probes are readily delivered and targeted to specific proteins within living cells using the Snap-tag approach [15,17], and optically manipulated with visible light to generate highly modulated cyanine fluorescence over many cycles of optical switching under normal conditions of cell culture. We also show that OLID can be used to improve image contrast in immunofluorescence (IF) microscopy (OLID-IF) through an OLID-based analysis of the modulated fluorescence of primary antibodies against F-actin co-labelled with Cy3 and NISO.
2. Material and methods
(a). Materials
Starting materials were purchased from Sigma-Aldrich and Fisher Scientific. Antibodies were purchased from Sigma-Aldrich and Invitrogen. 1H NMR spectra were recorded at 300 MHz on a Bruker AC-300 spectrometer or a Varian MercuryPlus 300. Mass spectra were recorded on a Micromass LCT for electrospray ionization or a Micromass AutoSpec for electron impact. UV spectra were determined with a Shimadzu 1601PC instrument. Fluorescence spectroscopy was performed on an SLM-AB2 instrument (Thermoelectron, Madison, WI, USA). Light-directed switching of the probe described in this work was achieved by irradiating the sample with the 365 or 546 nm lines of a hand-held UV lamp and a 100 W Hg-arc lamp (Zeiss).
(b). Synthesis of optical switch probes
Details will be presented elsewhere, although the synthetic methods used for the preparation of Cy-NISO, Cy-NISO-N-hydroxysuccinimide ester (NHS), Cy3-NISO-benzylguanosine (BG) and NISO-NHS are similar to those used in our earlier papers [18,19].
(c). Preparation of labelled antibodies
One hundred microlitres of Cy3-labelled anti-actin (Sigma-Aldrich) is diluted five times in phosphate-buffered saline, and treated with 20 µl NHS-NISO delivered from a 10 mM stock solution (dimethylformamide). After a 2-h reaction at 20°C in the dark, the mixture was applied to an Econo-PAC 10 DG column equilibrated in the same buffer to remove free NHS-NISO. The labelling ratio is determined by absorption spectroscopy using established methods [20].
(d). Cell culture, fixation and staining
NIH 3T3 cells (mouse embryonic fibroblast cell line) were grown in Dulbecco's modified Eagles medium (Invitrogen) supplemented with 10 per cent fetal bovine serum (Invitrogen), 1 per cent penicillin–streptomycin (Invitrogen) and 0.2 per cent Fungizone (Invitrogen). After incubation in 35 mm dishes for 24 h, cells were prepared for IF by using the −20°C methanol fixation approach and stained with antibodies according to the protocol from Sigma-Aldrich. NISO-labelled anti-actin-Cy3 was used to stain cellular actin in the studies reported herein.
(e). Optical lock-in detection immunofluorescence microscopy
Images were collected on an Olympus FluoView500 confocal microscope using a 60x, NA = 1.45 water immersion objective. UV light pulses from a 100 W Hg-arc lamp filtered through a 365 ± 25 bandpass filter were controlled with a time-varying shutter (Vincent Associates) to drive the spiro (SP) to merocyanine (MC) transition [15]. The duration of the 365 nm pulse was varied from 100 to 200 ms. Imaging of Alexa Fluor 488 or Cy3 and concomitant conversion of the MC excited state to SP was driven by using 488 or 543 nm laser scanning at a rate of 1–3 frame s–1 while the emission was collected by using a 510–520 nm pass filter or a 560 nm long pass filter. A typical optical switching cycle was composed of a 100 ms pulse at 365 nm during continuous scanning of the field using a 532 or 543 nm laser (schematized in figure 1c,f).
(f). Correlation analysis
A cross-correlation analysis is conducted within every pixel of the image field for the detected fluorescence emission and a reference signal of the optical switching, which represents the specific, time-varying intensity waveform of the switchable emission [6]. The internal reference waveform for the immunofluorescence study is obtained by averaging the fluorescence emission at a locus exhibiting the highest depth of Cy3-fluorescence intensity modulation (figure 5b). By cross-correlating the total emission signal within each pixel of an image over the five cycles of optical switching to the reference waveform, we obtain a correlation coefficient, a measure of correlation between the total emission and the reference waveform.
Figure 5.
(a) Schematic of photochromic FRET in an anti-actin antibody co-labelled with Cy3 and NISO. (b) Intensity trace of Cy3 fluorescence over the course of five cycles of optical switching between the SP and MC states on the antibody. The trace serves as the internal reference waveform in the OLID-FRET analysis. (c) Intensity image of Cy3 fluorescence in the SP state of an NIH 3T3 cell stained with the co-labelled antibody. (d) Image of the correlation coefficients in an OLID-FRET-based analysis of the Cy3/NISO-labelled antibody in the image shown in figure 5c.
The correlation coefficient, ρ(x,y), for any pixel location (x,y), is calculated as follows:
where I(x,y,t) is the detected fluorescence intensity at pixel (x,y) at time t during the switching cycle; R(t) is the reference waveform; μI and μR are the mean values; and σI and σR are the standard deviation (std) values of the pixel fluorescence intensity and the reference waveform, respectively.
The computed values of the correlation coefficient (0.0–1.0) are displayed on a pixel-by-pixel basis using either 8-bit grey or pseudo-colour scale (0–255), with near-zero value expected from background signals, highly mobile optical switch probes and emission from any other (conventional) fluorescence probe. Thus, the correlation value for an optical switch probe experiencing considerable translational motion over the course of an optical switching study is close to zero, whereas immobile probes will have a value closer to unity. The correlation value for an optical switch probe having very limited motion over the course of the optical switching study will fall between 0 and 1 [15]. Thus, the value of the correlation coefficient provides a measure of the persistence of the modulated signal within a given pixel area. This feature can be used to characterize dynamic properties of the optical switch probe, and may be further refined in studies that measure how the correlation value changes with the frequency of optical switching. The value of the correlation coefficient is independent of the actual intensity level of Cy3 fluorescence. Thus, an immobile optical switch probe whose fluorescence signal constitutes 1 or 100 per cent of the total intensity from a region in the image will have a similar correlation coefficient value, although more cycles of optical switching may be required to accurately obtain the correlation value from the weaker modulated signal.
3. Results
(a). Optimized design of cyanine naphthoxazine-based FRET probes
The hybrid fluorescent optical switch probe, schematized in figure 1b, is composed of a carbocyanine (Cy or Cy3) fluorophore (widely used for single molecule imaging) and NISO (a highly efficient optical switch [18–20]). Optical switching of the high quantum yield fluorescence emission of Cy in the hybrid probe is achieved through a photochromic FRET mechanism [16,19,21]. In particular, excitation of the highly fluorescent SP state (no-FRET) with a short (100 ms), low-power pulse between 365 and 405 nm generates the non-fluorescent MC state (FRET) of Cy-NISO (figure 1a,b). Excitation of the MC state of Cy-NISO using a low power of light between 535 and 632 nm regenerates the fluorescent SP state (figures 1a,b and 2a,c). As schematized in figure 1c, repeated exposure of Cy-NISO to a defined sequence of 365–405 nm and 543–632 nm light allows for a precise control of the SP and MC states in the hybrid probe, with concomitant modulation of FRET and the intensity of Cy-fluorescence.
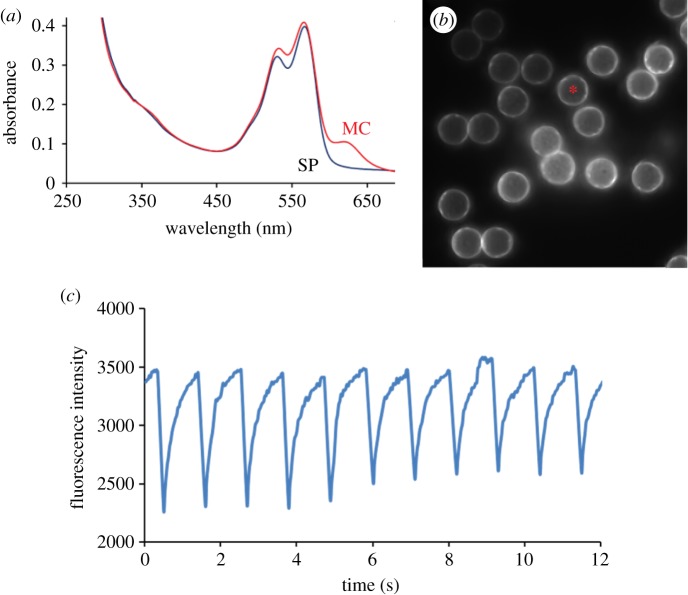
Figure 2.
(a) Absorption spectra and optical switching of Mal-Cy-NISO linked to G-actin in the SP and MC states; (b) A single image frame from a movie showing the Cy fluorescence on latex beads labelled with Cy-NISO-G-actin; (c) A trace of Cy-fluorescence intensity over eight cycles of optical switching of Cy-NISO-labelled G-actin on a single bead within the image field (asterisk shown in figure 2b).
The Cy-NISO class of photochromic FRET probe is optimized for optical switching of Cy-fluorescence in living cells (figure 1b) and has several advantages over other types of small molecule photochromic FRET probe [6]. These include: (i) truly orthogonal control of transitions between the SP and MC states, as the Cy dye does not absorb over wavelengths used to excite the SP state of Cy-NISO (365–405 nm); (ii) control of the SP to MC transition at a far more suitable excitation wavelength (405 nm) for studies in living cells, a claim supported by other studies that use 405 nm light to photo-activate Lov2-Rac [22] and paGFP [12]; (iii) Cy3-NISO has an increased solubility in water compared with other small molecule photochromes [6], owing to the presence of two sulphonate groups; and (iv) Cy and Cy3 have a higher extinction coefficient at 550 nm that is approximately 50 per cent higher than that measured for tetramethylrhodamine (TMR) (150 000 versus 96 000 M−1 cm−1).
(b). Optical switching of cyanine fluorescence in cyanine naphthoxazine probes covalently linked to actin
Optical switching of Cy fluorescence is next evaluated on proteins using a maleimido reactive form of Cy-NISO covalently attached to G-actin on cysteine-374. The G-actin–Cy-NISO conjugate is further linked to micrometre-sized carboxy-modified latex beads through coupling to 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) [15]. The manipulation and imaging of Cy emission on the surface of these beads is carried out on an Olympus confocal fluorescence microscope [15]. The intensity of Cy fluorescence, recorded on individual beads (the asterisk-labelled bead in figure 2b), decreases markedly following exposure to a single 100 ms pulse of 365–405 nm light (figure 2c). This quenching results from an efficient intra-molecular FRET between the Cy donor and the MC acceptor, which is expected owing to a favourable spectral overlap between the Cy emission and MC absorption spectra (figure 2a), and the close proximity (approx. 1 nm) between the FRET donor and acceptor groups. Subsequent exposure of the MC state to 15 scans at a low power of 543 nm light is sufficient to convert the MC state back to the SP state, resulting in an almost complete recovery of Cy-fluorescence signal (figure 2c). Repeated manipulation of the SP and MC states of NISO using a defined sequence of irradiation with near ultraviolet and visible light (figure 1c) results in a time-dependent change, or modulation, of the intensity of Cy fluorescence, with 10 cycles of optical switching being shown in the example in figure 2c. Analysis of the intensity profile of Cy fluorescence over the 10 cycles of optical switching indicates that little to no fatigue or bleaching of the Cy3 or NISO probes (figure 2c) occurs in this study.
(c). Optical switching of cyanine fluorescence in cells labelled with cyanine naphthoxazine
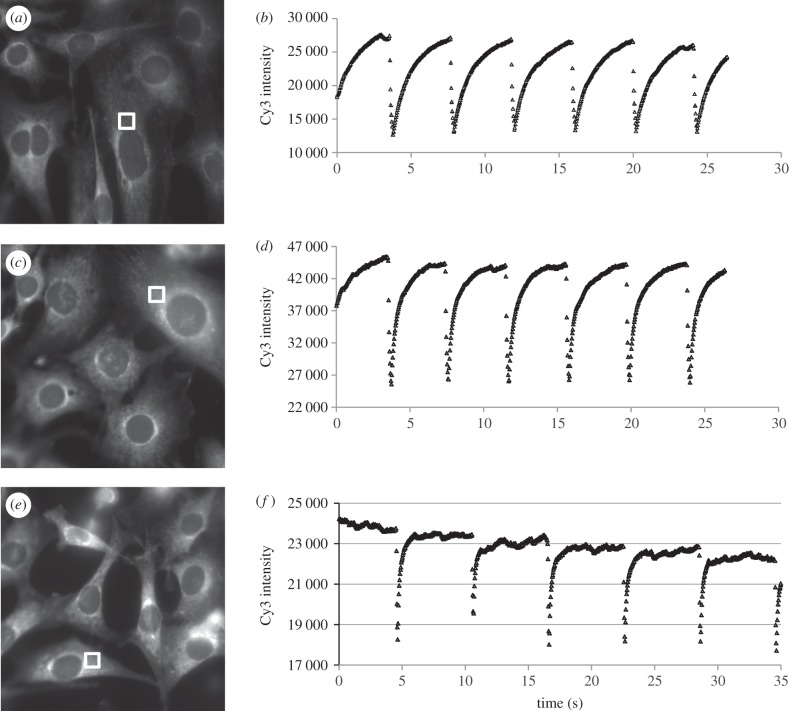
NIH 3T3 cells labelled with a cell permeable Cy-NISO probe are subject to wide-field optical switching of the SP and MC states of Cy-NISO and imaging of the Cy fluorescence. The excitation power of the 546 nm line of the 100 W Hg-arc lamp source is varied from 2.5, 12.5 and 50 per cent of the maximum output, with the corresponding Cy-fluorescence images shown in figure 3a,c,e, respectively. The intensity of Cy emission over the course of five to seven cycles of optical switching is shown for the three different excitation levels in figure 3b,d,f respectively. For the cell shown in figure 3a, the intensity of Cy emission is seen to decrease from 27 500 to 12 700 immediately following a 100 ms exposure to 365 nm, and recovers to less than the pre-365 nm pulse following exposure of the cell to 546 nm light at 2.5 per cent of the maximum output for 4 s (figure 3b). Although the FRET efficiency recorded immediately following the 365 nm pulse is measured at 54 per cent, it is likely to be much higher, given that appreciable amount of SP state forms before the first recording of the Cy-fluorescence signal (figure 3b). Next, the sample was exposed to a 100 ms pulse at 365 nm, resulting in a FRET efficiency of 46 per cent (approx. 45 500–25 500). Subsequent exposure of the image field to 546 nm at 12.5 per cent of the maximum output of the lamp leads to a more rapid recovery of Cy fluorescence compared with the previous case, with the plateau being reached within 3 s. In the third study, a FRET efficiency of 23 per cent is recorded immediately after the post-365 nm pulse recovers to the pre-365 nm irradiated state within 1 s, with subsequent exposure of the field to 546 nm light at 50 per cent of the maximum output (figure 3f). These studies also show that the Cy-NISO probes undergo robust and high-fidelity optical switching between the SP and MC states in fixed cells with little evidence of fatigue or photobleaching at the exposed levels of 365–405 nm and 546 nm light. The favourable proximity and spectral overlap between the Cy and MC states results in a very high efficiency of FRET in the MC state, whereas no FRET is recorded in the SP state. By alternating the population levels of the SP and MC states, one generates a high modulation Cy-fluorescence intensity waveform that is largely invariant within each cycle of a multiple switching study. The study also reveals that the power of the 546 nm light can be used to control the rate of the MC to SP transition, by increasing the number of MC molecules that enter the excited state during a fixed exposure to 546 nm, allowing the user to shape the waveform of the Cy signal.
Figure 3.
(a,c,e) Intensity images of Cy-NISO fluorescence in the SP state within fixed NIH 3T3 cells. (b,d,f) Intensity profiles of Cy fluorescence in fixed cells over several cycles of optical switching. The intensity traces shown in (b,d,f) are taken from regions within cells shown in (a,c,e) respectively. The intensity of the 546 nm light used to drive the MC to SP transition and to excite the Cy probe varies between 2.5%, 12.5% and 50% of the maximum output of the lamp for the traces shown in (b,d,f), respectively.
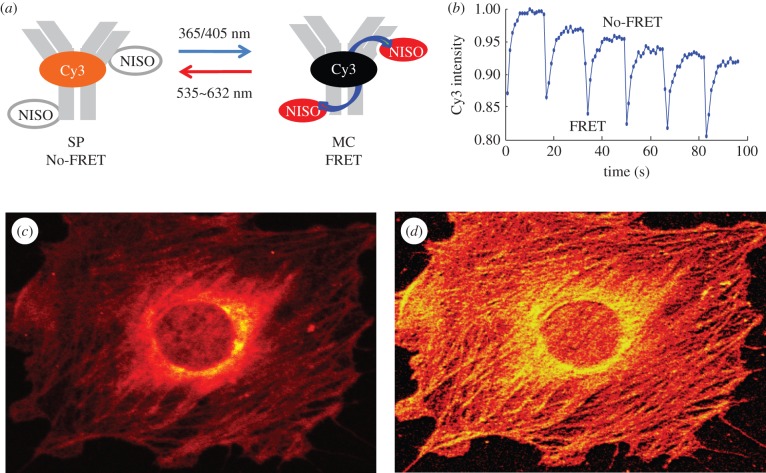
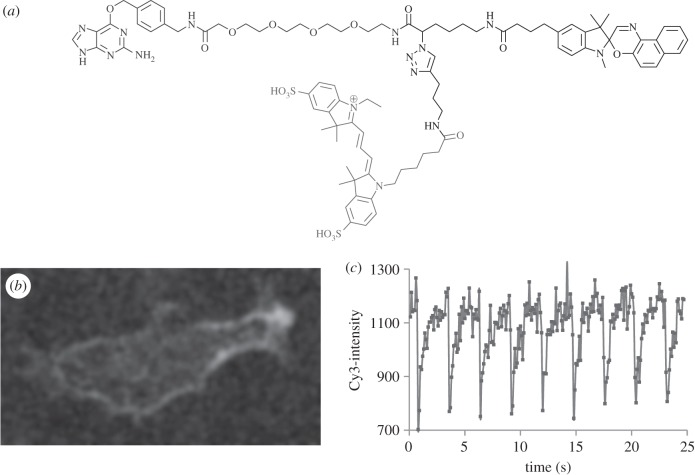
(d). Optical switching of cyanine fluorescence on the β2 adrenergic receptor
A Cy3-NISO-based suicide substrate is prepared for the Snap-tag (AGTase) [17,21], composed of a Cy3-NISO optical switch linked to BG, as shown in figure 4a. BG-Cy3-NISO is added to the culture medium of live NIH 3T3 cells expressing a SNAP-β2-adrenergic receptor (ADRB2) fusion protein [21]. The BG-Cy3-NISO acts as a suicide substrate and is specifically linked to the Snap-tag on the extracellular face of the cell membrane (figure 4b). An Olympus confocal microscope is used to manipulate the SP and MC states of the NISO probe and to image accompanying changes in the Cy3-fluorescence signal on labelled ADBR2 within living cells as detailed above for the Cy3-NISO probe. The profile of the Cy3-fluorescence intensity is identical within each of the eight cycles of optical switching (figure 4c), as are the minimum and maximum values of Cy3 fluorescence, and calculated FRET efficiencies. From an inspection of trace of the Cy3-fluorescence signal over the eight cycles of optical switching, one must conclude that there is little to no bleaching or fatigue over the course of the optical switching study.
Figure 4.
(a) Molecular structure of the Cy3-NISO-BG substrate used to label the Snap-tag. (b) A single image frame from a movie showing optical switching of Cy3 fluorescence in Cy3-NISO-BG-labelled SNAP-ADRB2 transfected in living NIH 3T3 cells. (c) The intensity profile of Cy3-fluorescence intensity on Cy3-NISO-labelled ADRB2 at the cell surface of a living cell over 10 cycles of optical switching.
(e). Optical lock-in detection-FRET immunofluorescence microscopy
The purpose of the following study is to use photochromic FRET between Cy3 and NISO and OLID to develop a new and general approach to improve image contrast in immunofluorescence microscopy (OLID-IF). A schematic of optical switching of the SP and MC states of NISO and Cy3 fluorescence within antibodies co-labelled with Cy3 and NISO is shown in figure 5a. Optical control of the SP and MC states of NISO in the antibody conjugate generates a modulated Cy3-fluorescence signal, which is subsequently isolated from background sources by using OLID [6,15]. OLID-IF is designed to work on any Cy3/NISO co-labelled primary or secondary antibody.
In the present application, OLID-IF is used to generate high-contrast imaging of the distribution of an anti-actin antibody within NIH 3T3 cells. The anti-actin antibody is co-labelled with Cy3 and NISO, as schematized in figure 5a—absorption spectral analysis is used to show that each antibody is labelled with an average of two molecules each of Cy3 and NISO. Optical switching of the Cy3 fluorescence on the antibody is realized by exposing the field of view to a defined sequence of 365 and 543 nm light within an Olympus confocal microscope [15], in order to manipulate the SP and MC levels on the antibody, with concomitant modulation of FRET and the Cy3 (donor) fluorescence signal (figure 5a). The efficiency of FRET in the antibody conjugate of approximately 14 per cent is less than that measured in the Cy-NISO probe (greater than 50%), which is expected based on the greater separation of the Cy3 and MC groups on the antibody.
The Cy3-fluorescence intensity image of the stained cell shown in figure 5c has low contrast because of a dominating background in the peri-nuclear region. While some actin filaments and stress fibres are apparent in the intensity image (figure 5c), actual details of their shape, length and fine structure are obscured by background signals. Optical switching of the Cy-fluorescence signal on the anti-actin antibody in the field of view is brought about following exposure to a 100 ms pulse at 365 nm to form the MC state while scanning the field at 543 nm at a rate of 1 scan/s, which brings about the MC to SP transition relieving the FRET condition. The intensity of the Cy3-fluorescence signal decreases by 13±0.1% immediately following the first and subsequent exposures of the field to 365 nm, and returns to approximately 3 per cent of the pre-365 nm level after 15 scans of the field at 543 nm (figure 5b). The normalized Cy3-intensity profile within each of the 5 cycles of optical switching of the preparation is identical (figure 5b), an indication that transitions between the SP and MC states of the NISO probe on the antibody are uniform and proceed via defined quantum yields [6]. The approximately 3 per cent decrease in the maximum intensity of red fluorescence for consecutive cycles of optical switching is likely due to photobleaching of Cy3, or the background, and not the NISO probe. This conclusion is supported by the finding that the FRET efficiency is constant over the five cycles of optical switching (13±0.1%) and the fact that photobleaching or fatigue of NISO would decrease FRET efficiency, owing to progressive loss of the MC acceptor.
We next compared the Cy3-fluorescence intensity image of the labelled NIH 3T3 cells recorded immediately prior to the first 365 nm exposure with the image of correlation coefficients (figure 5c,d, respectively). The correlation image (figure 5d) is completely devoid of the diffuse background that compromises the quality of the intensity image (figure 5c), especially in the per-nuclear region. Actin filaments are sharper and better-defined in the correlation image, revealing new details of their organization and fine details that are obscured in the intensity image (figure 5c). Another interesting feature of the correlation image is that surface-bound spotty regions that surround the cell body have high correlation values [23]. These spotty regions are most likely the actin-containing remnants of cell-substrate contacts that are barely visible in the intensity image as their intensity is close to the background level. This latter example nicely demonstrates how the OLID technique and images of the correlation coefficient can be used to improve image quality by amplifying modulated, ‘AC-like’, signals from the optical switch, even in cases where the signal is lower than the ‘DC’ background.
Thus, OLID-FRET is introduced as a new high-contrast imaging modality for immunofluorescence microscopy that is especially effective in resolving labelled antibodies whose fluorescence signal is similar to the background, a condition encountered in IF-based analysis of thick tissue samples. This feature may also be useful in imaging signals from rare antigens in cells or tissue.
4. Discussion
An ideal probe for OLID imaging of specifically labelled proteins within living cells is one that undergoes many cycles of orthogonal, rapid and reversible, optically driven transitions between the two states of the switch with high fidelity and over many cycles of optical switching [6,15]. For some types of reversible optical switch, typified by NitroBIPS, diarylethanes and Dronpa, the excited state of the coloured state of the switch can return to the same ground state with emission of fluorescence, or else isomerize to the non-coloured state of the switch [16,20,24–26]. It follows then that an optical switch probe having a quantum yield for fluorescence emission must be a poor switch, and vice versa [6]. We have previously shown that the fluorescence and switching functions of a probe can be uncoupled by imparting a switchable character on a conventional probe using a switchable acceptor probe via a FRET-based mechanism [18,19]. This concept is further optimized in this study by using probes harbouring a cyanine-derived fluorophore (as the donor probe) and NISO (as a photochromic FRET acceptor probe). The results presented in this study show that the combined spectroscopic, photochemical and fluorescence properties of the functionalized Cy-NISO and Cy3-NISO probes are much improved compared with other small molecule photochromes [3,27] and are more suitable for OLID-based high-contrast imaging of protein distribution within fixed and living cells.
Cyanine dyes have high fluorescence quantum yields and are bright and photostable. They also have a distinct advantage over TMR as the donor probe in photochromic FRET owing to the fact that they are transparent in the blue–near ultraviolet region of the spectrum. Consequently, unlike the TMR-NISO class of OLID-FRET probe [19], exposure of Cy-NISO to 405 nm, which brings about the SP to MC transition in NISO, does not excite the Cy probe. This feature increases the stability of the Cy-NISO probe compared with TMR-NISO and allows for efficient two-photon excitation-based control of the Cy fluorescence in the molecule. Optical modulation of FRET between Cy/Cy3 and the NISO optical switch occurs with high fidelity in hybrid probes as well as for protein conjugates individually labelled with Cy/Cy3 and NISO, albeit with lower modulation of the donor fluorescence signal.
The Cy-NISO and Cy3-NISO probes undergo robust, reversible, high-fidelity transitions between the SP and MC states of the NISO, with concomitant changes in the efficiency of FRET between the Cy/Cy3 and MC states. Multiple cycles of defined optical switching of the SP and MC states in these probes generate a highly modulated Cy/C3 fluorescence signal with little evidence of fatigue. Significantly, these probes allow for high-fidelity control of FRET, and the Cy/Cy3-fluorescence signal in living cells even when using low excitation powers (at 365–405 and approx. 550 nm) under standard conditions of cell culture and without the need for chemical additives. Orthogonal control of transitions between the SP and MC states can be brought about in the probe molecule by exciting the sample at 405 nm (SP to MC) and at 632 nm, which uniquely excites the MC state. Because the MC state has an absorption that extends to approximately 520 nm, the wavelength used to monitor Cy/Cy3 fluorescence can also bring about the MC to SP transition. This effect can be minimized by exciting the Cy probe at 532 nm where the MC absorption is low and by using low power compared with the 632 nm light source. Thus, by controlling the energy of different excitation sources (405, 532 and 632 nm), it is possible to control the shape of the Cy-fluorescence waveform in the sample. This feature is demonstrated in the present study by varying the power of a 546 nm light source from 2.5 to 50 per cent—many more excited-state MC probes are generated in a given exposure period at the higher power of 546 nm excitation, which accelerates the rate for the MC to SP transition. Our studies demonstrate that the Cy-NISO and Cy3 probes are the most optimized and useful of all small molecule optical switches reported in the literature as a result of the following improvements: (i) the action spectrum for the SP to MC transition that extends into the visible region of the spectrum (more than 400 nm); (ii) the Cy/Cy3 probe has the highest extinction coefficient of any donor probe in photochromic FRET probes; (iii) the NISO switch is robust and undergo multiple cycles of switching between the SP and MC states without significant fatigue; (iv) the absorption spectrum of the MC state of NISO is red-shifted to greater than 620 nm and allows for orthogonal optical control of FRET in the Cy/NISO probe; (v) the Cy-NISO and Cy3-NISO probes are easily coupled to specific reactive or protein targeting groups, as seen in the selective labelling of a Snap-tag fusion with the β2 adrenergic receptor in living cells. Interestingly, increasing the power of the 532–546 nm light source in the field of view increases the recovery of the Cy3-fluorescence signal owing to a relief of FRET (as seen in figure 3b,d,f), and at higher powers as those used in this study, we can expect that the rate of Cy3 photobleaching will also increase. In this regard, Cy-NISO functions more like a probe in the STORM technique. Significantly different, however, is the fact that the optical switching and eventual photobleaching of Cy probe occurs without the need for high levels of noxious additives (up to 100 mM mercaptoethanol) or removal of oxygen [5,10], and consequently should prove more suitable for live cell SR imaging [8,9,28–30].
Antibodies randomly co-labelled with an average of two molecules of Cy3 and NISO are also shown to undergo high-fidelity optical switching of their Cy3 fluorescence in response to multiple cycles of a defined sequence of low-power excitation with 365–405 nm and 546–632 nm. In this system, which can be generalized to any primary or secondary antibody, or used with related detection proteins, such as streptavidin Cy3/NISO, the average separation between the Cy3 and MC states on the antibody result in an approximate 13 per cent change in FRET between the SP and MC states of NISO. The red fluorescence signal decreases over five cycles of optical switching by approximately 3 per cent, primarily as a result of the photobleaching of background probes—the constant value of FRET efficiency (approx. 13%) measured in each of the five switching cycles in figure 5 strongly suggests that the NISO probe is highly resistant to fatigue. While it is possible that the Cy3 probe is also photobleached during the OLID study, the loss of donor probe will not affect the calculated FRET efficiency. The modulated Cy3-fluorescence signal on anti-actin labelled antibodies in the NIH 3T3 cell is subject to an OLID. Image contrast is significantly improved in the image of correlation coefficients compared with the raw Cy3-intensity image (figure 5c,d). This result is significant, as auto-fluorescence signals inherent or introduced to the preparation during chemical fixation of a cell or tissue represent a limiting factor in IF microscopy that can only be overcome by amplifying the signal associated with the primary antibody–antigen complex. This is usually achieved by using fluorescently labelled secondary antibodies, although there are limits to this approach as over-labelled antibodies may lead to non-specific binding in the sample. OLID analysis of the modulated Cy3 signal in the sample (OLID-IF) is very effective in isolating the modulated Cy3 component of the signal from the un-modulated background, or Cy3 probes that are not undergoing FRET. The high-contrast correlation coefficient image shown in figure 5d reveals features of the actin cytoskeleton that are within the range of the background signal in the intensity image (figure 5c). Because almost any primary or secondary antibody can be co-labelled with Cy3 and NISO, or equally well with amino or thiol reactive versions of the Cy-NISO and CY3-NISO probes, OLID can be applied to almost all types of IF microscopy, and may be adapted to a STORM-like platform for SR imaging.
Acknowledgements
This work was financially supported by grants from the NIH (PN2 EY018241 Nanomedicine Development Center for the optical control of biological function and R01 GM086233-01 to G.M. and GM086233-01 to Y.Y.)
References
- 1.Giepmans BNG, Adams SR, Ellisman MH, Tsien RY. 2006. The fluorescent toolbox for assessing protein location and function. Science 312, 217–224 10.1126/science.1124618 (doi:10.1126/science.1124618) [DOI] [PubMed] [Google Scholar]
- 2.Theriot JA, Mitchison TJ. 1991. Actin microfilament dynamics in locomoting cells. Nature 352, 126–131 10.1038/352126a0 (doi:10.1038/352126a0) [DOI] [PubMed] [Google Scholar]
- 3.Yan Y, Marriott G. 2003. Analysis of protein interactions using fluorescence technologies. Curr. Opin. Chem. Biol. 7, 1–6 10.1016/S1367-5931(03)00002-4 (doi:10.1016/S1367-5931(03)00002-4) [DOI] [PubMed] [Google Scholar]
- 4.Hell SW, Dyba M, Jakobs S. 2004. Concepts for nanoscale resolution in fluorescence microscopy. Curr. Opin. Neurobiol. 14, 599–609 10.1016/j.conb.2004.08.015 (doi:10.1016/j.conb.2004.08.015) [DOI] [PubMed] [Google Scholar]
- 5.Zhuang X. 2009. Nano-imaging with STORM. Nat. Photon. 3, 365–367 10.1038/nphoton.2009.101 (doi:10.1038/nphoton.2009.101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan Y, Marriott ME, Petchprayoon C, Marriott G. 2011. Optical switch probes and optical lock-in detection (OLID) imaging microscopy: high-contrast fluorescence imaging within living systems. Biochem. J. 433, 411–422 10.1042/BJ20100992 (doi:10.1042/BJ20100992) [DOI] [PubMed] [Google Scholar]
- 7.Hell SW, Wichmann J. 1994. Breaking the diffraction resolution limit by stimulated emission: stimulated emission depletion microscopy. Opt. Lett. 19, 780–782 10.1364/OL.19.000780 (doi:10.1364/OL.19.000780) [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann M, Eggeling C, Jakobs S, Hell SW. 2005. Breaking the diffraction barrier in fluorescence microscopy at low light intensities by using reversibly photoswitchable proteins. Proc. Natl Acad. Sci. USA 102, 17 565–17 569 10.1073/pnas.0506010102 (doi:10.1073/pnas.0506010102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. 2006. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 10.1126/science.1127344 (doi:10.1126/science.1127344) [DOI] [PubMed] [Google Scholar]
- 10.Rust MJ, Bates M, Zhuang X. 2006. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, 793–796 10.1038/nmeth929 (doi:10.1038/nmeth929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards CI, Hsiang JC, Dickson RM. 2010. Synchronously amplified fluorescence image recovery (SAFIRe). J. Phys. Chem. B 114, 660–665 10.1021/jp909167j (doi:10.1021/jp909167j) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lippincott-Schwartz J, Patterson GH. 2009. Photoactivatable fluorescent proteins for diffraction-limited and super-resolution imaging. Trends Cell Biol. 19, 555–565 10.1016/j.tcb.2009.09.003 (doi:10.1016/j.tcb.2009.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aubin JE. 1979. Autofluorescence of viable cultured mammalian cells. J. Histochem. Cytochem. 27, 36–43 10.1177/27.1.220325 (doi:10.1177/27.1.220325) [DOI] [PubMed] [Google Scholar]
- 14.Axelrod D. 2003. Total internal reflection fluorescence microscopy in cell biology. Methods Enzymol. 361, 1–33 10.1016/S0076-6879(03)61003-7 (doi:10.1016/S0076-6879(03)61003-7) [DOI] [PubMed] [Google Scholar]
- 15.Marriott G, Mao S, Sakata T, Jackson D, Gomez T, Aaron H, Isacoff EY, Yan Y. 2008. High contrast imaging based on optical lock-in detection imaging of synthetic and genetically encoded optical switches. Proc. Natl Acad. Sci. USA 46, 17 789–17 794 10.1073/pnas.0808882105 (doi:10.1073/pnas.0808882105) [DOI] [Google Scholar]
- 16.Song L, Jares-Erijman E, Jovin T. 2002. A photochromic acceptor as a reversible light-driven switch in fluorescence resonance energy transfer (FRET). J. Photochem. Photobiol. A 150, 177–185 10.1016/S1010-6030(02)00129-6 (doi:10.1016/S1010-6030(02)00129-6) [DOI] [Google Scholar]
- 17.Keppler A, Kindermann M, Gendreizig S, Pick H, Vogel H, Johnsson K. 2004. Labeling of fusion proteins of O6-alkylguanine-DNA alkyltransferase with small molecules in vivo and in vitro. Methods 32, 437–444 10.1016/j.ymeth.2003.10.007 (doi:10.1016/j.ymeth.2003.10.007) [DOI] [PubMed] [Google Scholar]
- 18.Sakata T, Yan Y, Marriott G. 2005. Optical switching of dipolar interactions on proteins. Proc. Natl Acad. Sci. USA 102, 4759–4764 10.1073/pnas.0405265102 (doi:10.1073/pnas.0405265102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petchprayoon C, Yan Y, Mao S, Marriott G. 2011. Rational design, synthesis, and characterization of highly fluorescent optical switches for high-contrast optical lock-in detection (OLID) imaging microscopy in living cells. Bioorg. Med. Chem. 19, 1030–1040 10.1016/j.bmc.2010.07.015 (doi:10.1016/j.bmc.2010.07.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakata T, Yan Y, Marriott G. 2005. A family of site selective optical switches. J. Organic Chem. 70, 2009–2013 10.1021/jo048207o (doi:10.1021/jo048207o) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao S, Benninger RKP, Yan Y, Petchprayoon C, Jackson D, Easley CJ, Piston DW, Marriott G. 2008. Optical lock-in detection of FRET using synthetic and genetically encoded optical switches. Biophys. J. 94, 4515–4524 10.1529/biophysj.107.124859 (doi:10.1529/biophysj.107.124859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn K. 2009. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 463, 104–108 10.1038/nature08241 (doi:10.1038/nature08241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choidas A, Jungbluth A, Sechi A, Ullrich A, Marriott G. 1998. The suitability and application of a GFP-actin fusion protein for long-term imaging of the organization and dynamics of the cytoskeleton in mammalian cells. Eur. J. Cell Biol. 77, 81–90 10.1016/S0171-9335(98)80075-7 (doi:10.1016/S0171-9335(98)80075-7) [DOI] [PubMed] [Google Scholar]
- 24.Kolmakov K, Belov VN, Bierwagen J, Ringemann C, Muller V, Eggeling C, Hell SW. 2010. Red-emitting rhodamine dyes for fluorescence microscopy and nanoscopy. Chem. Eur. J. 16, 158–166 10.1002/chem.200902309 (doi:10.1002/chem.200902309) [DOI] [PubMed] [Google Scholar]
- 25.Ando R, Flors C, Mizuno H, Hofkens J, Miyawaki A. 2007. Highlighted generation of fluorescence signals using simultaneous two-color irradiation on Dronpa mutants. Biophys. J. 92, 97–99 10.1529/biophysj.107.105882 (doi:10.1529/biophysj.107.105882) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stiel AC, et al. 2008. Generation of monomeric reversibly switchable red fluorescent proteins for far-field fluorescence nanoscopy. Biophys. J. 96, 2989–2997 10.1529/biophysj.108.130146 (doi:10.1529/biophysj.108.130146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Suarez M, Ting AY. 2008. Fluorescent probes for super-resolution imaging in living cells. Nat. Rev. Mol. Cell Biol. 9, 929–943 10.1038/nrm2531 (doi:10.1038/nrm2531) [DOI] [PubMed] [Google Scholar]
- 28.Hess ST, Gould TJ, Gudheti MV, Maas SA, Mills KD, Zimmerberg J. 2007. Dynamic clustered distribution of hemagglutinin resolved at 40 nm in living cell membranes discriminates between raft theories. Proc. Natl Acad. Sci. USA 104, 17 370–17 375 10.1073/pnas.0708066104 (doi:10.1073/pnas.0708066104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shroff H, Galbraith CG, Galbraith JA, Betzig E. 2008. Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nat. Methods 5, 417–423 10.1038/nmeth.1202 (doi:10.1038/nmeth.1202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones SA, Shim SH, He J, Zhuang X. 2011. Fast, three-dimensional super-resolution imaging of live cells. Nat. Methods 8, 499–508 10.1038/nmeth.1605 (doi:10.1038/nmeth.1605) [DOI] [PMC free article] [PubMed] [Google Scholar]